Abstract
Conditional mutations are essential for determining the stage- and tissue-specific functions of genes. Here we achieve conditional mutagenesis in zebrafish using FT1, a gene-trap cassette that can be stably inverted by both Cre and Flp recombinases. We demonstrate that intronic insertions in the gene-trapping orientation severely disrupt the expression of the host gene, whereas intronic insertions in the neutral orientation do not significantly affect host gene expression. Cre- and Flp-mediated recombination switches the orientation of the gene-trap cassette, permitting conditional rescue in one orientation and conditional knockout in the other. To illustrate the utility of this system we analyzed the functional consequence of intronic FT1 insertion in supv3l1, a gene encoding a mitochondrial RNA helicase. Global supv311 mutants have impaired mitochondrial function, embryonic lethality, and agenesis of the liver. Conditional rescue of supv311 expression in hepatocytes specifically corrected the liver defects. To test whether the liver function of supv311 is required for viability we used Flp-mediated recombination in the germline to generate a neutral allele at the locus. Subsequently, tissue-specific expression of Cre conditionally inactivated the targeted locus. Hepatocyte-specific inactivation of supv311 caused liver degeneration, growth retardation, and juvenile lethality, a phenotype that was less severe than the global disruption of supv311. Thus, supv311 is required in multiple tissues for organismal viability. Our mutagenesis approach is very efficient and could be used to generate conditional alleles throughout the zebrafish genome. Furthermore, because FT1 is based on the promiscuous Tol2 transposon, it should be applicable to many organisms.
High throughput functional genomic and informatic methods have been developed to interrogate the genome and extract functional predictions about many genes at a time. However, careful phenotypic analysis of genetic mutants remains the sine qua non of reductionist biological science. In most experimental organisms, random mutagenesis is the preferred or only mutagenic technique available. DNA alkylating agents, transposable elements, or retroviruses are traditionally used in these organisms. A major limitation of these traditional genetic methods is that they reveal only the earliest and/or most prominent function of a gene as later functions are masked by the earlier phenotype, which is often lethality. To assess later functions, for example in metabolism, aging, or behavior, conditional alleles are required.
The development of conditional alleles has proven a boon to studying gene function in temporally or spatially restricted contexts. Traditional conditional alleles disrupt gene function by changing the environment, for example by increasing the temperature. Engineered conditional alleles disrupt gene function by activating a recombination-mediated molecular switch that ablates gene function in one state, but has no functional consequences in the other state (1, 2). In the mouse, engineered conditional alleles can be generated by homologous recombination to insert the molecular switch at defined loci or by retroviral-mediated random insertion of the molecular switch (3, 4). The second approach leverages the orientation-dependent gene disruption of a gene trap and the ability of Flp/Cre recombinases to stably invert the gene trap. By strategically arranging dimers of heterotypical flp- and cre-recombinase binding sites flanking the gene trap, stable inversion is achieved in cis by recombinase-mediated Flip and Excision (FlEx) (5). However, this conditional gene-trap mutagen has not been validated at the organismal level.
A distinct advantage of FlEx-based conditional gene-trap mutations is the possibility of stage- and tissue-specific rescue or knockout of the mutated genes. In zebrafish, several gene-trap mutagenesis methods have been developed (6, 7), including the “gene-break” (6, 8) and “FlipTrap” (9) technologies. We set out to test whether the FlEx-based conditional gene-trap mutagenesis approach functions at the organismal level in zebrafish. We show here that a highly mutagenic transposable element can be used for conditional analysis of essential genes.
Results
Development of a Conditional Mutagen.
