Abstract
Antimicrobials have been used extensively as growth promoters (AGPs) in agricultural animal production. However, the specific mechanism of action for AGPs has not yet been determined. The work presented here was to determine and characterize the microbiome of pigs receiving one AGP, tylosin, compared with untreated pigs. We hypothesized that AGPs exerted their growth promoting effect by altering gut microbial population composition. We determined the fecal microbiome of pigs receiving tylosin compared with untreated pigs using pyrosequencing of 16S rRNA gene libraries. The data showed microbial population shifts representing both microbial succession and changes in response to the use of tylosin. Quantitative and qualitative analyses of sequences showed that tylosin caused microbial population shifts in both abundant and less abundant species. Our results established a baseline upon which mechanisms of AGPs in regulation of health and growth of animals can be investigated. Furthermore, the data will aid in the identification of alternative strategies to improve animal health and consequently production.
Keywords: intestinal microbiota, microbiome maturation, metagenomics
In the 1940s, it was observed that animals fed dried mycelia of Streptomyces aureofaciens that naturally contained chlortetracycline exhibited enhanced growth (1). Since the 1950s, antimicrobials have been used in agricultural animal production for the treatment, control, and prevention of infectious diseases, as well as for growth promotion (2, 3). The National Swine Survey showed that 41% of feeds included one or more individual antimicrobials and 19% included combinations of antimicrobials. Tylosin and bacitracin were most often used in feeds for grower and finisher pigs (4, 5). Because the use of antimicrobials leads to the emergence of resistance to antimicrobials (6, 7), efforts to reduce their use with the goal of preserving the efficacy of current antimicrobials are being implemented.
The specific mechanisms whereby antimicrobials act as growth promoters (AGPs) are unclear. Early studies demonstrated that oral antimicrobials did not have growth-promoting effects when given to germ-free animals (8). Therefore, it is likely that growth promotion is based on changes to the gut microbiota. Several mechanisms of how growth promoters act have been postulated including the prevention of subclinical infections and the reduction in microbial use of nutrients (2, 9). AGPs also could act by reducing the presence of opportunistic pathogens in animals fed AGPs. Increased immune mediators such as interleukin-1 induced by gut bacteria have been shown to reduce feed conversion in animals with a conventional microflora (10), which illustrate that the host’s response to the indigenous microflora could be a factor limiting growth efficiency. Direct effects of AGPs on the gut microflora might result in decreased competition for nutrients and a reduction in microbial metabolites that depress animal growth (9). Certain bacteria in the gut are known to be metabolically important for animal growth. For example, cellulolytic bacteria in ruminants digest cellulose into fermentable glucose that serves as a substrate for further microbial fermentation and for use by the animal. Cellulose would not otherwise be used by the host. Therefore, it is highly likely that growth promotion could be mediated by selection of specific bacterial populations that contribute to the metabolism of the animal thereby enhancing feed conversion.
It is our hypothesis that AGPs cause alterations of the gut microflora, and these changes bring increased feed efficiency to animals resulting in growth promotion. A first step to understanding the process of growth promotion is to identify and describe alterations in the gut microflora of the pig. Recently there have been three reports on microbial shifts in response to the use of AGPs using culture independent assessments (11–13). Overall, these studies were small in scale and used animals held in infectious disease isolation facilities and did not replicate conditions comparable to how commercial pigs are reared. The work presented here was designed to better understand the effects that a single growth promoter had on the microbiome of pig feces using a longitudinal study design that used pigs in typical commercial settings. Tylosin, a member of the macrolide family of antimicrobials, was selected for this study because it is thought to be the most commonly used AGP. The results presented here demonstrate that tylosin produces consistent and specific alterations of the distal intestinal microflora of the pig.
Results
DNA Sequence Data and Quality Control.
The studies described were designed to detect changes in the fecal microbiome of pigs being raised in conventional production units over time. The study design used two groups of pigs (10 pigs per group). One group received tylosin in their feed during the entire experimental period, whereas the second group did not receive tylosin. The protocol was performed using two independent farms. DNA sequences in each group and farm were analyzed as pooled groups based on our previous results that demonstrated strong similarities between the fecal microbiomes of pigs within the same pen (14). However, because samples from each pig had a bar code identifier, we were able to quantify the number of sequence reads per animal. A total of 504,204 and 1,543,479 DNA sequence reads were generated from farm 1 and farm 2, respectively. Over 86% and 94% of the total number of sequence reads from farm 1 and farm 2 passed the quality control implemented in this study, resulting in 435,225 and 1,445,371 sequences for farm 1 and farm 2. The total number of sequence reads from farm 2 was 3.3 times larger than that of farm 1 because of the improved sequencing chemistry used with samples from farm 2 (GS-FLX chemistry for farm 1 and titanium chemistry for farm 2). The average number of sequence reads generated per pig was 4,352 for farm 1 and 14,454 for farm 2. The median sequence read length for both the tylosin and no-tylosin groups on farm 1 and farm 2 was 137–138 bases with no ambiguous bases. The range of sequence reads was 81–196 bases and, when a homopolymer run was detected, the median length of the homopolymer was 5 bases (Table S1).
