Abstract
Endoderm-mesenchyme cross-talk is a central process in the development of foregut-derived organs. How signaling pathways integrate the activity of multiple ligands to guide organ development is poorly understood. We show that two Wnt ligands, Wnt2 and Wnt7b, cooperatively induce Wnt signaling without affecting the stabilization of the Wnt canonical effector β-catenin despite it being necessary for Wnt2–Wnt7b cooperativity. Wnt2–Wnt7b cooperation is specific for mesenchymal cell lineages and the combined loss of Wnt2 and Wnt7b leads to more severe developmental defects in the lung than loss of Wnt2 or Wnt7b alone. High-throughput small-molecule screens and biochemical assays reveal that the Pdgf pathway is required for cooperative Wnt2-Wnt7b signaling. Inhibition of Pdgf signaling in cell culture reduces Wnt2–Wnt7b cooperative signaling. Moreover, inhibition of Pdgf signaling in lung explant cultures results in decreased Wnt signaling and lung smooth-muscle development. These data suggest a model in which Pdgf signaling potentiates Wnt2–Wnt7b signaling to promote high levels of Wnt activity in mesenchymal progenitors that is required for proper development of endoderm-derived organs, such as the lung.
Keywords: mesenchyme, epithelium
Numerous studies have demonstrated that the patterning and organ specification events of the foregut endoderm are determined, in part, by signaling cues from the surrounding mesenchyme (1, 2). Of these cues, Wnt signaling plays a potent and essential role in specifying anterior foregut identity (3). Multiple Wnt ligands are expressed in spatially specific patterns along the anterior-posterior axis and in both the developing endodermal and mesenchymal components of the gut tube. However, little is understood concerning how this expression complexity promotes regional specific foregut development.
The Wnt pathway is composed of multiple secreted ligands, cell-surface receptors, and intracellular effector proteins that comprise a highly interactive network that intersects with other pathways and promotes multiple processes, including cell fate specification, differentiation, migration, and proliferation (reviewed in refs. 4 and 5). Wnt2 and Wnt2b are expressed in the developing lung mesenchyme and signal canonically in a paracrine manner to promote lung specification in the anterior foregut endoderm (3, 6). In contrast, Wnt7b is expressed in the adjacent developing lung endoderm from the earliest time points of development and plays an important role in regulating proliferation of the endodermal and mesenchymal lineages within the developing lung (7, 8). However, whether these two families of Wnt ligands cooperate to promote anterior foregut development is unknown. Recent studies have shown that Wnt5a and Wnt11 can interact and cooperate to promote Xenopus axis formation, raising the possibility that specific Wnt ligand interactions may promote important aspects of vertebrate development (9).
Given the complementary expression patterns of Wnt2 and Wnt7b ligands and the fact that genetic inactivation of either Wnt2 or Wnt7b leads to a similar hypoplastic lung phenotype with defects in smooth-muscle development (3, 7, 8, 10, 11), we sought to determine whether these two Wnt ligands cooperate to promote development of the anterior foregut and lung. Using a combination of genetic, biochemical, and high throughput screening approaches, we show that Wnt2 and Wnt7 subfamiles cooperate to drive high levels of Wnt signaling activity specifically in mesenchymal lineages mediated by the Frizzled (Fzd) receptors Fzd5 and Fzd8. Moreover, we show that Pdgf signaling is necessary and sufficient to promote this cooperation. Taken together, these studies define a unique mechanism whereby certain Wnt ligand subfamiles cooperate to promote high levels of signaling in a cell lineage specific manner, in part through Pdgf signaling to regulate foregut organogenesis.
Results
Specific Subfamilies of Wnt Ligands Drive Cooperative Signaling Activation.
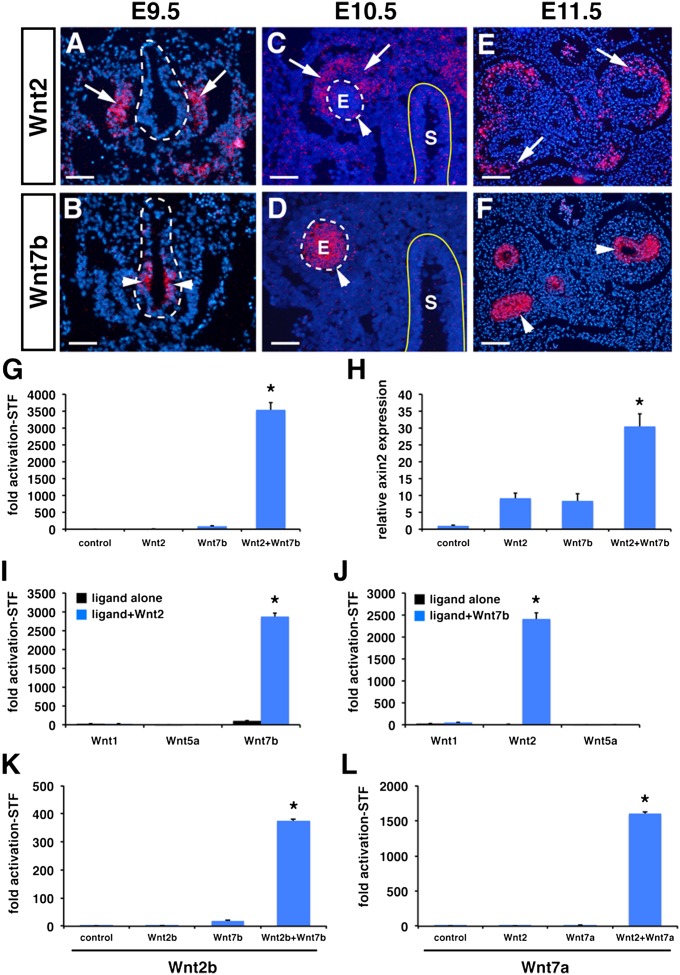
Previous work has shown Wnt2 and Wnt7b are expressed in mutually exclusive domains in the developing anterior foregut and lung (3, 7). Using in situ hybridization, we find that Wnt2 expression is observed in the mesenchyme on the ventral side of the anterior foregut at embryonic day (E) 9.5 (Fig. 1A). At this same time point, Wnt7b is expressed exclusively in the ventral anterior foregut endoderm where the lung endoderm progenitors are specified (Fig. 1B). This complementary expression pattern with Wnt2 expressed in the developing mesenchyme and Wnt7b expression restricted to the developing lung endoderm continues at E10.5 and E11.5 and throughout lung development (Fig. 1 C–F) (3, 7).
