Abstract
With frigid temperatures and virtually no in situ productivity, the deep oceans, Earth’s largest ecosystem, are especially energy-deprived systems. Our knowledge of the effects of this energy limitation on all levels of biological organization is very incomplete. Here, we use the Metabolic Theory of Ecology to examine the relative roles of carbon flux and temperature in influencing metabolic rate, growth rate, lifespan, body size, abundance, biomass, and biodiversity for life on the deep seafloor. We show that the relative impacts of thermal and chemical energy change across organizational scales. Results suggest that individual metabolic rates, growth, and turnover proceed as quickly as temperature-influenced biochemical kinetics allow but that chemical energy limits higher-order community structure and function. Understanding deep-sea energetics is a pressing problem because of accelerating climate change and the general lack of environmental regulatory policy for the deep oceans.
Keywords: metabolism, benthos, macroecology, ecosystem function
Life requires energy. The flux and transformation of energy influences processes and patterns across levels of biological organization. Three distinct types of energy affect biological systems: solar radiation in the form of photons, thermal kinetic energy as indexed by temperature, and chemical potential energy stored in reduced carbon compounds (1). Genomic, phenotypic, and taxonomic diversity and complexity are correlated with variation in energy availability in space and time (1, 2). For example, the acquisition of mitochondria through endosymbiosis allowed for increases in energy expenditure, which in turn, facilitated increases in coding genome size and complexity (3). Global variation in metabolic rates and life history traits, particularly in ectotherms, in part reflects variation in temperature (4). The tremendous range in body size among metazoans is tied both to patterns of carbon accessibility and temperature (5–7). The rapid proliferation of higher-order taxa during the Mesozoic Marine Revolution is posited to have been driven by increases in energy availability (8, 9).
The deep oceans, which encompass depths below 200 m, cover most of Earth and are especially energy-deprived systems. Globally, temperatures of most of the seafloor vary between −1 °C and 4 °C (10). These cold temperatures limit the biochemical kinetics of metabolism. Photosynthetically active radiation is nonexistent, and consequently, primary production is virtually absent, occurring only through alternative pathways, such as chemosynthesis. However, chemosynthesis represents a small percentage of total ocean production (0.02–0.03%) and a small percentage (3%) of carbon flux to nonchemosynthetic systems (11). The chemical energy that sustains most deep-sea organisms is sequestered from sinking particulate organic carbon (POC) derived from primary production in the euphotic zone hundreds of meters to kilometers above. POC flux decreases with depth in the water column, because material is remineralized, and distance seaward from productive coastal regions. At the abyssal seafloor, this downward flux represents less than 1% of surface production (12).
Although the availability of specific types of energy is important at some levels of biological organization in the deep sea, its effects at other levels are unknown. Body size and temperature are primary determinants of metabolic rate for benthic deep-sea organisms (13–15). Previous work indicates that, after accounting for these variables within clades, metabolic rates do not vary with depth (13, 14), which is inversely related to POC flux (16, 17). The influence of energy availability on individual growth rates and lifespans is unknown. At the community level, biomass and abundance generally decline with depth. Direct tests for the influences of POC and temperature on these community attributes are rare, but they suggest only weak effects for temperature (16, 18). Although broad-scale patterns of deep-sea biodiversity are well-established and presumably linked to POC, specific tests of this relationship remain limited (reviewed in ref. 19). In a recent study of species–energy relationships for modern deep-sea mollusks of the North Atlantic, POC had substantially greater predictive power than temperature (20), which is in contrast to findings from fossil assemblages of deep-sea ostracods, where temperature generally prevailed (21, 22). It remains unclear whether these results can be generalized to larger spatial scales and other taxa.
Our meager knowledge of energetics in the deep sea is unfortunate considering the rapid and accelerating climate change and the general lack of environmental policy for conserving deep ecosystems (23). Recent research indicates that global phytoplankton production has declined at a rate of ∼1% of the global median per year over the last century (24). Regional-scale changes have been more heterogeneous, with the equatorial Pacific experiencing overall declines of ∼50% over the last decade and polar regions experiencing increases of comparable magnitude (25). The deep sea is also warming. The deep Mediterranean water mass has warmed by 0.12 °C since the middle of the last century (26). Deep oceans now store 16–89% more heat (27). These modifications and redistribution of total energy in the oceans will inevitably impact the deep-sea fauna, perhaps rapidly (28–30). Clearly, there is a strong need for a more complete understanding of energetics at the deep-sea floor to enable greater understanding and predictive power for the consequences of forthcoming climate change.
Here, we use the Metabolic Theory of Ecology (MTE) (4) as a framework to understand and link energetics across multiple scales of biological organization in the deep sea. Specifically, we use MTE to examine the relative roles of carbon flux and temperature in influencing metabolic rate, growth rate, lifespan, body size, abundance, biomass, and biodiversity across broad taxonomic and geographic scales (Fig. 1). Specifically, we assess (i) the effects of the availability of thermal kinetic energy (i.e., temperature) and chemical potential energy (i.e., POC) on the deep-sea benthos at different levels of biological organization from individual to community to ecosystem; (ii) the extent that extremely energy-limited systems follow common macroecological patterns; and (iii) how deep-sea ecosystems may be affected by climate change. We show that the relative effects of thermal and chemical energy vary considerably across organizational scales. Consequently, climate change may greatly impact the capacity for biodiversity, carbon cycling, and general ecosystem function in the deep oceans.