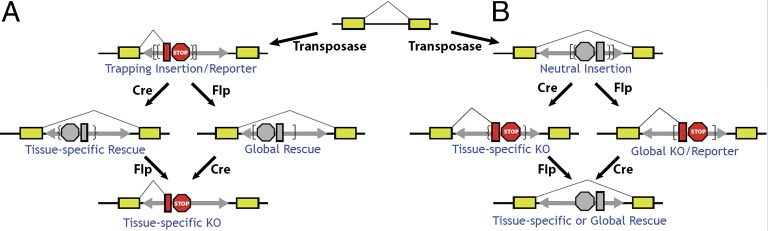
To adapt the FlEx-based conditional gene-trap mutagenesis (FlExTrap) approach to zebrafish, we generated FT1, a Tol2-based FlExTrap vector. It consists of a highly mutagenic gene trap contained within the FlEx cassette that can be stably inverted by both Cre and Flp recombinases (10, 11). FRT/F3 and loxP/lox5171 are incompatible recombination sites used for the Flp and Cre recombinases, respectively, to stably invert the cassette (10). The reporter gene, mCherry, has its own initiation codon. It is in the +2 reading frame, but the other reading frames can easily be generated by filling in a unique SalI/AccI site upstream of mCherry. The gene trap consists of five tandem copies of a polyadenylation signal sequence to achieve highly efficient transcriptional termination and this feature was optimized in zebrafish embryos (Fig. S1). Germline insertions were generated using standard Tol2 transgenesis and were identified in the F1 generation (7). Uniquely, this conditional transposable mutagen can be used for functional genomic studies regardless of orientation of the starting insertions. Cre- and Flp-mediated recombination generates both ubiquitous and conditional gene inactivation and ubiquitous and conditional rescue (Fig. 1) (11). We refer to the orientation of the FlExTrap in relation to the orientation of the tagged gene. When the gene trap is in the same orientation as the host gene, the insertion is in the gene-trapping (Gt) orientation and is denoted T. When the gene trap is in an inverted orientation relative to the host gene, the insertion is in the neutral orientation and it is denoted N. Intronic insertions in the T orientation are expected to be mutagenic, whereas insertions in the N orientation are expected to be functionally neutral. The orientation symbol may be followed by c and/or f to indicate that it has been inverted by Cre or Flp recombinase, respectively.
Fig. 1.
The FT1 mutagen functions regardless of the starting orientation and the trap is invertible with both the cre and flp recombinases. The FT1 mutagen consists of a FlEx-based invertible cassette that consists of lox sites (brackets) and frt sites (braces) that allow stable inversion of the cassette with cre and flp, respectively. The invertible cassette carries an mCherry reporter exon (red rectangle) downstream of a strong splice acceptor and upstream of five repeats of a transcriptional stop and polyadenylation sequence derived from the BGH gene (red octagon). This cassette is carried on a Tol2 transposon to facilitate transgenesis (double-headed gray arrow). (A) Insertion of the FT1 mutagen into an intron in the gene-trapping orientation (T) results in splicing from the endogenous 5′ exon to the strong splice acceptor and generation of an mCherry fusion transcript that possibly produces a fusion protein, transcriptional termination, and potentially, loss-of-function of the endogenous gene. The FlEx cassette may be inverted with either tissue-specific expression of cre or flp resulting in tissue-specific rescue (Left) or flp/cre mRNA injection at the one-cell stage, resulting in global inversion and rescue (Right). The cassette may be inverted one additional time (Bottom). (B) Insertion of the FT1 mutagen in the neutral (nontrapping) orientation (N). The gene trap cassette is not active in this orientation, allowing transcriptional read through and causing little effect on expression of the endogenous gene. The FlEx cassette may be inverted with either tissue-specific expression of cre/flp, resulting in tissue-specific gene knockout (KO) (Left) or flp/cre mRNA injection resulting in global KO. An additional cassette inversion can be performed for tissue-specific or global inversion of the cassette and subsequent rescue. Although cre and flp function equivalently for inversion in germline and somatic cells, only cre-mediated inversion in somatic cell is depicted for simplicity.
High-Efficiency Conditional Mutagenesis.
