Summary
Long-term plasticity contributes to memory formation and sleep plays a critical role in memory consolidation. However, it is unclear whether sleep slow oscillation by itself induces long-term plasticity that contributes to memory retention. Using in vivo pre-thalamic electrical stimulation at 1 Hz which itself does not induce immediate potentiation of evoked responses, we investigated how the cortical evoked response was modulated by different states of vigilance. We found that somatosensory evoked potentials during wake were enhanced after a slow-wave sleep episode (with or without stimulation during sleep) as compared to a previous wake episode. In vitro, we determined that this enhancement has a postsynaptic mechanism that is calcium-dependent, requires hyperpolarization periods (slow waves), and requires a co-activation of both AMPA and NMDA receptors. Our results suggest that long-term potentiation occurs during slow-wave sleep supporting its contribution to memory.
Introduction
An early study demonstrated that the rate of forgetting is lower during sleep as compared to wakefulness (Jenkins and Dallenbach, 1924). Recent advances propose that a major role of sleep is memory consolidation (Diekelmann and Born, 2010; Maquet, 2001; Siegel, 2005). Both slow-wave sleep (SWS) and rapid eye movement (REM) sleep can contribute to the memory consolidation, but early stages of sleep (mainly SWS) increased procedural memory (Gais et al., 2000), pharmacological blockage of REM sleep did not impair procedural memory (Rasch et al., 2009). Boosting slow oscillation with extracranial fields (Marshall et al., 2006) or training-related increase in slow-wave activity (Huber et al., 2004) correlated with an increased memory retention suggesting that SWS is critical for memory formation. A plausible physiological mechanism of memory is synaptic plasticity (Bear, 1996; Hebb, 1949; Steriade and Timofeev, 2003). Ocular dominance experiments on young cats demonstrated that sleep plays crucial role in brain development (Frank et al., 2001). If indeed SWS induces synaptic plasticity, the signal processing before and after the SWS period should be different, however physiological data on SWS-dependent modulation of signal processing during waking that follows sleep are missing.
Intracellular activities of cortical neurons during wake and REM sleep are characterized by steady depolarization and firing, while during SWS the depolarization and firing alternates with hyperpolarization and silence (Chauvette et al., 2010; Steriade et al., 2001; Timofeev et al., 2001). Mimicking neuronal firing during SWS, continuous rhythmic stimulation or repeated trains of cortical stimuli in brain slices were shown to induce steady-state synaptic depression, but synaptic responses were enhanced after the trains of stimuli (Galarreta and Hestrin, 1998, 2000). The repeated grouped firing during SWS resembles the classical long-term potentiation (LTP) protocol (Bliss and Lomo, 1973). Both AMPA and NMDA receptors are subject to long-term plasticity (Kirkwood et al., 1993; Zamanillo et al., 1999) and these receptors are also responsible for the sleep-dependent memory formation (Gais et al., 2008). A classical neocortical mechanism of postsynaptic LTP depends on NMDA receptor activation, which leads to calcium entry and an activation of kinase cascade including CaMKII that phosphorylate AMPA receptor and leads to the insertion of GluR1-containing AMPA receptor into synapses (Lisman et al., 2012; Malinow and Malenka, 2002). Increased or reduced activity during wake affected the physiological responses during subsequent sleep (Huber et al., 2006; Huber et al., 2008). Here we used a reversed approach observing if sleep will affect responses in the subsequent wake period. We tested the hypothesis that SWS enhances synaptic efficacy via its unique pattern of activities, namely neuronal depolarization and firing (active or UP states) intermingled with hyperpolarizing periods (silent or down states), will induce long-term changes in synaptic efficacy.
Results
Evoked responses are potentiated after slow-wave sleep
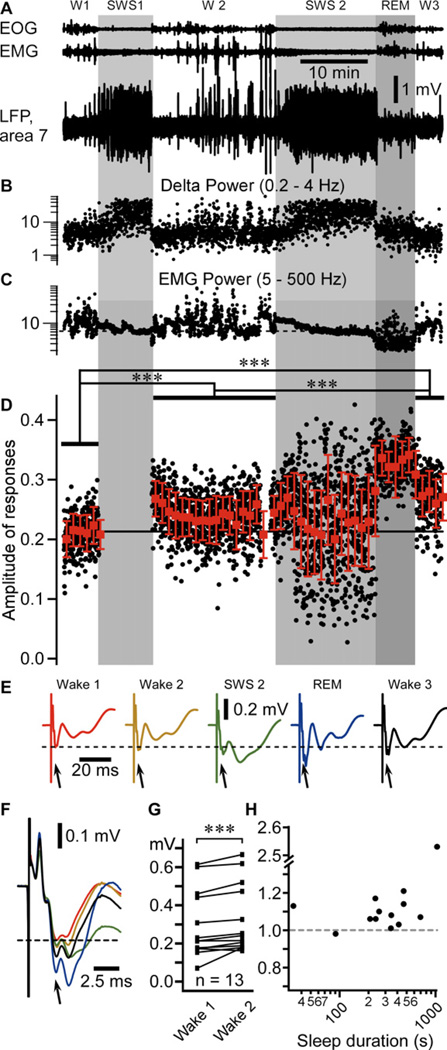
We recorded multiple electrographic signals from non-anesthetized head-restrained cats, including electro-oculogram (EOG), electromyogram (EMG), and local field potential (LFP) from different cortical areas (Figure 1A–C). States of vigilance were characterized as in our previous studies (Steriade et al., 2001; Timofeev et al., 2001). To study the effect of SWS on synaptic (network) plasticity, we used medial lemniscus stimulation (1Hz) and recorded the evoked potential responses in the somatosensory cortex during wake/sleep transitions (see Experimental procedures). In the example shown in figure 1, the mean amplitude of the N1 response was 0.213 ± 0.030 mV during the first wake episode (Figure 1D–F). As the first slow waves appeared in the LFPs, we stopped the stimulation for the whole first episode of SWS and restarted it as soon as the animal woke up (W2); the N1 response was transiently increased and then it was reduced, but it remained enhanced as compared to wake 1; the mean amplitude of N1 response was 0.241 ± 0.037 mV during the second wake episode (Figure 1D–F). Stimulations were applied in the following sleep episode, which was composed of SWS and REM sleep periods. The responses were highly variable during SWS (SWS2, 0.234 ± 0.073 mV) and showed the largest amplitude during REM sleep (0.330 ± 0.035 mV). The mean amplitude during the third wake episode was further increased (W3, 0.274 ± 0.039 mV) as compared to the first two wake episodes (Figure 1D–F). The amplitude of responses was significantly different in all waking periods (p<0.001 for all comparison, one way ANOVA Kruskal-Wallis with Dunn’s multiple comparison test). The SWS-dependent increase in evoked potential did not depend on whether stimulations occurred during SWS (Figure S1) or not (Figures 1, 2).