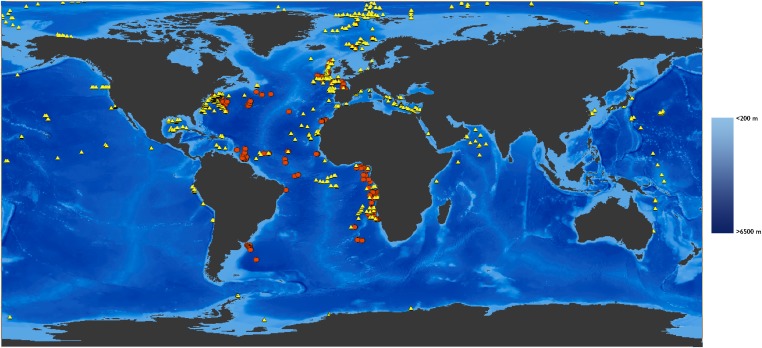
Fig. 1.
Sampling locations of bacteria, meiofauna, macrofauna, and megafauna used in the standing stock (yellow triangles) and mollusks used in the diversity analyses (orange circles). Areas shallower than 200 m (i.e., continental shelf) are indicated by pale blue.
Results
Metabolic Rate.
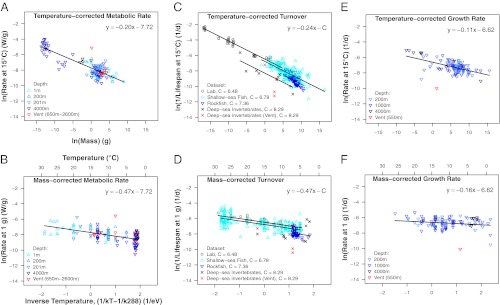
Body size and temperature account for significant fractions of the variance in metabolic rate for deep-dwelling taxa collected at depths > 200 m (R2 = 0.70; F = 86.11, df = 2, 75; P < 10−15) and shallow-dwelling taxa (R2 = 0.44; F = 15.69, degrees of freedom (df) = 2, 40; P < 10−5). The two groups exhibit no significant differences with respect to the exponent characterizing size dependence (F = 0.38, df = 2, 116; P = 0.69), the activation energy characterizing temperature dependence (F = 1.16, df = 2, 116; P = 0.32), or the metabolic normalization characterizing the size- and temperature-corrected rates (F = 0.13, df = 1, 117; P = 0.72). Thus, shallow- and deep-dwelling benthic organisms are well-characterized by a single metabolic rate model (solid lines in Fig. 2 A and B), with a mass exponent (−0.20) that is close to but slightly lower than the MTE-predicted value of −0.25 [95% confidence interval (CI) = −0.23 to −0.17), and an activation energy (0.47 eV) that includes the predicted range of 0.6–0.7 eV (95% CI = 0.32–0.62 eV) (Eq. S2). After accounting for size and temperature using these parameter estimates, metabolic rate does not vary significantly with depth (F = 0.04, df = 1, 119; P = 0.84) (Fig. S1), which serves as a proxy for carbon flux to benthic organisms, but it does vary significantly among taxonomic groups (F = 2.14, df = 9, 111, P = 0.03) (Fig. S1). Hydrothermal vent and methane seep taxa generally seem to have metabolic rates near other deep-sea taxa and shallow sea taxa when mass and temperature are accounted; the exception is Methanoaricia dendrobranchiata, which seems to have a higher than expected metabolic rate.
Fig. 2.
Body size and temperature scaling relationships for (A and B) mass-specific metabolic rate (W/g), (C and D) inverse lifespan (1/d), and (E and F) mass-specific growth rate (1/d). Data were obtained from diverse shallow and deep-sea organisms (data sources are given in Methods). Mass is expressed in grams, and temperature is expressed as 1/kT − 1/kTc (1/eV), where T is absolute temperature (in Kelvin) and Tc = 288 K (= 15 °C); therefore, the absolute values of the slopes of the temperature relationships correspond to the estimated activation energy, and the intercepts correspond to the logarithm of the rate for a 1 g organism at 15 °C. The models were fitted using common slopes in instances where ANOVA indicated that the estimated size exponent and/or estimated activation energy did not differ significantly among groups (P > 0.05). No line is depicted in D for deep-sea invertebrates, because temperature was insignificant (P = 0.78).
Individual Turnover and Growth.
