Abstract
Aberrant mitochondrial function, morphology, and transport are main features of neurodegenerative diseases. To date, mitochondrial transport within neurons is thought to rely mainly on microtubules, whereas actin might mediate short-range movements and mitochondrial anchoring. Here, we analyzed the impact of actin on neuronal mitochondrial size and localization. F-actin enhanced mitochondrial size and mitochondrial number in neurites and growth cones. In contrast, raising G-actin resulted in mitochondrial fragmentation and decreased mitochondrial abundance. Cellular F-actin/G-actin levels also regulate serum response factor (SRF)-mediated gene regulation, suggesting a possible link between SRF and mitochondrial dynamics. Indeed, SRF-deficient neurons display neurodegenerative hallmarks of mitochondria, including disrupted morphology, fragmentation, and impaired mitochondrial motility, as well as ATP energy metabolism. Conversely, constitutively active SRF-VP16 induced formation of mitochondrial networks and rescued huntingtin (HTT)-impaired mitochondrial dynamics. Finally, SRF and actin dynamics are connected via the actin severing protein cofilin and its slingshot phosphatase to modulate neuronal mitochondrial dynamics. In summary, our data suggest that the SRF-cofilin-actin signaling axis modulates neuronal mitochondrial function.
Keywords: neurodegeneration, neuronal gene expression
Mitochondria are dynamic organelles going through phases of morphological reorganization and subcellular relocalization (1). Mitochondria constantly reshape morphology by fusion and fission processes. This involves molecules such as mitofusins, optic atrophy 1 (OPA1) and dynamin-related protein 1, respectively (1, 2). Mitochondrial fragmentation in neurites and accumulation in the cell body are hallmarks of many neurodegenerative diseases, including ALS, Huntington disease, and Parkinson disease (3).
Many aspects of neuronal mitochondrial dynamics might be connected to the differential distribution and dynamics of cytoskeletal elements. F-actin is enriched in growth cones and spines, whereas microtubules are rather ubiquitously localized. In neurons, mitochondrial transport is largely accomplished through microtubule-assisted long-range movements (4). So far, there are only a few reports on actin function in neuronal mitochondrial transport and morphology. In general, actin is considered to mediate short-range mitochondrial movements and anchoring within growth cones and synaptic terminals (5–8). Indeed, following F-actin depolymerization, mitochondria fail to accumulate at areas of localized NGF signaling (5). Pharmacological interference with actin and microtubule function in neurons revealed that mitochondrial transport depends on microtubules and only to some extent on microfilaments (9, 10). In contrast to mitochondrial transport, in neurons, there are few data available on the role of the actin cytoskeleton on mitochondrial morphological alterations and localization.
In addition to actin’s role as a cytoskeletal scaffold, actin dynamics regulate activity of the transcription factor serum response factor (SRF). F-actin polymerization and depolymerization increase and reduce SRF activity, respectively (11, 12). SRF activity is not only adjusted by actin dynamics, but SRF, in turn, modulates actin by adjusting mRNA levels of actin isoforms (Acta, Actb, and Actc) and actin binding proteins (ABPs), such as myosins, tropomyosins, gelsolin, and actinin (11, 12). The latter is accomplished through SRF cofactors of the myocardin-related transcription factor (MRTF) family (11, 12). In addition, phosphorylation of the actin-severing protein cofilin is enhanced, and activity is thereby blocked in SRF-deficient neurons. This suggests that SRF/MRTF normally operates as cofilin activator (13, 14). Cofilin activity is inhibited by LIM domain containing kinase (LIM) phosphorylation and elevated by slingshot phosphatases (15). So far, nuclear SRF/MRTF signaling has been connected with cytoplasmic cofilin activity via a Pctaire1/Cdk5-Pak1-LIM kinase pathway resulting in cofilin activation (14). Nevertheless, it remains to be demonstrated whether cofilin activity alone is sufficient to rescue SRF-associated neuronal phenotypes, an important aspect considered in this study. SRF’s impact on actin dynamics is highlighted in conditional Srf KO neurons. Here, actin-dependent processes, such as neurite outgrowth, growth cone shape, and branch formation, are impaired (16). Similar phenotypes are observed in cofilin-deficient neurons (17).
In this study, we investigated the SRF-cofilin-actin regulatory unit in neuronal mitochondrial morphology and subcellular localization. In Srf mutants, axons displayed abnormal mitochondrial morphology with balloon-like inclusions, mitochondrial fragmentation, altered subcellular localization, and ATP metabolism. Thus, SRF deficiency resulted in mitochondrial structural and bioenergetic changes similar to those observed in neurodegenerative diseases, such as Huntington disease. Conversely, constitutively active SRF-VP16 stimulated formation of mitochondrial networks and alleviated huntingtin (HTT)-impaired mitochondrial dynamics. Using actin point mutations, we report a function of actin dynamics in regulation of mitochondrial size, neurite occupancy, and growth cone abundance. Further, cofilin and its activator, the slingshot phosphatase, were identified as regulators of mitochondrial morphology and distribution. Finally, we show that actin-cofilin interactions and SRF are interdependent in regulating mitochondrial dynamics and neuron function.
Results
Altered Mitochondrial Morphology, Distribution, and Activity in SRF-Deficient Neurons.
We used reported conditional neuronal Srf mouse mutants to investigate mitochondrial morphology and function in vivo (13, 18–20). In this mouse model, Cre recombinase expression is driven by the neuron-specific Camk2a promoter. This allows for SRF depletion in neurons but not in glial cells in forebrain regions, including cortex, striatum, and hippocampus. These SRF-deficient mice die at 3 wk of age. At this time point, no overall axonal loss but severe demyelination of axons is observed (21). Because mitochondrial dysfunction and axonal demyelination are connected (22), we investigated mitochondrial morphology and function in SRF-deficient mice.
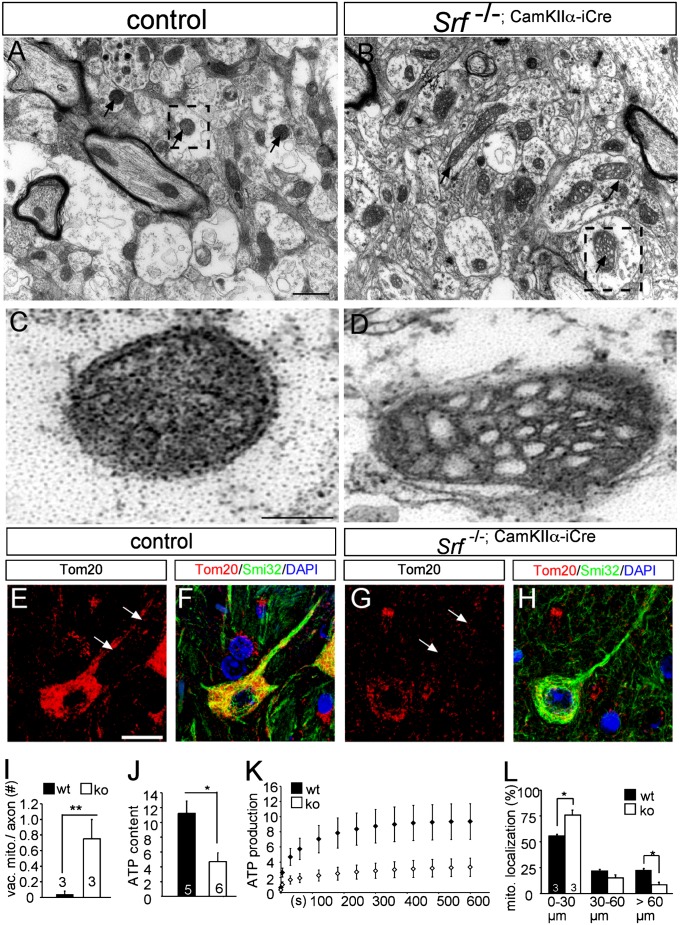
In an ultrastructural analysis of postnatal day (P) 14 SRF-deficient mice, alterations in mitochondrial morphology in callosal axons were observed (Fig. 1). In axons of WT neurons, mitochondria were round in shape and electron-dense, indicating dense packing of cristae [Fig. 1A (arrow) and C]. In contrast, axons of Srf mutant neurons had abnormal mitochondria with bottle-shaped morphologies and increased vacuolization [Fig. 1B (arrow) and D, quantified in Fig. 1I]. The size of mitochondria in Srf mutant neurons was slightly but not significantly reduced (WT: average diameter = 0.16 μm, KO: average diameter = 0.13 μm).
Fig. 1.
Altered mitochondrial morphology, distribution, and activity in SRF-deficient neurons. (A and B) Mitochondria (arrows) in P14 corpus callosal axons of WT neurons are electron-dense. (C) Higher magnification reveals a double membrane of a mitochondrion indicated by the dashed area in A. (B) In contrast, in axons of SRF-deficient neurons, mitochondria are vacuolized and frequently bottle-shaped (higher magnification flipped 90° in D). WT (E and F) and Srf mutant (G and H) P14 cortices were labeled for mitochondria (Tom20, red) and neurites (Smi32, green). In WT neurons, mitochondria were present in neurites (arrows in E), whereas abundance was reduced in SRF-deficient neurons (arrows in G). (I) Number of vacuolized mitochondria (vac. mito.) in an axon cross-section is depicted. ko, knockout; wt, wild type. (J) Basal ATP content in mitochondria of P14 Srf mutant brains is reduced compared with WT. (K) ATP production rate is decreased in mitochondria derived from SRF-deficient hippocampi compared with WT. (L) Percentage of mitochondria found in neurite segments ranging from 0 to ≥60 μm. In neurite parts >60 μm away from the cell body, mitochondria were reduced in Srf mutants compared with WT. Numbers in bars represent numbers of independent animals. (Scale bar: A–D, 1 μm; E–H, 10 μm.)
In addition, in P14 WT mice, Tom20-positive mitochondria were present in the cell body and neurites [Fig. 1E (arrows)] of cortical neurons in vivo (Fig. 1 E, F, and L). Conversely, inspection of SRF-deficient neurons revealed mitochondrial accumulation in the cell body and decreased abundance in the neurite [Fig. 1 G (arrow), H, and L]. The overall abundance of mitochondria and other cell organelles in total brain lysates of SRF-deficient mice was not obviously changed (Fig. S1). However, when comparing individual neurons, Tom20 abundance was decreased in Srf mutant compared with WT neurons (compare Fig. 1 G and E).