Figure 1. Amplitude of evoked potential responses (N1) to medial lemniscus stimuli throughout sleep-wake periods.
(A) Fragment of electro-oculogram (EOG), electromyogram (EMG), and local field potential (LFP) recorded in area 7 of a cat during sleep/wake transitions (W1–3 – Wake; SWS – Slow-wave sleep; REM – Rapid eye movement sleep. Light gray area depicts SWS episodes, darker gray area depicts a REM episode.
(B) Dots represent the delta power (area between 0.2 and 4 Hz in the Fast Fourier transform) of 1 second bins from the LFP segment shown in A.
(C) Dots represent the EMG power.
(D) Black dots represent individual evoked responses (N1) amplitude recorded in the LFP of somatosensory cortex (area 3) to the medial lemniscus stimuli (1Hz). Red squares are the running averages and the standard deviation for 60 responses (1 min). ***p<0.001, one way ANOVA Kruskal-Wallis with Dunn’s multiple comparison test.
(E) Averaged (area 3) evoked responses for each states of vigilance. The dotted line here and in panel F indicates the voltage of the mean response amplitude in wake 1, and the arrows point to the component of the response that was studied.
(F) Zoom-in and superimposition of averages presented in E (same color code).
(G) Group data from 13 “wake1-SWS-wake2” sequences, from 4 cats. Paired comparison of the mean response amplitude during wake1 and wake2 (pre-SWS and post-SWS) episodes. ***p<0.001, Wilcoxon matched-pairs signed rank test.
(H) Normalized (to wake 1 mean amplitude of evoked response) change in amplitude of response plotted against sleep duration.
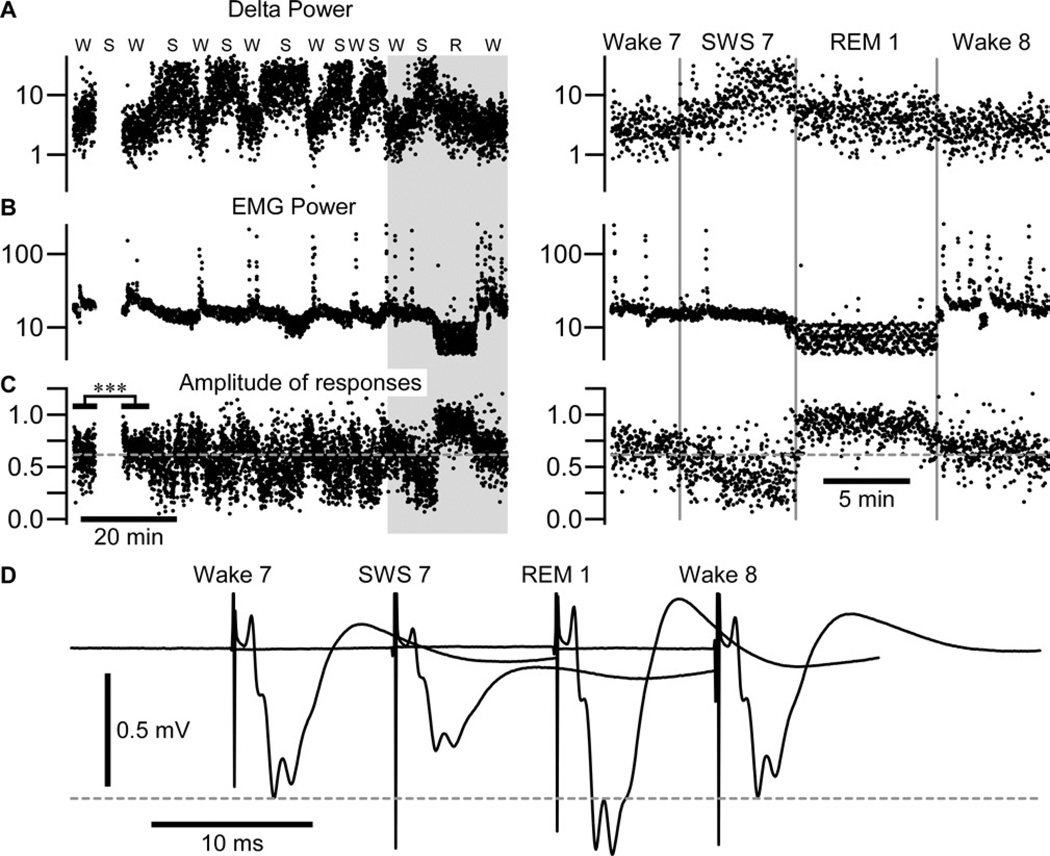
Figure 2. REM sleep does not potentiate somatosensory evoked potential in a following wake episode.
The delta power calculated from an area 7 local field potential (A) and EMG power (B) calculated around each medial lemniscal stimulus (±500 ms).
(C) The amplitude of somatosensory evoked potential. W – Wake, S – SWS, R – REM sleep. Note that no stimulation was delivered during the first slow-wave sleep episode and that responses were very significantly enhanced in the second wake episode as compared to the first wake episode; ***p<0.001, unpaired t-test with Welch’s correction. The right panel corresponds to the shaded area in (A, B, C) expanded. The amplitude of response was not enhanced after late REM sleep; p=0.7, unpaired t-test with Welch’s correction.
(D) Averaged response in each state of vigilance from wake 7 to wake 8 as indicated.
