Abstract
Notch (N) is a transmembrane receptor that mediates cell–cell interactions to determine many cell-fate decisions. N contains EGF-like repeats, many of which have an O-fucose glycan modification that regulates N-ligand binding. This modification requires GDP-l-fucose as a donor of fucose. The GDP-l-fucose biosynthetic pathways are well understood, including the de novo pathway, which depends on GDP-mannose 4,6 dehydratase (Gmd) and GDP-4-keto-6-deoxy-d-mannose 3,5-epimerase/4-reductase (Gmer). However, the potential for intercellularly supplied GDP-l-fucose and the molecular basis of such transportation have not been explored in depth. To address these points, we studied the genetic effects of mutating Gmd and Gmer on fucose modifications in Drosophila. We found that these mutants functioned cell-nonautonomously, and that GDP-l-fucose was supplied intercellularly through gap junctions composed of Innexin-2. GDP-l-fucose was not supplied through body fluids from different isolated organs, indicating that the intercellular distribution of GDP-l-fucose is restricted within a given organ. Moreover, the gap junction-mediated supply of GDP-l-fucose was sufficient to support the fucosylation of N-glycans and the O-fucosylation of the N EGF-like repeats. Our results indicate that intercellular delivery is a metabolic pathway for nucleotide sugars in live animals under certain circumstances.
Keywords: sugar metabolism, intercellular transport
Emerging evidence indicates that the glycosylation of transmembrane receptors is important for cell signaling (1–3). For example, the O-fucose glycan modifications of the Notch (N) receptor influence its activities, and the study of this system has led to new paradigms about how glycosylation affects cell-signaling mechanisms (4, 5). N is the transmembrane receptor for an evolutionarily conserved signaling pathway that regulates various cell specifications through cell–cell communication (6). N’s extracellular domain contains a tandem array of EGF-like repeats (7), many of which are O-glycosylated, including by O-fucose and O-glucose modifications (8). EGF repeats of N that contain the sequence Cys2-X4-5-Ser/Thr-Cys3 are O-fucosylated by O-fucosyltransferase 1 (O-fut1) (9–11), and a GlcNAc is specifically added to the O-linked fucose on these EGF-like repeats by Fringe family proteins, which are evolutionarily conserved β1,3 N-acetylglucosaminyltransferases (4, 5). In mammalian cells, Sia-α2,3-Gal-β1,4 is further added to the GlcNAc, and consequently an O-linked tetrasaccharide (Sia-α2,3-Gal-β1,4-GlcNAc-β1,3-Fuc) is formed on these EGF-like repeats (12).
N receptors are activated by binding ligands from one of two families, the Delta (Dl)- and Serrate (Ser)-type proteins (13, 14). The Fng-dependent O-fucose glycan modification of N promotes interactions between N and Dl-type ligands, but it suppresses N’s interactions with Ser-type ligands (4, 15). Because the expression of fng is highly region-specific, the fng-dependent regulation of N–ligand interactions is restricted to and crucial for N activation in specific developmental contexts, such as wing development in Drosophila, although this modification is not a general requirement for N activation (16). Although the O-fucosylation of N is required for its further GlcNAc modification by Fng, conflicting results have been reported for the specific contribution of O-fucose monosaccharide to N signaling in Drosophila (5, 17–19).
Fucose modifications, including protein O-fucosylation, require GDP-l-fucose as the fucose donor. In mammals, GDP-l-fucose is synthesized by both de novo and salvage pathways (20). However, in Drosophila, GDP-l-fucose is synthesized only by the de novo pathway (21, 22). Thus, Drosophila is a useful system for studying the requirement of GDP-l-fucose in various biological events (23). The enzymes essential for the de novo synthesis of GDP-l-fucose, GDP-mannose 4,6 dehydratase (Gmd), and GDP-4-keto-6-deoxy-d-mannose 3,5-epimerase/4-reductase (Gmer), are encoded by the Gmd and Gmer genes, respectively (24). The Gmd homozygote causes GDP-l-fucose starvation in Drosophila (22).
In general, the biosynthetic cascades of nucleotide sugars, including GDP-l-fucose, are considered intracellular events, although sugars can also be acquired from the extracellular space through specific transporters (25). However, a previous study demonstrated that UDP-galactose and UDP-GalNAc are transported intercellularly among cultured CHO cells through intercellular junctions (26). A CHO cell derivative that is deficient in the enzyme UDP-Gal/UDP-GalNAc 4-epimerase shows defective LDL receptor structure and activity, which are restored by cocultivation with cells expressing the normal activity of this enzyme (26). This restoration of LDL receptor activity is suppressed by adding retinoic acid, an inhibitor of junctional communication (26). These results suggest that UDP-galactose and UDP-GalNAc can be supplied through intercellular junctional communication. However, such intercellular transport of nucleotide sugars has not been demonstrated in vivo. Furthermore, the nature of the intercellular communication involved in this delivery of nucleotide sugars is not yet clear. To address these issues, here we studied the intercellular delivery of GDP-l-fucose into cells in vivo, using mutants of genes involved in the biosynthetic pathway of GDP-l-fucose in Drosophila.
Results
Gmd and Gmer Are Required for Fng-Dependent N Signaling and the Fucosylation of Bulk Proteins.
