Abstract
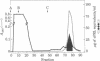
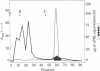
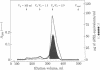
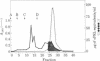
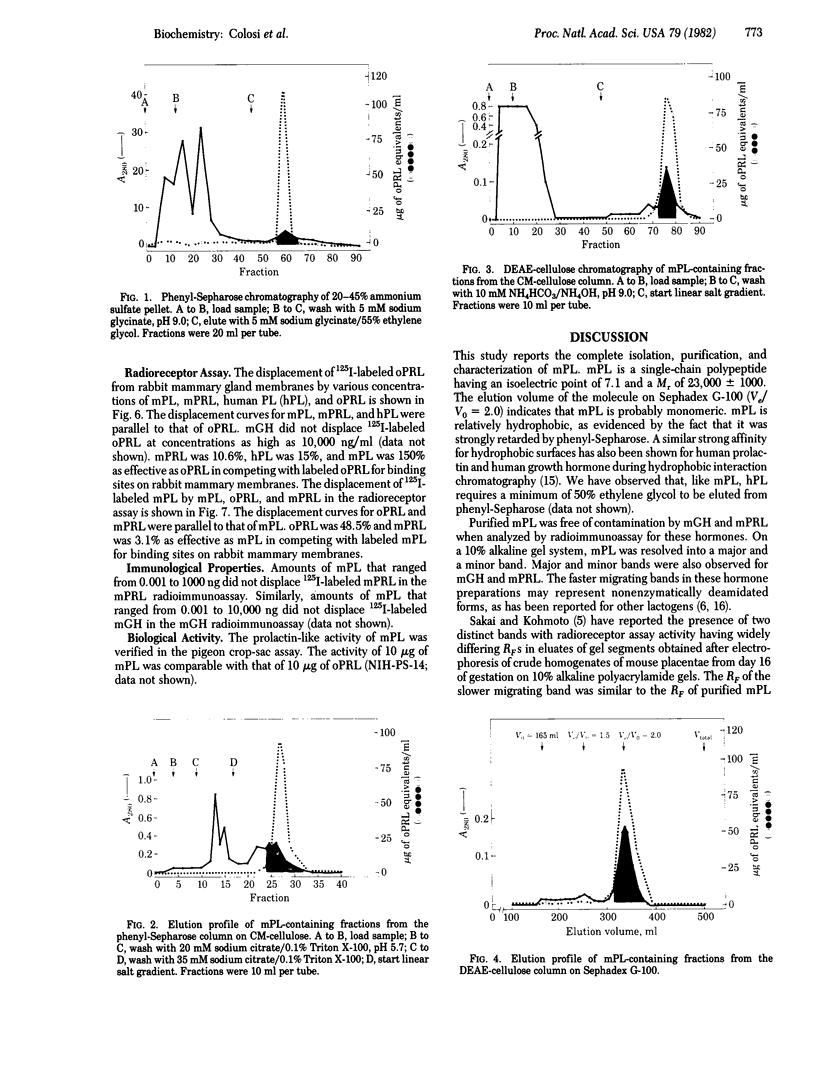
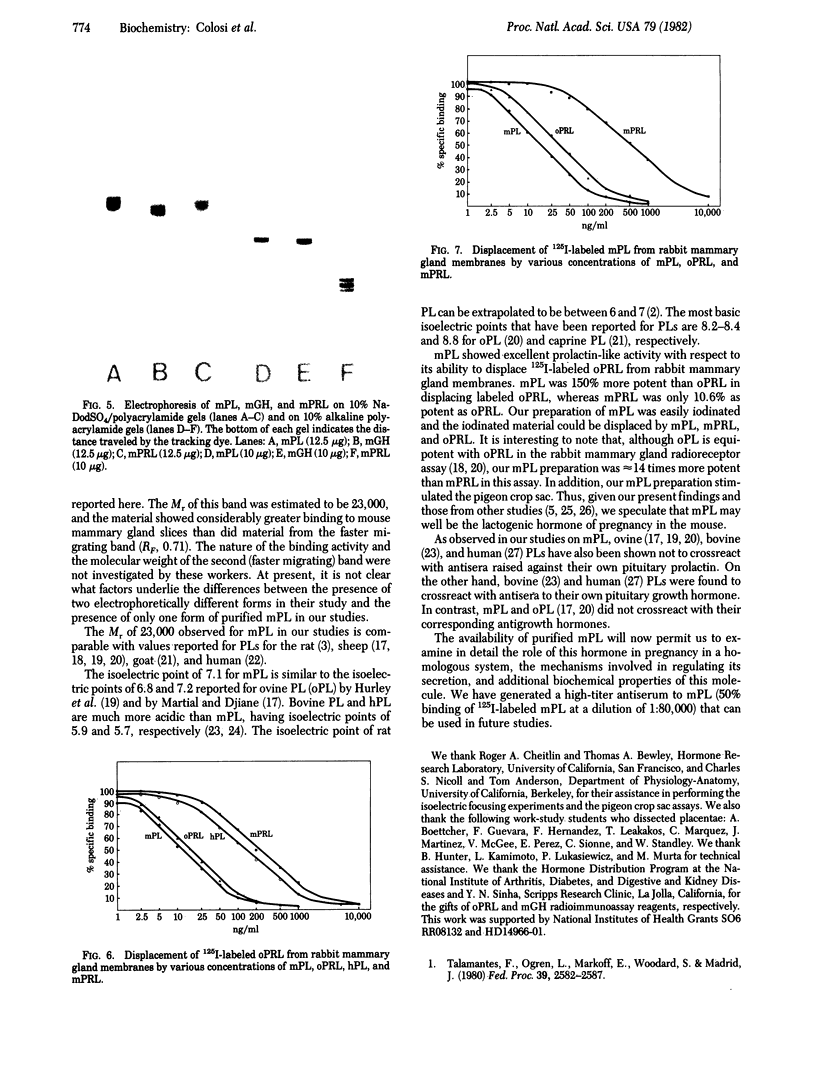
Mouse placental lactogen was purified 1840-fold from BALB/c placentae from days 14-18 of gestation with an overall yield of 29%. The purification procedure included alkaline homogenization and extraction, ammonium sulfate precipitation, hydrophobic interaction chromatography on Phenyl-Sepharose, ion-exchange chromatography on CM- and DEAE-cellulose, and gel exclusion chromatography on Sephadex G-100. On 10% alkaline polyacrylamide gels, mouse placental lactogen had an Rf of 0.19. Electrophoresis in gels containing NaDodSO4 showed a single band with a mobility corresponding to a Mr of 23,000 +/- 1000. The isoelectric point, determined by isoelectric focusing in 8 M urea/5% 2-mercaptoethanol, was 7.1. When tested in the pigeon crop sac assay, 10 micrograms of mouse placental lactogen produced stimulation comparable with that evoked by 10 micrograms of ovine prolactin. In the rabbit mammary gland radioreceptor assay, mouse placental lactogen was 150% more potent than ovine prolactin in displacing 125I-labeled ovine prolactin from rabbit mammary gland membranes. Iodinated purified mouse placental lactogen could be displaced from rabbit mammary gland membranes by mouse placental lactogen, mouse prolactin, and ovine prolactin. Ovine prolactin was 45% as avid as mouse placental lactogen is displacing 125I-labeled mouse placental lactogen from rabbit mammary gland membranes. Mouse placental lactogen did not crossreact with antisera to mouse prolactin or mouse growth hormone in a radioimmunoassay.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becka S., Bílek J., Slaba J., Skarda J., Mikulás I. Some properties of the goat placental lactogen. Experientia. 1977 Jun 15;33(6):771–772. doi: 10.1007/BF01944183. [DOI] [PubMed] [Google Scholar]