For the combined dataset, organisms that are smaller and living at warmer temperatures exhibit higher rates of individual turnover (R2 = 0.91; F = 1424, df = 2, 291; P < 10−15). The 95% CIs for the coefficients encompass MTE-predicted values of −0.25 for the mass exponent (−0.25; 95% CI = −0.26 to −0.24) and the predicted range of 0.6–0.7 eV for the activation energy (0.56 eV; 95% CI = 0.49–0.62 eV) (Eq. S2). Significant differences in turnover rate exist between data sources: size and temperature both have significant independent effects on longevity, and together, they account for the majority of the variance for laboratory-cultured organisms (both P < 10−15; R2 = 0.99), shallow-dwelling fish (both P < 10−15; R2 = 0.60), and deeper-dwelling rockfish (mass: P < 10−10, temperature: P = 0.01; R2 = 0.67). However, for deep-sea invertebrates, only mass is significant (mass: P = 0.005; temperature: P = 0.78; R2 = 0.50), despite the wide temperature range (2.5 °C to 17 °C). The exponent characterizing the size dependence does not differ significantly among the four data sources (F = 1.92, df = 3, 285; P = 0.13), and the activation energy does not differ significantly among the three datasets exhibiting significant temperature effects (F = 2.18, df = 2, 285; P = 0.12). Thus, all four datasets are well-characterized by a single mass exponent (−0.24) that is consistent with the MTE-predicted value of −0.25 (95% CI = −0.22 to −0.25) (four lines in Fig. 2C), and three of four datasets are well-characterized by a single activation energy (0.47 eV) that is slightly lower than the predicted range of 0.6–0.7 eV (95% CI = 0.40–0.54 eV) (three lines in Fig. 2D). The methane seep worm Lamellibrachia sp. (represented by two outlying red symbols in Fig. 2 C and D) seems to exhibit lower than expected rates of turnover.
For rockfish, bathymetric distribution data were available that allowed testing average depth as a proxy for POC flux. After accounting for mass and temperature, depth explains significant variation in individual turnover, with deeper rockfish possessing significantly lower rates of turnover (F = 4.9, df = 1, 33; P = 0.03).
For growth rate, the combined mass-temperature model accounts for significant variation among deep-sea organisms found at depths >200 m (R2 = 0.28; F = 21.23, df = 2,107; P < 10−7; Fig. 2 E and F), although the explanatory power of temperature in the model is marginally nonsignificant (F = 3.74, df = 1, 107; P = 0.06). The mass exponent (−0.11; 95% CI: −0.14 to −0.07) and activation energy (0.16 eV; 95% CI: 0.00–0.32 eV) both differ from the respective values of 0.25 and 0.65 eV predicted by MTE (Eq. S2). Overall, smaller species grow faster than their larger counterparts on a mass-specific basis. Again, the methane seep worm Lamellibrachia sp. has a comparatively low rate after accounting for size and temperature. Depth does not predict variation in growth rates (F = 2.36, df = 1, 106; P = 0.13).
Biomass, Abundance, and Body Size.
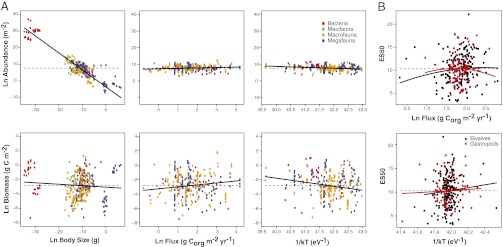
After accounting for spatial autocorrelation, carbon flux, but not temperature, is a significant predictor of body size such that higher fluxes lead to greater average body sizes in a locality (Fig. 3A and Table 1). For abundance and biomass, body size, temperature, and flux are all significant (Fig. 3A and Table 1). Abundance and biomass share similar coefficients, because abundance = biomass/mass; we present both for clarity. Body mass (−1.02; 95% CI = −1.00 to 1.04) scales with abundance at values near MTE predictions of −1 (Eq. S6). For temperature, the coefficient scales (−0.56; 95% eV CI = −0.29 to 0.83 eV) in the opposite direction of the value predicted by MTE (Eqs. S4 and S5). Overall, greater abundances are observed at higher carbon fluxes and among smaller body sizes; the same finding holds true for biomass.
Fig. 3.
(A) Partial regression plots of body size, carbon flux, and temperature scaling of abundance and biomass in benthic deep-sea bacteria, meiofauna, macrofauna, and megafauna. Partial regression plots are provided instead of raw data to visualize the independent contributions of each factor. Mean values of predictor and response variables were added to residual values for the x and y axes, respectively. Solid lines denote fitted regressions, with slope equal to the parameter estimate in the spatial eigenvector model. Dashed lines indicate response variable mean. Corg = organic carbon. (B) Partial regression plots of carbon flux and temperature scaling of diversity (measured as the expected number of species at 50 individuals) for benthic deep-sea gastropods and bivalves of the Atlantic Ocean. Partial regression plots are provided instead of raw data to visualize the independent contributions of each factor. Mean values of predictor and response variables were added to residual values for the x and y axes, respectively. Solid lines denote fitted regressions, with slope equal to the parameter estimate in the spatial eigenvector model. Dashed lines indicate response variable mean. Full results for the models can be found in Tables 1 and 2.
Table 1.