Microbial Diversity.
The diversity of microbial communities using pooled sequence reads from all ten pigs in each group was measured using Shannon–Weaver and Simpson (1-D) diversity indices (15). The diversity indices used represent how many different taxa are present in a sample, and higher numbers indicate higher diversity. The average Shannon–Weaver and Simpson (1-D) index values showed highly diverse microbial communities with mean values per group of 4.87 and 0.96 for farm 1, and 5.28 and 0.97 for farm 2. The range of these calculated values over the five sampling times (3-wk intervals starting at 10 wk of age and ending 22 wk of age) was 4.39–5.50 for Shannon–Weaver and 0.95–0.98 for Simpson (1-D). Both diversity indices were greater in younger animals than in older animals (Table S2).
Microbial Shifts in Response to the Use of Tylosin as an AGP: Taxon-Based Analysis.
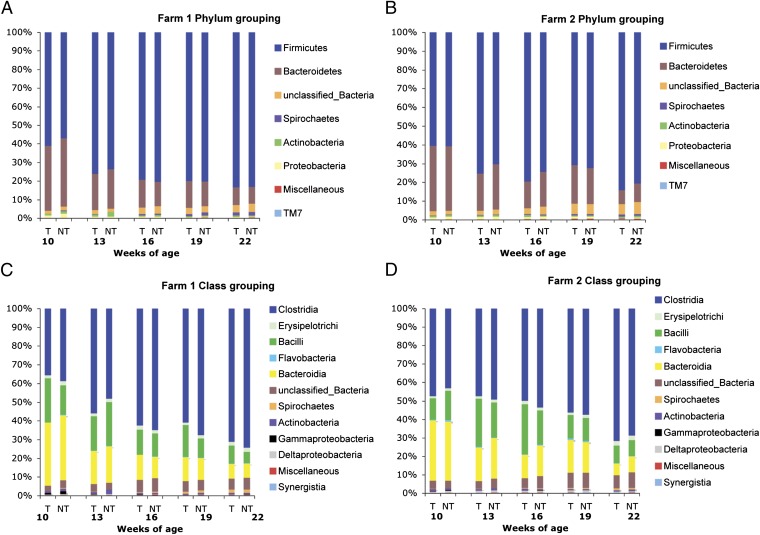
A taxon-dependent analysis using the Ribosomal Database Project (RDP) classifier was conducted to describe the composition of the fecal microbiome between animals receiving tylosin and those that did not receive tylosin and how it changed over time (16). The results of phylum and class distributions are shown in Fig. 1. Two phyla, Firmicutes and Bacteroidetes, were the most dominant in the fecal samples regardless of age of pigs or treatment group, and comprised more than 92% of the total sequences. The proportion of sequences that could not be assigned to a phylum using RDP classifier ranged from 1.61% to 6.37%. Bacteria in the phylum Spirochaetes increased over time ranging from 0.07% to 1.85%. The other phyla represented less than 1% of the total at all time points. The proportion of bacteria in the phyla Firmicutes, Spirochaetes, and unclassified phyla increased as the pigs aged, whereas the proportion of bacteria in the phyla Bacteroidetes, Actinobacteria, and Proteobacteria decreased. These changes were consistently observed in both farms. When the relative abundance of bacteria at the phylum level was compared between tylosin and no-tylosin groups of the same age, there were no significant differences (Fig. 1 A and B).
Fig. 1.
RDP classification of the sequences at phylum and class levels. RDP classifier was used with a bootstrap cutoff of 50. Pooled sequence reads from all 10 pigs in each group were used. (A) RDP classification of the sequence reads from farm 1 at phylum level. (B) RDP classification of the sequence reads from farm 2 at phylum level. (C) RDP classification of the sequence reads from farm 1 at class level. (D) RDP classification of the sequence reads from farm 2 at class level.
Bacteria in the classes Clostridia, Erysipelotrichi, Bacilli, Bacteroidia, Spirochaetes, and unclassified classes made up 95.58% of the total bacteria at all times. Bacteria in other classes represented less than 1% at most time points. Two classes, Clostridia and Bacilli represented the most common classes within the phylum Firmicutes. The proportion of Clostridia increased as pigs got older, whereas the proportion of Bacilli decreased. The increase in the proportion of Clostridia was greater than the decrease in the proportion of Bacilli resulting in an overall increase in the population of Firmicutes over time. The class Bacteroidia was the major class in the phylum Bacteroidetes, and the proportion of Bacteroidia decreased over time (Fig. 1 C and D).