- Bolander F. F., Jr, Fellows R. E. Purification and characterization of bovine placental lactogen. J Biol Chem. 1976 May 10;251(9):2703–2708. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chan J. S., Robertson H. A., Friesen H. G. The purification and characterization of ovine placental lactogen. Endocrinology. 1976 Jan;98(1):65–76. doi: 10.1210/endo-98-1-65. [DOI] [PubMed] [Google Scholar]
- Colosi P., Markoff E., Levy A., Ogren L., Shine N., Talamantes F. Isolation and partial characterization of secreted hamster pituitary prolactin. Endocrinology. 1981 Mar;108(3):850–854. doi: 10.1210/endo-108-3-850. [DOI] [PubMed] [Google Scholar]
- Hurley T. W., Handwerger S., Fellows R. E. Isolation and structural characterization of ovine placental lactogen. Biochemistry. 1977 Dec 13;16(25):5598–5604. doi: 10.1021/bi00644a033. [DOI] [PubMed] [Google Scholar]
- KAPLAN S. L., GRUMBACH M. M. STUDIES OF A HUMAN AND SIMIAN PLACENTAL HORMONE WITH GROWTH HORMONE-LIKE AND PROLACTIN-LIKE ACTIVITIES. J Clin Endocrinol Metab. 1964 Jan;24:80–100. doi: 10.1210/jcem-24-1-80. [DOI] [PubMed] [Google Scholar]
- Lewis U. J., Cheever E. V., Hopkins W. C. Kinetic study of the deamidation of growth hormone and prolactin. Biochim Biophys Acta. 1970 Sep 29;214(3):498–508. doi: 10.1016/0005-2795(70)90310-7. [DOI] [PubMed] [Google Scholar]
- Li C. H., Dixon J. S., Chung D. Amino acid sequence of human chorionic somatomammotropin. Arch Biochem Biophys. 1973 Mar;155(1):95–110. doi: 10.1016/s0003-9861(73)80012-8. [DOI] [PubMed] [Google Scholar]
- Markoff E., Colosi P., Talamantes F. Homologous radioimmunoassay for secreted mouse prolactin. Life Sci. 1981 Jan 12;28(2):203–211. doi: 10.1016/0024-3205(81)90554-3. [DOI] [PubMed] [Google Scholar]
- Markoff E., Talamantes F. Serum placental lactogen in mice in relation to day of gestation and number of conceptuses. Biol Reprod. 1981 May;24(4):846–851. doi: 10.1095/biolreprod24.4.846. [DOI] [PubMed] [Google Scholar]
- Markoff E., Talamantes F. The lactogenic response of mouse mammary explants to mose prolactin and growth hormone. Endocr Res Commun. 1980;7(4):269–278. doi: 10.3109/07435808009065978. [DOI] [PubMed] [Google Scholar]