Spatially explicit model fits using an SEVM approach for body size, biomass, and abundance (n = 442)
| Variable | Slope coefficient | SE | t Value | Pr(>|t|) | Upper 95% CI | Lower 95% CI |
| Body size | ||||||
| Intercept | 23.07 | 20.20 | 1.14 | 0.254 | 62.66 | −16.52 |
| 1/kT | −0.85 | 0.48 | −1.78 | 0.0765 | 0.09 | −1.79 |
| Ln flux | 1.17 | 0.26 | 4.61 | 5.31e-06 | 1.68 | 0.66 |
| Biomass | ||||||
| Intercept | 20.01 | 5.71 | 3.51 | 0.005 | 31.17 | 8.83 |
| Ln mass | −0.02 | 0.01 | −2.12 | 0.035 | 0.00 | −0.04 |
| 1/kT | −0.56 | 0.14 | 4.15 | 3.96e-05 | −0.29 | −0.83 |
| Ln flux | 0.27 | 0.07 | 3.63 | 0.0003 | 0.41 | 0.13 |
| Abundance | ||||||
| Intercept | 20.01 | 5.71 | 3.51 | 0.0005 | 31.19 | 8.81 |
| Ln mass | −1.02 | 0.01 | −106.63 | <2.00e-16 | −1.00 | −1.04 |
| 1/kT | −0.56 | 0.14 | −4.15 | 3.96e-05 | −0.29 | −0.83 |
| Ln flux | 0.27 | 0.07 | 3.63 | 0.0003 | 0.41 | 0.13 |
Bold values indicated significance at the α = 0.05 level.
Species Diversity.
After accounting for spatial autocorrelation in the data, the quadratic of carbon flux is significant for both Bivalvia and Gastropoda (Fig. 3B and Table 2). Thus, for mollusks, diversity peaks at intermediate chemical energy availability. Temperature does not predict variation in rarified diversity in any case.
Table 2.
Spatially explicit model fits using an SEVM for biodiversity in bivalves and gastropods
| Variable | Slope coefficient | SE | t Value | Pr(>|t|) | Upper 95% CI | Lower 95% CI |
| Bivalvia (n = 191) | ||||||
| Intercept | 10.05 | 0.32 | 31.45 | <2e-16 | 10.68 | 9.43 |
| Ln (depth) | −0.48 | 1.13 | −0.43 | 0.6712 | 1.73 | −2.69 |
| Ln (depth)2 | −0.08 | 0.35 | −0.23 | 0.8174 | 0.60 | −0.77 |
| 1/kT | 2.86 | 2.21 | 1.29 | 0.1974 | 7.19 | −1.47 |
| (1/kT)2 | 0.11 | 1.36 | 0.08 | 0.9348 | 1.47 | −1.25 |
| Ln(flux) | 0.88 | 0.59 | 1.49 | 0.1384 | 2.04 | −0.28 |
| Ln(flux)2 | −0.73 | 0.33 | −2.21 | 0.0287 | −0.08 | −1.38 |
| Gastropoda (n = 84) | ||||||
| Intercept | 14.36 | 0.71 | 20.25 | <2e-16 | 15.77 | 12.94 |
| Ln (depth) | −3.15 | 1.26 | −2.50 | 0.0146 | 5.66 | 0.64 |
| Ln (depth)2 | −1.09 | 0.96 | −1.13 | 0.2605 | 3.00 | −0.82 |
| 1/kT | 1.14 | 2.43 | 0.47 | 0.6400 | 5.98 | −3.70 |
| (1/kT)2 | 0.23 | 2.17 | 0.11 | 0.9145 | 4.55 | −4.09 |
| Ln(flux) | −0.50 | 0.79 | −0.64 | 0.5259 | 2.07 | −1.07 |
| Ln(flux)2 | −1.61 | 0.49 | −3.30 | 0.0015 | 2.59 | 0.63 |
Bold values indicated significance at the α = 0.05 level.
Discussion
The biological responses of organisms living in extreme environments, such as the deep ocean, deserts, and caves, to energy flux have not been well documented. Given the substantial energetic constraints involved, it might be expected that the patterns would differ from more energy-rich environments (31), but communities from extreme habitats may be analogous in their adaptations and response to this mode of life. Among deep-sea benthic organisms, the relative roles of chemical and thermal energy vary considerably across levels of biological organization (Table 3).
Table 3.
The direct effects of body mass, temperature, and particulate carbon flux (or depth as proxy) on processes and patterns across levels of complexity in deep-sea organisms
| Body mass | Temperature | POC/depth | |
| Individual level | |||
| Mass-specific metabolic rate | ↓ | ↑ | NS |
| Mass-specific turnover | ↓ | ↑* | ↑ Rockfish |
| Mass-specific growth rate | ↓ | Marginally NS | NS |
| Body size | NA | Marginally NS | ↑ |
| Community level | |||
| Biomass | ↓ | ↑ | ↑ |
| Abundance | ↓ | ↑ | ↑ |
| Diversity | NA | NS | Quadratic |
Note that carbon flux and temperature will have indirect effects on many of these processes, because they are influenced by body size. NA, not applicable; NS, not significant.
*Only for rockfish and not for deep-sea invertebrates.