Altered mitochondrial shape frequently correlates with the respiratory capability of mitochondria (3). Consistently, the basal ATP content (Fig. 1J) as well as new ATP production (Fig. 1K) of purified mitochondrial fractions derived from SRF-deficient brains was reduced. Of note, because these brain mitochondrial fractions contain mitochondria from both SRF-depleted neurons and “wild-type” glial cells, SRF’s impact on ATP metabolism might actually be underestimated in these experiments (Fig. 1 J and K).
In summary, SRF deficiency impairs mitochondrial morphology and energy production reminiscent of neurodegenerative disorders (23–26).
SRF Deficiency Modulates Mitochondrial Morphology and Distribution in Vitro.
To analyze mechanisms by which SRF modulates mitochondrial dynamics, we used primary mouse hippocampal neurons (Fig. 2). Mitochondria were visualized with a pDSRed2Mito vector harboring a cytochrome C mitochondrial import sequence.
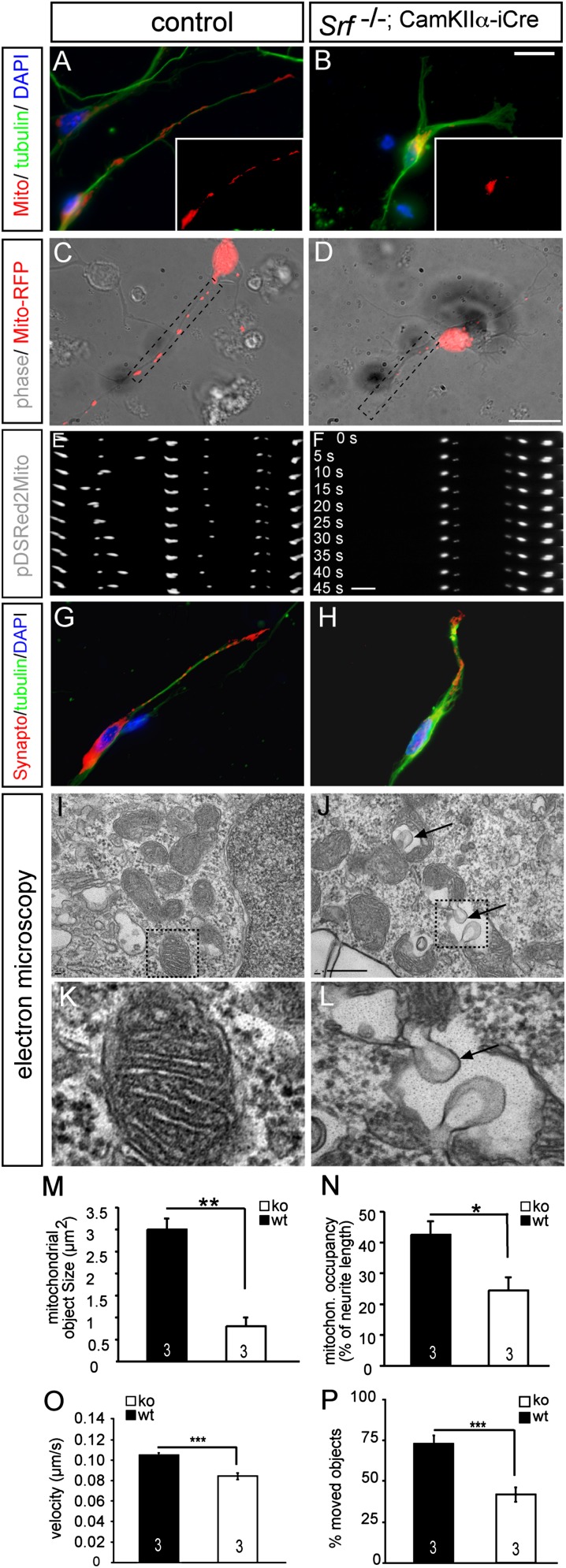
Fig. 2.
SRF deficiency modulates mitochondrial size, localization, and shape in vitro. (A and B) Primary mouse hippocampal neurons express pDSRed2Mito, allowing for mitochondrial visualization (red, Insets). (A) In WT neurons, mitochondria of variable size were present throughout the neurite. (B) In neurons lacking SRF, mitochondria accumulated in the cell body and only rarely entered neurites. (Insets) pDSRed2Mito signal of the entire neuron is shown. A WT neuron (C) and Srf mutant neuron (D) expressing pDSRed2Mito were used for time-lapse videomicroscopy. (E and F) Dashed areas show the position of individual frames. (E) In WT neurons, mitochondria move between frames taken every 5 s. (F) In contrast, on SRF ablation, almost no mitochondrial movement was observed. Size and distribution of synaptophysin were comparable between WT (G) and mutant (H) neurons. EM pictures of WT (I and K) and Srf mutant (J and L) hippocampal neurons in culture. (I and K) In WT neurons, double membranes and cristae structures are discernible. In Srf mutant neurons, balloon-shaped inclusions were observed (arrows in J and L). K and L are higher magnifications of dashed areas in I and J. (M) Quantification of mitochondrial object size in WT (wt) and SRF-deficient neurons. ko, knockout. (N) Percentage of neurite length occupied by mitochondria (mitochron.) is decreased in SRF-deficient neurons compared with WT. (O) Velocity of mitochondrial transport in WT and Srf mutant neurons. (P) Percentage of mitochondrial objects engaged in transport is depicted. (*P < 0.05; **P < 0.01; ***P < 0.001.) Numbers in bars represent numbers of independent experiments. (Scale bar: A–D, G, and H, 10 μm; E and F, 3 μm; I and J, 1 μm; K and L, 100 μm.)
In a WT neuron, mitochondrial objects of variable size were present throughout the entire neurite (Fig. 2 A and C). In contrast, in Srf mutant neurons, mitochondrial objects were smaller and accumulated, similar to the in vivo situation (Fig. 1), in the cell body (Fig. 2 B, D, and M). Such mitochondrial fragmentation is also reported in cell culture models of Huntington disease (24). Further, occupancy (i.e., the percentage of a neurite occupied with mitochondria) was reduced (Fig. 2 A–D, quantified in Fig. 2N). This parameter normalizes for the reduced neurite length observed in SRF-deficient neurons (19, 27, 28). In addition to mitochondrial size and occupancy, we assessed transport parameters (Figs. 2 and 3). Using time-lapse videomicroscopy (Fig. 2 C–F), we observed a reduction in velocity and percentage of moving mitochondria on SRF ablation (Fig. 2 O and P and Movies S1 and S2).
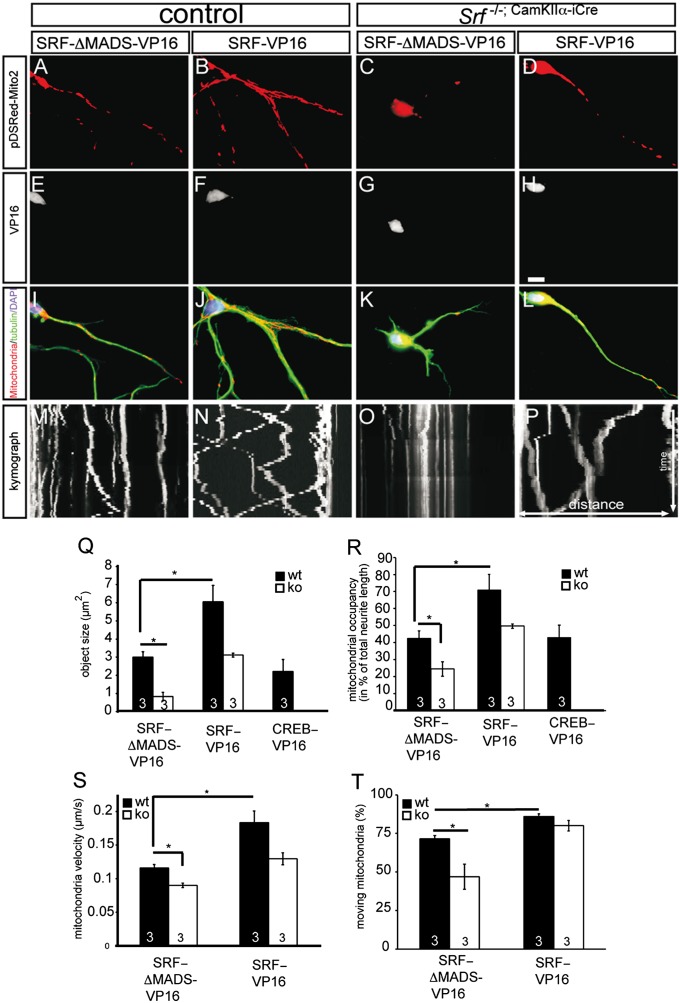
Fig. 3.
SRF-VP16 induces mitochondrial network formation and alters transport parameters. In WT neurons, constitutively active SRF-VP16–induced mitochondrial networks (B, F, and J) compared with SRF-ΔMADS-VP16–expressing neurons (A, E, and I) are shown. (C, G, and K) In an Srf mutant neuron, mitochondria accumulate around the cell body. (D, H, and L) SRF-VP16 expression in Srf mutant neurons increased mitochondrial object size and neurite occupancy. (M–P) Kymographs depict distance traveled by mitochondria (white bars) within 5 min. SRF-VP16 in WT neurons increased the number and velocity of moving mitochondria (N) compared with an SRF-ΔMADS-VP16–expressing neuron (M). On SRF ablation, mitochondria fail to move (O), a phenotype alleviated by SRF-VP16 (P). SRF-VP16 but not CREB-VP16 changed object size (Q) and occupancy (R). ko, knockout; wt, wild type. (S and T) SRF-VP16 increased the velocity and percentage of mitochondria being transported. (*P < 0.05.) Numbers in bars represent numbers of independent experiments. (Scale bar: A–L, 10 μm.)
Because SRF ablation modulates neuronal morphology (i.e., neurite length, growth cone structure) (16), alterations in mitochondrial size and localization might reflect a secondary effect also affecting other cell organelles. To rule out a major impact of such a secondary effect, we inspected synaptic vesicles (Fig. 2 G and H), Golgi apparatus, and lysosomes in Srf mutant neurons. None of these organelles were obviously affected, arguing that SRF has a rather specific influence on mitochondria. In addition, we ablated SRF by adenoviral Cre recombinase delivery in Srfloxp/loxp neurons after neurite extension was completed. Here, mitochondrial morphology was similarly impaired as before (Fig. 2). This supports a neurite length-independent SRF impact on mitochondria.