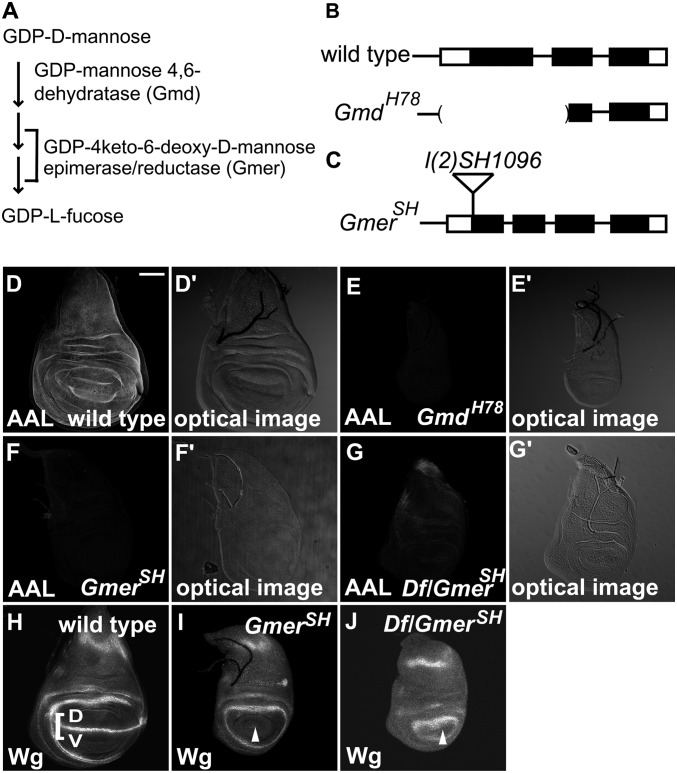
Gmd and Gmer are essential for GDP-l-fucose biosynthesis in Drosophila (Fig. 1A) (22, 23). GmdH78 is a null allele of Gmd (Fig. 1B), and GmdH78 homozygotes survive until the third larval instar (22). However, GmdH78 homozygotes lacking the maternal contribution of this gene’s product die as embryos, indicating that the maternal contribution of Gmd allows them to survive until the third instar (22). Gmer encodes the final enzyme of the GDP-l-fucose biosynthesis cascade (Fig. 1A). For this study, we used a fly line, GmerSH, which has a P-element insertion in the Gmer locus [l(2)SH1096], as an allele of Gmer (Fig. 1C). The P-element was inserted 29 bp downstream of the Gmer gene initiation codon (Fig. 1C). GmerSH homozygotes and transheterozygotes between GmerSH and Df(2R)BSC783, a deficiency uncovering the Gmer locus, died as third-instar larvae. The recessive lethal phenotype was no worse in these transheterozygotes than in the GmerSH homozygotes, suggesting that GmerSH is a null allele.
Fig. 1.
Gmd and Gmer are required for the fucosylation of bulk proteins and for Fng-dependent Notch signaling. (A) Schematic of GDP-l-fucose biosynthesis showing the roles of Gmd and Gmer, which are essential enzymes for its de novo synthesis in Drosophila. (B) Genomic organization of the Drosophila Gmd locus. The exons of the Gmd gene are shown as boxes, and the predicted coding regions are shaded black. A 0.8-kb deletion in GmdH78 is indicated by parenthesis. (C) Genomic organization of the Drosophila GmerSH locus. The exons are shown as boxes, with the predicted coding regions shaded black. A P-element, l(2)SH1096, is inserted 29-bp downstream of the predicted initiation codon. (D–G) AAL staining and (D′–G′) bright-field images of late third-instar wing imaginal discs. (D and D′) Wild-type wing disc. (E and E′) GmdH78 homozygous wing disc. (F and F′) GmerSH homozygous wing disc. (G and G′) GmerSH/Df(2R)BSC783 wing imaginal disc. (H–J) Anti-Wg antibody staining of late third-instar wing imaginal discs. (H) Wild-type wing imaginal disc. D, dorsal compartment; V, ventral compartment. Wg expression along the D/V boundary is indicated by a square bracket. (I) GmerSH homozygous wing imaginal disc. (J) GmerSH/Df(2R)BSC783 wing imaginal disc. White arrowheads indicate the D/V boundary in I and J. (Scale bar in D, 50 μm, applicable to D–J.)
To evaluate the roles of Gmd and Gmer in fucose modifications, we examined the fucosylation of bulk proteins in these mutants. Aleuria Aurantia Lectin (AAL) is an l-fucose lectin that recognizes α1,3- and α1,6-linked fucose residues (27, 28); thus, it should recognize the fucose moieties of N-glycans and possibly O-fucose. However, the AAL staining was not reduced in cells homozygous for O-fut14R6, a null mutant, in vivo (n = 17) (Fig. S1 A–C), suggesting that O-fucose’s contribution to the AAL staining was minor. In wild-type wing imaginal discs from late third-instar larvae, the AAL staining was ubiquitous (Fig. 1D). However, in the wing imaginal discs of GmdH78 or GmerSH homozygotes, the AAL staining was severely reduced (Fig. 1 E and F). To further characterize the GmerSH allele, we compared the intensity of AAL staining between the wing imaginal discs of GmerSH homozygotes and of transheterozygotes between GmerSH and Df(2R)BSC783 (Fig. 1 F, F′, G, and G′). In both cases, the AAL staining was similarly reduced, consistent with GmerSH being a null allele (Fig. 1 F and G). These results indicate that the fucosylation of bulk proteins was significantly reduced in the GmdH78 and GmerSH mutants.
In late third-instar larvae, wingless (wg) expression is activated in the wing imaginal disc by N signaling along the dorsal–ventral compartment boundary (D/V boundary) (Fig. 1H, white square bracket) (29). wg expression is not detected in the wing imaginal discs of Gmd homozygotes (22), and here we found that Wg protein was also not detectable in the late third-instar wing imaginal discs of GmerSH homozygotes or in transheterozygotes between GmerSH and Df(2R)BSC783 (Fig. 1 I and J, white arrowhead). In contrast, Wg expression encircling the wing pouch, which is independent of N signaling, was normal in these wing imaginal discs (Fig. 1 I and J). These results are consistent with the previous proposal that GDP-l-fucose biosynthesis is required for Fng-dependent N signaling (9–11).
Functions of Gmd and Gmer Are Cell-Nonautonomous.