At the level of the organism, size and temperature play primary roles in controlling individual metabolic rate and turnover. We find no evidence that the availability of chemical energy influences metabolic rates of deep-sea taxa, consistent with earlier work (32). Likewise, the lack of a role for depth precludes the effect of pressure on metabolic rate, which was previously noted (32). Hydrothermal vent and methane seep organisms experiencing high in situ primary production (orders of magnitude greater than the background abyssal plains) have rates comparable with other deep-sea organisms. The exception seems to be methane seep worms, providing an intriguing avenue of future investigation. We do, however, find some evidence that energy availability, as indexed by depth, influences lifespans of rockfish, perhaps in part because caloric restriction can increase in lifespan (33). Although the size–temperature scaling relationships that we observe for rates of individual metabolism and turnover agree with each other and the predictions of MTE, they differ from those relationships observed for growth rate. These findings are inconsistent, because growth rate is ultimately constrained by metabolic rate and therefore, should exhibit similar scaling. A potential explanation for this discrepancy is that the growth data compiled here include both estimates calculated during some portion of ontogeny and estimates time-averaged over the entire lifespan. Clearly, more work is needed to understand growth rate and its relationship to metabolic rate and lifespan in deep-sea organisms.
Our results suggest that individual metabolic rate proceeds as quickly as allowed by body size and temperature-influenced biochemical kinetics, implying that chemical energy limits biota through its effects at higher levels of biological organization. Variation in carbon flux is the primary driver of patterns in biomass, abundance, and biodiversity. The exception is abundance and biomass, where temperature has a minor but significant role.
Previous work hypothesized a link between body size and carbon flux in the deep sea (7, 34–36), but explicit tests are rare and limited to mollusks (37, 38). We present evidence of a link between broad-scale variation in body size and carbon flux among contemporary deep-sea organisms. Thus, size, a significant predictor in all of the relationships seen here, introduces additional indirect chemical energy constraints among all levels of biological organization. Therefore, although direct effects of chemical energy are not detectable for lower levels of biological organization, indirect chemical energy effects impact the processes through controlling body size.
Our results indicate that chemical energy plays a primary role in determining spatial patterns of α-diversity in the deep sea, consistent with other recent studies (22, 39–42). We show that oceanic-scale patterns of biodiversity in the deep sea are directly related to carbon flux. Deep-sea biodiversity exhibits a unimodal relationship with chemical energy availability over both regional (20) and oceanic scales. We do not find that temperature effects scale up to biodiversity patterns, despite hypotheses suggesting a relationship (43) and empirical evidence of its importance in shallow water systems (44). The decline of species diversity with decreased carbon flux may be related to Allee effects, because population numbers decrease under extreme food limitation, with highly oligotrophic regions representing sinks (45). The decline of diversity with increasing carbon flux may reflect guild interactions, where larger mobile deposit feeders monopolize carbon flux while degrading the environment for smaller organisms (40). Intermediate levels of carbon availability may afford a balance between increased species coexistence and decreased niche overlap (46). A considerable amount of variation exists in biodiversity that is not accounted for by the factors here, and it may be related to spatial and temporal variation in environmental factors that are not measured (47, 48).
Despite the deep sea clearly operating under extreme conditions of energy availability, inviting a tendency to regard it as a unique environment, it responds to the same energetic rules as other systems. The deep sea simply represents the extreme end of known processes. Across scales of organization, macroecological patterns in the deep sea are largely consistent with predictions of MTE (4). Across levels, with the notable exception of biodiversity and standing stock, temperature and body size scale near and often encompass MTE predictions for the scaling of mass and temperature. We also find that biological differences among taxa, although significantly elevating or diminishing overall rates, do not change the scaling parameters. The largest inconsistencies with MTE are the lack of temperature scaling in biodiversity and reverse scaling in standing stock. These inconsistencies may result, because a true temperature signal is completely swamped by variation in carbon flux. However, many systems do not exhibit temperature–biodiversity relationships (1, 49). The reverse scaling of temperature with standing stock remains an intriguing finding that warrants additional research.
Previous commentary concluded the prominence of chemical energy as a driving factor for abyssal regions, the deepest, largest, and most food-poor regions of the deep oceans (23). Here, we show that, for the entire deep oceans, the importance of thermal energy prevails at lower levels of biological organization; however, processes and patterns at higher levels are increasingly dominated by chemical energy. Direct effects of body size on these processes may introduce additional indirect effects of chemical energy across biological levels. Thus, climate change will greatly impact ecosystem functioning through impacts on food and temperature regimens operating at different scales of organization. Our findings indicate that the deep sea, once thought to be remote and buffered against climatic change, may function quite differently in the future.
Methods
MTE.
MTE has attracted both criticism and excitement (50–62). Much of the debate on MTE focuses on aspects of the value of scaling exponents or the importance of fractal networks in predicting scaling with body size (63). Although interesting, these issues are not relevant to this study, because we are less concerned with whether slopes precisely match theoretical predictions. Rather, we use MTE as a useful analytical and hierarchical framework to explore the relative contributions of thermal and chemical energy across scales of biological organization. This application of MTE has proven valuable for explaining variation in a wide range of biological processes (64–67). MTE proposes that individual metabolic rates, by setting the rates of resource uptake from the environment and resource allocation to survival, growth, and reproduction, control ecological processes at all levels of organization from individuals to the biosphere (4). Individual metabolic rate, B (W), is governed largely by the combined effects of body size, M (g), and absolute temperature, T (K), with mass scaling in multiples of 1/4 and temperature scaling near the activation energy of the respiratory complex (∼0.6–0.7 eV) (4). Additional explanation, equations, and predictions across scales of biological complexity can be found in SI Text.