In vivo, vacuolized mitochondria were observed in SRF-deficient neurons (Fig. 1). We also analyzed mitochondrial ultrastructure in SRF-deficient neurons in vitro (Fig. 2 I–L). In WT neurons, mitochondrial cristae were readily visible (Fig. 2 I and K). In contrast, in Srf mutant neurons, we observed that the cristae structure was disrupted by large, balloon-like vacuole inclusions [Fig. 2 J and L (arrows)].
Constitutively Active SRF-VP16 Enhances Mitochondrial Network Formation in Neurons.
SRF loss of function resulted in aberrant mitochondrial morphology, localization, and size (Figs. 1 and 2). To complement these findings, we used SRF gain-of-function (GOF) experiments (Fig. 3). Here, neurons overexpressed constitutively active SRF-VP16, a fusion protein of SRF and the viral VP16 transactivation domain (29). As a control, we included SRF-ΔMADS-VP16, which lacks parts of SRF’s DNA binding domain. In addition, we included a related gene regulator, cAMP responsive element binding protein (CREB)-VP16, to investigate the SRF specificity of obtained results.
In WT neurons expressing SRF-ΔMADS-VP16 (Fig. 3 A, E, and I), the size and localization of mitochondrial objects were similar to those of mock and GFP-expressing neurons (Fig. 2A). Thus, in line with previous reports (19, 29), SRF-ΔMADS-VP16 did not obviously influence mitochondrial or other parameters tested. In contrast, SRF-VP16 expression in WT neurons induced the formation of large mitochondrial networks throughout the neurites (Fig. 3 B and J) rather than the isolated smaller objects observed in neurons expressing SRF-ΔMADS-VP16 (Fig. 3A). Such induction of neuronal mitochondrial networks appears quite specific for SRF, because CREB failed to modulate mitochondrial dynamics (Fig. 3 Q and R).
Next, we analyzed whether SRF-VP16 rescues mitochondrial fragmentation associated with SRF deficiency. In Srf mutant neurons expressing SRF-ΔMADS-VP16 (Fig. 3 C, G, and K), mitochondria were reduced in size as observed before (Fig. 2). Conversely, SRF-VP16 was able to rescue mitochondrial morphology and localization in Srf mutant neurons (Fig. 3 D, H, and L). The distribution and size of mitochondria in SRF-VP16–expressing Srf mutant neurons were comparable to those of WT neurons (Fig. 3 Q and R).
In addition to SRF-VP16, we investigated whether WT SRF was able to rescue phenotypes associated with SRF deficiency (Fig. S2). Indeed, WT SRF fused to GFP (SRF-GFP) increased mitochondrial size, neurite occupancy, and neurite length in a Srf mutant background (Fig. S2). In WT neurons, SRF-GFP was unable to induce such large mitochondrial networks as observed for SRF-VP16 (Fig. S2 and Fig. 3B).
We also examined mitochondrial transport dynamics in living neurons using time-lapse videomicroscopy. These results are summarized in kymographs depicting the distance traveled by individual objects during the 5-min recording session (Fig. 3 M–P). This analysis measured mitochondrial velocity (Fig. 3S) and the percentage of objects being transported (Fig. 3T). In WT neurons, SRF-VP16 increased transport velocity and percentage of moving mitochondrial objects (Fig. 3N). Because SRF-VP16 induced mitochondrial network formation (Fig. 3B), only individual objects excluded from networks were analyzed. In SRF-deficient neurons expressing SRF-ΔMADS-VP16, mitochondria were rarely engaged in movement (Fig. 3O). SRF-VP16 expression enhanced the velocity (Fig. 3S) and number (Fig. 3T) of moving mitochondria in Srf mutant neurons (Fig. 3P).
In summary, SRF GOF enhanced mitochondrial size, occupancy, and transport velocity as well as the percentage of mitochondria engaged in movement.
SRF-VP16 Alleviates Huntingtin-Induced Mitochondrial Dynamics.
Because SRF-VP16 induced mitochondrial network formation and modulates mitochondrial transport (Fig. 3), we analyzed whether SRF-VP16 might alleviate impaired mitochondrial dynamics associated with neurodegeneration in vitro (Fig. 4). To this end, we used a GFP-tagged huntingtin construct consisting of 130 glutamine residues (GFP-HTT130), which induces huntingtin protein aggregates resembling those observed in the disease. As a control, a GFP-HTT23 construct that does not form aggregates was used (24).
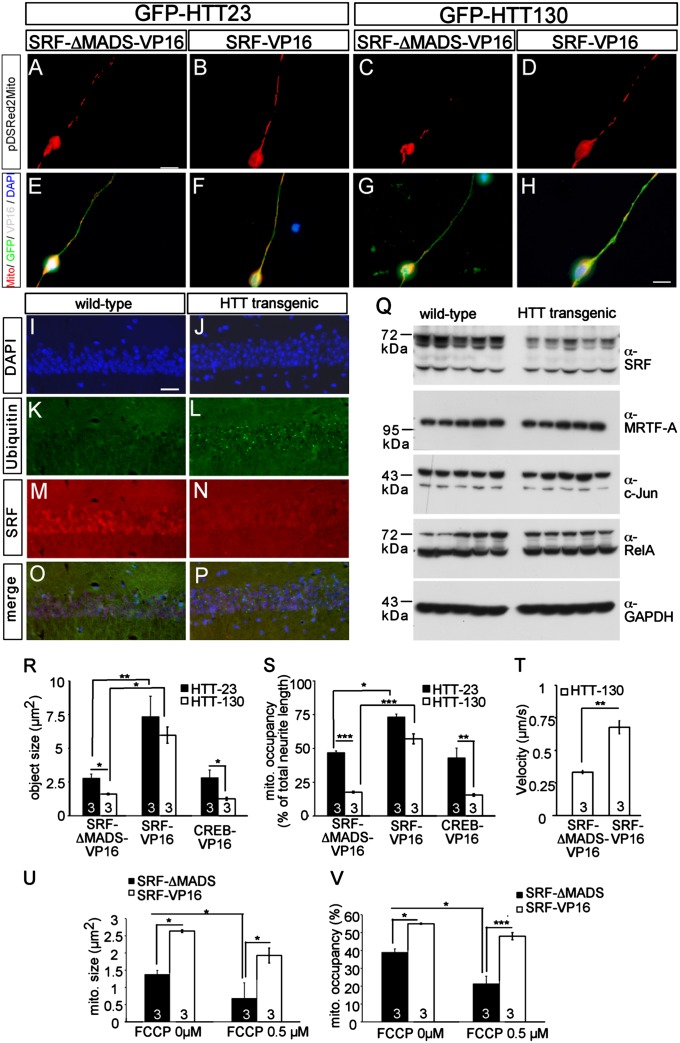
Fig. 4.
SRF-VP16 modulates neurodegeneration-inflicted mitochondrial dynamics. (A–H) In primary neurons, GFP-HTT130, an aggregation-competent huntingtin mutant, induced mitochondrial fragmentation and decreased occupancy (C and G) compared with the control GFP-HTT23 (A and E) in an SRF-ΔMADS-VP16–expressing neuron. (D and H) SRF-VP16 inhibited mitochondrial fragmentation and increased mitochondrial occupancy in the presence of aggregation-competent HTT130. (I–P) SRF (N) is down-regulated in the hippocampal CA1 region of adult huntingtin transgenic mice compared with a nuclear SRF stain in WT mice (M). Antiubiquitin staining depicts huntingtin aggregates in HTT transgenic mice (L) but not in controls (K). (Q) Lysates of hippocampi derived from WT and HTT transgenic mice were blotted for the indicated antibodies. Full-length SRF (approximately 67 kDa), in contrast to other gene regulators (c-Jun, MRTF-A, RelA), is down-regulated in huntingtin transgenic mice. SRF-VP16 but not CREB-VP16 modulated mitochondrial (mito.) dynamics inflicted by HTT130 quantified by object size (R) and occupancy (S). (T) Mitochondrial transport velocity known to be decreased by aggregation-competent HTT130 is almost doubled by SRF-VP16 but not by the SRF control construct (SRF-ΔMADS-VP16). FCCP decreases size (U) and occupancy of mitochondria (V) in WT neurons. SRF-VP16 enhanced mitochondrial size (U) and neurite occupancy (V) in the presence of FCCP. (*P < 0.05; **P < 0.01; ***P < 0.001.) Numbers in bars represent numbers of independent experiments. (Scale bar: A–H, 10 μm; I–P, 20 μm.)
Consistent with previous reports (24), HTT130 expression reduced mitochondrial size and occupancy in neurons positive for SRF-ΔMADS-VP16 (Fig. 4 C and G) compared with neurons expressing HTT23 (Fig. 4 A, E, R, and S). As before (Fig. 3), SRF-VP16 enhanced mitochondrial object size and occupancy in an HTT23-positive neuron (Fig. 4 B and F). Notably, in neurons expressing aggregation-causing HTT130, SRF-VP16 strongly enhanced mitochondrial size and mitochondrial occupancy along neurites (Fig. 4 D and H). Once again, this effect appears specific to SRF-VP16 because CREB-VP16 failed to rescue huntingtin-inflicted mitochondrial pathology (Fig. 4 R and S). HTT130 decreases mitochondrial transport velocity (24). Such decreased velocity of mitochondrial transport inflicted by aggregation-competent huntingtin can be alleviated by SRF-VP16 but not by SRF-ΔMADS-VP16 (Fig. 4T).
Huntingtin, as well as SRF, localizes to the nucleus and modulates gene regulation (30, 31). Therefore, we asked whether nuclear SRF might be targeted by huntingtin. For this, we analyzed a transgenic Huntington mouse model (R6/2) expressing an aggregation-competent exon1 fragment of huntingtin (32). SRF was reduced in the CA1 region of huntingtin mice (Fig. 4 I–P). In addition, full-length SRF (approximately 67 kDa), but not a potential splice variant (approximately 50 kDa), was reduced in hippocampal lysates (Fig. 4Q) compared with the control. This reduction is rather SRF-specific, because protein levels of other gene regulators (MRTF-A, c-Jun, and RelA) remained unaltered (Fig. 4Q).