Although the enzymes required for the biosyntheses of various nucleotide sugars have been elucidated (30), very few studies have examined whether nucleotide sugars are exchanged intercellularly (31), and none have investigated such exchanges in vivo. To address these issues, we next examined whether Gmd and Gmer function cell-autonomously or cell-nonautonomously in vivo, because these mutants should behave cell-nonautonomously if GDP-l-fucose is transferred intercellularly. Although the cell-nonautonomous behavior of Gmd was previously reported (18, 19, 32), this phenomenon has not been examined in detail.
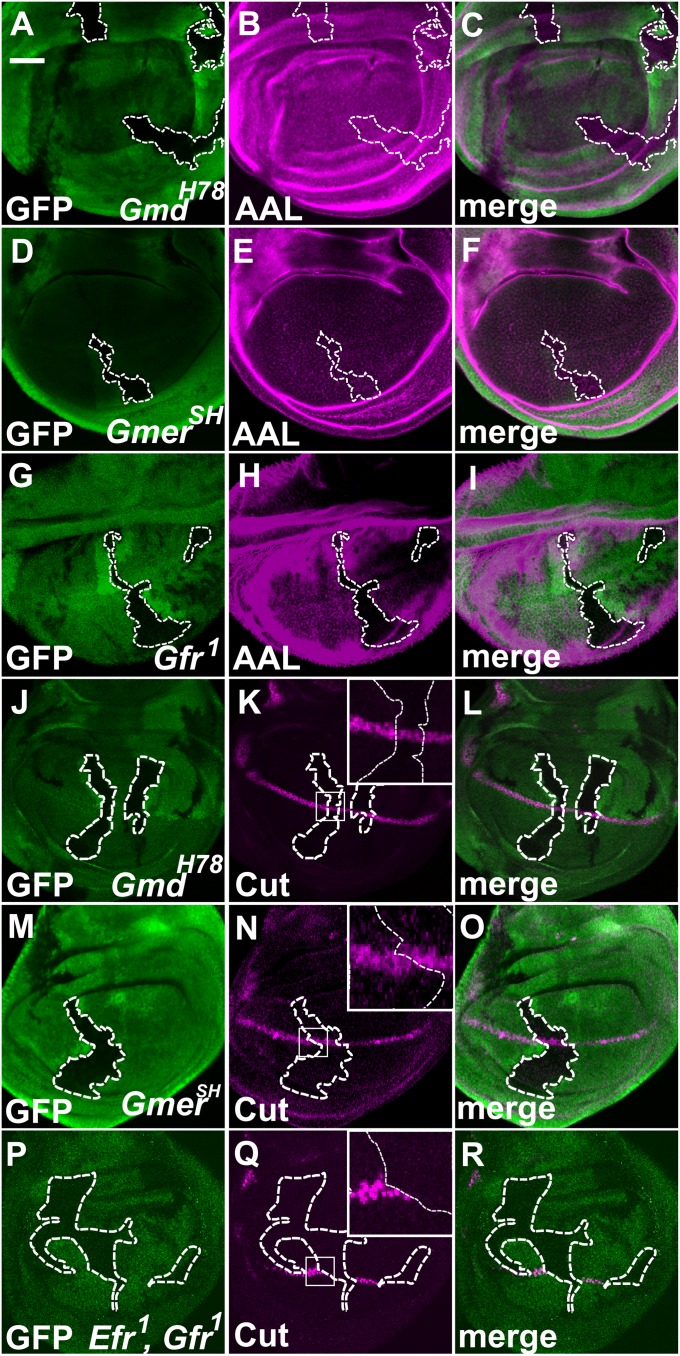
Using the Flippase/Flippase Recombination Target (FLP/FRT) system, we generated somatic mosaic clones of Gmd or Gmer homozygous cells (indicated by the absence of GFP expression) in late third-instar wing imaginal discs of heterozygotes (Fig. 2 A–F). AAL staining was not reduced in the homozygous somatic clones of the GmdH78 or GmerSH alleles (Fig. 2 A–F). This result suggested that the surrounding heterozygous cells supplied the GDP-l-fucose intercellularly to the homozygous mutant cells. In contrast, the AAL staining was diminished in cells homozygous for Gfr, which encodes a GDP-l-fucose transporter (Fig. 2 G–I). Gfr transports GDP-l-fucose, which is essential for the fucosylation of N-glycans, into the Golgi from the cytoplasm (23).
Fig. 2.
Gmd and Gmer function cell-nonautonomously. (A–I) Late third-instar wing imaginal discs carrying somatic clones homozygous for GmdH78 (A–C), GmerSH (D–F), or Gfr1 (G–I) were stained with AAL. (A, D, and G) Regions lacking GFP (green) are GmdH78 (A), GmerSH (D), or Gfr1 (G) homozygous cells. (B, E, and H) AAL-staining (magenta) of wing imaginal discs carrying somatic clones homozygous for GmdH78 (B), GmerSH (E), or Gfr1 (H). (C, F, and I) Merged images of A and B, D and E, and G and H, respectively. (J–R) Late third-instar wing imaginal discs carrying somatic clones homozygous for GmdH78 (J–L), GmerSH (M–O), or Efr1 and Gfr1 (P–R) and stained with an anti-Cut antibody. (J, M, and P) Regions lacking GFP (green) are GmdH78 (J), GmerSH (M), or Efr1 and Gfr1 (P) homozygous cells. (K, N, and Q) Anti-Cut antibody staining (magenta) of wing imaginal discs carrying somatic clones homozygous for GmdH78 (K), GmerSH (N), or Efr1 and Gfr1 (Q). (L, O, and R) Merged images of J and K, M and N, and P and Q, respectively. Insets: Higher magnifications of the regions indicated by white squares (K, N, and Q). The boundaries of the somatic clones are indicated by white broken lines. (Scale bar in A, 50 μm, applicable to A–R.)