A total of 158 and 187 genera were identified from farm 1 and farm 2, respectively. Of the total number of genera identified in both farms, 16 abundant genera were detected. The abundant genera were defined as having more than 1% of the total DNA sequences. The 16 most abundant genera were: Anaerobacter, Prevotella, Streptococcus, Lactobacillus, Coprococcus, Sporacetigenium, Megasphaera, Blautia, Oscillibacter, Subdoligranulum, Faecalibacterium, Dialister, Pseudobutyrivibrio, Roseburia, Sarcina, and Butyricicoccus. These 16 genera plus the unclassified genera accounted for 90.3% and 90.5% of the total sequences from farms 1 and 2, respectively. One genus, Prevotella, was a member of phylum Bacteroidetes; the other 15 genera belonged to the phylum Firmicutes. Among the 16 abundant genera, the detection frequencies of the genera Anaerobacter, Streptococcus, Coprococcus, Sporacetigenium, Oscillibacter, Roseburia, and Sarcina showed increases over time, whereas those in the genera Prevotella, Lactobacillus, Megasphaera, Blautia, Subdoligranulum, Faecalibacterium, Dialister, Pseudobutyrivibrio, and Butyricicoccus decreased (Tables S3, S4, and S5).
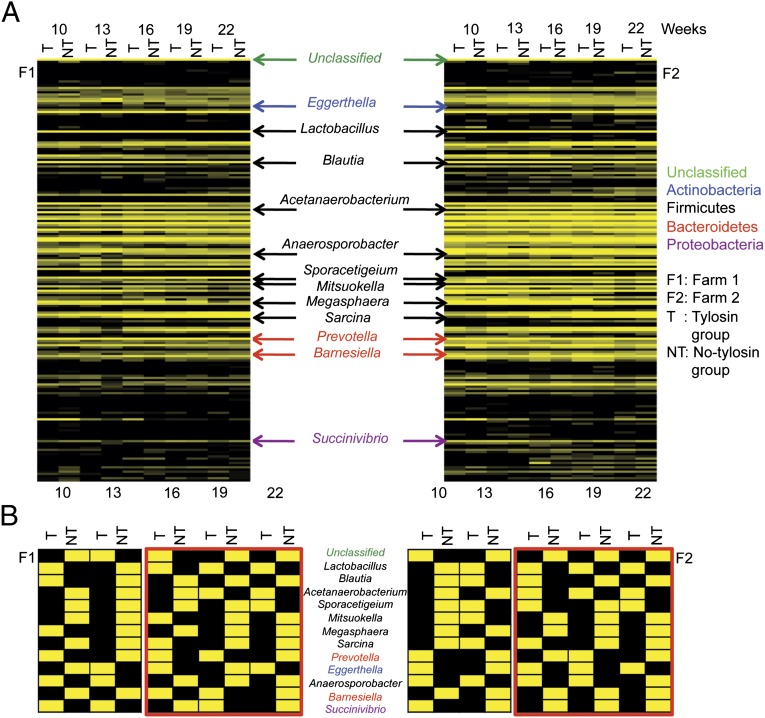
To compare how the composition of the fecal bacteria differed between treatment groups, Metastats was used to identify differentially abundant genera. Although there were no differences between treatment groups at the phylum level, a total of 12 differentially abundant genera were identified from both farms at the genus level (Fig. 2 A and B). These included six abundant (>1% of the total sequences) and six less abundant genera. The six abundant genera consisted of Prevotella, Lactobacillus, Sporacetigenium, Megasphaera, Blautia, and Sarcina, and the less abundant genera consisted of Barnesiella, Mitsuokella, Acetanaerobacterium, Anaerosporobacter, Succinivibrio, and Eggerthella. Eight of the 12 differentially abundant genera belonged to the phyla Firmicutes and two of 12 belonged to Bacteroidetes. Each of the other two genera was in the phyla Proteobacteria, and Actinobacteria. Lactobacillus, Sporacetigenium, Acetanaerobacterium, and Eggerthella were detected more frequently in the tylosin group than in no-tylosin group. The others were present more frequently in the no-tylosin group than the tylosin group. Unclassified bacteria at the genus level also were differentially represented in pigs from both farms and were observed more frequently in the no-tylosin group. The same pattern of population distribution of the differentially abundant genera was observed from both farms at 22 wk. The population distribution patterns of differentially abundant genera at 22 wk began to emerge from 16 wk and continuing through 19 wk of age (Fig. 2 A and B).
Fig. 2.
Differentially abundant genera between tylosin and no-tylosin groups. Pigs in the tylosin groups received tylosin in their feed during the whole experimental period (10–22 wk of age), whereas pigs in the no-tylosin groups did not receive tylosin. The heat map was created using genus based results after normalization. Yellow indicates abundant genera and black indicates less abundant genera. Each column represents groups and each row indicates genus. F1 and F2 indicate farm 1 and farm 2, respectively, and T and NT represent each tylosin group and no-tylosin group. (A) Differentially abundant genera were indicated by arrows and color-coded. Genus names indicate by the color their relationship to the phylum level. (B) Twelve differentially abundant genera and unclassified genera.
Microbial Shifts in Response to the Use of Tylosin as an AGP: Operational Taxonomic Unit (OTU)-Based Analysis.