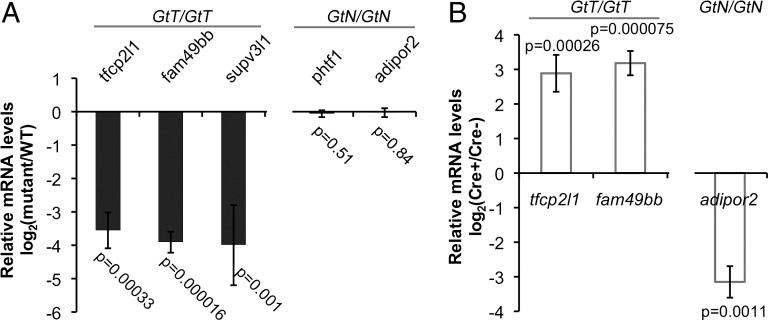
Integration of FT1 into the genome is predicted to generate mCherry expression if the gene-trap insertion is in frame with the host gene. We identified five founders with germline transmission of embryonic mCherry expression among 17 FT1-injected fish that were screened. From four founders that survived our laboratory relocation, we identified seven genic insertions by linker-mediated PCR (LM-PCR): three in the T orientation (Fig. S2), three in the N orientation, and one in an exon (Table 1). The exonic insertional allele, ift122GtT should not be conditional. Additionally, we identified gene-trapping loci where the insertion was in frame (e.g., supv3l1GtT), out of frame (e.g., tfcp2l1GtT), or upstream of the first coding exon (e.g., fam49bbGtT). They all decreased transcript levels by ∼15-fold (Fig. 2A and Table 1) at 3.5 days postfertilization (dpf). None of the insertions in the N orientation generated detectable mCherry expression, but the three in the T orientation generated mCherry expression (Fig. S2). The exonic insertion also generated mCherry expression. As predicted, inversion of phtf1GtN/+ or Felix-inaGtN/+ with Cre or Flp recombinase did not activate mCherry expression because the fluorescent protein is out of frame. Highlighting the increased sensitivity of LM-PCR compared with fluorescent detection, mCherry expression was not detected in adipor2GtN embryos following inversion even though it was fused in frame, which was likely due to the low level of expression at this locus (12). We analyzed the effects of intronic insertions on host gene mRNA expression by quantitative RT-PCR (qRT-PCR) (primers listed in Table S1). Compared with wild-type siblings, homozygotes for the three T insertional alleles had dramatically lower host gene expression (Fig. 2A and Table 1), whereas FT1 insertions in the N orientation did not result in significant loss of mRNA expression (Fig. 2A and Table 1). Thus, the FT1 gene trap is unidirectional and its mutagenicity is specific to insertions in the T orientation.
Table 1.
Summary of FT1 insertion alleles
| Locus | Insert site | Orientation | Reading frame in T orientation | mCherry signal in T orientation | LOF phenotype | Transcript, % WT |
| ift122 | Exon 10 | T | In | + | Ciliary defects | NA |
| supv3l1 | Intron 7 | T | In | + | Lethal | <7 |
| tfcp2l1 | Intron 1 | T | Out | + | ND | <7 |
| fam49bb | Intron 1 | T | 5′ UTR | + | ND | <7 |
| felix-ina | Intron 15 | N | Out | − | ND | NA |
| phtf1 | Intron 14 | N | Out | − | ND | 96 |
| adipor2 | Intron 4 | N | In | − | ND | 98 |
Fig. 2.
Mutagenicity and interconversion of T and N alleles. (A) Bar graph (mean ± SD) of qRT-PCR data showing the transcript levels of the affected genes in homozygotes relative to wild-type siblings at 3.5 dpf. The mutagenic state of the insertions is indicated at the Top of the graph. (B) Bar graph (mean ± SD) of qRT-PCR data showing the transcript levels of affected genes in 3.5-dpf homozygotes of the insertion alleles with Tg(hsp70l:Cre)VU297 relative to homozygotes without Tg(hsp70l:Cre)VU297. The mutagenic state of the insertions is indicated at the Top of the graph. Embryos were heat shocked at 38.5 °C for 40 min starting at 9 hpf.
Stable Interconversion of FT1 Insertion Alleles Between Mutagenic and Neutral States.
To determine whether neutral and mutagenic FT1 insertions are interconvertible, we crossed carriers of the insertion alleles to Tg(hsp70l:Cre)VU297 and identified double carriers [e.g., tfcp2l1GtT/+; Tg(hsp70l:Cre)]. Our previous studies have shown that Tg(hsp70l:Cre)VU297 efficiently induces inversion of FlEx-flanked fluorescent reporters in somatic cells upon heat shock (10). Double carriers [e.g., tfcp2l1GtT/+; Tg(hsp70l:Cre)] were then crossed to heterozygous carriers of the corresponding allele (e.g., tfcp2l1GtT/+). Embryos were heat shocked at 38.5 °C for 40 min starting at 9 hours postfertilization (hpf) to induce Cre and drive inverting recombination. Transcript levels of the mutant allele in homozygous progeny in the presence or absence of Cre were compared by qRT-PCR at 3.5 dpf. We first tested whether inversion of T alleles restores gene expression of the mutagenized gene. In the presence of Tg(hsp70l:Cre)VU297, heat shock increased the tfcp2l1 transcript levels in tfcp2l1GtT/GtT embryos by 7.5-fold. Likewise, in the presence of Tg(hsp70l:Cre)VU297 heat shock increased fam49bb transcript levels in fam49bbGtT/GtT embryos by ninefold (Fig. 2B). Notably, heat shock did not achieve the degree of reduction observed in homozygotes of germline T alleles. This is likely due to incomplete inversion. We next tested whether N alleles reduce host gene expression after inversion, using adipor2GtN as an example. After heat shock, the adipor2 mRNA levels in the adipor2 GtN/GtN; Tg(hsp70l:Cre) embryos was reduced ninefold compared with adipor2 GtN/GtN (Fig. 2B). Injection of Cre and Flp mRNA into oocytes rather than heat-shock induction of Cre produced similar results for both T and N alleles. Thus, T-to-N inversion of the FT1 insertions greatly restores the mRNA levels of the host locus, whereas N-to-T inversion significantly reduces mRNA levels of the host locus. Taken together, our data show that intronic FT1 insertions are conditional and can be used for reversible inactivation of targeted genes.