In addition to huntingtin-dependent neurodegeneration, SRF may function more generally in counterbalancing the impact of other neurodegenerative stimuli on mitochondrial function. To test this, a second neurodegenerative stimulus, carbonyl cyanide-p-trifluoro-methoxy phenylhydrazone (FCCP), an uncoupling reagent disrupting mitochondrial function, was used (33). In control neurons, FCCP reduced mitochondrial size (Fig. 4U) and occupancy (Fig. 4V). Indeed, SRF-VP16 also rescued FCCP-induced mitochondrial fragmentation and increased neurite occupancy (Fig. 4 U and V).
SRF-VP16 enhances the survival of neurons exposed to injury and neurotoxic insults, as demonstrated recently (34). This neuroprotective SRF-VP16 potential might be exerted, at least in part, via SRF-VP16’s impact on mitochondria (Fig. 4).
SRF Is Not a Major Transcriptional Regulator of Mitochondrial Genes.
SRF controls at least two major gene expression programs: (i) immediate early genes and (ii) genes encoding components of the actin cytoskeleton (11). Most of the mitochondrial phenotypes evoked by SRF ablation reported herein (Figs. 1–3) involved morphological changes of mitochondrial size and localization. SRF might affect these parameters by directly transcriptionally regulating genes encoding, for example, for the fusion or fission machinery (1). All genes annotated for mitochondrial function were screened for SRF binding sites (CArG boxes) in their promoter regions (21). SRF was not predicted to have a strong affinity for any of the known fusion or fission. Because SRF might indirectly regulate mRNA abundance of such genes, quantitative PCR analysis of SRF-deficient brain tissue and primary neurons was performed (Fig. S3). None of the fusion genes (Mfn1, Mfn2, and Opa1), fission genes (Fis1 and Dnml1), motor proteins (Kif1b and Kif5b), structural genes (Immt), mitochondrial enzymes (Coxa and Aldh2), or gene regulators of mitochondrial function (Nrf1, Ppargc1a, and Ppargc1b) tested was obviously regulated by SRF (Fig. S3).
Moreover, we tested whether SRF might impinge on mitochondrial dynamics by transcriptional regulation of genes associated with neurodegeneration and mitochondrial function (Fig. S4). For this, we selected a set of CArG box-containing genes identified in a genome-wide search for previously undescribed SRF target genes (35). This gene set includes well-known genes associated with neurodegeneration and mitochondrial function, such as the Parkinson genes Park2 and Lrrk2 and the optic atrophy genes Opa 3 and Spg7 involved in spastic paraplegia (Fig. S4). However, none of these genes was obviously affected by either SRF-VP16 or SRF deficiency compared with established SRF target genes used as a positive control, such as Acta2, Egr1, and gelsolin (Fig. S4). Thus, SRF appears not to modulate mitochondria by regulating an entire gene set encoding mitochondrial genes.
Actin Dynamics Modulate Neurite and Growth Cone Mitochondrial Dynamics.
Many neuronal phenotypes associated with SRF deficiency appear to originate from SRF’s regulation of genes encoding actin isoforms and activity of ABPs, such as cofilin and gelsolin (16) (Fig. S1). In contrast, microtubule alterations are not reported so far. In agreement, total and acetylated tubulin levels in vivo (Fig. S1) were not altered on SRF ablation, in contrast to actin (Fig. S1). In addition, tubulin abundance and distribution in neurites and growth cones in vitro were not obviously modulated by SRF deficiency (e.g., Figs. 2 and 3), actin, cofilin, or Ssh mutant overexpression (Figs. 5–7). This argues against impaired microtubule function as a prime cause of mitochondrial changes inflicted by SRF deficiency.
Fig. 5.
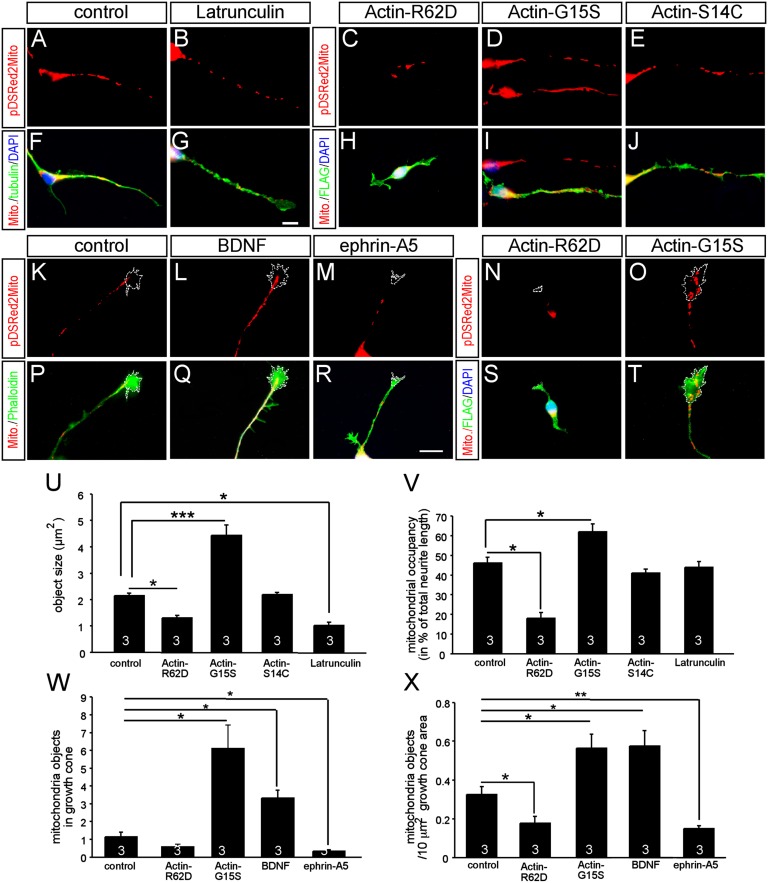
Actin tread-milling modulates neurite and growth cone mitochondrial dynamics. WT neurons were treated with latrunculin (B and G) or expressed actin mutant proteins favoring G-actin (R62D; C and H) or F-actin (G15S, D and I; S14C, E and J). Latrunculin shortened mitochondrial length (B and G) compared with controls (A and F). Actin R62D (C and H) decreased mitochondrial size and occupancy compared with controls (A and F). In contrast, actin G15S (D and I) but not S14C (E and J) increased mitochondrial size and occupancy. Growth cone stimulated with the attractive guidance cue BDNF (L and Q) harbored more mitochondria than a nontreated growth cone (K and P). (M and R) In contrast, ephrin-A5, a guidance cue disrupting F-actin, reduced growth cone mitochondrial number. Actin R62D (N and S) reduced mitochondrial number in growth cones compared with controls (K and P). (O and T) Actin G15S-enhanced mitochondrial number in growth cones. Individual growth cones labeled with a dashed line are indicated. Quantification of object size (U) and occupancy (V) was modulated by actin mutants and latrunculin. Quantification of mitochondria in growth cones, without (W) and with (X) normalization for growth cone area, is shown. (*P < 0.05; **P < 0.01; ***P < 0.001.) Numbers in bars represent numbers of independent experiments. (Scale bar: A–T, 10 μm.)
Fig. 7.
Cofilin S3E affects mitochondrial dynamics and counteracts SRF-VP16. In primary hippocampal neurons (A–H), SRF-VP16 enhanced mitochondrial size and number in neurites (B and F). (C and G) Cofilin S3E reduced mitochondrial size and neurite occupancy. (D and H) Coexpression of cofilin S3E and SRF-VP16 prevented SRF-VP16’ s potential to enhance mitochondrial size and number in neurites. In Srf mutant neurons expressing SRF-ΔMADS-VP16 (K and O), P-cofilin levels were up-regulated compared with WT (I and M). SRF-VP16 reduced P-cofilin levels in WT (J and N) and more pronouncedly in Srf mutant (L and P) neurons. Cofilin S3E reduced mitochondrial (mito.) size (Q) and neurite occupancy (R) and counteracted SRF-VP16’s impact on mitochondria. Quantification of phosphocofilin (S) and total cofilin (T) levels in individual neurons is shown. (*P < 0.05; **P < 0.01; ***P < 0.001.) Numbers in bars represent numbers of independent experiments. ko, knockout; n.s., not significant; wt, wild type. (Scale bar: A–P, 10 μm.)
Next, we asked whether the underlying cause of impaired mitochondrial dynamics on SRF ablation might be linked to actin. So far, the role of actin on mitochondrial size and neurite and growth cone occupancy has not been thoroughly investigated. Therefore, we first analyzed the consequence of enhanced or decreased actin polymerization on these parameters (Fig. 5). We interfered with actin tread-milling by overexpression of well-characterized actin mutant proteins favoring either increased or decreased F-actin abundance (36, 37). The actin point mutants G15S and S14C favor F-actin assembly yet differ with regard to cofilin interaction. Actin R62D is excluded from F-actin, thereby increasing monomeric G-actin (36, 37). In addition, actin G15S stimulates, whereas actin R62D reduces, SRF-mediated gene expression in neurons (27).
Latrunculin application, increasing monomeric G-actin levels, decreased mitochondrial size (Fig. 5 B and G, quantified in Fig. 5 U and V). Similarly, expression of polymerization-incompetent actin R62D decreased mitochondrial size and occupancy in neurites (Fig. 5 C and H, quantified in Fig. 5 U and V). The actin R62D mutant also counteracted the SRF-VP16–mediated increase of mitochondrial length and neurite occupancy (Fig. S5). Thus, actin R62D, inhibiting SRF gene regulation (27), phenocopies Srf mutant neurons with regard to mitochondrial dynamics (compare Figs. 2B and 5 C and N). Polymerization-competent actin G15S (Fig. 5 D and I) increased mitochondrial object size and occupancy, as shown for SRF-VP16 (Fig. 3). This is in keeping with SRF-VP16’s potential to raise F-actin levels (29). Actin S14C did not obviously modulate mitochondrial dynamics (Fig. 5 E and J).
In neurons, F-actin is enriched in growth cones, a prime compartment of mitochondrial activity. To analyze actin’s role in growth cone mitochondria, growth cones were stimulated with guidance cues, such as BDNF and ephrin-A5, which are known modulators of growth cone actin dynamics (38).