Fig. 1.
Wnt2 and Wnt7b are expressed in complementary expression patterns in the lung and promote cooperative signaling. Wnt2 is expressed in the developing lung mesenchyme from E9.5–E11.5 (A, C, E, arrows), but Wnt7b is expressed in the developing lung endoderm from E9.5–E11.5 (B, D, F, arrowheads). Wnt2 and Wnt7b promote high levels of cooperative signaling activity using the STF reporter (G) and by measuring endogenous Axin2 expression by qPCR (H). Wnt2 does not cooperate with Wnt1 or Wnt5a to promote STF activity (I). Wnt7b does not cooperate with Wnt1 or Wnt5a to promote STF activity (J). Wnt2b can cooperate with Wnt7b (K) and Wnt7a can cooperate with Wnt2 (L) to drive high levels of Wnt signaling. E, lung endoderm; S, stomach endoderm. Reporter assay results are mean of three replicates ± SD (*P < 0.05). (Scale bars: 75 μm in A–D; 150 μm in E and F.)
To test whether Wnt2 and Wnt7b can cooperate in promoting Wnt signaling, we expressed Wnt2 and Wnt7b, alone or in combination, along with the SuperTopFlash (STF) luciferase reporter to detect canonical Wnt signaling activity in the rat lung mesenchymal cell line RFL6 (12). Although either Wnt2 or Wnt7b expression alone results in reproducible but low-level STF activation, the combination of Wnt2 and Wnt7b results in a dramatic cooperative activation of STF (Fig. 1G). To determine whether Wnt2 and Wnt7b could also activate endogenous targets of Wnt signaling in a cooperative manner, we assessed expression of endogenous axin2, a well-described direct target of Wnt signaling, using quantitative PCR (qPCR) (13). These data show that Wnt2 and Wnt7b can activate endogenous axin2 in a cooperative manner in RFL6 cells (Fig. 1H). To determine whether the cooperative Wnt activitation by Wnt2 and Wnt7b is specific to these ligands, we tested whether Wnt1, a traditionally canonical Wnt ligand, or Wnt5a, a traditionally noncanonical Wnt ligand, could cooperate with Wnt2 or Wnt7b (14, 15). Our studies show that neither Wnt1 nor Wnt5a cause cooperative activation of Wnt signaling with Wnt2 or Wnt7b (Fig. 1 I and J). However, Wnt2b and Wnt7a are able to cooperatively activate STF, indicating that other members of the Wnt2 and Wnt7 families are capable of cooperation (Fig. 1 K and L).
Wnt2–Wnt7b Cooperative Signaling Is Essential for Early Lung Development.
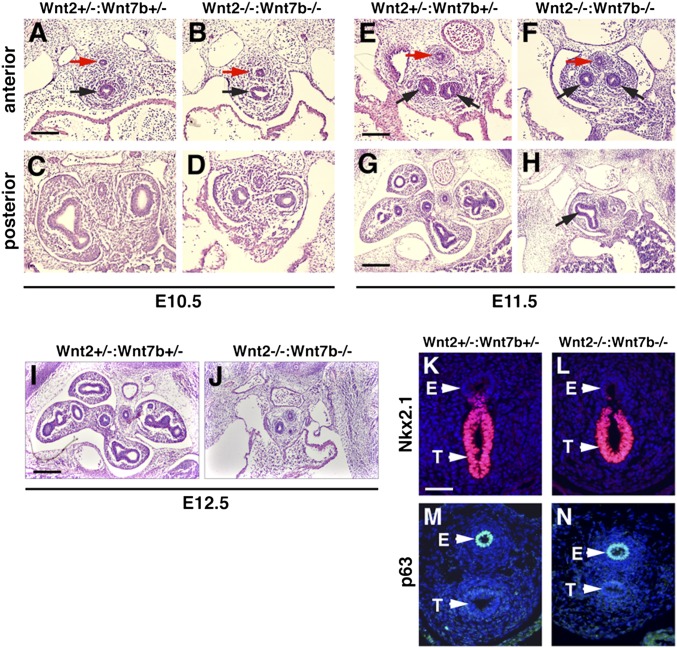
To determine whether the cooperation observed between Wnt2 and Wnt7b plays a significant role in vivo, we determined the consequences of loss of both Wnt2 and Wnt7b on early lung development. Of note, loss of either Wnt2 or Wnt7b alone results in a similar lung hypoplasia phenotype (3, 7, 8, 10). Lungs from Wnt2−/−:Wnt7b−/− double mutants show severe defects in branching morphogenesis, with an almost complete loss of secondary branching after trachea separation from the esophagus (Fig. 2 A–J). Because the Wnt2−/−:Wnt7b−/− mutants die by E13.5, our analysis was restricted to E12.5 and earlier time points. Although trachea-esophagus separation was apparent in the histological sections of Wnt2−/−:Wnt7b−/− double-mutants at all time points tested, we assessed expression of Nkx2.1 and p63, markers of lung and esophagus endoderm, respectively, to determine if lung specification occurred normally. Expression of Nkx2.1 was restricted to the developing trachea and expression of p63 was restricted to the developing esophagus in both control and Wnt2−/−:Wnt7b−/− mutants (Fig. 2 K–N) (16, 17). Thus, although the loss of Wnt2 or Wnt7b alone leads to a minor lung hypoplasia phenotype (7, 10), the combined loss of both Wnt2 and Wnt7b leads to a dramatic loss of lung branching morphogenesis but normal lung specification.