FT1 Insertion in supv3l1 Causes Recessive Embryonic Lethality.
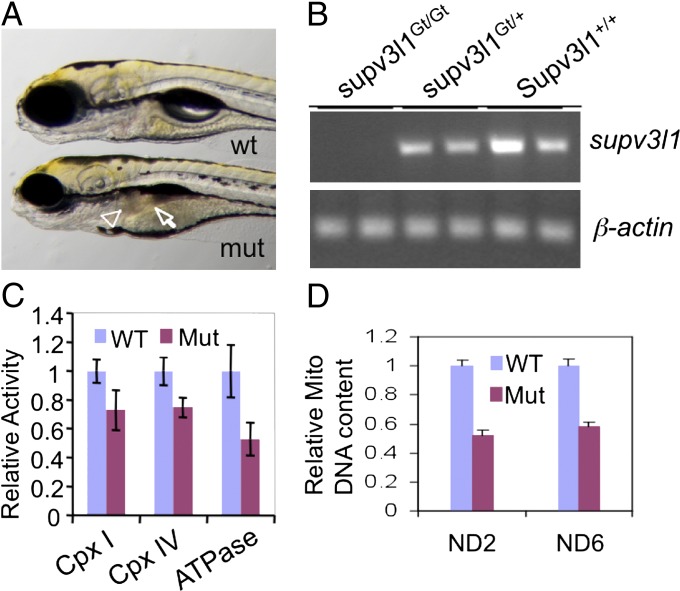
To determine whether the FT1 mutagen can be used to study later developmental functions of genes that are required for embryonic viability, we analyzed a gene-trapping FT1 insertion in supv3l1. supv3l1 is a zebrafish ortholog of the yeast suv3 gene that encodes an essential mitochondrial RNA helicase. Loss of SUPV3L1 function in mice results in embryonic lethality (13, 14). An FT1 insertion in the seventh intron of zebrafish supv3l1 resulted in recessive embryonic lethality (Fig. 3A). Quantitative RT-PCR analysis using primers spanning exons 6 and 7 confirmed that the FT1 insertion in supv3l1 decreased the expression of supv3l1 by more than 15-fold compared with wild type (Table 1, Fig. 2A, and Fig. 3B).
Fig. 3.
Phenotypes of supv3l1GtT/GtT mutants. (A) Gross morphological defects at 5 dpf in supv3l1Gt/Gt showing smaller eyes, lack of an inflated swim bladder, small and dark liver (arrowhead), and underdeveloped and dark intestine (arrow). (B) RT-PCR analysis showing undetectable supv3l1 mRNA in the mutant (Upper ) with normal β-actin mRNA levels (Lower). (C) Bar graph (mean ± SE) of whole-animal Western blot analysis of the levels of components of mitochondrial complexes showing a decrease in the levels of NDUFB6 (complex I), CoxVa (complex IV), and F-ATPase (complex V) in the mutants (n = 4). P < 0.05 for complex IV and ATPase. The difference for complex I did not reach statistical significance (P = 0.12). (D) Bar graph (mean ± SEM) showing a decrease in mitochondrial DNA relative to nuclear DNA (β-actin 2) (n = 4). P < 0.05 for both ND2 and ND6. n = 4.