We next examined whether the intercellular supply of GDP-l-fucose is sufficient for the O-fucosylation of N, which is required for the Fng-dependent activation of N signaling. The expression of cut along the D/V boundary depends on the activation of N signaling (33). cut encodes a transcription factor that specifically localizes to the nucleus. Thus, the expression of cut detected by anti-Cut antibody staining is a useful marker for determining the cell-autonomous behavior of GmdH78 or GmerSH mutants. We found that the expression of cut was not reduced in the somatic clones of cells homozygous for GmdH78 or GmerSH (Fig. 2 J–O). The expression of Wg along the D/V boundary was also not affected in these cells (Fig. S2 A–F). In contrast, in cells homozygous for Gfr1 and Efr1, the expression of Cut and Wg along the D/V boundary was largely abolished (Fig. 2 P–R and Fig. S2 G–I). This result is consistent with a previous report showing that Gfr and Efr are redundantly required for the transport of GDP-l-fucose into the lumen of the endoplasmic reticulum, where O-fucosylation takes place (34). Therefore, the intercellular supply of GDP-l-fucose is sufficient for the O-fucosylation of N.
Intercellular Delivery of GDP-l-Fucose Is Limited to a Given Organ.
The intercellular transport of GDP-l-fucose could be mediated by several possible mechanisms. For example, GDP-l-fucose could be transported among the cells of a single organ through the extracellular space or without entering the extracellular space. Alternatively, it could be transported via the body fluids and enter cells through transporters in the plasma membrane. To distinguish between these possibilities, Gmd or Gmer was specifically overexpressed using the Gal4-Upstream Activation Sequence (UAS) system in a limited part of wing imaginal discs or in different organs, in its respective mutant background. We then examined whether the Wg expression along the D/V boundary of the late third-instar wing imaginal discs was rescued.
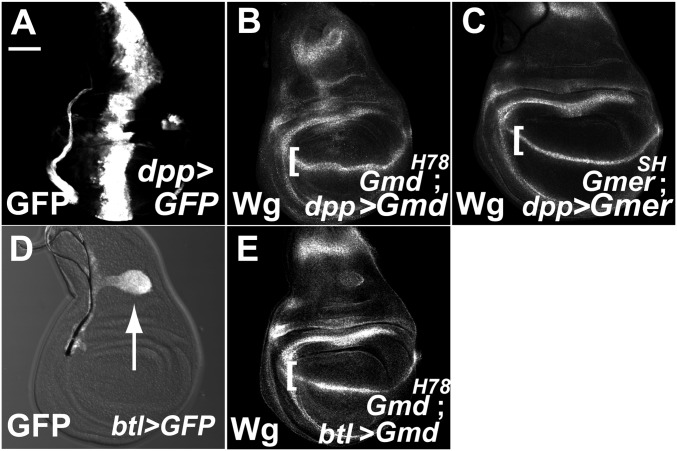
First, we overexpressed Gmd or Gmer in its respective homozygous mutant along the anterior–posterior compartment boundary (A/P boundary) of wing imaginal discs (GmdH78 and GmerSH; Fig. 3 B and C). The overexpressed constructs were driven by decapentaplegic (dpp)-Gal4 in the region indicated by the expression of GFP (Fig. 3A). Despite the restricted expression of these genes in the wing imaginal discs, the expression of Wg was rescued along the entire D/V boundary in all cases examined (n > 20) (Fig. 3 B and C). We also found that the specific expression of Gmd driven by breathless (btl)-Gal4 in the trachea blast, which is attached to the narrow portion of the wing imaginal disc (Fig. 3D, GFP-positive region indicated by arrow), was sufficient to rescue the Wg expression along the entire wing imaginal disc D/V boundary in GmdH78 homozygotes, in all cases examined (n > 20) (Fig. 3E). The AAL staining of these wing imaginal discs was also restored (Fig. S3 A, C, and E). These results indicate that GDP-l-fucose can spread throughout the epithelium of the wing imaginal discs.
Fig. 3.
GDP-l-fucose is delivered intercellularly within an organ. (A) GFP labeling showing the UAS-GFP expression pattern along the A/P boundary of third-instar wing imaginal discs under the dpp-Gal4 driver, which was used in B and C. (B, C, and E) Wg expression along the D/V boundary of the third-instar wing imaginal discs (square brackets in B, C, and E). (B) Wing disc of a GmdH78 homozygote overexpressing UAS-Gmd along the A/P boundary. (C) Wing disc of a GmerSH homozygote overexpressing UAS-Gmer along the A/P boundary. (D) Expression pattern of UAS-GFP in third-instar wing imaginal discs (white arrow), under control of a btl-Gal4 driver. (E) Wing disc of a GmdH78 homozygote overexpressing Gmd driven by btl-Gal4. (Scale bar in A, 50 μm, applicable to A–E.)