Data and Analyses.
Datasets were constructed for individual metabolic rates, turnover, and growth as well as biomass, abundance, mean body size, and diversity for communities for a variety of invertebrates and vertebrates inhabiting the shallow and deep oceans. For individual measurements, data were collected on temperature, both in situ and experimental, and body size of the individual. For many of the individual measurements, specific geographic information was not provided that would allow us to derive site-specific estimates of POC flux. As an alternative, we used depth as a proxy for POC. For standing stock and diversity, estimates of in situ temperature and POC flux were used. Specific details of datasets can be found in SI Text, and the data can be found online at http://dx.doi.org/10.5061/dryad.78nt1.
For metabolic rate, growth rate, and lifespan analyses, we fit linear models using R statistical software. In explicitly geographic analyses, applying standard statistical analysis approaches, such as ordinary least squares, can result in spatial autocorrelation remaining in the residuals, leading to increased type I error rates, biased parameter estimates, and spatial pseudoreplication (68). We, therefore, used a spatial eigenvector mapping (SEVM) approach for the size, abundance, biomass, and diversity analyses to explicitly account for the potential effects of spatial autocorrelation on inference (68). Environmental variables were log-transformed and where necessary, centered before incorporation into SEVM models. Linear (biomass, body size, and abundance) and quadratic (diversity) SEVM models were fitted using the R package spdep (http://cran.r-project.org/web/packages/spdep/index.html). Moran’s I tests indicated that, in contrast to models without the spatial eigenvector components, no significant spatial autocorrelation remained in the residuals of the spatial models, indicating that the SEVM achieved the aim of accounting for spatial autocorrelation.
Supplementary Material
Acknowledgments
C.R.M. thanks Michelle Gaither-McClain. C.R.M. was supported by National Evolutionary Synthesis Centre National Science Foundation Grant EF-0905606. A.P.A. was supported under Australian Research Council’s Discovery Projects Funding Scheme DP-110105280. M.A.R. is supported by National Science Foundation Grant OCE-1129612.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Datasets are deposited in Dryad, http://dx.doi.org/10.5061/dryad.78nt1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208976109/-/DCSupplemental.
References
- 1.Clarke A, Gaston KJ. Climate, energy and diversity. Proc Biol Sci. 2006;273:2257–2266. doi: 10.1098/rspb.2006.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nevo E. Evolution of genome-phenome diversity under environmental stress. Proc Natl Acad Sci USA. 2001;98:6233–6240. doi: 10.1073/pnas.101109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 4.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 5.Freckleton RP, Harvey PH, Pagel M. Bergmann’s rule and body size in mammals. Am Nat. 2003;161:821–825. doi: 10.1086/374346. [DOI] [PubMed] [Google Scholar]
- 6.Hunt G, Roy K. Climate change, body size evolution, and Cope’s Rule in deep-sea ostracodes. Proc Natl Acad Sci USA. 2006;103:1347–1352. doi: 10.1073/pnas.0510550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClain CR, Boyer A, Rosenberg G. The island rule and the evolution of body size in the deep sea. J Biogeogr. 2006;33:1578–1584. [Google Scholar]
- 8.Bambach RK. Seafood through time: Changes in biomass, energetics, and productivity in the marine ecosystem. Paleobiology. 1993;19:372–397. [Google Scholar]
- 9.Finnegan S, McClain CR, Kosnik M, Payne JL. Escargots through time: An energetic comparison of marine gastropod assemblages before and after the Mesozoic Marine Revolution. Paleobiology. 2011;37:252–269. [Google Scholar]
- 10.Locarnini RA, et al. 2010. World Ocean Atlas 2009, Volume 1: Temperature, NOAA Atlas NESDIS 68, ed Levitus S (US Government Printing Office, Washington, DC)
- 11.Van Dover CL. The Ecology of Deep-Sea Hydrothermal Vents. Princeton: Princeton Univ Press; 2000. [Google Scholar]
- 12.Lampitt RS, Anitia AN. Particle flux in the deep seas: Regional characteristics and temporal variability. Deep Sea Res Part 1 Oceanogr Res Pap. 1997;44:1377–1403. [Google Scholar]
- 13.Childress JJ, Cowles DL, Favuzzi JA, Mickel TJ. Metabolic rates of benthic deep-sea decapod crustaceans decline with increasing depth primarily due to the decline in temperature. Deep Sea Res Part A. Oceanogr Res Pap. 1990;37:929–949. [Google Scholar]