F-actin polymerization in growth cones is enhanced by BDNF (27, 39). Notably, BDNF increased the number of mitochondrial objects in growth cones about threefold (Fig. 5 L, Q, and W). Eph receptor activation by ephrins results in a transient F-actin decrease (19, 40). Under these conditions, ephrin-A5 decreased mitochondrial number in growth cones (Fig. 5 M, R, W, and X). Thus, attractive (BDNF) and repulsive (ephrin-A5) axon guidance cues enhanced and decreased growth cone occupancy of mitochondria, respectively.
To analyze more directly whether actin tread-milling might alter mitochondrial growth, cone occupancy actin mutant proteins were used (Fig. 5 N, O, S, T, W, and X). As observed with ephrin-A5 treatment, actin R62D diminished mitochondrial objects in growth cones (Fig. 5 N and S). Conversely, F-actin elevation via actin G15S (Fig. 5 O and T) resulted in a fivefold increase in growth cone mitochondrial localization (Fig. 5W) similar to BDNF treatment. This also holds true when normalizing for the actual growth cone area (Fig. 5X).
In sum, these data suggest that shifting actin tread-milling toward polymerization increases mitochondrial size, neurite occupancy, and number of mitochondria in growth cones. Conversely, elevating G-actin results in mitochondrial fragmentation, decreased occupancy, and exclusion of mitochondria from growth cones.
Active Cofilin and Slingshot Rescue Mitochondrial Dynamics and Neuronal Motility Inflicted on SRF Deficiency.
Actin dynamics are modulated by cofilin, an ABP that severs and disassembles F-actin and directly or indirectly generates new seeds for F-actin assembly. Cofilin is primarily activated via regulation of a critical serine residue (S3). LIM kinase phosphorylation inactivates cofilin, whereas dephosphorylation by slingshot phosphatases activates cofilin (15). Enhanced phosphorylation, and thereby cofilin inactivation, is induced by SRF inhibition (13, 14). Hence, we asked whether the underlying mechanism of SRF’s impact on mitochondrial function and neuronal motility in general might be connected to cofilin (Figs. 6 and 7). Modulation of cofilin function was achieved by the expression of two GFP-tagged cofilin point mutations (41): cofilin S3A, a putative activated cofilin variant that is unphosphorylatable (Fig. 6), and a phosphomimetic cofilin variant, cofilin S3E, that is a bona fide actin-severing incompetent protein (Fig. 7).
Fig. 6.
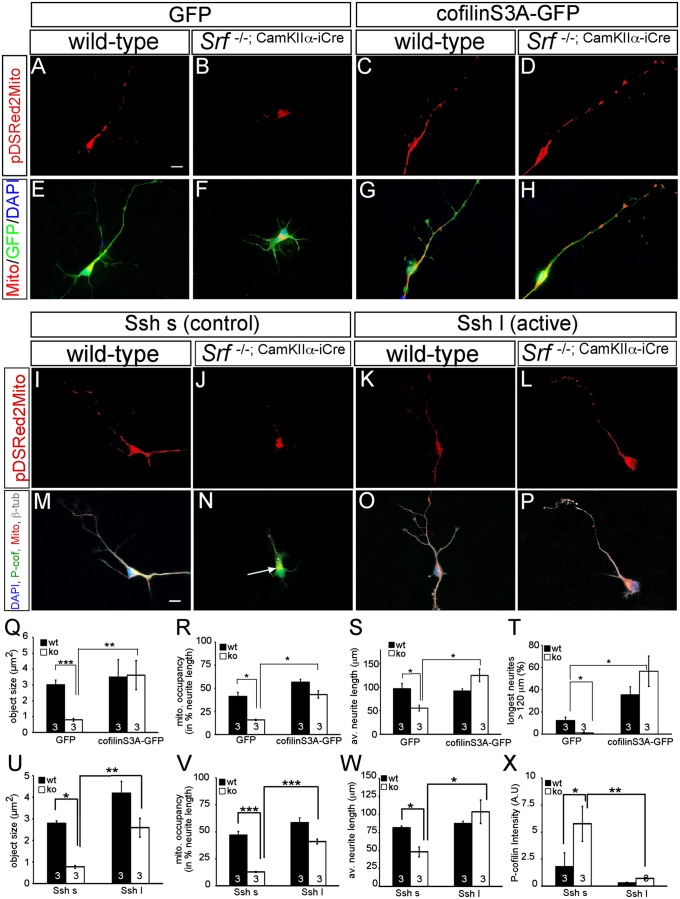
Active cofilin and slingshot rescue mitochondrial motility and neurite growth impaired by SRF ablation. In primary neurons, SRF ablation resulted in mitochondrial fragmentation (B and F) compared with WT (A and E) as before (Fig. 2). (C and G) Overexpression of cofilin S3A in WT neurons only slightly enhanced mitochondrial size and neurite occupancy (compare with Q and R). (D and H) Active cofilin S3A rescued impaired mitochondrial dynamics induced by SRF deficiency and elevated neurite growth. (I–P) In WT neurons, active Ssh l (K and O) in comparison to the control Ssh s (I and M) increased mitochondrial size and neurite occupancy, although not statistically significantly (compare with U and V). (L and P) Active Ssh enhanced mitochondrial size and occupancy as well as neurite growth in Srf mutant neurons. In addition, Ssh l (P) suppressed cofilin phosphorylation in SRF-deficient neurons (compare N with P). Cofilin S3A rescued mitochondrial (mito.) size (Q) and neurite occupancy (R) in Srf mutants. (S and T) SRF-deficient neurons expressing cofilin S3A achieved neurite length comparable to WT neurons (S). av., average; ko, knockout; wt, wild type. (T) Cofilin S3A also enhanced neurite growth in WT neurons. Active Ssh l rescued mitochondrial size (U), neurite occupancy (V), and neurite length (W) in Srf mutant neurons. (X) Ssh reduced P-cofilin in WT and more strongly in Srf mutants. (*P < 0.05; **P < 0.01; ***P < 0.001.) Numbers in bars represent numbers of independent experiments. (Scale bar: A–P, 10 μm.)
First, we analyzed whether increasing cofilin activity with cofilin S3A was able to rescue SRF-associated phenotypes (Fig. 6). Overexpression of cofilin S3A did not obviously alter mitochondrial size and occupancy in WT neurons (Fig. 6 A, C, E, and G). However, introduction of cofilin S3A in SRF-deficient neurons rescued mitochondrial fragmentation and neurite occupancy (Fig. 6 B, D, F, H, Q, and R). Now, the average mitochondrial size and neurite occupancy in SRF-deficient neurons expressing cofilin S3A (Fig. 6 D and H) were comparable to those of GFP-expressing WT neurons (Fig. 6 A and E). In addition to mitochondrial function, we inspected whether cofilin S3A rescued neurite length decreased on SRF deletion (19). In WT neurons, cofilin S3A enhanced neurite growth (Fig. 6T), as reported previously (41). In SRF-deficient neurons, cofilin S3A expression resulted in neurite outgrowth comparable to that of WT neurons (Fig. 6 S and T). Thus, cofilin S3A rescued mitochondrial parameters (i.e., shape, localization, neurite outgrowth inflicted by SRF deficiency).
In Srf mutants, cofilin phosphorylation (P-cofilin) is enhanced (13). Thus, we reasoned that overexpression of the cofilin phosphatase slingshot (Ssh) might rescue SRF-associated phenotypes (Fig. 6 I–P and U–X). For these experiments, two Ssh proteins, truncated short Ssh (Ssh s) and the full-length (long) active Ssh (Ssh l), were used. In WT neurons, active Ssh l enhanced mitochondrial object size, although not in a statistically significant manner (Fig. 6 I, K, M, O, and U). Importantly, active Ssh l rescued mitochondrial size (Fig. 6U) and neurite occupancy (Fig. 6V), and neurite length (Fig. 6W) decreased on SRF deficiency (Fig. 6 L and P). In addition, we inspected P-cofilin levels in these primary neurons (Fig. 6 M–P, quantified in Fig. 6X). P-cofilin was up-regulated in Srf mutant neurons expressing inactive Ssh s [Fig. 6N (arrow)] as shown in vivo previously (13). These elevated P-cofilin levels in Srf mutant neurons were suppressed on Ssh l expression (Fig. 6 P and X).
Thus, overexpression of active cofilin or slingshot rescued phenotypes inflicted on SRF depletion, suggesting that cofilin is an important effector to convey SRF-mediated activities, such as mitochondrial dynamics and neurite length, in neurons.
Inactive Cofilin Modulates Mitochondrial Shape and Counteracts SRF-VP16.
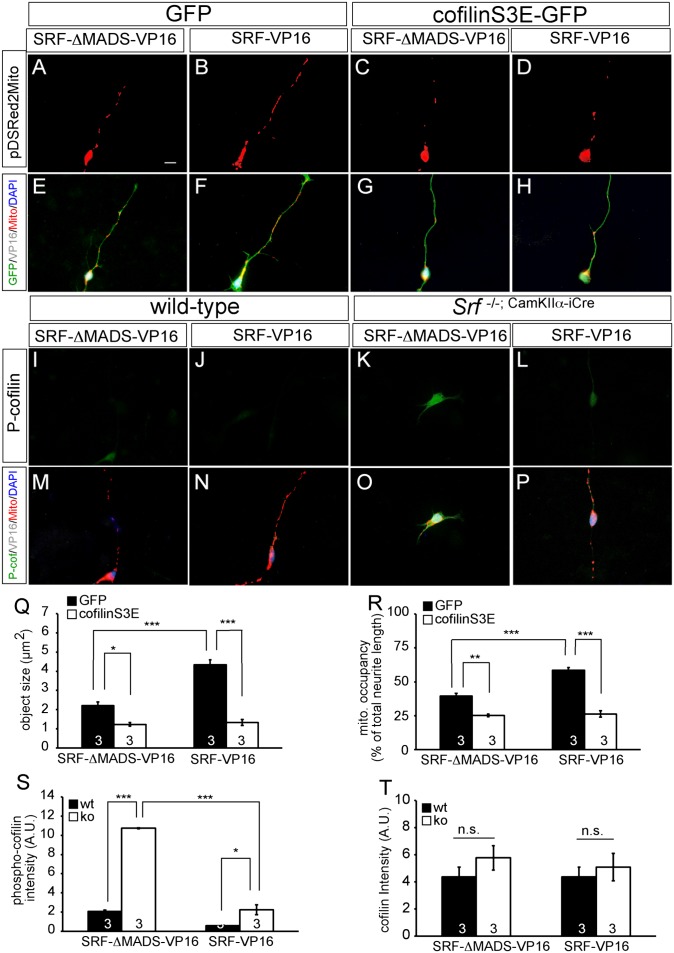
To corroborate data obtained with active cofilin S3A mutant (Fig. 6), we expressed the inactive cofilin mutant S3E in WT neurons (Fig. 7). Cofilin S3E reduced mitochondrial size and neurite occupancy in WT neurons (Fig. 7 C and G) compared with a GFP control (Fig. 7 A and E, quantified in Fig. 7 Q and R).