REM sleep does not play a significant role in the enhancement of response
On an experimental day, the increase always occurred between the first and the second period of wake and often between the second and the third period of wake. When the increased amplitude of evoked potential saturated after few SWS/wake transitions, the presence of REM sleep did not lead to further enhancement (Figure 2) as it appears in the figure 1D–F. In that example, responses were significantly enhanced after the first sleep episode (0.615 ± 0.144 mV in wake 1 vs. 0.666 ± 0.112 mV in wake 2, p<0.001, unpaired t-test with Welch’s correction) but responses were not further enhanced after the following sleep episodes (no statistical differences in consecutive waking state from wake 2 to wake 8, Figure 2C, p>0.05, one way ANOVA Kruskal-Wallis with Dunn’s multiple comparison test). However, the response during all waking periods remained significantly different from wake 1 (p<0.01, one-way ANOVA, Dunnett’s multiple comparison test). The overall mean amplitude for a first wake episode (n=13, from 3 different cats) was 0.297 ± 0.176 mV and it was 0.328 ± 0.177 mV during wake2; this difference was highly significant (Figure 1G, p<0.001, Wilcoxon matched-pairs signed rank test). Even relatively short periods of SWS were sufficient to enhance responses in the second wake episode (Figure 1H).
Intracellular responses during wake – slow-wave sleep – wake transitions
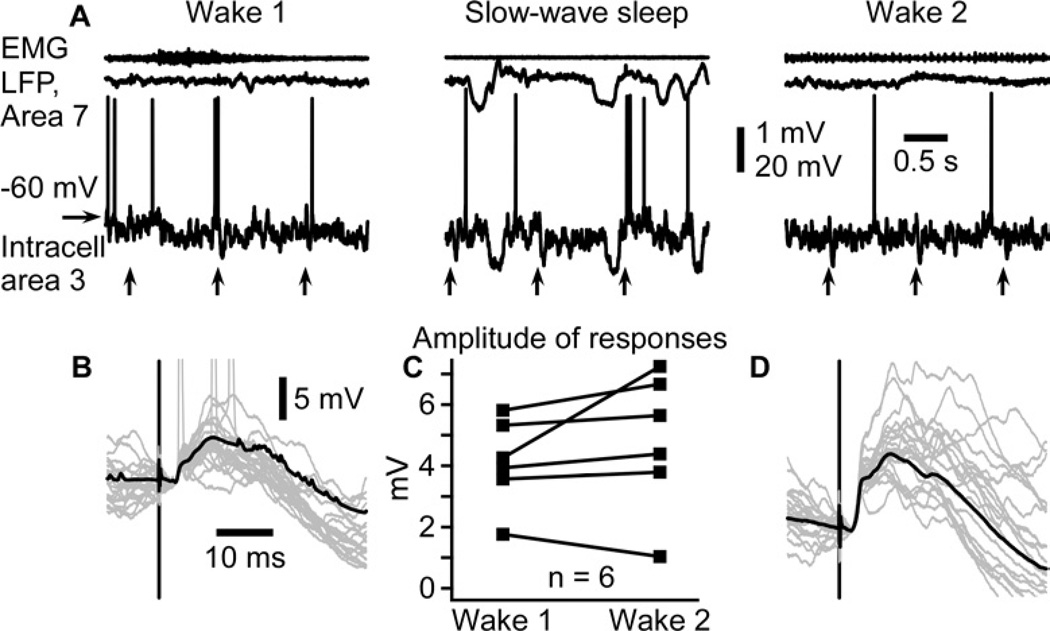
We obtained six intracellular recordings from somatosensory cortical neurons in which we recorded evoked responses to medial lemniscus stimuli during two consecutives wake episodes separated by a period of SWS, although it was not always the first and second wake episodes (Figure 3). As expected, membrane potential recordings showed the presence of prolonged silent states during SWS, which were absent during wake (Figure 3A). Responses were highly variable during SWS (not shown), while they were stable during wake (Figure 3B,D). In five out of the six intracellular recordings in which we were able to obtain stable recording throughout wake-SWS-wake transitions (85 recording sessions, near 200 wake-SWS-wake transitions, 2 animals), the response amplitude was increased in the second wake episode (post-SWS) as compared to the first episode (pre-SWS), however due to the small number of recordings, the difference was not significant (Figure 3C, p=0.2, Wilcoxon matched-pairs signed rank test). Therefore, intracellular recordings support field potential observations.
Figure 3. Intracellularly recorded evoked responses are enhanced after a period of slow-wave sleep.
(A) Electromyogram (EMG) from neck muscle, surface local field potential (LFP) from area 7, and intracellular recording from somatosensory cortex in consecutive states of vigilance as indicated. Vertical arrows indicate the time of medial lemniscus stimulation.
(B, D) Superimposition of 20 individual responses (gray traces) and the averaged response (black trace) during the first (B) and the second (D) episode of wake. Note that responses are ampler in the second episode of wake.
(C) Paired comparison of intracellular response amplitude of 6 neurons during two consecutive wake episodes separated by a slow-wave sleep episode. Each symbol represents the averaged response amplitude of one neuron during either wake 1 (left) or wake 2 (right). Lines indicate responses of the same neuron in two conditions.