Fig. 2.
Wnt2−/−:Wnt7b−/− mutants have severe defects in early lung branching that are not observed in single mutants. Wnt2−/−:Wnt7b−/− mutants form a separate trachea/lung and esophagus (A and B, E and F, red versus black arrows), but the lung fails to undergo extensive subsequent branching after the first airway bifurcation (H, black arrow). By E12.5, Wnt2−/−:Wnt7b−/− mutant lungs have not progressed but control (Wnt2+/−:Wnt7b+/−) lungs have branched extensively (I and J). The expression patterns of Nkx2.1 and p63 at E10.5, which mark the primitive trachea (T) and esophagus (E), respectively, are normal in Wnt2−/−:Wnt7b−/− mutants compared with controls (K–N). (Scale bars: 75 μm in A–F; 150 μm in G–J; 50 μm in K–N.)
Wnt2–Wnt7b Cooperation Is Required for Development of Distal Lung Endoderm Progenitors.
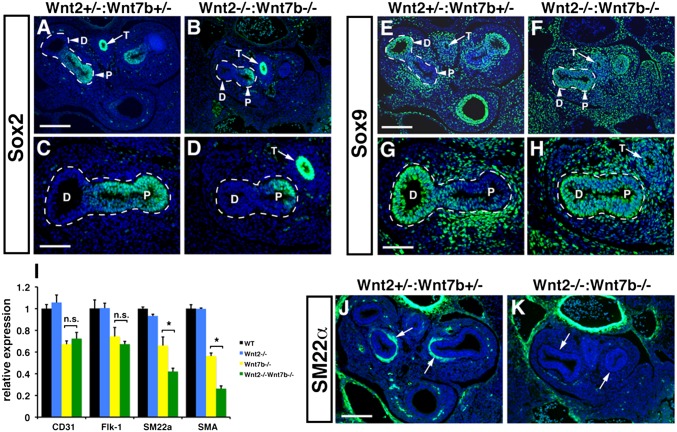
Given the significant defects in early branching of the lung, we examined whether there were alterations in proximal and distal progenitor development in the Wnt2−/−:Wnt7b−/− mutants. We assessed expression of Sox2, a marker of proximal endoderm progenitors, and Sox9, a marker of distal endoderm progenitors, in the developing lung (18, 19). Although Sox2 expression was confined to the proximal regions of the developing airway branches as well as the esophagus in both Wnt2+/−:Wnt7b+/− controls and Wnt2−/−:Wnt7b−/− double-mutants (Fig. 3 A–D), Sox9 expression was expressed at low levels and diffusely throughout the defective airway branches in the Wnt2−/−:Wnt7b−/− mutants and did not exhibit the polarized expression pattern apparent in the control lungs (Fig. 3 E–H). These data suggest that proximal-distal patterning of the distal lung endoderm progenitors is disrupted in Wnt2−/−:Wnt7b−/− mutants.
Fig. 3.
Defective distal progenitor and lung mesenchyme development in Wnt2−/−:Wnt7b−/− mutants. The Sox2 expression pattern is normal in Wnt2−/−:Wnt7b−/− mutants compared with controls (A–D). However, the Sox9 expression pattern is more diffuse indicating defective distal progenitor development in Wnt2−/−:Wnt7b−/− mutants (E–H). qPCR analysis shows that smooth-muscle markers SM22α and smooth-muscle α-actin (SMA) are decreased in a cooperative manner by loss of both Wnt2 and Wnt7b expression (I). SM22α expression reveals a significant loss in smooth muscle development in Wnt2−/−:Wnt7b−/− mutants (J and K, arrows). Histology and qPCR were performed on E11.5 embryos. D, distal lung endoderm; P, proximal lung endoderm; T, trachea. qPCR results are average of three samples ± SEM (n.s, no significance; *P < 0.01). (Scale bars 150 μm in A, B, E, F, J, K; 75 μm in C, D, G, H.)
Because endoderm-mesenchyme signaling is essential for patterning of lung mesodermal derivatives, we examined expression of endothelial and smooth-muscle markers in Wnt2−/−:Wnt7b−/− mutant lung buds. Combined loss of Wnt2 and Wnt7b leads to a cooperative loss of smooth-muscle gene expression (Fig. 3I). Immunostaining for SM22α expression shows a dramatic loss, and in some cases absence, of smooth-muscle development in Wnt2−/−:Wnt7b−/− mutant lungs (Fig. 3 J and K). Interestingly, although loss of Wnt7b leads to decreased expression of endothelial cell markers CD31 and Flk-1, the additional loss of Wnt2 did not further decrease expression of these genes, suggesting that smooth-muscle differentiation was particularly sensitive to the cooperative affects of Wnt2 and Wnt7b signaling (Fig. 3I).