Although most sequences were classified at the phylum and class levels, a high proportion of sequences could not be classified at the genus level. Therefore, a taxon-independent analysis using OTUs as the unit of analysis was conducted for the in depth ecological analysis of the bacterial communities. We defined an OTU as having 95% sequence identity. Compared with a total of 158 and 187 genera identified in farm 1 and farm 2, a total of 5,499 and 12,637 OTUs were identified in farm 1 and farm 2, respectively. Of those OTUs, 421 and 870 OTUs on farm 1 and farm 2, respectively, were defined in our study as core OTUs because they existed in all groups at all five time points in each farm. Although core OTUs comprised only 7.68% and 6.88% of the total OTUs in farms 1 and 2, these core OTUs contained the majority of the sequences, 82.4% and 85.4% of the total sequences from farms 1 and 2. These results indicate that animals share a core set of bacteria that are the most prevalent microbes. This core represents a small number of species regardless of their ages. Therefore, the major variation of bacterial populations is mainly a result of the less dominant species present in different individuals. Representative sequences for each core OTU were retrieved and subjected to a taxonomic analysis using RDP classifier. As would be expected, most of the core OTUs belonged to the phyla Firmicutes and Bacteroidetes, accounting for 86.22% and 87.01% of the total core OTUs in farms 1 and 2, respectively.
Venn diagrams were generated to compare OTUs between groups at the same time points (Fig. S1). An average of 66.48% and 68.60% of the total OTUs were shared between groups at the same age, and an average of 97.89% and 98.47% of the total sequence reads belonged to the shared OTUs in farm 1 and farm 2, respectively. Unique OTUs, which accounted for an average of 33.52% and 31.40% of the total OTUs at the same age, contained just 2.11% and 1.53% of the total sequence reads in farm 1 and farm 2 (Fig. S1). Although shared OTUs were comprised of both abundant and less abundant OTUs, unique OTUs to each group were mainly less abundant OTUs. Sequence reads belonging to unique OTUs at different time points were subjected to analysis using RDP classifier. RDP classifier is a web based program that assigns 16S rRNA sequences to phylogenetically consistent bacterial taxonomy. Unclassified bacteria at the genus level were the most abundant at 64.11% and 63.89% of the total unique sequence reads to each group in farms 1 and farm 2. Among the OTUs classified at the genus level, Prevotella was the most abundant genus accounting for 7.67% and 9.04% of the total unique sequence reads in farms 1 and 2 (Table S6).