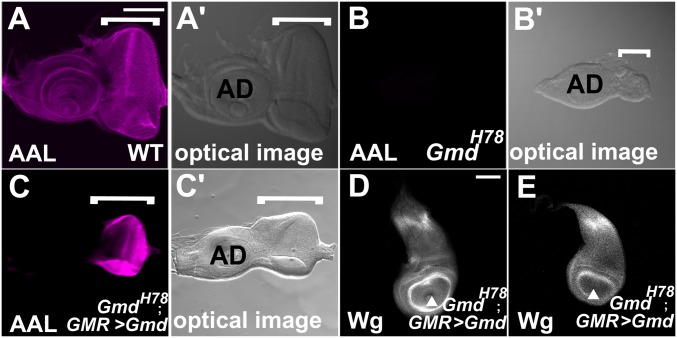
It was also possible that GDP-l-fucose is transported between organs in the larval body fluid. To test this possibility, we expressed Gmd under the control of GMR-Gal4 and Lsp2-Gal4 in the eye imaginal disc and fat body, respectively, in GmdH78 homozygotes (35). These two organs are isolated in the body from the wing imaginal discs, where the Fng-dependent activation of N was examined. In wild-type eye imaginal discs, AAL staining was detected uniformly (Fig. 4 A and A′, white square bracket). However, in the Gmd H78 homozygotes, AAL staining was largely diminished in the eye imaginal disc as in the wing imaginal discs (Fig. 4 B and B′, white square bracket). The expression of Gmd driven by GMR-Gal4 recovered the AAL staining in the entire eye imaginal discs (Fig. 4 C and C′, square bracket), even though GMR-Gal4 expresses Gal4 only in the differentiated photoreceptor and pigment cells (the right half of the eye imaginal disc in Fig. 4C) (35). However, AAL staining was not restored in the antenna imaginal disc even though these two imaginal discs are interconnected, suggesting that some variation in molecular transport was present (Fig. 4C). We also noted that the development of the eye imaginal discs was not completely rescued, probably because the expression of GMR-Gal4 is initiated only after the differentiation of eye imaginal disc cells (compare Fig. 4 A′ and C′) (36). Importantly, we found that Wg expression along the D/V boundary of the GmdH78 homozygote wing imaginal discs was not rescued in any of these larvae (n > 20) (Fig. 4D), nor was it rescued by the expression of Gmd driven by Lsp2-Gal4 in the fat body of GmdH78 homozygotes (Fig. 4E). In these wing imaginal discs, a reduced level of AAL staining was still observed (Fig. S3 G and I). Thus, GDP-l-fucose is not exchanged through the body fluid among isolated organs in the Drosophila body.
Fig. 4.
GDP-l-fucose is not supplied through body fluids. (A–C) AAL staining and (A′–C′) bright-field images of late third-instar eye imaginal discs (indicated by white square brackets). (A and A′) Wild-type eye imaginal disc. (B and B′) GmdH78 homozygous eye imaginal disc. (C and C′) Eye imaginal disc of a GmdH78 homozygote overexpressing UAS-Gmd driven by GMR-Gal4. (D and E) Anti-Wg antibody staining of late third-instar wing imaginal discs. AD, antenna imaginal discs. (D) Wing imaginal disc isolated from the larva of a GmdH78 homozygote overexpressing UAS-Gmd in the eye imaginal disc driven by GMR-Gal4. (E) Wing imaginal disc isolated from the larva of a GmdH78 homozygote overexpressing UAS-Gmd in the fat body driven by Lsp-Gal4. White arrowheads indicate the D/V boundary. (Scale bar in A, 50 μm, applicable to A–C′; in D, 50 μm, applicable to D and E.)
GDP-l-Fucose Is Supplied Intercellularly by a Gap Junction-Mediated Mechanism.
Gap junctions are channels formed by the direct intercellular apposition of oligomeric transmembrane proteins, such as the Connexin family proteins in mammals, which permit the direct exchange of ions and small molecules (less than 1 kDa) between cells without passage into the extracellular space (37). In invertebrates, Innexin family proteins form the gap junctions (38). Eight innexin genes have been found in Drosophila melanogaster (38, 39). To assess the involvement of gap junctions in the intercellular delivery of GDP-l-fucose, we performed a knockdown of innexin genes, using RNAi, in vivo. Among the innexin genes, we selected optic ganglion reduced (ogre), innexin2 (inx2), innexin3 (inx3), and innexin4 (inx4) as knockdown targets, because they are expressed in epithelia, which comprises the wing imaginal disc where N signaling is activated along the D/V boundary (40).
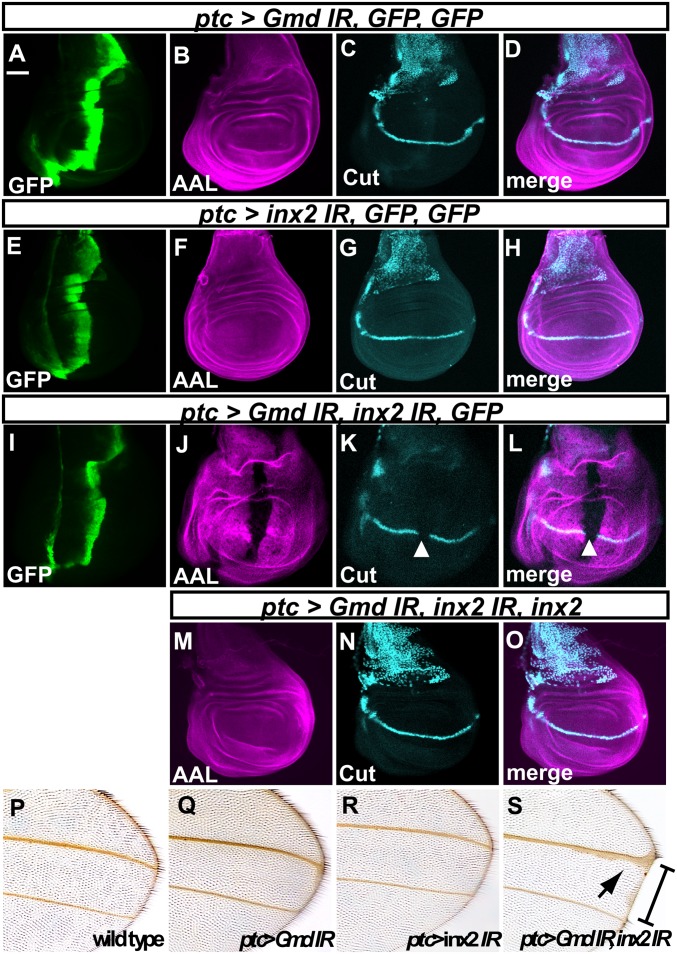
A hairpin double-stranded RNA (dsRNA) corresponding to part of the Gmd or inx2 cDNA was produced under the control of patched (ptc)-Gal4 in the region along the A/P boundary of late third-instar wing imaginal discs that were otherwise wild type (indicated by GFP expression in Fig. 5 A, E, and I). The AAL staining and cut expression along the D/V boundary were unaffected by the knockdown of either Gmd or inx2 (BL29306) by RNAi, in all cases examined (n = 30 for Gmd and n = 27 for inx2) (Fig. 5 B–D and F–H). However, the knockdown of inx2 and Gmd together reduced the level of AAL staining and cut expression, in all cases examined (n = 23) (Fig. 5 J–L). These defects were completely rescued by the simultaneous overexpression of wild-type inx2 (n = 27) (Fig. 5 M–O) (41). Furthermore, a different inx2 RNAi line (v102194), which produced a hairpin dsRNA corresponding to a different part of the inx2 cDNA, showed the same result (n = 27 for inx2 RNAi and n = 32 for inx2 and Gmd RNAi) (Fig. S4 B–J). These results suggest that the losses of AAL staining and cut expression were not due to an off-target effect of the RNAi against inx2.