Wnt2–Wnt7b Cooperative Signaling Occurs in Mesenchymal but Not Epithelial Cell Lineages.
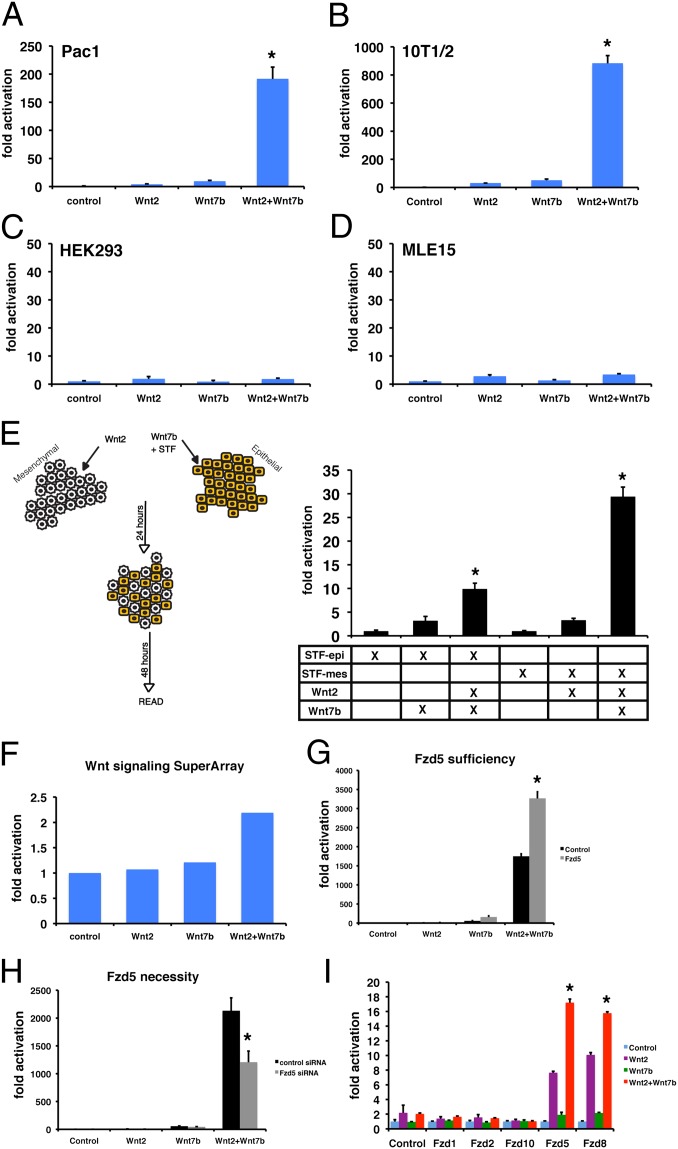
Because Wnt2 is expressed specifically in the developing mesenchyme of the lung and Wnt7b is expressed specifically in the developing epithelium of the lung (3, 7), we wanted to determine whether the cooperation between Wnt2 and Wnt7b functioned in both cell lineages. Therefore, we expressed Wnt2, Wnt7b, or both in two additional mesenchymal cells types, Pac1 and 10T1/2 cells, which represent rat vascular smooth muscle and mouse embryonic fibroblasts, respectively. We also performed the same experiments in two epithelial cell types, HEK-293 and MLE15 cells, which represent human kidney and mouse lung epithelium, respectively. These studies showed that coexpression of Wnt2 and Wnt7b resulted in cooperative Wnt signaling in both Pac1 and 10T1/2 cells but no cooperative signaling was observed in HEK-293 or MLE15 cells (Fig. 4 A–D). To assess whether the Wnt2–Wnt7b cooperative signaling occurred in a paracrine manner, we expressed Wnt2 and Wnt7b in RFL6 and HEK-293 cells, respectively, but transfected the STF reporter in only one of the cell types to assess cell-specific Wnt activity. When the STF reporter was transfected into HEK-293 cells, Wnt2–Wnt7b cooperative signaling was not observed (Fig. 4E). However, when the STF reporter was transfected into RFL6 cells, we observed high levels of Wnt2–Wnt7b cooperative signaling (Fig. 4E). Taken together, these data indicate that mesenchymal cell lineages respond to Wnt2–Wnt7b cooperative signaling in a paracrine manner, which explains the loss of airway smooth-muscle development in Wnt2−/−:Wnt7b−/− mutants, and suggests that the defects in patterning of Sox9+ progenitors is secondary to defects in the adjacent mesenchyme and its ability to direct proximal-distal progenitor development.
Fig. 4.
Wnt2–Wnt7b cooperative signaling occurs in mesenchymal but not epithelial cell lineages and Fzd5 and Fzd8 are critical receptors for Wnt2–Wnt7b cooperative signaling. Wnt2–Wnt7b cooperative signaling occurs in Pac1 and 10T1/2 cells but no cooperation is observed in HEK293 and MLE15 cells (A–D). Wnt2–Wnt7b cooperative signaling occurs robustly in a paracrine fashion in mesenchymal cells (RFL6) but less so in epithelial cells (HEK293) in a cell mixture assay (E). Analysis of a qPCR Superarray assay shows that Fzd5 is induced by Wnt2–Wnt7b cooperative signaling (F). Overexpression of Fzd5 in RFL6 cells can enhance Wnt2–Wnt7b cooperative signaling (G), but siRNA knockdown of Fzd5 expression inhibits this cooperation (H). Among Fzds tested, only Fzd5 and the highly related Fzd8, can confer Wnt2–Wnt7b cooperative signaling to nonresponsive HEK293 cells (I). Reporter assay results are mean of three replicates ± SD (*P < 0.01).