In vitro, only the full sleep-like pattern of stimulation replicates the in vivo results
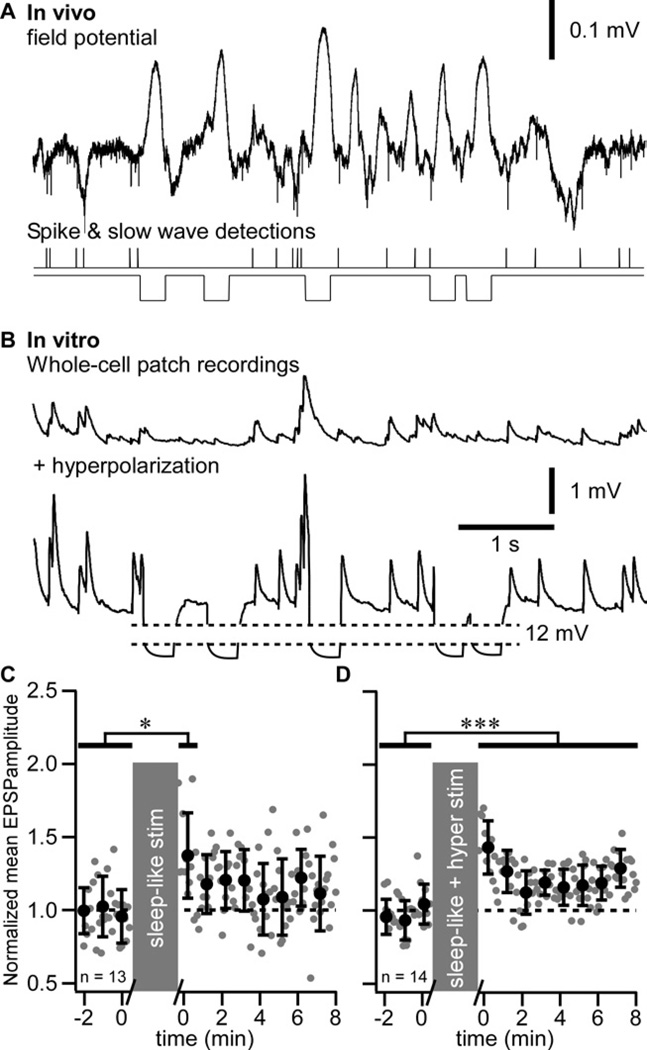
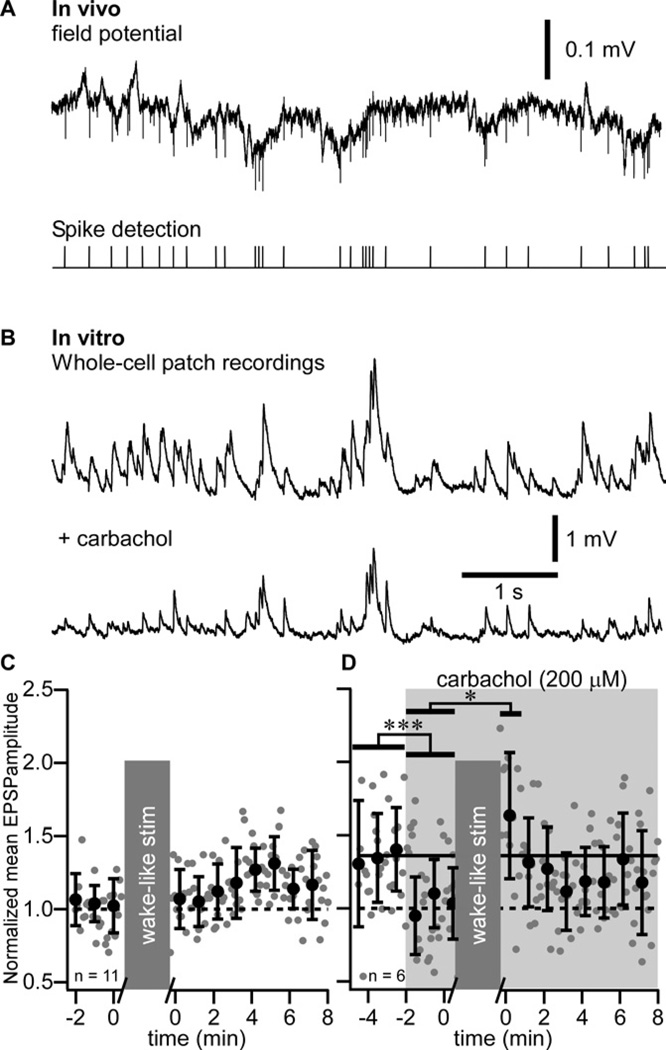
To characterize the mechanisms implicated in the potentiation of evoked responses, we performed whole-cell recordings from layer 2–3 pyramidal regular-spiking neurons in vitro. From in vivo LFP recordings, we extracted the timing of a single unit firing recorded during SWS (Figure 4A) and during wake (Figure 5A) and used that timing to build sleep-like (Figure 4B) and wake-like (Figure 5B) pattern of synaptic stimulation; the timing of slow waves was also detected to build the intracellular hyperpolarizing current pulses stimulation pattern replicating hyperpolarizing (silent) states of SWS (Figure 4B, see Experimental procedures). The stimulation protocols are detailed in (Figure S2). The mean membrane potential of neurons recorded in vitro was maintained to about −65 mV to mimic the membrane potential of cortical neurons during wake or active phases of SWS. Minimal intensity stimuli were applied in the vicinity of recorded neurons. The sleep-like pattern of synaptic stimulation induced a transient facilitation only ((Figure 4C) 0.640 ± 0.245 mV in control vs. 0.817 ± 144 mV in the first minute after conditioning, p<0.05, Mann-Whitney test). A long-lasting facilitation of responses was induced using a combination of sleep-like synaptic stimulation and intracellular hyperpolarizing current pulses with the timing of slow waves (full sleep-like pattern, (Figure 4D), 0.994 ± 0.527 mV in control vs. 1.184 ± 0.833 mV after conditioning, p<0.001, Mann-Whitney test). The pattern of facilitation was identical to the one observed after a period of SWS (compare Figure 4D with Figure 1D, wake2) suggesting similar process leading to this facilitation.
Figure 4. Slow-wave sleep pattern of synaptic stimulation combined to intracellular hyperpolarization pulses induces long-term potentiation in vitro.
(A) In vivo field potential recording during a SWS period of a cat. Extracellular spikes were detected and their timing was used for the synaptic stimulation pattern. Slow waves were also detected during the same time period and their timing was used for intracellular membrane potential hyperpolarization.
(B) In vitro recordings during sleep-like synaptic stimulation pattern (upper trace) and during the full sleep-like pattern of stimulation (synaptic + hyperpolarizing pulses, lower trace).
(C) Group data of normalized EPSP amplitude of in vitro whole-cell recordings in control and after sleep-like synaptic pattern of stimulation.
(D) Group data of normalized EPSP amplitude in control and after the full sleep-like pattern of stimulation. Gray dots are individual responses amplitude and black circles are the running averages and standard deviation for 12 consecutive responses (1 min). *p<0.05, ***p<0.001, Mann-Whitney test. Grey boxes indicate the 10 minutes stimulation protocol that was used.
Figure 5. Absence of long-term potentiation after wake pattern of stimulation in vitro.
(A) In vivo field potential recording during waking period of a cat. Extracellular spikes were detected and their timing was used as synaptic stimulation pattern.