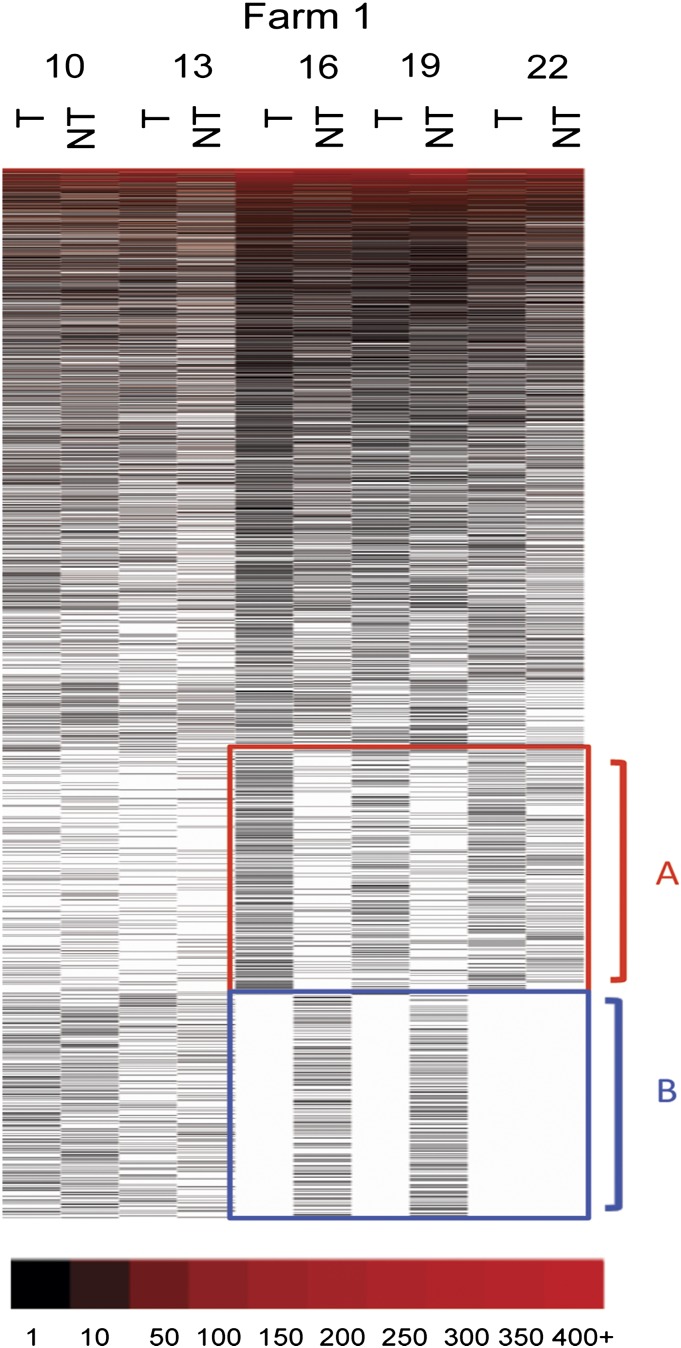
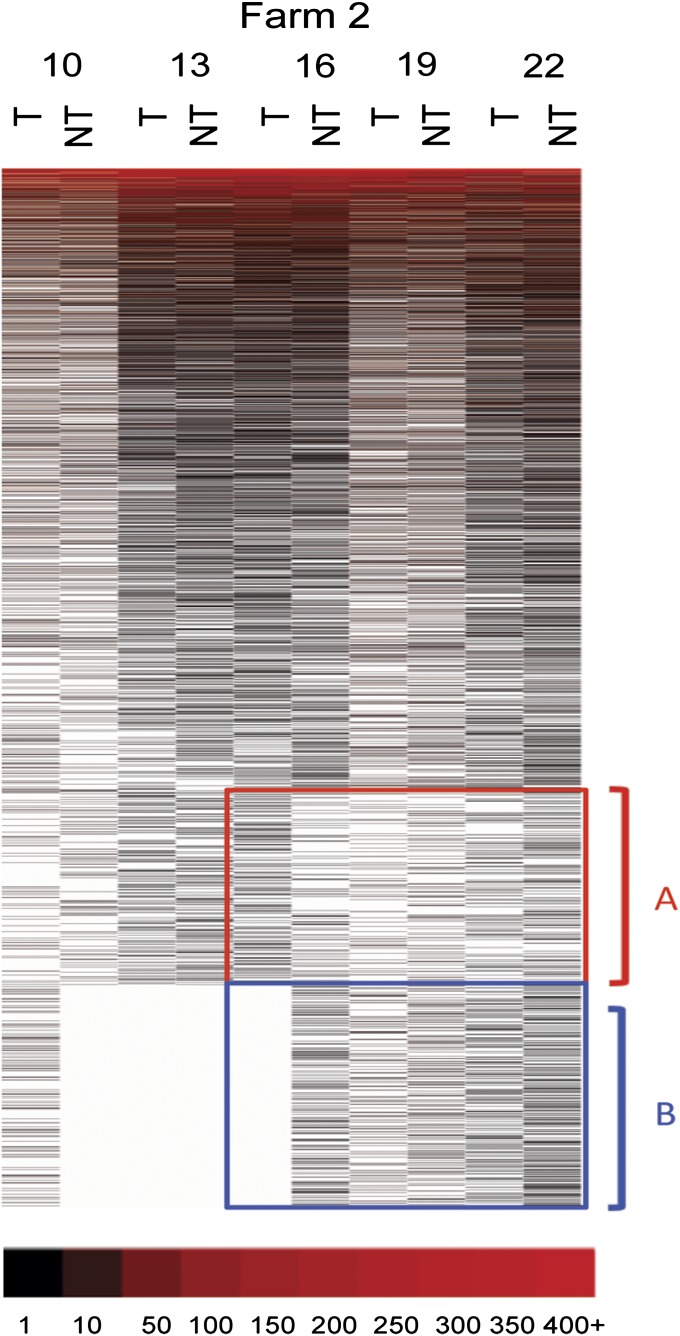
To visualize the distribution of the different OTUs in the two treatment groups, a heat map was prepared (Fig. 3). The OTUs were ordered by abundance with the most abundant sequences being at the top of the heat map. Abundant OTUs were red color-coded and white blanks indicated missing OTUs. Two regions of the heat map, labeled A and B, contained close to 40% of the total OTU sequences. These regions were highlighted because of the apparent differences in their distributions between the pigs in the two treatment groups. The fecal microbiomes of pigs that were 10 wk of age had nearly identical heat map patterns regardless of treatment group. This finding would be expected because pigs only began to receive tylosin at this time. However, by 16 wk and continuing through 19 wk of age, there was an obvious shift in microbial content in the feces in pigs treated with tylosin with the depletion of OTUs in bracket B and an increase in the OTUs in bracket A. When pigs reached 22 wk of age, a similar shift was found in pigs that did not receive tylosin (Fig. 3). When the same process was used to analyze pigs from farm 2, a more complicated pattern emerged (Fig. 4) that did not mimic what was observed with farm 1. We did detect differences in the compositions of the fecal microbiomes between the two treatment groups. However, the ordering of the OTUs did not result in the same heat map patterns in the regions designated A and B. This could result because the depth of sequence coverage was much greater for farm 2 and thus the ordering of the sequence abundance was different for farm two, the two farms could not be directly compared using heat maps for visualization.
Fig. 3.
Distribution of OTUs in the two treatment groups in farm 1. The heat map for farm 1 was created using OTUs with an OTU definition at a similarity cutoff of 95%. Each column represents groups and each row indicates OTUs. OTUs were sorted with the most abundant OTUs displayed at the top and the least abundant OTUs at the bottom. T and NT indicate tylosin and no-tylosin group, respectively, and numbers indicates weeks of age. Abundant OTUs were red color-coded and white blanks indicate missing OTUs.
Fig. 4.