Fzd5 and Fzd8 Promote Wnt2–Wnt7b Cooperative Signaling.
To assess Wnt signaling components that may promote or inhibit Wnt2–Wnt7b cooperative signaling, we performed a qPCR screen for genes known to be involved in the Wnt pathway. From this screen, Fzd5 was identified as a receptor that was strongly induced by Wnt2–Wnt7b cooperative signaling (Fig. 4F). Overexpression of Fzd5 in RFL6 cells enhanced Wnt2–Wnt7b cooperation (Fig. 4G). Moreover, siRNA knockdown of Fzd5 inhibited Wnt2–Wnt7b cooperative signaling in RFL6 cells (Fig. 4H and Fig. S1). To test whether expression of other Fzds were able to confer Wnt2–Wnt7b cooperation in nonresponsive epithelial cells, we transfected expression vectors for Fzd1, Fzd2, Fzd8, and Fzd10 along with Wnt2 and Wnt7b expression vectors into HEK-293 cells, which are nonresponsive to Wnt2–Wnt7b cooperation (Fig. 4C). These data show that expression of Fzd5 or Fzd8 was able to confer Wnt2–Wnt7b cooperative signaling to HEK-293 cells (Fig. 4I). Fzd5 and Fzd8 are highly related Fzds and both are expressed in the lung (20, 21). Interestingly, Fzd5 increased Wnt2 but not Wnt7b signaling suggesting that Fzd5 promotes the Wnt2–Wnt7b cooperative signaling by enhancing Wnt2 activity, which is in line with previous work showing that Wnt2 synergizes with the Fzd5 receptor in Xenopus axis development (22). The finding that Fzd5 and Fzd8 can promote Wnt2–Wnt7b cooperative signaling suggests that receptor availability may partly explain the cooperative signaling in mesenchymal but not epithelial cell lineages.
Wnt2–Wnt7b Cooperative Signaling Does Not Lead to Increased Nuclear β-Catenin Levels.
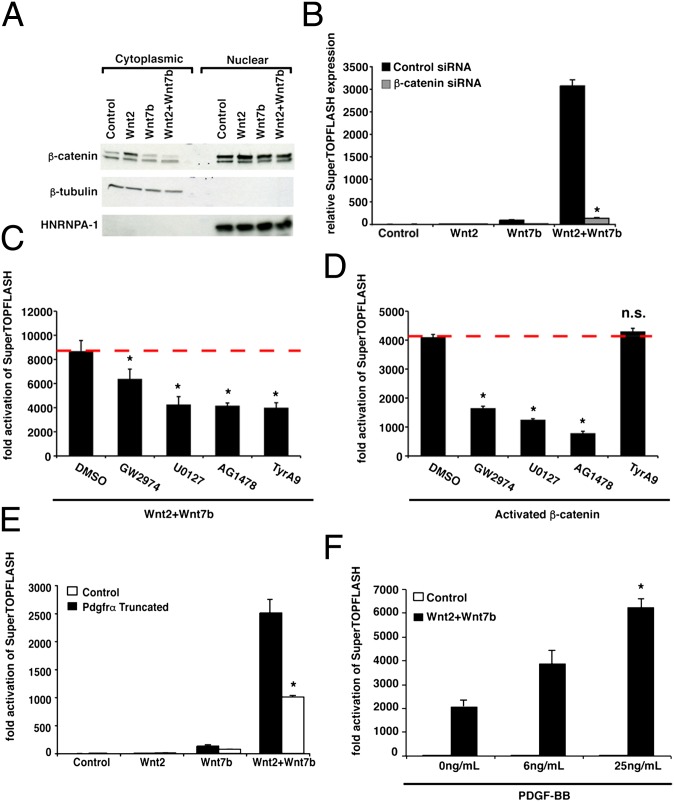
The strong induction in canonical Wnt signaling observed by Wnt2–Wnt7b signaling suggests that this cooperativity leads to a concordant increase in nuclear β-catenin levels. Therefore, we performed Western blots for β-catenin on both cytoplasmic and nuclear fractions of RFL6 cells expressing Wnt2, Wnt7b, or Wnt2 and Wnt7b (Fig. 5A). These experiments revealed a lack of increased nuclear or cytoplasmic β-catenin levels by coexpression of Wnt2 and Wnt7b, although a slight increase was observed by expression of Wnt2 alone (Fig. 5A). In contrast, siRNA-mediated inhibition of β-catenin expression resulted in decreased Wnt2–Wnt7b cooperative signaling (Fig. 5B and Fig. S1). Thus, although Wnt2–Wnt7b cooperative signaling did not lead to increased levels of β-catenin, steady-state expression of β-catenin was necessary for the signaling to occur.
Fig. 5.