supv3l1GtT/GtT mutants were indistinguishable from wild-type during the first 2 d of development. However, by 3 dpf supv3l1GtT/GtT mutants had slightly smaller eyes. The difference in eye size became more apparent by 4 and 5 dpf (Fig. 3A), and the supv3l1GtT/GtT mutants also exhibited underdeveloped liver and intestine. This phenotype was associated with a largely unconsumed yolk sac and an uninflated swim bladder (Fig. 3A). supv3l1GtT/GtT mutants did not form the second liver lobe by 7 dpf and usually died by 8 dpf. To determine whether loss of supv3l1 impairs mitochondria, we measured the levels of mitochondrial complexes I (NADH coenzyme Q reductase), IV (cytochrome c oxidase), and V (ATP synthase) by Western blot. Complexes in mutants decreased to 75, 75, and 55%, respectively, compared with controls (Fig. 3C and Fig. S3). Further, qPCR analysis revealed an approximately twofold reduction in mitochondrial DNA copy number (Fig. 3C). Together, these data suggest that zebrafish supv3l1 is important for mitochondrial function.
Liver-Specific Rescue of supv3l1GtT.
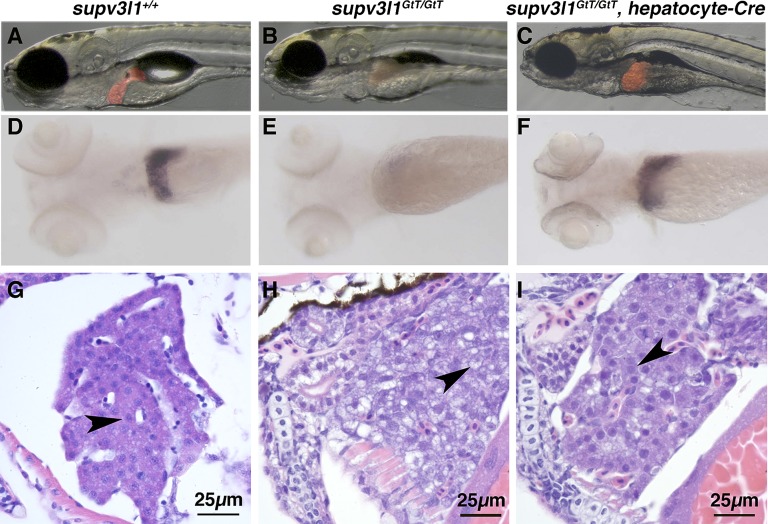
We next examined whether hepatocyte-specific inversion of the gene trap could rescue the liver defects in supv3l1GtT/GtT mutants. We generated a hepatocyte-specific Cre line Tg(−2.8fabp10:Cre; −0.8cryaa:Venus)S955 (referred to as hepatocyte-Cre). Crossing hepatocyte-Cre to a previously characterized Cre/Flp fluorescent reporter line showed that inversion is restricted to the liver (Fig. S4A) (10). To better visualize the liver, we crossed supv3l1GtT onto the LiPan background [Tg(fabp10:DsRed; ela:EGFP)] (15), which expresses DsRed in the liver and EGFP in the pancreas. At 5 dpf, hepatocyte-Cre+/−, supv3l1GtT/GtT animals exhibited a normal liver size. Yellow bile in the intestine suggested that the liver was functional. Animals with liver-specific rescue still exhibited small eyes and an underdeveloped intestine, as observed in supv3l1GtT/GtT global mutants (Fig. 4 A–C and Fig. S4B). Whole mount in situ hybridization analysis for the differentiated hepatocyte marker transferrin a showed that hepatocyte-specific expression of supv311 rescued the size and differentiation state of supv3l1GtT/GtT mutant livers (Fig. 4 D–F). Histological analysis of the liver by H&E staining revealed that, unlike the vacuolated hepatocytes in supv3l1GtT/GtT fish, hepatocytes in hepatocyte-Cre+/−, supv3l1GtT/GtT fish have distinct nuclear and cytoplasmic staining similar to wild type (Fig. 4 G–I), further confirming the rescue. Taken together, we conclude that tissue-specific rescue can be achieved using the FT1 gene trap. Not surprisingly, liver-specific rescue is insufficient to overcome the lethality of a broadly expressed mitochondrial gene and these embryos die at 8 dpf.
Fig. 4.