Because we demonstrated rescue of SRF-inflicted phenotypes by active cofilin (Fig. 6), we investigated whether inactive cofilin S3E abrogates SRF-VP16’s potential to modulate mitochondrial dynamics (Fig. 7). The coexpression of cofilin S3E with SRF-VP16 prevented SRF-VP16–mediated increases in mitochondrial size and neurite occupancy (Fig. 7 D and H, quantified in Fig. 7 Q and R). This suggests that SRF-VP16 exerts its impact on mitochondria via cofilin (Fig. 7) and functional actin tread-milling (Fig. S5). To strengthen this finding further, we investigated SRF-VP16’s potential to alter endogenous cofilin activity (Fig. 7 I–P, S, and T). As with brain tissue previously (13), we observed enhanced cofilin phosphorylation (i.e., cofilin inactivation) in Srf mutant neurons (Fig. 7 K and O). This suggests that SRF might reduce phosphorylation, and thereby activate cofilin in WT cells. Indeed, SRF-VP16 suppressed P-cofilin in WT neurons, an effect even more pronounced in SRF-deficient neurons (Fig. 7 J, L, N, P, and S). Total cofilin levels were unchanged (Fig. 7T).
In summary, SRF appears to form a signaling axis with cofilin to modulate neuronal mitochondrial shape and neurite occupancy.
Discussion
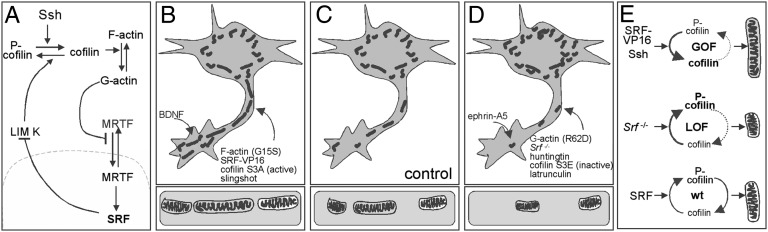
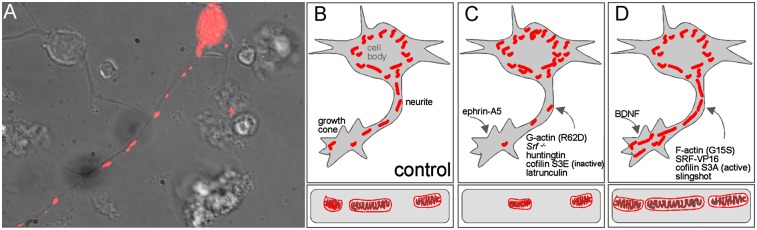
In this study, we demonstrate that SRF-cofilin-actin signaling affects mitochondrial dynamics (i.e., size, subcellular distribution to neurites and growth cones, mitochondrial energy metabolism). SRF (SRF-VP16, Fig. 3), the F-actin favoring mutant actin G15S (Fig. 5), an active cofilin protein (S3A, Fig. 6), or the cofilin activator slingshot (Fig. 6) enhanced mitochondrial size and neurite occupancy (Fig. 8B) compared with controls (Fig. 8 C and E). In contrast, SRF deletion resulted in altered mitochondrial ultrastructure and fragmentation reminiscent of neurodegeneration (Figs. 1, 2, and 8 D and E). Similar to SRF deficiency, we observed that huntingtin (Fig. 4), G-actin (R62D and latrunculin, Fig. 5), and the inactive cofilin protein S3E (Fig. 7) induced mitochondrial fragmentation and reduced neurite occupancy (Fig. 8D). Taken together, these results indicate that misregulation of an SRF-cofilin signaling axis may underlie disrupted mitochondrial dynamics and energy metabolism during neurodegeneration.
Fig. 8.
Summary of SRF-cofilin-actin signaling in neuronal mitochondrial dynamics. (A) Summary of the SRF-cofilin-actin interplay. (B–D) Compared with the control (C), F-actin, SRF-VP16, cofilin S3A, and slingshot (summarized in B) enhanced mitochondrial size and neurite occupancy. (D) In contrast, G-actin, SRF deficiency, huntingtin, and cofilin S3E decreased both parameters. BDNF (B) increased, whereas ephrin-A5 (D) decreased, mitochondrial number in growth cones. (E) (Top) SRF GOF via SRF-VP16 decreased P-cofilin levels and enhanced mitochondrial size. (Middle) SRF loss of function (LOF) enhanced P-cofilin and reduced mitochondrial size. (Bottom) In sum, this suggests that fine-tuning of cofilin activity by WT SRF is important toward modulation of mitochondrial size.
Role of the Actin Cytoskeleton in Neuronal Mitochondrial Dynamics.
Microtubules steer long-range transport of mitochondria in neurites. In contrast, actin has not been investigated in the same depth and has been implicated in local anchoring of mitochondria in growth cones (2, 4).
In this study, we show that polymerization-competent actin (G15S) enhanced, whereas G-actin (R62D) decreased, mitochondrial presence in growth cones (Figs. 5 and 8). In line with this finding, BDNF, an attractive guidance cue inducing F-actin–rich filopodia (38), enhanced mitochondrial translocation into growth cones (Fig. 5). In contrast, ephrin-A5, a repulsive guidance cue causing F-actin disruption, decreased mitochondrial occupancy in growth cones (Fig. 5). Thus, F-actin, per se, or F-actin assembly induced by attractive guidance cues might promote anchoring of mitochondria in growth cones. Conversely, raising G-actin by repulsive molecules, such as ephrin-A5, resulted in clearance of mitochondria from growth cones. Such a model is in agreement with previous reports, because NGF enhanced mitochondrial anchoring depending on F-actin (5) and Wiskott-Aldrich syndrome protein (WASP) inhibition, a stimulator of F-actin assembly, prevented activity-dependent mitochondrial trafficking to dendritic spines (8).
In addition to growth cones, we show that actin modulates mitochondria in neurites (Fig. 8). First, F-actin (G15S) enhanced mitochondrial size and neurite abundance (Fig. 5). Second, and in line with the actin G15S findings, SRF-VP16, which increases F-actin (29), increased mitochondrial size, resulting in extensive mitochondrial networks (Fig. 3). Third, the active cofilin mutant S3A or slingshot (Fig. 6) enhanced mitochondrial size and localization, particularly in Srf mutants. Such cofilin activation most likely enhances actin tread-milling, because cofilin-mediated F-actin severing increases actin turnover and generates new seeds for F-actin polymerization (15). Conversely, increased G-actin (R62D or latrunculin, Fig. 5), SRF ablation (Figs. 1 and 2), or the inactive cofilin mutant (S3E, Fig. 7) resulted in mitochondrial fragmentation and neurite clearance (Fig. 8D). Thus, F-actin appears to enhance, whereas G-actin decreases, mitochondrial size and neurite occupancy. Results obtained with actin R62D are in contrast to those of previous reports (10) demonstrating that pharmacological interference with actin dynamics did not impair subcellular mitochondrial localization. This discrepancy might be explained by actin R62D’s potential to inhibit neuronal SRF-mediated gene transcription directly, thereby inducing phenotypes similarly evoked by SRF deficiency, such as altered mitochondrial localization.
How would actin accomplish its effect on mitochondria? F-actin is concentrated in growth cones, whereas neurites are largely devoid of F-actin. Naturally, this suggests that F-actin might mainly affect mitochondria in growth cones (Fig. 5). Nevertheless, stimulation of neurons with guidance cues, such as NGF, also induces actin patches in neurites, which are then filled with mitochondria (42). Similarly (e.g., on induction of neuronal apoptosis), cofilin translocates to mitochondria in neurites (43, 44). Thus, under certain cellular conditions, cofilin and actin might directly or indirectly associate with neurite mitochondria. Mechanistically, it is currently not understood how cofilin-actin signaling might affect mitochondrial size and subcellular localization. Recent data suggest that myosin motor proteins affect neuronal mitochondrial size (45). Thus, in one possible scenario, myosins might recruit adaptor and anchoring molecules linking actin/cofilin to mitochondria. In a next step, fission or fusion proteins might be recruited, adjusting mitochondrial size. Such recruitment of, for example, fission proteins to mitochondria requires F-actin polymerization (46).
In summary, our data suggest that the actin cytoskeleton can adjust mitochondrial size and localization in neurons. Many external stimuli, such as guidance cues, modulate neuronal actin dynamics. Thus, actin might be particularly important to regulate mitochondrial size and subcellular localization during signaling processes of morphological neuron remodeling, such as neurite growth, growth cone guidance, and synapse formation.
SRF’s Implication in Mitochondrial Biology and Neurodegeneration.
SRF deficiency resulted in mitochondrial fragmentation and reduced mitochondrial transport and neurite occupancy reminiscent of various neurodegenerative diseases. In addition, cristae swelling and impaired ATP metabolism are indicative of degenerating neurons (3) (Figs. 1 and 2). Because bioenergetic impairments and altered size/localization of mitochondria are most likely molecularly coupled, it is difficult to single out the primary cause of these phenotypes.
Such neurodegeneration inflicted by SRF ablation might be connected to cofilin inhibition observed in Srf mutant neurons (13). Indeed, cofilin aggregates reported in neurodegeneration (15, 47) block mitochondrial trafficking (48). SRF deficiency suggests that SRF may exert a neuroprotective function in WT neurons. In accordance, raising SRF activity via SRF-VP16 induced mitochondrial networks (Fig. 3) and exerted a neuroprotective function by alleviating the impact of two neurotoxic stimuli, huntingtin and FCCP (Fig. 4).