Nuclear β-catenin levels are not altered by Wnt2–Wnt7b cooperative signaling and Pdgf signaling is essential for Wnt2–Wnt7b cooperation. Western blots were performed on nuclear and cytoplasmic fractions of RFL6 cells expressing Wnt2, Wnt7b, or Wnt2+Wnt7b. A slight increase in β-catenin levels was observed for Wnt2-expressing cells but no further increase was observed in Wnt2+Wnt7b-expressing cells (A). The nuclear and cytoplasmic fractions were pure as noted by sequestered expression of β-tubulin and HNRNPA-1, respectively. siRNA knockdown of β-catenin in RFL6 cells inhibits the Wnt2–Wnt7b cooperation (B). From the LOPAC screen four compounds—AG1478, U0126, GW2974, and Tryphostin A9—targeted the Egfr or Pdgfr pathways. The effects of AG1478, U0126, GW2974, and Tryphostin A9 on Wnt2–Wnt7b signaling and activated β-catenin signaling were compared using a 1-μM dose for each compound. Although AG1478, U0126, and GW2974 all inhibited β-catenin signaling (D), Tryphostin A9 affected Wnt2–Wnt7b signaling but not activated β-catenin signaling (C). A dominant-negative truncated Pdgfrα decreased STF activation (E). The addition of Pdgf-BB ligand to Wnt2+Wnt7b-transfected RFL6 cells results in increased STF activation (F). Reporter assay results are mean of three replicates ± SD (n.s., no significant difference; *P < 0.05).
High-Throughput Small-Molecule Screen Reveals the Importance of Pdgf Signaling in Wnt2–Wnt7b Cooperative Signaling.
To identify potential pathways that promote Wnt2–Wnt7b signaling independent of β-catenin signaling, we performed a high-throughput small-molecule screen using 1,280 pharmacologically active compounds (LOPAC, Library of Pharmacologically Active Compounds) that selectively inhibit components of most major signaling pathways to detect pathways important for Wnt2–Wnt7b cooperation. We excluded hits that promoted cell-cycle arrest, cell death, or proliferation based on software accompanying the library and focused on multiple hits toward common signaling pathways that inhibited signaling by at least 35% (Fig. S2). This screen identified four compounds that target the Egf/Pdgf signaling pathways: (i) Tyrphostin AG1478, a selective inhibitor of EGFR activity (23); (ii) U0126, a MEK1/2 inhibitor (24); (iii) GW2974, a dual EGFR and ErbB-2 receptor tyrosine kinase inhibitor (25); and (iv) Tyrphostin A9, a selective Pdgfr tyrosine kinase receptor inhibitor (26). Interestingly, the Egf and Pdgf pathways have been implicated in regulating Wnt signaling activity (11, 27). All four of these compounds inhibited Wnt2–Wnt7b cooperative signaling in a dose-dependent manner (Fig. S2).
Based on our findings that expression of β-catenin is necessary for Wnt2–Wnt7b cooperative signaling but that steady-state levels of β-catenin do not change significantly in this cooperative signaling (Fig. 5 A and B), we assessed whether inhibition of Egf or Pdgf signaling inhibited Wnt/β-catenin signaling in general, or specifically inhibited the Wnt2–Wnt7b cooperative signaling. A pathway inhibitor that affected Wnt2–Wnt7b cooperative signaling without affecting activated β-catenin signaling would implicate this pathway in the specific regulation of Wnt2–Wnt7b signaling. These studies showed that only the Pdgf signaling inhibitor Tyrphostin A9 inhibited Wnt2–Wnt7b cooperative signaling without effecting activated β-catenin signaling (Fig. 5 C and D). To further support the Pdgf chemical inhibitor data, we expressed a dominant-negative truncated form of Pdgfrα (Pdgfrα truncated) in RFL6 cells, along with Wnt2, Wnt7b, and STF. We observed a similar decrease in the cooperation between Wnt2 and Wnt7b with the expression of a truncated form of Pdgfrα as we observed with Tyrphostin A9 (Fig. 5E). To test whether activation of Pdgf signaling could enhance Wnt2–Wnt7b cooperative signaling, RFL6 cells expressing Wnt2 and Wnt7b were treated with recombinant Pdgf-BB ligand. These studies show that Pdgf-BB caused a significant increase in Wnt2–Wnt7b cooperative signaling (Fig. 5F).