Liver-specific expression of supv3l1 in supv3l1GtT/GtT mutants rescues hepatocyte defects. (A–C) Images of Lipan (A), LiPan/supv3l1GtT/GtT (B), LiPan/hepatocyte-Cre+/−/supv3l1GtT/GtT (C) showing liver agenesis in supv3l1GtT/GtT and liver-specific rescue by hepatocyte-Cre. Other defects in supv3l1GtT/GtT remain in hepatocyte-Cre+/−/supv3l1GtT/GtT. Each image is a merge of a bright-field image and a red fluorescent image of the same larva. (D–F) In situ hybridization analysis of transferrin a mRNA expression in 5-dpf larvae showing the specific expression in the liver of wild-type and heterozygous larva (90/114) (D), the absence in supv3l1GtT/GtT (12/114) (E), and the restoration in the liver-specific rescue hepatocyte-Cre+/−/supv3l1GtT/GtT (12/114) (F). Eye size in E and F remains smaller than in D. Observed numbers are not different from expectation (χ2 test, P = 0.623). (G and H) Liver H&E staining of wild-type (G), supv3l1GtT/GtT (H), and hepatocyte-Cre+/−/supv3l1GtT/GtT (I) larvae at 5 dpf, indicating the poorly differentiated hepatocytes with vacuolated cytoplasm and weak nuclear staining in global mutants (H) is fully restored by hepatocyte-Cre (I). Arrowheads indicate hepatocytes.
Conditional Inactivation of supv3l1.
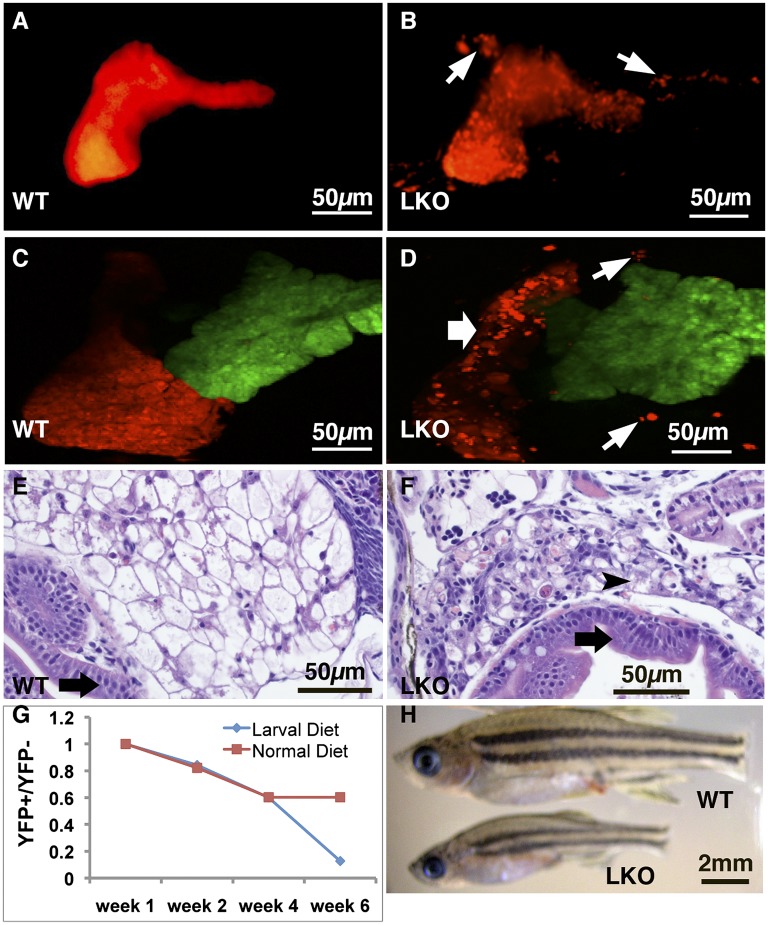
supv3l1GtT/GtT mutants exhibit defects in multiple tissues, a situation common to homozygous mutants of many genes. Often, these pleiotropic effects confound functional analysis. The complex and pleiotropic phenotype of global supv3l1 inactivation provides an opportunity to determine whether tissue-specific mutant phenotypes can be studied using the FT1 mutagen. To test whether the loss of supv3l1 function in the liver contributes to embryonic lethality, we first generated a germline supv3l1GtN allele. We injected 100 pg of Flp mRNA at the one-cell stage into supv3l1GtT embryos to invert the gene-trap cassette, which resulted in nearly 100% germline transmission of supv3l1GtNf. We bred supv3l1GtNf onto the LiPan background to facilitate morphological analysis of the endodermal organs. To achieve liver-specific knockout (LKO) of supv3l1 functions, we generated hepatocyte-Cre+/−/supv3l1GtNf/GtNf animals, which reactivated the gene-trap cassette specifically in the liver. Henceforth, we will refer to these animals as supv3l1LKO. LiPan/supv3l1LKO larvae did not exhibit abnormal liver morphology before 10 dpf. However, at 10 dpf, hepatocytes in LiPan/supv3l1LKO larvae started to detach from the liver body and red fluorescence accumulated in the pronephric heads, consistent with liver degeneration. The liver phenotype became increasingly more severe and widespread with time. By 14–18 dpf, massive detachment of hepatocytes occurred in ∼97% (67/69) of LiPan/supv3l1LKO animals, whereas only 1% (1/79) of wild-type animals had any detached hepatocytes (Fig. 5 A–D). Conversion of the GtNf allele to the gene-disrupting GtTfc allele in the liver was confirmed at 18 dpf by hepatocyte-specific expression of mCherry as detected