SRF is down-regulated by huntingtin in vivo (Fig. 4 I–Q). Of note, the SRF cofactor Elk-1 has recently been found in human huntingtin aggregates (49). Thus, SRF, along with Elk-1, might also be a direct target of neurodegenerative diseases (Fig. 4 I–Q). In turn, as suggested by our in vitro data, SRF-VP16 might be beneficial to alleviate certain hallmarks of neurodegenerative diseases, such as impaired mitochondrial function (Fig. 4).
How does SRF affect neuronal mitochondrial function? Although we cannot completely rule it out, our data do not favor a mechanism of SRF transcriptionally regulating a whole set of mitochondrial genes (Figs. S3 and S4). In contrast, the cofilin-actin signaling axis emerged as an important SRF effector mediating SRF’s impact not only on mitochondria but on neuronal motility (e.g., neurite growth) (Fig. 6). Cofilin is inactivated on SRF deletion (13). Of note, our data do not support direct transcriptional regulation of cofilin by SRF (Fig. S4). SRF is found at the cofilin promoter (Fig. S4A). However, in line with ES cells (13), cofilin mRNA levels in neurons were neither modulated by SRF-VP16 nor by SRF ablation (Fig. S4B). This supports recent data suggesting that SRF regulates cofilin activity through a signaling cascade involving Pctaire1/Cdk5-Pak1-LIM kinase (14).
So far, it was unclear whether cofilin was essential to convey SRF activity in neurons. Mitochondrial fragmentation, reduced neurite occupancy, and neurite growth can be rescued by cofilin activation in Srf mutant neurons. This was achieved either by an active cofilin mutant (S3A, Fig. 6) or by reducing inactivated P-cofilin by means of slingshot overexpression (Fig. 6). Conversely, interference with cofilin function (S3E) prevented SRF-VP16 from enhancing mitochondrial size (Fig. 7). Thus, we show that cofilin is necessary and sufficient for SRF to exert its function. In contrast to cofilin, actin G15S and WT actin cannot rescue neurite growth in Srf mutant neurons (19, 27; mitochondria were not analyzed in these earlier studies). This further suggests that the underlying cause of an SRF-impaired actin cytoskeleton is mainly distorted dynamics rather than abundance of actin filaments.
SRF might team up with MRTF partner proteins to exert its impact on cofilin as previously suggested (14, 16). Of note, similar to SRF ablation (Fig. 1), MRTF-A ablation results in mitochondrial vacuolization (50). Thus, MRTFs might connect SRF with the cofilin-actin pathway, and thereby modulate mitochondrial function.
Materials and Methods
Mice.
We used Srf−/−;Camk2a-iCre mutant mice in which Cre recombinase expression was induced in neurons by the Camk2a locus. As controls, we used Srf+/−;Camk2a-iCre or Srf−/−;Camk2a-iCre Cre-negative animals (13). The Srf gene was excised via loxP sites. All the mouse experiments used in this study were approved by the Regierungspräsidium Tübingen. R6/2 mice (32) were purchased from Jackson Laboratories.
EM.
Mice were perfused or cell cultures were fixed with 2.5% (vol/vol) glutaraldehyde and 2.5% (wt/vol) paraformaldehyde (PFA) in PBS. Vibratome sections of 500 μm were postfixed with 1% (vol/vol) osmium tetroxide in 100 mM PO4 buffer at pH 7.2 for 1 h on ice, washed with H2O, treated with 1% (vol/vol) aqueous uranyl acetate for 1 h at 4 °C, dehydrated through a graded series of ethanol, washed with propylene oxide as an intermedium, infiltrated with propylene oxide/resin mixtures, and finally embedded in Epon [using glycidether 100 (Carl Roth, Germany)]. Ultrathin sections were collected on coated slot grids, stained with uranyl acetate and lead citrate, and viewed in a Tecnai G2 electron microscope.
Plasmids.
SRF plasmids were described previously (29). The vector for mitochondrial-targeted RFP (pDSRed2Mito) was provided by Doron Rapaport (Interfaculty Institute for Cell Biology, University of Tübingen, Tübingen, Germany). The synaptophysin vector was provided by Eckart Gundelfinger (Leibniz Institute, Magdeburg, Germany). The His-tagged slingshot constructs (pcDNA 3.1myc/his+) used in this study were described previously (51) and were provided by James Bamburg (Colorado State University, Fort Collins, CO). The GFP-tagged cofilin plasmids (cofilin S3A and cofilin S3E) were prepared as described previously (41) but in the pEGFP-N1 vector (Clontech). R62D, G15S, and S14C actin plasmids were provided by Guido Posern (Max Planck Institute of Biochemistry, Munich, Germany). Huntingtin plasmids were provided by Xiao-Jiang Li (Emory University School of Medicine, Atlanta, GA). The SRF-GFP vector was kindly provided by J. Solway (University of Chicago, Chicago, IL).
Neuronal Cell Culture.
P1–P3 hippocampal cultures were incubated in neuronal minimal essential medium (NMEM)/B27 medium as described previously (19). In brief, neurons (5 × 103–104) were cultured on poly-l-lysine–coated (100 μg/mL; Sigma) and laminin-coated (20 μg/mL; Gibco) coverslips (13 mm) for 2–3 d in vitro. To visualize mitochondria, we used nucleofection (Amaxa) with 3 μg of pDSRed2Mito to deliver a mitochondrial-targeted RFP into hippocampal neurons. In coexpression experiments, 1.5 μg of pDSRed2Mito and 1.5 μg of a second vector (cofilin, HTT, SRF, or actin) were used. For triple transfections, 1 μg of each vector was used. For slingshot experiments, we used 0.3 μg of pDSRed2Mito and 3 μg of Ssh l or Ssh s, respectively. To fragment mitochondria, neurons were stimulated with FCCP (0.5 μM; Sigma) for 150 min, followed by fixation. For growth cone stimulation experiments, ephrin-A5-Fc (R&D Systems) or Fc alone (Sigma) was applied to the culture medium at 1 μg/mL; both were preclustered with 10 μg/mL anti-human IgG Fc-specific (Sigma) for 10 min at 37 °C. BDNF (Peprotech) was applied at 10 ng/mL for 60 min at 37 °C.
Immunocytochemistry.
Cells were fixed for 15 min in 4% (wt/vol) PFA/5% (wt/vol) sucrose/PBS, permeabilized for 5 min in 0.1% Triton X-100/PBS, and blocked for 30 min in 2% (wt/vol) BSA/PBS. The following primary antibodies were incubated overnight at 4 °C: mouse α-class III β-tubulin (1:1,000; Covance), rabbit α-VP16 (1:1,000; Abcam), mouse α-VP16 (1:500; Santa Cruz), mouse α-FLAG (1:300; Sigma), rabbit α-His (1:10,000; Bethyl), and rabbit α-cofilin and α-phospho-cofilin (both 1:500, gifts from James Bamburg). Primary antibodies were detected with Alexa 488- or Alexa 660-conjugated secondary antibodies (1:1,000; Molecular Probes), followed by DAPI staining. Cells were stained for F-actin with Texas Red-Phalloidin or Phalloidin 505 (1:100; Molecular Probes).
Immunohistochemistry.
Brains were fixed in 4% (vol/vol) PFA, and 5 μm paraffin sections were prepared. Slices were incubated overnight at 4 °C with rabbit α-SRF (1:500; Santa Cruz), rabbit α-Tom20 (1:500; Santa Cruz), mouse α-Smi32 (1:1,000; Covance), and mouse α-ubiquitin (1:1,000; Abd Serotec) primary antibodies. Sections were incubated for 1 h at room temperature with Alexa-conjugated secondary antibodies (1:300) and counterstained with DAPI.
Biochemistry.
Tissue was lysed in 100 mM Tris (pH 7.8), 150 mM NaCl, 1 mM EDTA, 1% (vol/vol) Triton X-100, 0.1% SDS, 1 mM PMSF, 1 mM sodium vanadate, 1 mM NaF, 1 mM sodium pyrophosphate, and protease inhibitors (Roche). Samples were resolved on 10% (wt/vol) SDS/PAGE, followed by transfer on PVDF membranes (Amersham). After blocking, primary antibodies were applied overnight at 4 °C: mouse α-pan-actin (1:500; Linaris), mouse α-phospho-cofilin (1:1,000; Cell Signaling), mouse α-cofilin (1:1,000; Cell Signaling), mouse α-β-tubulin (1:2,000; Sigma), mouse α-acetyl-tubulin (1:2,000; Sigma), rabbit α-SRF (1:500; Santa Cruz), rabbit α-GM130 (1:1,000; Abcam), rabbit α-Tom20 (1:500; Santa Cruz), α-SOD2 (1:500; Millipore), rabbit α c-Jun (1:500; Santa Cruz), rabbit α-RelA (1:500; Santa Cruz), and mouse anti-GAPDH (1:50,000; Acris). Detection of first antibodies was achieved with HRP-conjugated secondary antibodies (1:3,000) and the ECL Western Blotting Substrate (Millipore).
ATP Production Assay.
Hippocampal tissue from P12–P14 mice was cut into pieces and homogenized in 1 mL of isolation medium [1 mM EDTA, 10 mM Tris⋅HCl, 320 mM sucrose, and Roche complete protease inhibitor (pH 7.4)] using a Potter homogenizer with a Teflon-coated pestle on ice. The homogenate was washed twice at 1,500 × g for 10 min at 4 °C, and the supernatant was centrifuged at 14,000 × g for 15 min at 4 °C. The pellet was washed twice and finally resuspended in 200 μL of isolation buffer. Supernatants were collected as cytosolic fractions. The protein amount of mitochondrial and cytosolic fractions was determined using Bradford reagent. ATP production was measured using the ATP determination kit (Molecular Probes). In brief, 10 μL of the mitochondrial and cytosolic fractions was added to 270 μL of luciferase reaction solution (Molecular Probes) and 20 μL of substrate solution (malate and pyruvate, final concentrations of 3.3 mM). The reaction was started by adding 2.5 mM ADP and recorded for 5 min with a luminometer (Lumat LB 9507; Berthold). ATP production was determined by calculating the initial slope of the graph with Excel (Microsoft Corporation) and normalized to the protein amount of the mitochondrial or cytosolic fraction. ATP content and production (nmol of ATP/mg of protein) are depicted in Fig. 1.