Distribution of OTUs in the two treatment groups in the farm 2. The heat map for the farm 2 was created using OTUs with an OTU definition at a similarity cutoff of 93%. Each column represents groups and each row indicates OTUs. OTUs were sorted with the most abundant OTUs displayed at the top and the least abundant OTUs at the bottom. T and NT indicate tylosin and no-tylosin group, respectively, and numbers indicates weeks of age. Abundant OTUs were red color-coded and white blanks indicate missing OTUs.
Dendrograms were prepared by using the Bray–Curtis index to compare the similarity of the bacterial communities between groups. Whereas microbial population shifts in both abundant and less abundant genera in pig intestines were detected, the samples clustered based on sampling time point (Fig. S2).
Discussion
We hypothesized that the beneficial effects of tylosin would be mediated by compositional changes of the pig gut bacterial communities (2, 11, 17, 18). This hypothesis is partly based on the understanding that AGPs lack growth promoting effects in germ-free animals (8, 19) and because many antimicrobials with different modes of action promote animal growth. The work presented here was designed to better understand the effects that a single AGP, tylosin, has on the fecal microbiome of the pig. We showed that tylosin consistently and specifically altered the microbiome of feces of pigs.
Using 16S rRNA gene sequencing we previously showed profound changes in the pig fecal microbiome over time (14). Here we showed that tylosin results in significant changes in specific genera and OTUs and that some of these changes occur at unique times within the growth of pigs (Figs. 2 and 3). What was most striking were the changes in the less abundant OTUs in pigs from farm 1 over time (see groups bracketed with A and B in Fig. 3). Before exposure to tylosin, the fecal microbiomes of pigs in the two groups were quite similar. However, there was a pronounced shift in the distribution and quantity of microbes in regions A and B with treatment with tylosin being correlated with a shift of OTUs in the B region of the heat map to the A region (Fig. 3). As the untreated pigs reached maturity, their fecal microbiomes, particularly in the A and B regions become more like pigs in the tylosin treatment group. Our interpretation of these results is that tylosin speeds the development or maturation of the unique “adult-like” fecal microbiome but that eventually pigs in the nontreatment groups finally catch up. However, the same pattern of redistribution of OTUs was not overtly seen when a similar heat map of OTUs in pigs from farm 2 was observed (Fig. 4). This difference could be the result of several possibilities. The first possibility is a technical issue that is based on the fact that much deeper sequencing was performed for samples from farm 2. This resulted from the shift to “titanium” chemistry use during sequencing of samples from farm 2. Thus, because of the difference in coverage the actual ordering of the OTUs in the two heat maps could be different. A second possibility is that, because the data were obtained from pigs from two different farms, their microbiomes were not identical and the compositional changes caused by tylosin therefore were different. However, the genus level analysis (Fig. 2) did show compositional changes in common to both farms when pigs treated with tylosin were compared with nontreated pigs. A third possibility is that our interpretation based on farm 1 that tylosin speeds up the maturation of the gut microbiome is not correct and that the actual compositional changes identified through the genus level analysis are microbes closely linked to feeding of tylosin. To distinguish these possibilities additional sampling from additional farms will be necessary.
There was no significant difference in the proportion of taxonomic groupings of the fecal microbiomes at the phylum level regardless of treatment group at all sampling times. However, there were statistical differences when measured at the genus level. A total of twelve differentially abundant genera were observed in both farms at the genus level (Fig. 2 A and B). Lactobacillus, Sporacetigenium, Acetanaerobacterium, and Eggerthella were detected more frequently in the tylosin group than in no-tylosin group, and the others were present more frequently in the no-tylosin group than the tylosin group (Fig. 2B).
In previous studies using pigs as an animal model, certain species of Lactobacillus have been shown to increase in concentration in AGP-treated pigs (11, 12, 20). In this study we also detected a relative increase in the concentration of the genus Lactobacillus. A positive relation between the increase in abundance of Lactobacillus spp. population and an increase in weight gain has been shown in other animal models (18, 21).
Sporacetigenium, Acetanaerobacterium, and Eggerthella were more abundant in pigs with tylosin treatment. In vitro studies showed that Sporacetigenium and Acetanaerobacterium spp ferment mono, di-, or oligosaccharides into acetic acid, ethanol, hydrogen and carbon dioxide (22, 23). Sporacetigenium spp also has been identified as one of common bacterial population in fermentation reactors where the main volatile fatty acids were acetic acid and propionic acid (24). It should be noted that about 5–20% of the total energy of the pigs is provided by fermentation end products including acetic and propionic acids in the cecum and colon (9). Eggerthella spp is a Gram-positive bacteria, which is known to be involved in metabolism of catechin and lignan that are plant origin (25, 26). However, physiological and functional roles of Eggerthella in the intestines of food animals are unknown.
In comparing the results reported here with those from other studies using AGPs, differences in the microbial population shifts did occur (11, 12, 20). It is unclear why these differences occurred, but is likely related to differences in the AGP used. The AGP used in our study was tylosin, whereas the other three studies used combination of chlortetracycline with different AGPs. Likewise, we elected to use feces as the collected sample so that we could repeatedly sample the same pigs, whereas Rettedal et al. and Collier et al. collected tissue or digesta in their studies. We also elected to use commercial pigs in a production setting instead of experimental isolation facilities where access to different sets of microbes in different environments might be responsible for this inconsistency. Furthermore, in our study two farms were used and the microbial shifts between the two farms were quite similar.