by immunofluorescence (Fig. S5 A and B), as well as the marked reduction of supv3l1 mRNA in the liver as measured by qRT-PCR (Fig. S5C). The death of hepatocytes that are homozygous for the GtTfc allele was confirmed by quantitative PCR analysis of liver mRNA and genomic DNA from 1-mo-old fish. The ratio of gene-trap cassette in the T and N orientation was 3:1 in control sibling LiPan+/−/hepatocyte-Cre+/−/supv3l1GtNf/+ fish, indicating that hepatocytes constitute ∼75% of all liver cells. In contrast, the T and N ratio was 0.26:1 in LiPan/supv3l1LKO animals, which corresponds to a >10-fold decrease in hepatocyte mass. If the nonhepatocyte cell number is unaffected, then >90% of the hepatocytes degenerated in supv3l1LKO animals (Fig. S5C). Histopathology of supv3l1LKO livers confirmed a reduced number of hepatocytes, with many swollen cells lacking clear nuclei, suggestive of necrosis (Fig. 5 E and F).
Fig. 5.
Phenotype of liver-specific supv3l1 inactivation. (A–D) Confocal images of the Left (A and B) and Right (C and D) sides of 21-d-old LiPan (A and C) and LiPan/supv3l1LKO (B and D) fish showing detached hepatocytes (white arrows) (B and D) and smaller right liver lobe (white arrow) in supv3l1LKO fish (D). (E and F) Histological analysis of wild-type (E) and supv3l1LKO (F) fish indicate disorganized liver with dead cells (arrowhead) but normal intestinal lumen (black arrows) in supv3l1LKO fish (F). (F) Survival curve of supv3l1LKO fish (lens YFP+) compared with supv3l1GtNf/GtNf siblings (lens YFP−) during the first 6 wk of life. One group was fed a larval diet until 28 dpf followed by a juvenile diet (red) and the other group was kept on the larval diet (blue). (G) Photograph of a wild-type and a supv3l1LKO mutant at 5 wk of age showing the smaller size of the mutant.
Survival of supv3l1LKO mutants was reduced compared with controls. Under our typical feeding regimen ∼40% of the supv3l1LKO mutants died by 28 dpf (Fig. 5G). supv3l1LKO fish that survived beyond 28 dpf were markedly smaller than their supv3l1GtNf/GtNf siblings (Fig. 5H). At 2 mo of age, the average body length (excluding tail fin) is 1.61 cm for supv3l1GtNf/GtNf fish (± 0.06 cm, SE, n = 24) and 1.28 cm for supv3l1LKO (± 0.06 cm, SE, n = 27) (P < 0.001, T test). Animals that survived past 28 dpf did not exhibit any additional excess lethality compared with controls. This coincides with the switching from a larval diet to a juvenile diet. To determine whether changing food composition affects survival, we compared supv3l1LKO and supv3l1GtNf/GtNf animals reared exclusively on a larval diet to those that were switched to juvenile food at 28 dpf. Whereas supv3l1LKO did not exhibit any further lethality between 28 dpf and 42 dpf on the juvenile diet, there was a marked dropoff in survival among supv3l1LKO animals that were kept on the larval diet compared with supv3l1GtNf/GtNf controls by 42 dpf (Fig. 5G). We conclude that a juvenile diet places a reduced metabolic load on the compromised supv3l1LKO hepatocytes.