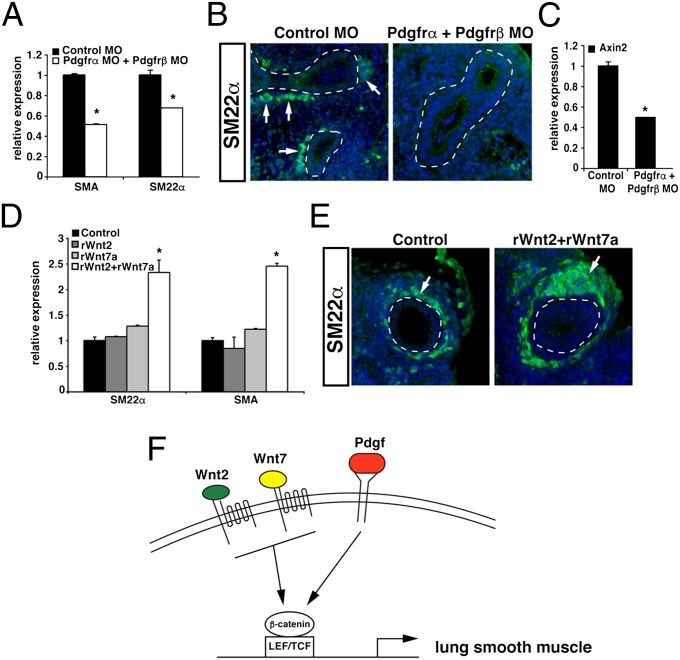
To determine if this potent role for Pdgf signaling in the Wnt2–Wnt7b cooperation was necessary for smooth-muscle development in the lung, lung explants were treated with splice-blocking morpholinos against Pdgfrα and Pdgfrß, which resulted in significantly decreased expression of these two receptors (Fig. S3). Knockdown of Pdgfrα and Pdgfrß resulted in a significant decrease in smooth-muscle gene expression as well as a decreased smooth-muscle development surrounding the lung airways (Fig. 6 A and B). Moreover, decreased Pdgfrα/β expression in the lung leads to decreased axin2 gene expression, suggesting that Pdgfrα/β are required for endogenous levels of Wnt activity in the lung (Fig. 6C). To further assess whether the combined activity of the Wnt2 and Wnt7 ligand families could cooperatively increase smooth-muscle development in the lung, we treated lung explants with recombinant Wnt2, Wnt7a, or Wnt2 plus Wnt7a. Recombinant Wnt7a was used in these studies as recombinant Wnt7b was not available and Wnt7a cooperates with Wnt2 (Fig. 1L). These data show that Wnt2 and Wnt7a can cooperatively increase both smooth muscle gene expression and development in the lung (Fig. 6 D and E). Taken together, these data reveal a unique Wnt signaling mechanism whereby Pdgf signaling promotes a specific Wnt2–Wnt7b cooperative signaling mechanism required for mesenchymal cell differentiation in the lung (Fig. 6F).
Fig. 6.
Pdgfr signaling is necessary for lung smooth-muscle development and Wnt2–Wnt7b cooperative signaling promotes lung smooth-muscle development in lung explants. Knockdown of Pdgfrα/β in E11.5 lung explants resulted in decreased smooth-muscle gene expression by qPCR (A). Immunohistochemistry for SM22α showed decreased smooth-muscle formation surrounding the airways of Pdgfrα/β knockdown in E11.5 lung explants, compared with control explants; n = 3 lung explants for each condition (B). The decreased Pdgfrα/β expression resulted in decreased axin2 gene expression (C), suggesting cross-talk between the Pdgf and Wnt signaling pathways. Addition of recombinant Wnt2 and Wnt7a to E11.5 lung explants resulted in increased smooth-muscle gene expression by qPCR (E) and increased smooth-muscle development surrounding the airways (F); n = 3 lung explants for each condition. Model of Wnt2–Wnt7b cooperative signaling and the promotion of lung mesenchymal development. Epithelial Wnt7b signals in a paracrine manner to Wnt2 expressing mesenchymal cells. The combined action of Wnt2 and Wnt7b, in combination with Pdgf signaling, results in increased canonical Wnt signaling, which allows for proper smooth-muscle development (D). qPCR results are average of three samples ± SEM (*P < 0.01). (Magnification in B and E, 200×.)
Discussion
In this study, we show that specific Wnt ligands can cooperatively induce high levels of Wnt signaling to regulate proximal distal patterning and mesenchyme development in the lung. We found that the combinatorial actions of Wnt2 and Wnt7b promote mesenchymal cell-specific Wnt activity that is mediated, in part, through the Pdgf pathway. The necessity of this Wnt2–Wnt7b cooperation is demonstrated by the dramatic lung phenotype in Wnt2−/−:Wnt7b−/− mutant lungs, which display a severe truncation of branching morphogenesis along with disrupted distal endoderm progenitor patterning. Our results highlight the importance of reciprocal epithelial/mesenchymal signaling for lung development and show the necessity of specific combinations of Wnt ligands to promote foregut-derived organ development.
Endoderm–mesenchymal interactions are a recurrent theme throughout embryonic development. Although the foregut endoderm begins as an undifferentiated sheet of epithelium, the ventral aspect of the foregut endoderm will eventually give rise to multiple organs, including the thyroid, lung, liver, and ventral pancreas. An abundance of literature highlights the in vivo importance of epithelial–mesenchymal interactions for lung specification and development. During lung endoderm specification, Wnt2/Wnt2b function in a paracrine fashion to specify Nkx2.1+ lung endoderm progenitors within the ventral anterior foregut endoderm (3). Conditional deletion of β-catenin activity from the foregut endoderm phenocopies the Wnt2/Wnt2b lung agenesis phenotype indicating that Wnt2/2b act in a canonical fashion to specify Nkx2.1+ lung progenitors (3, 6). Based on the present and previous studies, we propose a model in which expression of Wnt2 and Wnt7b, along with Pdgfr activity, promotes maximal Wnt signaling activity in the lung mesenchyme, resulting in proper differentiation of specific cell lineages, such as smooth muscle. How Pdgfr signaling promotes maximal signaling in cooperation with Wnt2 and Wnt7b is unclear, but could involve several different mechanisms, including posttranslational modifications of β-catenin, that enhance its activity by releasing the repressive activity of a repressor, or enhancing the activity of a β-catenin coactivator.
Our work has shown that the combination of Wnt2 and Wnt7b does not result in a concordant increase in β-catenin accumulation that could explain the high levels of reporter activity observed. Examples of increased β-catenin signaling, with modest to no effects on β-catenin accumulation, have been seen in previous studies where modifications to β-catenin or the binding of coactivators to promoter regions enhances canonical signaling. The transcriptional coactivator p300 has been shown to affect the activity of β-catenin through acetylation. This acetylation results in an increased β-catenin affinity for Tcf4 and higher levels of signaling, without affecting nuclear β-catenin accumulation (28). Protein kinase A has been shown to phosphorylate β-catenin, which results in β-catenin binding to the transcriptional coactivator cyclic AMP-responsive element-binding protein, leading to increased signaling without affecting β-catenin accumulation (29). A similar mechanism could occur in the cooperation between Wnt2 and Wnt7b, in which Pdgf signaling results in coactivator activation leading to high levels of canonical signaling, without a reciprocal increase in nuclear β-catenin accumulation.