Fig. 5.
inx2 is required for the intercellular delivery of GDP-l-fucose. (A–O) Late third-instar wing imaginal discs stained with an anti-GFP antibody (A, E, and L, green), AAL (B, F, J, and M, magenta), and an anti-Cut antibody (C, G, K, and N, turquois). (A–D) ptc-Gal drove the expression of two UAS-GFP transgenes where a hairpin dsRNA of Gmd (Gmd IR) was produced. (E–H) ptc-Gal drove the expression of two UAS-GFP transgenes where a hairpin dsRNA of inx2 (inx2 IR) was produced. (I–L) ptc-Gal drove the expression of a UAS-GFP transgene where hairpin dsRNAs of Gmd and inx2 were produced. (M–O) ptc-Gal drove the expression of UAS-inx2 where hairpin dsRNAs of Gmd and inx2 were produced. The total number of UAS promoters in each experiment was adjusted to be the same by introducing UAS-GFP. D, H, L, and O are merged images of B and C, F and G, J and K, and M and N, respectively. White arrowheads indicate the regions of reduced AAL staining (J and K) and cut expression (K and L). (P–S) Adult wings. (P) Wild-type. (Q–S) Wings producing hairpin dsRNAs targeting Gmd (Q), inx2 (R), or Gmd and inx2 (S) under the control of ptc-Gal4. Square bracket and black arrow indicate a wing blade notch and wing vein-thickening, respectively, in S. (Scale bar in A, 50 μm, applicable to A–O.)
These results were consistent with the adult wing phenotype induced by the RNAi against these genes. Diminished cut expression along the D/V boundary is associated with the disruption of N signaling and results in a loss of wing-margin tissue (42). The knockdown of either Gmd or inx2 by RNAi did not affect the wing margin (n = 41 for Gmd and n = 52 for inx2) (Fig. 5 Q and R), but knocking down both together resulted in the loss of wing margin tissue in all cases examined (n = 30) (Fig. 5S).
In contrast to the results for inx2, the knockdown of ogre (n = 23), inx3 (n = 31), or inx4 (n = 22) together with Gmd by RNAi did not reduce the AAL staining or cut expression (Fig. S5 A–I). These results suggest that GDP-l-fucose is transported although gap junctions composed of Inx2 in the epithelium of the wing imaginal disc. Our results also showed that the GDP-l-fucose synthesized in individual cells is sufficient for the fucosylation of bulk proteins and for the activation of Fng-dependent N signaling in the absence of the intercellular supply.
Discussion
The nucleotide sugars UDP-galactose and UDP-GalNAc were previously shown to be transported although intercellular junctions in mammalian cultured cells, although the involvement of intercellular junctional communication was based only on an inhibitor with low specificity, which was available at that time (26). In this study, we demonstrated that GDP-l-fucose is intercellularly exchanged among epithelial cells within an organ through inx2-dependent gap junctions in vivo. Thus, this is evidence for the intercellular exchange of a nucleotide sugar through gap junctions in a living animal. This intercellular supply of GDP-l-fucose is sufficient for the O-fucosylation of N, even in the absence of its cell-autonomous supply by de novo biosynthesis.
The molecular weight of GDP-l-fucose is 589.34 Da. Given that gap junctions transport small molecules whose molecular weight is less than 1 kgDalton (37), GDP-l-fucose is small enough to be transported although gap junctions. In Drosophila, a few reports have demonstrated that genes encoding enzymes involved in the biosynthesis of nucleotide sugars behave cell-nonautonomously (31), although the nucleotide sugars transported among cells were not identified in these experiments, and the mechanisms of such nucleotide sugar delivery have not been studied. These potential nucleotide sugars are also small enough to be transported although gap junctions. Therefore, intercellular exchange might be a general property by which various nucleotide sugars enter animal cells that form gap junctions.
The kinetics of diffusion through inx2-dependent gap junctions was measured in the epithelium of the Drosophila proventriculus (43). Injected lucifer yellow diffused into approximately 20 cells after 16 h (43). In the present study, the region-specific expression of Gmd or Gmer in its respective mutant background was sufficient to rescue the fucosylation of the entire wing imaginal discs. There are 100–200 epithelial cells across the wing pouch region of a wing imaginal disc. Our results suggested that GDP-l-fucose is delivered into a number of cells although inx2-dependent gap junctions. Considering that the development of the wing imaginal discs takes several days, the diffusion speed of GDP-l-fucose roughly coincides with that of lucifer yellow, reported previously (43).