(B) In vitro recordings during wake-like pattern of synaptic stimulation in control (Upper trace) and after adding 200 µM of carbachol (Lower trace).
(C) Group data of normalized EPSP amplitude of in vitro whole-cell recordings in control and after wake-like pattern of synaptic stimulation.
(D) Group data of normalized EPSP amplitude of in vitro whole-cell recordings in control and after wake-like pattern of synaptic stimulation in presence of carbachol (shaded area). Gray dots are individual responses amplitude and black circles are the running averages and standard deviation for 12 consecutive responses (1 min). *p<0.05, ***p<0.001, Mann-Whitney test. Grey boxes indicate the 10 minutes stimulation protocol that was used.
The wake-like synaptic stimulation pattern did not show any facilitation of evoked responses (Figure 5C). To model the neuromodulation activities present during waking state, we added the cholinergic agonist carbachol in the bath (200 µM), which in agreement with previous observations (Gil et al., 1997) significantly decreased the amplitude of responses in control conditions ((Figure 5D), 0.596 ± 0.361 mV vs. 0.454 ± 0.123 mV, p<0.001, Mann-Whitney test). After the wake-like synaptic stimulation pattern on the background of carbachol action, we observed only a transient enhancement of responses (0.679 ± 0.179 mV, p<0.05, Mann-Whitney test). These results demonstrated that a synaptic activation with the sleep-like pattern of spiking, accompanied with postsynaptic hyperpolarizations (full sleep-like protocol) corresponding to silent states of SWS was the only tested condition that induced LTP of evoked responses.
Mechanisms of the enhancement of responses during the full sleep-like stimulation
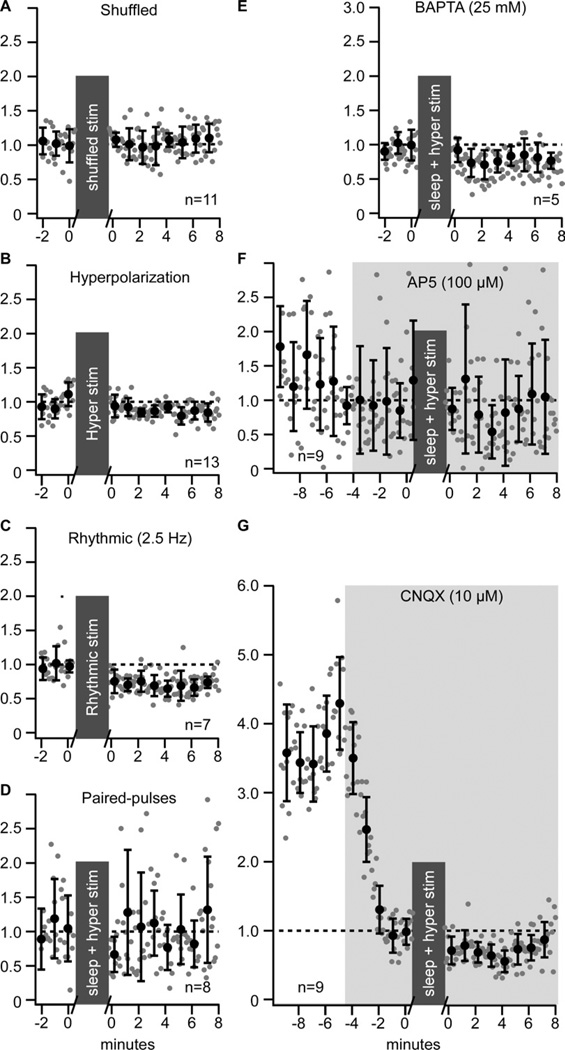
Shuffling the timing of synaptic stimulations from the sleep-like pattern, application of intracellular hyperpolarizing current pulses alone, or rhythmic (2.5 Hz) synaptic stimulations did not reveal any long-term plasticity (Figure 6A–C). The paired-pulse (ISI 50 ms) test showed (a) an enhancement of responses to stimuli after the full sleep-like protocol of stimulation (not shown), but (b) the paired-pulse ratio did not change (Fig. 6D). This combined with the fact that intracellular hyperpolarizing potentials were needed to induce LTP of evoked responses suggests that the enhancement was postsynaptic. Using the full sleep-like protocol of stimulation with BAPTA (25 mM) added to the patch solution to block calcium postsynaptic mechanisms abolished the enhancement of the response (Figure 6E). Adding the NMDA receptor antagonist AP5 (100 µM) or the AMPA receptor antagonist CNQX (10 µM) to the bath solution blocked the enhancement of response by either drugs suggesting that the investigated form of LTP requires a co-activation of both receptor types (Figure 6F,G). These results indicate that the mechanism of enhancement of responses during the full sleep-like stimulation is compatible with the classical LTP.
Figure 6. Properties of long-term plasticity induced by sleep pattern of stimulation.
(A) Group data of normalized EPSP amplitude of in vitro whole-cell recordings in control and after shuffled timing of sleep pattern of synaptic stimulation.
(B) Group data of normalized EPSP amplitude in control and after a period with the hyperpolarization pattern of stimulation only (no synaptic stimulation).
(C) Group data of normalized EPSP amplitude in control and after rhythmic pattern of synaptic stimulation (2.5 Hz).
(D) Paired-pulse (ISI 50 ms) test applied before and after the full sleep-like stimulation pattern (synaptic + hyperpolarization). Group data of normalized EPSP amplitude in control and after period with the full sleep-like stimulation pattern in presence of the calcium chelator BAPTA (25 mM) (E), the NMDA antagonist AP5 (100 µM) (F), or the AMPA antagonist CNQX (10 µM)
(G). For all panels, gray dots are individual responses amplitude and black circles are the running averages and standard deviation for 12 consecutive responses (1 min). Grey boxes indicate the 10 minutes stimulation protocol that was used.
Discussion
Our in vivo results showed that cortical evoked response to medial lemniscal stimuli during wake was enhanced in a subsequent wake episode whether stimuli were applied or not during SWS supporting the hypothesis of memory consolidation during SWS. Our in vitro results showed that only the full sleep-like pattern of stimulation (synaptic + hyperpolarization) mimicking SWS was able to induce the initial transient and the longer-lasting enhancement of responses. Importantly, in vitro results showed that this enhancement was postsynaptic, calcium-dependent, and required an activation of both NMDA and AMPA receptors matching the classical neocortical postsynaptic LTP.