Venn diagrams were generated to make qualitative comparisons between tylosin and no-tylosin groups at the same age. It should be noted that almost all of the sequences were shared between tylosin and no-tylosin groups at the same age. An average of 97.89% and 98.47% of the total bacterial sequences belonged to the shared OTUs. These OTUs represented an average of 66.48% and 68.60% of the total OTUs in farm 1 and farm 2, respectively (Fig. S1). This indicates that unique OTUs to each group were more likely to be found as less abundant OTUs because unique OTUs that accounted for average of 33.52% and 31.40% of the total OTUs at the same age contained just 2.11% and 1.53% of the bacterial sequences in farm 1 and farm 2 (Fig. S1). This result is consistent with the data visualization based on heat maps where the greatest number of differences appear toward the bottom of the heat maps.
Given that unique OTUs are less abundant OTUs, abundant and beneficial functions from less abundant microbes can be considered (27, 28). Sequence reads belonging to OTUs unique to each tylosin and no-tylosin groups were subjected to the RDP classifier analysis. RDP classifier analysis indicated that the majority of unique OTUs were unclassified bacteria at genus level, accounting for 64.11% and 63.89% of the total unique sequence reads in farm 1 and farm 2 (Table S6). However, it remains unknown at this point whether unclassified bacteria contributed to fundamental metabolic functions within gastrointestinal tract of the animals.
Materials and Methods
Full protocols are available in SI Materials and Methods.
Animals and Sample Collection.
Two independent commercial farms were used in this study. Ten pigs from the two pens were randomly selected and ear tagged for identification. Pigs in one pen received tylosin [40 ppm (40 g/ton)] in their feed, whereas pigs in the other pen did not. Fresh fecal samples were individually collected from the rectum of each of the ear tagged animals at 3-wk intervals starting when the pigs were 10 wk old.
Isolation of DNA.
Total DNA containing the fecal microbial communities was extracted from individual fecal samples using an established method (29). The protocol was modified by including 1,000 μL of ASL buffer and InhibitEX tablets. Both reagents are components of the QIAgen DNA Mini Stool kit (QIAGEN). DNA quantity was measured using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific).
PCR Amplicon Production and Sequencing.
PCR primers that flanked the V3 hypervariable region of bacterial 16S rRNA genes were designed. The primer sequences were 5′-(Roche A)-10-base barcode-CCTACGGGAGGCAGCAG-3′ (forward) and 5′-(Roche B)-ATTACCGCGGCTGCTGG-3′ (reverse) (14, 30, 31). (The forward primers were composed of the Roche A sequence followed by a unique 10 base pair barcode and then the region flanking V3.) The PCR amplicon products were generated by 20 cycles of PCR reactions. Only PCR products without primer dimers and contaminant bands were used for pyrosequencing. The PCR products from different pigs in the same time group were pooled together in equimolar ratios. The pooled PCR amplicons were sequenced at the Biomedical Genomic Center at the University of Minnesota using a Roche 454 GS-FLX sequencer (454 Life Sciences).
Data Analysis.
Only high-quality sequences obtained after quality control analysis were used in our analysis (full protocols are described in SI Materials and Methods). Phylogenetic assessments were performed using RDP classifier with a bootstrap cutoff of 50% (16). Metastats (32) was used to detect differentially abundant genera in samples after each sequence read was assigned to a taxon by RDP classifier. Richness and diversity indices were generated using Mothur (version 1.21.1) with an OTU definition at an identity cutoff of 95% after having implemented a pseudosingle linkage algorithm (15, 33, 34). Heat maps were generated using Mothur (version 1.21.1) and Java TreeView (15, 35).
Supplementary Material
Acknowledgments
We thank Dr. Russ Bey and Dr. Keith Wilson from Newport Laboratories (Worthington, MN) and Dr. Kwang-Soo Lyoo for their support and help. We thank Minnesota Supercomputing Institute of the University of Minnesota for their technical support. H.B.K. was supported by a Doctoral Dissertation Fellowship provided by the University of Minnesota. This work was supported by US Department of Agriculture/National Research Initative Grant 2007-35212-18046.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequences used in this paper are publicly available at Galaxy, https://main.g2.bx.psu.edu/u/kim-et-al/h/kim-et-al.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205147109/-/DCSupplemental.