- 14.Seibel BA, Childress JJ. Metabolism of benthic octopods (Cephalopoda) as a function of habitat depth and oxygen concentration. Deep Sea Res Part 1 Oceanogr Res Pap. 2000;47:1247–1260. [Google Scholar]
- 15.Mahaut ML, Sibuet M, Shirayama Y. Weight-dependent respiration rates in deep-sea organisms. Deep Sea Res Part 1 Oceanogr Res Pap. 1995;42:1575–1582. [Google Scholar]
- 16.Johnson NA, et al. The relationship between the standing stock of deep-sea macrobenthos and surface production in the western North Atlantic. Deep Sea Res Part 1 Oceanogr Res Pap. 2007;54:1350–1360. [Google Scholar]
- 17.Pace ML, Knauer GA, Karl DM, Martin JH. Primary production, new production and vertical flux in the Eastern Pacific. Nature. 1987;325:803–804. [Google Scholar]
- 18.Wei C-L, et al. Global patterns and predictions of seafloor biomass using random forests. PLoS One. 2010;5:e15323. doi: 10.1371/journal.pone.0015323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex MA, Etter RJ. Deep-Sea Biodiversity: Pattern and Scale. Cambridge, MA: Harvard Univ Press; 2010. [Google Scholar]
- 20.Tittensor DP, Rex MA, Stuart CT, McClain CR, Smith CR. Species-energy relationships in deep-sea molluscs. Biol Lett. 2011;7:718–722. doi: 10.1098/rsbl.2010.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt G, Cronin TM, Roy K. Species-energy relationships in the deep sea: A test using the Quaternary fossil record. Ecol Lett. 2005;8:739–747. [Google Scholar]
- 22.Yasuhara M, Cronin TM. Climatic influences on deep-sea ostracode (Crustacea) diversity for the last three million years. Ecology. 2008;89(11 Suppl):S53–S65. doi: 10.1890/07-1021.1. [DOI] [PubMed] [Google Scholar]
- 23.Smith CR, De Leo FC, Bernardino AF, Sweetman AK, Arbizu PM. Abyssal food limitation, ecosystem structure and climate change. Trends Ecol Evol. 2008;23:518–528. doi: 10.1016/j.tree.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Boyce DG, Lewis MR, Worm B. Global phytoplankton decline over the past century. Nature. 2010;466:591–596. doi: 10.1038/nature09268. [DOI] [PubMed] [Google Scholar]
- 25.Behrenfeld MJ, et al. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444:752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 26.Bethoux J-P, Gentili B, Raunet J, Tailliez D. Warming trend in the western Mediterranean deep water. Nature. 1990;347:660–662. [Google Scholar]
- 27.Levitus S, Antonov JI, Boyer TP, Stephens C. Warming of the world ocean. Science. 2000;287:2225–2229. [Google Scholar]
- 28.Danovaro R, Dell'Anno A, Pusceddu A. Biodiversity response to climate change in a warm deep sea. Ecol Lett. 2004;7:821–828. [Google Scholar]
- 29.Ruhl HA. Community change in the variable resource habitat of the abyssal northeast Pacific. Ecology. 2008;89:991–1000. doi: 10.1890/06-2025.1. [DOI] [PubMed] [Google Scholar]
- 30.Ruhl HA, Smith KL., Jr Shifts in deep-sea community structure linked to climate and food supply. Science. 2004;305:513–515. doi: 10.1126/science.1099759. [DOI] [PubMed] [Google Scholar]
- 31.Poulson TL. Adaptations of cave fishes with some comparisons to deep-sea fishes. Environ Biol Fishes. 2001;62:345–364. [Google Scholar]
- 32.Childress JJ. Are there physiological and biochemical adaptations of metabolism in deep-sea animals? Trends Ecol Evol. 1995;10:30–36. doi: 10.1016/s0169-5347(00)88957-0. [DOI] [PubMed] [Google Scholar]
- 33.Bishop NA, Guarente L. Genetic links between diet and lifespan: Shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 34.Thiel H. The size structure of the deep-sea benthos. Int Rev Gesamten Hydrobiol. 1975;60:575–606. [Google Scholar]
- 35.McClain CR, Rex MA, Jabbour R. Deconstructing bathymetric patterns of body size in deep-sea gastropods. Mar Ecol Prog Ser. 2005;297:181–187. [Google Scholar]
- 36.Rex MA, Etter RJ. Bathymetric patterns of body size: Implications for deep-sea biodiversity. Deep Sea Res Part 2 Top Stud Oceanogr. 1998;45:103–127. [Google Scholar]
- 37.McClain CR, Stegen JC, Hurlbert AH. Dispersal, environmental niches and oceanic-scale turnover in deep-sea bivalves. Proc Biol Sci. 2012;279:1993–2002. doi: 10.1098/rspb.2011.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClain CR, Gullet T, Jackson-Ricketts J, Unmack PJ. Increased energy promotes size-based niche availability in marine mollusks. Evolution. 2012;66:2204–2215. doi: 10.1111/j.1558-5646.2012.01580.x. [DOI] [PubMed] [Google Scholar]
- 39.Glover AG, et al. Polychaete species diversity in the central Pacific abyss: Local and regional patterns, and relationships with productivity. Mar Ecol Prog Ser. 2002;240:157–170. [Google Scholar]