The cortical slow oscillation has a frequency of about 1 Hz (Steriade et al., 1993). One Hz stimulation usually induces long-term depression in neocortex, but irregular pattern of low-frequency stimulation does not (Perrett et al., 2001). During the silent phase, neurons are hyperpolarized and no firing occurs. During the active phase, neurons are depolarized and multiple presynaptic spikes occur early after the onset of depolarization (Chauvette et al., 2010; Luczak et al., 2007). The network activities during sleep and the experimental protocol of the full sleep-like stimulation used in this study are compatible with protocols of induction of spike-timing dependent synaptic facilitation (Sjöström et al., 2008). The transition from hyperpolarized to depolarized states coupled with synaptic activities during active states is a natural pattern for spike-timing dependent plasticity. Therefore the presence of hyperpolarizing (silent) states appears to be a key component for the induction of LTP during sleep.
According to the sleep synaptic homeostasis hypothesis (Tononi and Cirelli, 2003, 2006), SWS results in a general synaptic downscaling because of a strong reduction in gene expression contributing to LTP (Cirelli et al., 2004; Cirelli and Tononi, 2000a, b). However, the total cortical level of kinase (CaMKII) does not change between sleep and waking state (Guzman-Marin et al., 2006; Vyazovskiy et al., 2008). Other studies have demonstrated that sleep-dependent memory consolidation requires the co-activation of both AMPA and NMDA receptors (Gais et al., 2008) and that sleep promotes LTP using a parallel involvement of protein kinase A, CaMKII, and ERK (Aton et al., 2009). Sleep also promotes the translation of mRNAs related to plasticity (Seibt et al., 2012). Classical LTP consists in a calcium entry via NMDA receptors that will activate different kinase cascades among which CaMKII would play a critical role by phosphorylating AMPA receptor. Once phosphorylated, GluR1-containing AMPA receptors are translocated to the synapse leading to LTP. Also, the translocation of AMPA receptors to the synapse (Lisman et al., 2012; Malinow and Malenka, 2002) that likely occurs during SWS does not require new gene expression. This indicates that synaptic potentiation leading to memory formation can occur during SWS despite a reduction in the expression of genes responsible for LTP.
Are there inconsistencies of our results with previous studies? (a) After prolonged waking periods the slope of callosal evoked responses increases (Vyazovskiy et al., 2008). The earliest phases of evoked potential responses induced by callosal stimulation is composed of antidromic spikes followed by orthodromic spikes (Chang, 1953). This indicates that waking period can increase the excitability of callosal axons. (b) The frequency and amplitude of mEPSCs is higher in slices collected from animals after prolonged waking as compared to sleep (Liu et al., 2010). This is likely due to a rebound over excitability in the absence of acetylcholine. Indeed, waking state is characterized by activities of cholinergic system and acetylcholine reduces the amplitude of EPSPs [(Gil et al., 1997) and Fig. 5D]. (c) In somatosensory cortical slices of juvenile rats, calcium-permeable AMPA receptors were shown to be present at the synapse when animals were sacrificed after the wake period and it was absent after the sleep period, (Lante et al., 2011). Obviously, not all type of AMPA receptors are removed from synapses during sleep, thus it does not preclude the insertion of other AMPA receptors types at synapses during sleep. A recent study in cats showed that intracortical inhibition of m-TOR signaling abolished sleep-dependent plasticity, while no effects were observed in the plasticity induced during wake (Seibt et al., 2012). Therefore, it is very likely that plasticity induced during wake or during sleep have different mechanisms.
The stimulation of medial lemniscal fibers is not a learning task per se, thus it is difficult to affirm whether this experiment simulates a declarative or a non-declarative learning task. However, most of procedural learning tasks implicate the somatosensory system and procedural memory was shown to benefit from SWS (Huber et al., 2004; Rasch et al., 2009), which is also in agreement with our results. The enhancement of responses was always present after the first SWS episode and often also after the second SWS episode, but then the response was saturated. Our results suggest that once potentiated the response cannot be further potentiated for a certain time window. This is in agreement with studies on humans showing that mainly early sleep and naps, rich in slow waves, are important for memory improvement (Gais et al., 2000; Mednick et al., 2003; Nishida and Walker, 2007). Our results show a potentiation of cortical responsiveness after a period of SWS and that an imitation of sleep slow oscillation in vitro was sufficient to strengthen the cortical synapses providing a physiological mechanism for sleep-dependent memory formation.
Experimental Procedures
Experiments were carried out in accordance with the guideline of the Canadian Council on Animal Care and approved by the Laval University Committee on Ethics and Animal Research.
In vivo experiments
Experiments were conducted on 4 adult non-anesthetized cats. The cats were purchased from an established animal breeding supplier. Good health conditions of all animals were certified by the supplier and determined upon arrival to animal house by physical examination, which was performed by animal facilities technicians and a veterinarian in accordance with requirements of the Canadian Council on Animal Care. The surgery was performed on animals in 5–20 days from their arrival to the local animal house. We recorded field potentials and intracellular activities of cortical neurons from somatosensory cortex of cats during natural sleep/wake transitions. We also recorded field potentials from other cortical areas.