References
- 1.Jones FT, Ricke SC. Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult Sci. 2003;82:613–617. doi: 10.1093/ps/82.4.613. [DOI] [PubMed] [Google Scholar]
- 2.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: History and mode of action. Poult Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- 3.Niewold TA. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult Sci. 2007;86:605–609. doi: 10.1093/ps/86.4.605. [DOI] [PubMed] [Google Scholar]
- 4.McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34(Suppl 3):S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- 5.Dewey CE, Cox BD, Straw BE, Bush EJ. Use of antimicrobials in swine feeds in the United States. Swine Health and Production. 1999;7:19–25. [Google Scholar]
- 6.Schwarz S, Chaslus-Dancla E. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet Res. 2001;32:201–225. doi: 10.1051/vetres:2001120. [DOI] [PubMed] [Google Scholar]
- 7.Phillips I, et al. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J Antimicrob Chemother. 2004;53:28–52. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- 8.Coates ME, Fuller R, Harrison GF, Lev M, Suffolk SF. A comparison of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. Br J Nutr. 1963;17:141–150. doi: 10.1079/bjn19630015. [DOI] [PubMed] [Google Scholar]
- 9.Gaskins HR, Collier CT, Anderson DB. Antibiotics as growth promotants: Mode of action. Anim Biotechnol. 2002;13:29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- 10.Roura E, Homedes J, Klasing KC. Prevention of immunologic stress contributes to the growth-permitting ability of dietary antibiotics in chicks. J Nutr. 1992;122:2383–2390. doi: 10.1093/jn/122.12.2383. [DOI] [PubMed] [Google Scholar]
- 11.Looft T, et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc Natl Acad Sci USA. 2012;109:1691–1696. doi: 10.1073/pnas.1120238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rettedal E, et al. Alteration of the ileal microbiota of weanling piglets by the growth-promoting antibiotic chlortetracycline. Appl Environ Microbiol. 2009;75:5489–5495. doi: 10.1128/AEM.02220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen HK, et al. Antibiotics in feed induce prophages in swine fecal microbiomes. MBio 2. 2011;2 doi: 10.1128/mBio.00260-11. e00260-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HB, et al. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet Microbiol. 2011;153:124–133. doi: 10.1016/j.vetmic.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Schloss PD, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole JR, et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37(Database issue):D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman HD, Johnson ZB. Use of antibiotics and roxarsone in broiler chickens in the USA: analysis for the years 1995 to 2000. Poult Sci. 2002;81:356–364. doi: 10.1093/ps/81.3.356. [DOI] [PubMed] [Google Scholar]
- 18.Lin J. Effect of antibiotic growth promoters on intestinal microbiota in food animals: A novel model for studying the relationship between gut microbiota and human obesity? Front Microbiol. 2011;2:53. doi: 10.3389/fmicb.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coates ME, Davies MK, Kon SK. The effect of antibiotics on the intestine of the chick. Br J Nutr. 1955;9:110–119. doi: 10.1079/bjn19550016. [DOI] [PubMed] [Google Scholar]
- 20.Collier CT, et al. Molecular ecological analysis of porcine ileal microbiota responses to antimicrobial growth promoters. J Anim Sci. 2003;81:3035–3045. doi: 10.2527/2003.81123035x. [DOI] [PubMed] [Google Scholar]
- 21.Dumonceaux TJ, Hill JE, Hemmingsen SM, Van Kessel AG. Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken. Appl Environ Microbiol. 2006;72:2815–2823. doi: 10.1128/AEM.72.4.2815-2823.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Song L, Dong X. Sporacetigenium mesophilum gen. nov., sp. nov., isolated from an anaerobic digester treating municipal solid waste and sewage. Int J Syst Evol Microbiol. 2006;56:721–725. doi: 10.1099/ijs.0.63686-0. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Dong X. Acetanaerobacterium elongatum gen. nov., sp. nov., from paper mill waste water. Int J Syst Evol Microbiol. 2004;54:2257–2262. doi: 10.1099/ijs.0.63212-0. [DOI] [PubMed] [Google Scholar]
- 24.Ren N, et al. Microbial community structure of ethanol type fermentation in bio-hydrogen production. Environ Microbiol. 2007;9:1112–1125. doi: 10.1111/j.1462-2920.2006.01234.x. [DOI] [PubMed] [Google Scholar]
- 25.Mabrok HB, et al. Lignan transformation by gut bacteria lowers tumor burden in a gnotobiotic rat model of breast cancer. Carcinogenesis. 2012;33:203–208. doi: 10.1093/carcin/bgr256. [DOI] [PubMed] [Google Scholar]
- 26.Kutschera M, Engst W, Blaut M, Braune A. Isolation of catechin-converting human intestinal bacteria. J Appl Microbiol. 2011;111:165–175. doi: 10.1111/j.1365-2672.2011.05025.x. [DOI] [PubMed] [Google Scholar]
- 27.Walker A. Say hello to our little friends. Nat Rev Microbiol. 2007;5:572–573. doi: 10.1038/nrmicro1720. [DOI] [PubMed] [Google Scholar]
- 28.Arumugam M, et al. MetaHIT Consortium Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36:808–812. doi: 10.2144/04365ST04. [DOI] [PubMed] [Google Scholar]
- 30.Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parameswaran P, et al. A pyrosequencing-tailored nucleotide barcode design unveils opportunities for large-scale sample multiplexing. Nucleic Acids Res. 2007;35:e130. doi: 10.1093/nar/gkm760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLOS Comput Biol. 2009;5:e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schloss PD, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol. 2005;71:1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol. 2010;12:1889–1898. doi: 10.1111/j.1462-2920.2010.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.