In contrast to our observation in the epithelium of wing imaginal discs, Gmd homozygous clones behave cell-autonomously in the stem cells of the adult fly intestine (32). Thus, the intercellularly supplied GDP-l-fucose is not a general property of Drosophila tissues and cells. This discrepancy could be explained if these stem cells do not form gap junctions with the surrounding cells, although this hypothesis remains to be tested. It is also likely that the number of gap junctions and their Innexin-subunit composition affect the efficiency of intercellular GDP-l-fucose diffusion (44). These possibilities may explain our observation that GDP-l-fucose did not diffuse from the eye imaginal disc to the antenna imaginal disc even though these two imaginal discs are continuous.
In the present study, we failed to show physiological roles of the intercellular delivery of GDP-l-fucose, mainly because disruption of the innexin genes should abolish the transportation of various small molecules, not only GDP-l-fucose, through gap junctions. Thus, we could not distinguish specific effects associated with the absence of GDP-l-fucose from other defects induced by the lack of other molecules’ intercellular transport. On the other hand, the consumption rate of nucleotide sugars could differ locally, even within an organ. Thus, the intercellular supply of nucleotide sugars may serve to compensate for such uneven consumption rates, although further quantitative analyses are needed to address this possibility. Nevertheless, our results introduce the novel viewpoint that the intercellular exchange of nucleotide sugars must be considered as an aspect of sugar metabolism in vivo.
Materials and Methods
Fly Stocks.
We used Canton-S as the wild-type stock. The following mutant alleles were used: GmdH78, a null mutant allele of Gmd (22, 23), and l(2)SH1931, a P-element insertion mutant of Gmer. Expression of the UAS lines was driven by dpp-Gal4, ptc-Gal4, btl-Gal4, Lsp2-Gal4, or GMR-Gal4. Knockdown of Gmd was performed using 8890R-2, which is an RNAi line of Gmd (NIG-FLY, http://www.shigen.nig.ac.jp/fly/nigfly/index.jsp). Knockdown of inx2 was performed using BL29306 (TRiP) and v102194 (VDRC). For knockdown of the other innexin family genes, we used the following RNAi lines from VDRC: v7136 for ogre, v39095 for inx3, and v33277 for inx4. UAS-inx2 was described previously (41). All flies were raised at 25 °C, except for the experiments involving RNAi, which were carried out at 30 °C.
Expression Constructs of Gmd and Gmer.
cDNAs encompassing the entire ORFs of Gmd (GM12762) and Gmer (GM03782) were inserted into the BglII and XhoI sites or the EcoRI and XhoI sites of pUAS-T, and the resulting constructs were designated as UAS-Gmd and UAS-Gmer, respectively. Transgenic fly lines were established by a standard procedure.
Generation of Mosaics.
Somatic clones were generated by Flp-mediated mitotic recombination in y w hs-flp; Gmd FRT40A/Ubi-GFP FRT40A and y w hs-flp; FRTG13l(2)SH1931/FRTG13 Ubi-GFP larvae. The expression of Flp was induced in the second-instar larvae by a 30-min heat shock at 37 °C.
Immunohistochemistry.
Late third-instar eye imaginal discs and late third-instar wing imaginal discs were immunostained as previously described. The following antibodies were used: mouse anti-Wg (1:250; 4D4), mouse anti-Cut (1:250; 2B10), and rabbit anti-GFP (1:1,000; MBL). Alexa 488- (Molecular Probes) and Cy3- (Rockland) conjugated secondary antibodies were used at a dilution of 1:500. For lectin staining, biotin-conjugated AAL (1 μg/mL; Seikagaku) was used as previously described (28). All images were obtained by confocal microscopy (Pascal and LSM700, Zeiss).
Supplementary Material
Acknowledgments
We thank the Bloomington Stock Center, Drosophila Genetic Resource Center, National Institute of Genetics, and Vienna Drosophila RNAi Center for flies, and the Developmental Studies Hybridoma Bank for antibodies. UAS-inx2 was a kind gift from M. Mukai. This work was supported by grants-in-aid from the Japanese Ministry of Education, Culture, Sports and Science (to T.A. and K. Matsuno), grants from Precursory Research for Embryonic Science and Technology, Japan Science and Technology Corporation (to K. Matsuno), and the Naito Foundation (K. Matsumoto).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202369109/-/DCSupplemental.
References
- 1.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 2.Luther KB, Haltiwanger RS. Role of unusual O-glycans in intercellular signaling. Int J Biochem Cell Biol. 2009;41:1011–1024. doi: 10.1016/j.biocel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009;139:1229–1241. doi: 10.1016/j.cell.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brückner K, Perez L, Clausen H, Cohen S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406:411–415. doi: 10.1038/35019075. [DOI] [PubMed] [Google Scholar]
- 5.Moloney DJ, et al. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406:369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- 6.Bonegio R, Susztak K. Notch signaling in diabetic nephropathy. Exp Cell Res. 2012;318:986–992. doi: 10.1016/j.yexcr.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris RJ, Spellman MW. O-linked fucose and other post-translational modifications unique to EGF modules. Glycobiology. 1993;3:219–224. doi: 10.1093/glycob/3.3.219. [DOI] [PubMed] [Google Scholar]
- 8.Acar M, et al. Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell. 2008;132:247–258. doi: 10.1016/j.cell.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okajima T, Irvine KD. Regulation of notch signaling by o-linked fucose. Cell. 2002;111:893–904. doi: 10.1016/s0092-8674(02)01114-5. [DOI] [PubMed] [Google Scholar]
- 10.Sasamura T, et al. neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development. 2003;130:4785–4795. doi: 10.1242/dev.00679. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J Biol Chem. 2001;276:40338–40345. doi: 10.1074/jbc.M107849200. [DOI] [PubMed] [Google Scholar]
- 12.Moloney DJ, et al. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J Biol Chem. 2000;275:9604–9611. doi: 10.1074/jbc.275.13.9604. [DOI] [PubMed] [Google Scholar]