Preparation
Chronic experiments were conducted using an approach similar to that previously described (Steriade et al., 2001; Timofeev et al., 2001). For implantation of recording chamber and electrodes, cats were anesthetized with isoflurane (0.75–2%). Prior to surgery, the animal was given a dose of pre-anesthetic, which was composed of ketamine (15 mg/kg), buprenorphine (0.01 mg/kg), and acepromazine (0.3 mg/kg). After site shaving and cat intubation for gaseous anesthesia, the site of incision was washed with at least three alternating passages of a 4% chlorexidine solution and 70% alcohol. Lidocaine (0.5 %) and/or marcaïne (0.5 %) was injected at the site of incision and at all pressure points. During surgery, electrodes for LFP recordings, electromyogram (EMG) from neck muscle, and electro-oculogram (EOG) were implanted and fixed with acrylic dental cement. Custom-made recording chambers were fixed over somatosensory cortex for future intracellular recordings. Eight to ten screws were fixed to the cranium. To allow future head-restrained recordings without any pressure point, four bolts were covered in the dental cement that also covered bone-fixed screws, permanently implanted electrodes, and fixed recording chamber. Throughout the surgery, the body temperature was maintained at 37°C using a water circulating thermo-regulated blanket. Hearth beat and oxygen saturation were continuously monitored using a pulse oximeter (Rad-8, MatVet, Montreal, Canada) and the level of anesthesia was adjusted to maintain a hearth beat at 110–120 per minute. A lactate ringer solution (10 ml/kg/h, i.v.) was given during the surgery. Following the surgery, cats were given buprenorphine (0.01 mg/kg) or anafen (2 mg/kg) twice a day for three days, and baytril (5 mg/kg) once a day for seven days. About a week was allowed to animals to recover from the surgery before the first recording session occurred. Usually, 2–3 days of training were sufficient for cats to remain in head-restrained position for 2–4 hours and display several periods of quiet wakefulness, SWS, and REM sleep. The recordings were performed up to 40 days after the surgery. In this study the LFP data were analyzed from the first recording session of the day only. Animals were kept awake for at least one hour prior to recording session.
Recordings and in vivo stimulation
All in vivo recordings were done in a Faraday chamber. LFPs were recorded using tungsten electrodes (2 MΩ, bandpass filter 0.1 Hz to 10 kHz) and amplified with AM 3000 amplifiers (A-M systems, Sequim, WA, USA) with custom modifications. We aimed to implant electrodes at 1 mm below the cortical surface. A coaxial electrode (FHC, Bowdoin, ME, USA) was implanted in the medial lemniscus fibers for stimulation. Electric stimuli were delivered at 1 Hz in all states of vigilance (wake, SWS, REM). Given the high spontaneous (≈5 Hz) and evoked firing rates (up to 125 Hz) in cuneatothalamic pathway (medial lemniscus) and the high efficacy of synaptic transmission in this pathway (Alloway et al., 1994), 1 Hz stimulation could not induce synaptic plasticity per se. Intracellular recordings were performed using glass micropipettes filled with 2.5 M of potassium acetate and having a resistance of 30–70 MΩ. A high-impedance amplifier with active bridge circuitry (Neurodata IR-283 amplifiers, Cygnus Technology, PA, USA, lowpass filter 10 kHz), was used to record the membrane potential and to inject current into neurons. Intracellular recordings were performed from somatosensory cortex according to the atlas (Reinoso-Suarez, 1961). A silver wire was fixed either in the frontal bone over the sinus cavity or in the occipital bone over the cerebellum and was used as a reference electrode. All electrical signals were digitally sampled at 20 kHz on Vision (Nicolet, Wisconsin, USA) and stored for off-line analysis. At the end of experiments, the cats were euthanized with a lethal dose of pentobarbital (100 mg/kg i.v.).
In vitro experiments
Experiments were conducted on 30 Sprague Dawley rats (P21–P30, Charles River Laboratories International, Inc.).
Slice preparation
Rats were first anesthetized with ketamine-xylazine (40 and 10 mg/kg). The brain was then quickly dissected and maintained in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 2.8, CaCl2 1.2, MgSO4 2, NaH2PO4 1.25, NaHCO3 26, and D-glucose 10 (Sigma-Aldrich Canada, Canada), pH 7.4, aerated with 95% O2 and 5% CO2. Osmolarity was 300 ± 5 mOsm. Coronal slices (350–400 µm) from one hemisphere were cut with a vibratome to obtain complete sections containing the somatosensory cortex. Slices were transferred to a holding chamber where they were kept at room temperature for at least 1h in the same ACSF and aerated with 95% O2, 5% CO2. The brain slices were transferred into a submerged recording chamber maintained at 34°C, containing the perfusion ACSF at a rate of 3 ml/min. The perfusion solution was identical to the cutting solution.
Recordings
Pyramidal neurons in layers II/III were preselected using an infrared differential interference contrast camera microscopy on an upright microscope based on their triangular shape and on their morphology after Lucifer Yellow 0.2% staining (LY, Sigma Aldrich Canada, Canada). We obtained somatic whole-cell current-clamp recordings (10–20 MΩ access resistances) with patch pipettes (resistance between 3–5 MOhm) containing (mM): Potassium D-gluconate 130, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10, KCl 10, MgCl2 2, ATP 2, and GTP 2 (Sigma-Aldrich Canada, Canada) at pH 7.2 and 280 mOsm. In separate experiments, BAPTA 25mM (Sigma-Aldrich Canada, Canada) was added to the patch solution to block calcium. AP5 (100 µM) or CNQX (10 µM) (both from Sigma-Aldrich Canada, Canada) were also added to the bath solution to block AMPA and NMDA receptors respectively, and carbachol (200 µM) (Sigma-Aldrich Canada, Canada) was also added to model cholinergic activities during wake. To model wake-like membrane potential values, a steady depolarizing current was injected to maintain the membrane potential near −65 mV.
Cortical slices stimulation
Two tungsten electrodes (1–2 MΩ) were placed in layers II/III for extracellular electrical stimulation. Pulses of 0.01−0.02 ms duration and of 0.01−0.15 mA intensity were delivered at a minimal intensity in order to obtain EPSPs and some failures. This intensity of stimulation reproduces the basic properties of single-axon EPSPs in vivo (Crochet et al., 2005). Minimal intensity stimuli were delivered every 5 sec in control and after conditioning, because that frequency of microstimulation does not induce synaptic plasticity in cortical slices (Seigneur and Timofeev, 2010).
LFP recordings during natural sleep and waking states were used to extract the timing of a unit firing during wake and during SWS (about 10 minutes for each state); the timing of onset of slow waves was also extracted from LFP recordings as described previously (Mukovski et al., 2007). To model silent states in patch-clamp recordings in vitro we applied hyperpolarizing current pulses of 200 ms [mean silent states during SWS (Chauvette et al., 2011)] starting at the exact timing estimated from in vivo LFP recordings. To isolate extracellular spikes the LFP was band-pass filtered (60 Hz - 10 kHz). We used only spikes from single unit recordings. As the unit was well isolated and that the spike amplitude was well above the noise level, a threshold was manually set to detect the timing of spikes. An example of such detection can be found in our previous publication (Chauvette et al., 2011). We used the exact timing of spikes detected in vivo to microstimulate electrically cortical slices. Binary files used for stimulation were generated and run in Clampex software (Axon pClamp 9, Molecular Devices, USA) to trigger the stimulators for wake-like, sleep-like and full sleep-like stimulation pattern applied in vitro (Figure S2). Obviously, no stimuli were delivered during hyperpolarizing states. The sleep-like, full sleep-like, or wake-like stimulation sessions lasted for about 10 min.