Quantitative Real-Time PCR.
Total RNA was isolated with the RNeasy kit (Qiagen). RT was performed with 1 μg of RNA using reverse transcriptase (Promega) and random hexamers. Quantitative real-time PCR was performed on an ABI PRISM 7700 Sequence Detector with Power PCR SYBR green PCR master mix (Applied Biosystems). Expression was determined in relation to Gapdh RNA levels. Primer sequences are provided in Table S1.
Image Acquisition and Statistics.
Images were acquired on a Zeiss Axiovert 200M or Zeiss LSM710 confocal microscope using an Axiocam camera and Axiovision (Zeiss, Germany) or ZEN (Zeiss, Germany) software. We used 10×, 20×, 63×, and 100× Zeiss objective lenses with an N.A. of 0.3, 0.8 and 1.3 (oil) and 1.3 (oil), respectively. Pictures were further processed using SlideBook 5 software (Intelligent Imaging Innovation) and Photoshop software (Adobe Systems).
The numbers in the bar graphs indicate numbers of independent experiments performed (at least 3 experiments). In each experiment, 15–20 neurons per condition were analyzed. Axiovision software was used to determine single neurite and mitochondrial length. Mitochondrial occupancy (percentage) was calculated by the ratio of the summated mitochondrial length and neurite length. Mitochondrial object size was determined using SlideBook 5 software by setting equal signal intensity thresholds for all experiments and automated calculation of the residual mitochondrial objects. P-cofilin and cofilin levels for individual cells were determined by Slidebook 5 software by setting equal signaling intensity thresholds and calculation of the sum of intensity.
Statistical analysis was assessed using t tests or, for multiple comparisons, one-way ANOVA with a Bonferroni post hoc test, with *, **, and *** indicating P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively. Error bars depict SD.
Live Cell Imaging and Time-Lapse Analysis.
Three days after transfection, mitochondrial transport was analyzed by imaging primary hippocampal neurons on an Axiovert 200M. Images were acquired using a 100×/1.3 N.A. oil immersion objective every 5 s for 5 min. The generated time series were further analyzed using SlideBook 5 software applying the particle tracking tool. Mitochondrial objects were considered to be moving if they moved more than 1.5 μm in three consecutive frames. This resulted in a saltatory trafficking pattern with phases of moving and stopping mitochondria. Mitochondria without any movement for more than three consecutive frames throughout the whole recording time were counted as stationary. The average velocities of all moving phases of moving mitochondria were measured as well as the percentage of moving mitochondria. For each condition, experiments were repeated three times recording mitochondria movement in 10 neurons in every experiment, resulting in analysis of at least 300 mitochondrial objects for each condition. To visualize mitochondrial movements, kymographs were generated from time-lapse image series with the “MultipleKymograph” plug-in in ImageJ (National Institutes of Health). Resulting kymographs display the distance on the x axis and recorded time (5 min) on the y axis.
Supplementary Material
Acknowledgments
B.K. is supported by the Deutsche Forschungsgemeinschaft and Schram, Gottschalk, and Gemeinnützige Hertie Foundations. H.B. is supported by the Gemeinnützige Hertie Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 15090 (volume 109, number 38).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208141109/-/DCSupplemental.
References
- 1.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 2.MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20:102–112. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Knott AB, Bossy-Wetzel E. Impairing the mitochondrial fission and fusion balance:A new mechanism of neurodegeneration. Ann N Y Acad Sci. 2008;1147:283–292. doi: 10.1196/annals.1427.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Frederick RL, Shaw JM. Moving mitochondria: Establishing distribution of an essential organelle. Traffic. 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CW, Peng HB. Mitochondrial clustering at the vertebrate neuromuscular junction during presynaptic differentiation. J Neurobiol. 2006;66:522–536. doi: 10.1002/neu.20245. [DOI] [PubMed] [Google Scholar]
- 8.Sung JY, et al. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc Natl Acad Sci USA. 2008;105:3112–3116. doi: 10.1073/pnas.0712180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ligon LA, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:351–361. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posern G, Treisman R. Actin’ together: Serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Alberti S, et al. Neuronal migration in the murine rostral migratory stream requires serum response factor. Proc Natl Acad Sci USA. 2005;102:6148–6153. doi: 10.1073/pnas.0501191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokalled MH, Johnson A, Kim Y, Oh J, Olson EN. Myocardin-related transcription factors regulate the Cdk5/Pctaire1 kinase cascade to control neurite outgrowth, neuronal migration and brain development. Development. 2010;137:2365–2374. doi: 10.1242/dev.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein BW, Bamburg JR. ADF/cofilin: A functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knöll B, Nordheim A. Functional versatility of transcription factors in the nervous system: The SRF paradigm. Trends Neurosci. 2009;32:432–442. doi: 10.1016/j.tins.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Bellenchi GC, et al. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev. 2007;21:2347–2357. doi: 10.1101/gad.434307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casanova E, et al. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 2001;31(1):37–42. doi: 10.1002/gene.1078. [DOI] [PubMed] [Google Scholar]
- 19.Knöll B, et al. Serum response factor controls neuronal circuit assembly in the hippocampus. Nat Neurosci. 2006;9:195–204. doi: 10.1038/nn1627. [DOI] [PubMed] [Google Scholar]
- 20.Wiebel FF, Rennekampff V, Vintersten K, Nordheim A. Generation of mice carrying conditional knockout alleles for the transcription factor SRF. Genesis. 2002;32(2):124–126. doi: 10.1002/gene.10049. [DOI] [PubMed] [Google Scholar]
- 21.Stritt C, et al. Paracrine control of oligodendrocyte differentiation by SRF-directed neuronal gene expression. Nat Neurosci. 2009;12:418–427. doi: 10.1038/nn.2280. [DOI] [PubMed] [Google Scholar]
- 22.Mahad D, Lassmann H, Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol Appl Neurobiol. 2008;34:577–589. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin LJ, et al. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: Mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500:20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- 24.Orr AL, et al. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy PH, Mao P, Manczak M. Mitochondrial structural and functional dynamics in Huntington’s disease. Brain Res Brain Res Rev. 2009;61(1):33–48. doi: 10.1016/j.brainresrev.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song W, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern S, et al. A nuclear actin function regulates neuronal motility by serum response factor-dependent gene transcription. J Neurosci. 2009;29:4512–4518. doi: 10.1523/JNEUROSCI.0333-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickramasinghe SR, et al. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schratt G, et al. Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells. J Cell Biol. 2002;156:737–750. doi: 10.1083/jcb.200106008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: An alternative approach to Huntington’s disease. Nat Rev Neurosci. 2005;6:919–930. doi: 10.1038/nrn1806. [DOI] [PubMed] [Google Scholar]
- 31.Roze E, Bonnet C, Betuing S, Caboche J. Huntington’s disease. Adv Exp Med Biol. 2010;685:45–63. [PubMed] [Google Scholar]
- 32.Mangiarini L, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 33.Lee CW, Peng HB. The function of mitochondria in presynaptic development at the neuromuscular junction. Mol Biol Cell. 2008;19:150–158. doi: 10.1091/mbc.E07-05-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern S, Sinske D, Knöll B. Serum response factor modulates neuron survival during peripheral axon injury. J Neuroinflammation. 2012;9:78. doi: 10.1186/1742-2094-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benson CC, Zhou Q, Long X, Miano JM. Identifying functional single nucleotide polymorphisms in the human CArGome. Physiol Genomics. 2011;43:1038–1048. doi: 10.1152/physiolgenomics.00098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posern G, Miralles F, Guettler S, Treisman R. Mutant actins that stabilise F-actin use distinct mechanisms to activate the SRF coactivator MAL. EMBO J. 2004;23:3973–3983. doi: 10.1038/sj.emboj.7600404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posern G, Sotiropoulos A, Treisman R. Mutant actins demonstrate a role for unpolymerized actin in control of transcription by serum response factor. Mol Biol Cell. 2002;13:4167–4178. doi: 10.1091/mbc.02-05-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meier C, Anastasiadou S, Knöll B. Ephrin-A5 suppresses neurotrophin evoked neuronal motility, ERK activation and gene expression. PLoS ONE. 2011;6:e26089. doi: 10.1371/journal.pone.0026089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen TJ, Gehler S, Shaw AE, Bamburg JR, Letourneau PC. Cdc42 participates in the regulation of ADF/cofilin and retinal growth cone filopodia by brain derived neurotrophic factor. J Neurobiol. 2006;66:103–114. doi: 10.1002/neu.20204. [DOI] [PubMed] [Google Scholar]
- 40.Knöll B, Drescher U. Ephrin-As as receptors in topographic projections. Trends Neurosci. 2002;25:145–149. doi: 10.1016/s0166-2236(00)02093-2. [DOI] [PubMed] [Google Scholar]
- 41.Garvalov BK, et al. Cdc42 regulates cofilin during the establishment of neuronal polarity. J Neurosci. 2007;27:13117–13129. doi: 10.1523/JNEUROSCI.3322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ketschek A, Gallo G. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci. 2010;30:12185–12197. doi: 10.1523/JNEUROSCI.1740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chua BT, et al. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol. 2003;5:1083–1089. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- 44.Rehklau K, et al. ADF/cofilin proteins translocate to mitochondria during apoptosis but are not generally required for cell death signaling. Cell Death Differ. 2012;19:958–967. doi: 10.1038/cdd.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 47.Bamburg JR, et al. ADF/Cofilin-actin rods in neurodegenerative diseases. Curr Alzheimer Res. 2010;7:241–250. doi: 10.2174/156720510791050902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cichon J, et al. Cofilin aggregation blocks intracellular trafficking and induces synaptic loss in hippocampal neurons. J Biol Chem. 2012;287:3919–3929. doi: 10.1074/jbc.M111.301911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma A, et al. A neurotoxic phosphoform of Elk-1 associates with inclusions from multiple neurodegenerative diseases. PLoS ONE. 2010;5:e9002. doi: 10.1371/journal.pone.0009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y, et al. Acute myeloid leukemia-associated Mkl1 (Mrtf-a) is a key regulator of mammary gland function. Mol Cell Biol. 2006;26:5809–5826. doi: 10.1128/MCB.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soosairajah J, et al. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005;24:473–486. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]