- 40.McClain CR, Barry J. Habitat heterogeneity, biogenic disturbance, and resource availability work in concert to regulate biodiversity in deep submarine canyons. Ecology. 2010;91:964–976. doi: 10.1890/09-0087.1. [DOI] [PubMed] [Google Scholar]
- 41.McClain CR, Nekola JC, Kuhnz L, Barry JP. Local-scale turnover on the deep Pacific floor. Mar Ecol Prog Ser. 2011;442:193–200. [Google Scholar]
- 42.Wei C-L, et al. Bathymetric zonation of deep-sea macrofauna in relation to export of surface phytoplankton production. Mar Ecol Prog Ser. 2010;399:1–14. [Google Scholar]
- 43.Allen AP, Brown JH, Gillooly JF. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science. 2002;297:1545–1548. doi: 10.1126/science.1072380. [DOI] [PubMed] [Google Scholar]
- 44.Tittensor DP, et al. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466:1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- 45.Rex MA, et al. A source-sink hypothesis for abyssal biodiversity. Am Nat. 2005;165:163–178. doi: 10.1086/427226. [DOI] [PubMed] [Google Scholar]
- 46.McClain CR. Bathymetric patterns of morphological disparity in deep-sea gastropods from the western North Atlantic basin. Evolution. 2005;59:1492–1499. [PubMed] [Google Scholar]
- 47.Levin LA, et al. Environmental influences on regional deep-sea species diversity. Annu Rev Ecol Syst. 2001;32:51–93. [Google Scholar]
- 48.Sun X, Corliss B, Brown C, Showers W. The effect of primary productivity and seasonality on the distribution of deep-sea benthic foraminifera in the North Atlantic. Deep Sea Res Part 1 Oceanogr Res Pap. 2006;53:28–47. [Google Scholar]
- 49.Hawkins BA, et al. A global evaluation of metabolic theory as an explanation for terrestrial species richness gradients. Ecology. 2007;88:1877–1888. doi: 10.1890/06-1444.1. [DOI] [PubMed] [Google Scholar]
- 50.O'Connor MP, et al. Reconsidering the mechanistic basis of the metabolic theory of ecology. Oikos. 2007;116:1058–1072. [Google Scholar]
- 51.Algar AC, Kerr JT, Currie DJ. A test of Metabolic Theory as the mechanims underlying broad-scale species-richness gradients. Glob Ecol Biogeogr. 2007;16:170–178. [Google Scholar]
- 52.Allen AP, Gillooly JF. The mechanistic basis of the metabolic theory of ecology. Oikos. 2007;116:1073–1077. [Google Scholar]
- 53.Harte J. The value of null theories in ecology. Ecology. 2004;85:1792–1794. [Google Scholar]
- 54.Marquet PA, Labra FA, Maurer BA. Metabolic ecology: Linking individuals to ecosystems. Ecology. 2004;85:1794–1796. [Google Scholar]
- 55.Kaspari M. Using the metabolic theory of ecology to predict global patterns of abundance. Ecology. 2004;85:1800–1802. [Google Scholar]
- 56.Cyr H, Walker SC. An illusion of mechanistic understanding. Ecology. 2004;85:1802–1804. [Google Scholar]
- 57.Cottingham KL, Zens MS. Metabolic rate opens a grand vista on ecology. Ecology. 2004;85:1805–1807. [Google Scholar]
- 58.Koehl MAR, Wolcott BD. Can function at the organimsal level explain ecological patterns? Ecology. 2004;85:1808–1810. [Google Scholar]
- 59.Horn HS. Commentary on Brown et al.'s “Toward a metabolic theory of ecology.”. Ecology. 2004;85:1816–1818. [Google Scholar]
- 60.Bokma F. Evidence against universal metabolic allometry. Funct Ecol. 2004;18:184–187. [Google Scholar]
- 61.Clarke A. Is there a universal temperature dependence of metabolism? Funct Ecol. 2004;18:252–256. [Google Scholar]
- 62.Clarke A, Fraser KPP. Why does metabolism scale with temperature? Funct Ecol. 2004;18:243–251. [Google Scholar]
- 63.Isaac NJB, Carbone C. Why are metabolic scaling exponents so controversial? Quantifying variance and testing hypotheses. Ecol Lett. 2010;13:728–735. doi: 10.1111/j.1461-0248.2010.01461.x. [DOI] [PubMed] [Google Scholar]
- 64.López-Urrutia A, San Martin E, Harris RP, Irigoien X. Scaling the metabolic balance of the oceans. Proc Natl Acad Sci USA. 2006;103:8739–8744. doi: 10.1073/pnas.0601137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Connor MI, et al. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci USA. 2007;104:1266–1271. doi: 10.1073/pnas.0603422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ernest SKM, et al. Thermodynamic and metabolic effects on the scaling of production and population energy use. Ecol Lett. 2003;6:990–995. [Google Scholar]
- 67.Munch SB, Salinas S. Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proc Natl Acad Sci USA. 2009;106:13860–13864. doi: 10.1073/pnas.0900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dorman CF, et al. Methods to account for spatial autocorrelation in the analysis of species distributiona data: A review. Ecography (Cop.) 2007;30:609–628. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.