To test whether a specific pattern of sleep-like stimuli was needed to induce LTP we either shuffled the timing of interstimuli intervals using “Randbetween” function from Microsoft Office Excel (Shuffled test [Fig. 6A]) or we stimulated at 2.5 Hz continuously for 10 minutes to deliver the same number of stimuli as in the sleep-like protocol (Rhythmic test [Fig. 6C]). To test alterations in presynaptic release probability, the paired-pulse protocol (50 ms interstimuli interval) was used prior and after the full sleep-like stimulation (Fig. 6D).
Data analysis
Electrographic recordings were analyzed offline using custom-written routines in IgorPro (Lake Oswego, Oregon, USA). The delta power was calculated from 1 sec sliding time window as the integral power between 0.2 and 4 Hz of full spectrogram; the EMG power was calculated as the integral power between 5 and 500 Hz.
Statistical analysis
All numerical values are given as mean ± standard deviation. Specific statistical tests are indicated in the text and in figures legend and were performed in Prism5 (Graphpad software Inc., La Jolla, CA, USA). Briefly, data was first tested for normal distribution and if data had a normal distribution, parametric tests were used otherwise; the equivalent non-parametric test was used. If only two groups of data were compared, two-tailed unpaired t-test with Welch’s correction (parametric) or two-tailed Mann-Whitney test (non-parametric) was used. When the data was paired, then two-tailed Wilcoxon matched-pairs signed rank test (non-parametric) was applied. When more than two groups of data were compared, one way ANOVA Kruskal-Wallis (non-parametric) with Dunn’s multiple comparison test was applied.
Supplementary Material
Acknowledgments
We thank Sergiu Ftomov for his excellent technical support. This study was supported by CHIR, NIH, NSERC, and FRSQ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
S.C. and I.T. performed in vivo experiments and the related analysis; J.S. performed in vitro experiments and the related analysis. S.C. and J.S. share co-first authorship. S.C., J.S., and I.T. designed the experiments and contributed to writing the manuscript.
Competing financial interest
The authors declare no competing financial interests.
References
- Alloway KD, Wallace MB, Johnson MJ. Cross-correlation analysis of cuneothalamic interactions in the rat somatosensory system: influence of receptive field topography and comparisons with thalamocortical interactions. J Neurophysiol. 1994;72:1949–1972. doi: 10.1152/jn.1994.72.4.1949. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of Sleep-Dependent Consolidation of Cortical Plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT. Cortical response to activity of callosal neurons. J Neurophysiol. 1953;16:117–131. doi: 10.1152/jn.1953.16.2.117. [DOI] [PubMed] [Google Scholar]
- Chauvette S, Crochet S, Volgushev M, Timofeev I. Properties of Slow Oscillation during Slow-Wave Sleep and Anesthesia in Cats. J Neurosci. 2011;31:14998–15008. doi: 10.1523/JNEUROSCI.2339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex. 2010;20:2660–2674. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000a;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000b;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- Crochet S, Chauvette S, Boucetta S, Timofeev I. Modulation of synaptic transmission in neocortex by network activities. Eur J Neurosci. 2005;21:1030–1044. doi: 10.1111/j.1460-9568.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Gais S, Rasch B, Wagner U, Born J. Visual-procedural memory consolidation during sleep blocked by glutamatergic receptor antagonists. J Neurosci. 2008;28:5513–5518. doi: 10.1523/JNEUROSCI.5374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Burst firing induces a rebound of synaptic strength at unitary neocortical synapses. J Neurophysiol. 2000;83:621–624. doi: 10.1152/jn.2000.83.1.621. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol (Lond) 2006;575:807–819. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. Organization of behavior. New York: Wiley; 1949. [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huber R, Maatta S, Esser SK, Sarasso S, Ferrarelli F, Watson A, Ferreri F, Peterson MJ, Tononi G. Measures of Cortical Plasticity after Transcranial Paired Associative Stimulation Predict Changes in Electroencephalogram Slow-Wave Activity during Subsequent Sleep. J Neurosci. 2008;28:7911–7918. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JG, Dallenbach KM. Obliviscence during Sleep and Waking. Am J Psychol. 1924;35:605–612. [Google Scholar]
- Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- Lante F, Toledo-Salas JC, Ondrejcak T, Rowan MJ, Ulrich D. Removal of synaptic Ca(2)+permeable AMPA receptors during sleep. J Neurosci. 2011;31:3953–3961. doi: 10.1523/JNEUROSCI.3210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z-W, Faraguna U, Cirelli C, Tononi G, Gao X-B. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci U S A. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Mukovski M, Chauvette S, Timofeev I, Volgushev M. Detection of active and silent states in neocortical neurons from the field potential signal during slow-wave sleep. Cereb Cortex. 2007;17:400–414. doi: 10.1093/cercor/bhj157. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PloS one. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Dudek SM, Eagleman D, Montague PR, Friedlander MJ. LTD induction in adult visual cortex: role of stimulus timing and inhibition. J Neurosci. 2001;21:2308–2319. doi: 10.1523/JNEUROSCI.21-07-02308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci. 2009;12:396–397. doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- Reinoso-Suarez F. Topographischer Hirnatlas der Katze, fur Experimental-Physiologische Untersuchungen. Darmstadt: E. Merck; 1961. [Google Scholar]
- Seibt J, Dumoulin MC, Aton SJ, Coleman T, Watson A, Naidoo N, Frank MG. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneur J, Timofeev I. Synaptic impairment induced by paroxysmal ionic conditions in neocortex. Epilepsia. 2010;52:132–139. doi: 10.1111/j.1528-1167.2010.02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Rancz EA, Roth A, Häusser M. Dendritic Excitability and Synaptic Plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo : depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–576. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: An intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.