- Martal J., Djiane J. Purification of a lactogenic hormone in sheep placenta. Biochem Biophys Res Commun. 1975 Jul 22;65(2):770–778. doi: 10.1016/s0006-291x(75)80212-9. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Yanai R. Quantitative participation of placental mammotropic hormones in mammary development during pregnancy of mice. Endocrinol Jpn. 1971 Dec;18(6):507–510. doi: 10.1507/endocrj1954.18.507. [DOI] [PubMed] [Google Scholar]
- Nicoll C. S. Bio-assay of prolactin. Analysis of the pigeon crop-sac response to local prolactin injection by an objective and quantitative method. Endocrinology. 1967 Apr;80(4):641–655. doi: 10.1210/endo-80-4-641. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Reddy S., Watkins W. B. Purification and some properties of ovine placental lactogen. J Endocrinol. 1978 Jul;78(1):59–69. doi: 10.1677/joe.0.0780059. [DOI] [PubMed] [Google Scholar]
- Roberston M. C., Friesen H. G. The purification and characterization of rat placental lactogen. Endocrinology. 1975 Sep;97(3):621–629. doi: 10.1210/endo-97-3-621. [DOI] [PubMed] [Google Scholar]
- Robertson M. C., Friesen H. G. Two forms of rat placental lactogen revealed by radioimmunoassay. Endocrinology. 1981 Jun;108(6):2388–2390. doi: 10.1210/endo-108-6-2388. [DOI] [PubMed] [Google Scholar]
- Roos P., Nyberg F., Wide L. Isolation of human pituitary prolactin. Biochim Biophys Acta. 1979 Dec 11;588(3):368–379. doi: 10.1016/0304-4165(79)90345-3. [DOI] [PubMed] [Google Scholar]
- Sakai S., Kohmoto K. Binding of mouse choriomammotropin to prolactin receptors in the mouse mammary gland. Endocrinol Jpn. 1976 Dec;23(6):499–503. doi: 10.1507/endocrj1954.23.499. [DOI] [PubMed] [Google Scholar]
- Sinha Y. N., Selby F. W., Lewis U. J., VanderLaan W. P. Studies of GH secretion in mice by a homologous radioimmunoassay for mouse GH. Endocrinology. 1972 Sep;91(3):784–792. doi: 10.1210/endo-91-3-784. [DOI] [PubMed] [Google Scholar]
- Talamantes F., Jr Comparative study of the occurrence of placental prolactin among mammals. Gen Comp Endocrinol. 1975 Sep;27(1):115–121. doi: 10.1016/0016-6480(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Talamantes F., Ogren L., Markoff E., Woodard S., Madrid J. Phylogenetic distribution, regulation of secretion, and prolactin-like effects of placental lactogens. Fed Proc. 1980 Jun;39(8):2582–2587. [PubMed] [Google Scholar]