Our finding that Wnt2 and Wnt7b specifically cooperate during lung development to uniquely enhance transcriptional activation suggests that there exists distinct functions for specific Wnt ligands, the combinatorial actions of which may explain how broad domains of canonical Wnt activity during embryonic development affect only specific cell lineages to allow for organ specification and development.
Materials and Methods
Cell Culture and Transient Transfection Assays.
RFL6, Pac1, HEK293, and 10T1/2 cells were cultured as previously described (30, 31). Cells were transfected with the indicated expression plasmids using Fugene 6 (Roche). Forty-eight hours following transfection, luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega). For cell-mixture experiments, RFL6 and HEK293 cells were transfected with the indicated plasmids for 24 h, after which they were mixed in equal numbers. Forty-eight hours following mixture, luciferase assays were performed. For siRNA knockdown experiments, 24 h following transfection with expression vectors, cells were transfected with siRNAs directed against β-catenin or Fzd5 (Dharmacon) using Lipofectamine 2000 (Invitrogen). Luciferase assays were performed 48 h following transfection of siRNA. For chemical inhibitor assays, 24 h following transfection with expression vectors, chemical inhibitors were added at the indicated concentrations for 24 additional hours, followed by luciferase assays.
qPCR Wnt Signaling Array.
Total RNA was isolated from control and Wnt2+Wnt7b transfected RFL6 cells and used to generate cDNA, which was then used in a Rat Wnt signaling pathway PCR array which screens for 84 known components of the Wnt pathway (SABiosciences). See Table S1 for a list of qPCR primers.
LOPAC High-Throughput Small-Molecule Screen.
RFL6 cells were transfected with CMV-βgal or pcDNA3.1-Wnt2 and Wnt7b expression plasmids, along with STF. Twenty-four hours following transfection, cells were replated into 384-well tissue culture plates at 3 × 104 cells per well and compounds from the LOPAC library (Sigma-Aldrich) were added at a final concentration of 10 μM. Twenty-four hours following plating and addition of the LOPAC library, luciferase assays were performed using BriteLite reagent (Perkin-Elmer). The assay was repeated and data were normalized to the median of each 384 well plate. The publically available Sigma-Aldrich LOPAC1280 navigator was used to determine the targets and effects of the compounds (http://www.sigmaaldrich.com/chemistry/drug-discovery/validation-libraries/lopac1280-navigator.html).
Lung Explant Culture.
Lung buds were dissected from E11.5 embryos and cultured as previously described (10). Morpholinos and recombinant proteins were added for the indicated times and explants were isolated for either qPCR (48 h incubation) or histology (72 h incubation).
Supplementary Material
Acknowledgments
We thank Jeanne Geskes for her help in performing the Library of Pharmacologically Active Compounds screen and Andrius Kazlauskas for the truncated, dominant-negative human Pdgfrα plasmid. The E.E.M. Laboratory is supported by funding from National Institutes of Health Grants HL087825 and HL100405.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201583109/-/DCSupplemental.
References
- 1.Masters JR. Epithelial-mesenchymal interaction during lung development: The effect of mesenchymal mass. Dev Biol. 1976;51:98–108. doi: 10.1016/0012-1606(76)90125-1. [DOI] [PubMed] [Google Scholar]
- 2.Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Goss AM, et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. Beta-catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci USA. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopal J, et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–1634. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development. 2008;135:3719–3729. doi: 10.1242/dev.029025. [DOI] [PubMed] [Google Scholar]
- 10.Goss AM, et al. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol. 2011;356:541–552. doi: 10.1016/j.ydbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen ED, et al. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119:2538–2549. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 13.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol. 1995;15:2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- 16.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 17.Daniely Y, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–C181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 18.Tian Y, et al. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development. 2011;138:1235–1245. doi: 10.1242/dev.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 20.De Langhe SP, et al. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol. 2005;277:316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Huang HC, Klein PS. The Frizzled family: Receptors for multiple signal transduction pathways. Genome Biol. 2004;5:234. doi: 10.1186/gb-2004-5-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa T, et al. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- 23.Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur J Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 24.Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 25.Rusnak DW, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: Potential therapy for cancer. Cancer Res. 2001;61:7196–7203. [PubMed] [Google Scholar]
- 26.Bilder GE, et al. Tyrphostins inhibit PDGF-induced DNA synthesis and associated early events in smooth muscle cells. Am J Physiol. 1991;260:C721–C730. doi: 10.1152/ajpcell.1991.260.4.C721. [DOI] [PubMed] [Google Scholar]
- 27.Kim SE, Choi KY. EGF receptor is involved in WNT3a-mediated proliferation and motility of NIH3T3 cells via ERK pathway activation. Cell Signal. 2007;19:1554–1564. doi: 10.1016/j.cellsig.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Lévy L, et al. Acetylation of beta-catenin by p300 regulates beta-catenin-Tcf4 interaction. Mol Cell Biol. 2004;24:3404–3414. doi: 10.1128/MCB.24.8.3404-3414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25:5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wikenheiser KA, et al. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.