- 13.Brou C, et al. A novel proteolytic cleavage involved in Notch signaling: The role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 14.Mumm JS, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 15.Panin VM, Papayannopoulos V, Wilson R, Irvine KD. Fringe modulates Notch-ligand interactions. Nature. 1997;387:908–912. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- 16.Irvine KD. Fringe, Notch, and making developmental boundaries. Curr Opin Genet Dev. 1999;9:434–441. doi: 10.1016/S0959-437X(99)80066-5. [DOI] [PubMed] [Google Scholar]
- 17.Stahl M, et al. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem. 2008;283:13638–13651. doi: 10.1074/jbc.M802027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glavic A, López-Varea A, de Celis JF. The balance between GMD and OFUT1 regulates Notch signaling pathway activity by modulating Notch stability. Biol Res. 2011;44:25–34. doi: 10.4067/S0716-97602011000100004. [DOI] [PubMed] [Google Scholar]
- 19.Okajima T, Reddy B, Matsuda T, Irvine KD. Contributions of chaperone and glycosyltransferase activities of O-fucosyltransferase 1 to Notch signaling. BMC Biol. 2008;6:1. doi: 10.1186/1741-7007-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker DJ, Lowe JB. Fucose: Biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 21.Roos C, Kolmer M, Mattila P, Renkonen R. Composition of Drosophila melanogaster proteome involved in fucosylated glycan metabolism. J Biol Chem. 2002;277:3168–3175. doi: 10.1074/jbc.M107927200. [DOI] [PubMed] [Google Scholar]
- 22.Sasamura T, et al. The O-fucosyltransferase O-fut1 is an extracellular component that is essential for the constitutive endocytic trafficking of Notch in Drosophila. Development. 2007;134:1347–1356. doi: 10.1242/dev.02811. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa HO, et al. Notch deficiency implicated in the pathogenesis of congenital disorder of glycosylation IIc. Proc Natl Acad Sci USA. 2005;102:18532–18537. doi: 10.1073/pnas.0504115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tonetti M, et al. The metabolism of 6-deoxyhexoses in bacterial and animal cells. Biochimie. 1998;80:923–931. doi: 10.1016/s0300-9084(00)88889-6. [DOI] [PubMed] [Google Scholar]
- 25.Barrett MP, Walmsley AR, Gould GW. Structure and function of facilitative sugar transporters. Curr Opin Cell Biol. 1999;11:496–502. doi: 10.1016/s0955-0674(99)80072-6. [DOI] [PubMed] [Google Scholar]
- 26.Hobbie L, Kingsley DM, Kozarsky KF, Jackman RW, Krieger M. Restoration of LDL receptor activity in mutant cells by intercellular junctional communication. Science. 1987;235:69–73. doi: 10.1126/science.3798096. [DOI] [PubMed] [Google Scholar]
- 27.Kochibe N, Furukawa K. Purification and properties of a novel fucose-specific hemagglutinin of Aleuria aurantia. Biochemistry. 1980;19:2841–2846. doi: 10.1021/bi00554a004. [DOI] [PubMed] [Google Scholar]
- 28.Lühn K, et al. Identification and molecular cloning of a functional GDP-fucose transporter in Drosophila melanogaster. Exp Cell Res. 2004;301:242–250. doi: 10.1016/j.yexcr.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 29.Rulifson EJ, Blair SS. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development. 1995;121:2813–2824. doi: 10.1242/dev.121.9.2813. [DOI] [PubMed] [Google Scholar]
- 30.ten Hagen KG, Zhang L, Tian E, Zhang Y. Glycobiology on the fly: Developmental and mechanistic insights from Drosophila. Glycobiology. 2009;19:102–111. doi: 10.1093/glycob/cwn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haerry TE, Heslip TR, Marsh JL, O’Connor MB. Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila. Development. 1997;124:3055–3064. doi: 10.1242/dev.124.16.3055. [DOI] [PubMed] [Google Scholar]
- 32.Perdigoto CN, Schweisguth F, Bardin AJ. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development. 2011;138:4585–4595. doi: 10.1242/dev.065292. [DOI] [PubMed] [Google Scholar]
- 33.Micchelli CA, Rulifson EJ, Blair SS. The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development. 1997;124:1485–1495. doi: 10.1242/dev.124.8.1485. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa HO, et al. Two pathways for importing GDP-fucose into the endoplasmic reticulum lumen function redundantly in the O-fucosylation of Notch in Drosophila. J Biol Chem. 2010;285:4122–4129. doi: 10.1074/jbc.M109.016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 36.Adamson AL, Wright N, LaJeunesse DR. Modeling early Epstein-Barr virus infection in Drosophila melanogaster: The BZLF1 protein. Genetics. 2005;171:1125–1135. doi: 10.1534/genetics.105.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loewenstein WR. Junctional intercellular communication: The cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 38.Phelan P. Innexins: Members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–245. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Phelan P, Starich TA. Innexins get into the gap. Bioessays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- 40.Stebbings LA, et al. Gap junctions in Drosophila: developmental expression of the entire innexin gene family. Mech Dev. 2002;113:197–205. doi: 10.1016/s0925-4773(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 41.Mukai M, et al. Innexin2 gap junctions in somatic support cells are required for cyst formation and for egg chamber formation in Drosophila. Mech Dev. 2011;128:510–523. doi: 10.1016/j.mod.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 42.de Celis JF, García-Bellido A. Roles of the Notch gene in Drosophila wing morphogenesis. Mech Dev. 1994;46:109–122. doi: 10.1016/0925-4773(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 43.Lechner H, Josten F, Fuss B, Bauer R, Hoch M. Cross regulation of intercellular gap junction communication and paracrine signaling pathways during organogenesis in Drosophila. Dev Biol. 2007;310:23–34. doi: 10.1016/j.ydbio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Bauer R, et al. Intercellular communication: The Drosophila innexin multiprotein family of gap junction proteins. Chem Biol. 2005;12:515–526. doi: 10.1016/j.chembiol.2005.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.