Discussion
We have generated conditional mutations in the zebrafish germline using a FlEx-based gene trap. Because our method is independent of ES cells and homologous recombination, it is applicable to other genetic model organisms where homologous recombination is difficult or not yet possible. These include slime molds, plants, insects, fish, and frogs. The key to the success of our approach is the use of a highly efficient gene-trap cassette that blocks the transcriptional machinery in one orientation but is not mutagenic in the other orientation. This cassette can be stably inverted using both the Cre and Flp recombinases and it is carried in a transposon vector of low intrinsic mutagenicity.
Several other approaches for making conditional alleles using transposons have been recently reported. A series of gene-breaking cassettes, particularly GBT-RP2, have been shown to be highly mutagenic, likely because of the use of an enhanced polyadenylation signal and a putative boundary element (6). The recently described FlipTrap alleles are also highly mutagenic (9). Our data indicate that the mutagenicity of our gene-trap cassette is comparable to that of GBT-RP2 and FlipTrap. Moreover, unlike retroviral insertions that are mutagenic primarily when integrated in the first intron (16, 17), the mutagenicity of GBT, FlipTrap, and FlExTrap insertions is far less position dependent, rendering many insertions in early and late introns highly mutagenic.
There are differences in the function and configuration of the gene-trapping cassette between the GBT, FlipTrap, and FlExTrap mutagens that reflect differences in experimental strategies. Each approach relies on strong splice acceptors to drive splicing of the host transcript to a trapping terminal exon containing a fluorescent reporter. This approach permits detection of cells where the trapping exon is in frame to the host transcript. In the GBT and FlipTrap approaches, the transposon must integrate in the correct orientation within the host transcription unit to be functional. The GBT mutagen constitutes a gene trap in its normal state and to the mutagenicity can be reversed by either Cre-mediated excision of the gene-trap cassette or a splice-blocking morpholino. In contrast, the FlipTrap system is designed to be nonmutagenic in the original state but can be rendered mutagenic by a Cre-mediated FlEx event. A significant advantage of the FT1 mutagen is that the original orientation of the insertion does not matter. Insertion in the trapping and nontrapping orientation can be reversed by either Cre- or Flp-mediated recombination. Furthermore, sequential inversions can be performed, which greatly increases the flexibility of the genetic manipulations that can be achieved. Thus, the FT1 mutagen can generate both conditional rescue and conditional knockout alleles from a single integration event. FT1 is unique among these mutagens in this respect.
Two general approaches have been used to identify insertion mutant alleles; reporter expression and linker-mediated PCR. Reporter expression permits identification of cellular expression domains and even subcellular localization, but only for genes in which the gene trap integrates in the correct orientation and reading frame (in theory, one-sixth of the insertion events). For this approach to be viable in large-scale forward genetic screens, significant improvements in mutagenesis efficiency will be required. Moreover, for integration events in genes that are expressed at low levels, or are expressed at times that are not assayed, detection of the integration event is difficult or impossible. We used both reporter expression and a LM-PCR–based insertion detection approach to identify FlExTrap integrations. The LM-PCR–based approach identified several insertions that did not express the mCherry reporter. Thus, the LM-PCR approach should have long-term utility, particularly for genes that are expressed late in development or only under certain physiological conditions.
Zebrafish supv3l1 is an evolutionarily conserved ortholog of the yeast supv3 gene that encodes a putative mitochondrial RNA (mtRNA) helicase (14), which has been identified in many species, including humans (18). It is an essential component of the mitochondrial degradosome complex that regulates mtRNA decay and mRNA surveillance (19). Human SUPV3L1 is important for maintaining mitochondrial homeostasis (20) and has been shown to degrade double-stranded RNA (21). However, SUPV3L1 also appears to function in the cytoplasm, interacting with the WRN and BLM DNA helicases to suppress mitotic homologous recombination (13). Mice homozygous for a gene-trap insertion in the SUPV3L1 gene die in utero before midgestation (13). Conditional inactivation of murine SUPV3L1 by Mx1-Cre leads to a defective skin barrier and premature aging (22). In zebrafish, supv3l1 is also an essential gene. The longer survival of supv3l1GtT/GtT mutants is likely due to maternal supply of mRNA and protein. Our analysis of supv3l1GtT/GtT provides additional support for its conserved mitochondrial function in vertebrates. The pronounced defects in the retina, liver, and intestines are likely due to the rapid growth in these tissues during development, which likely requires elevated mitochondrial activity. Similar defects have been reported for mutations in the mitochondrial import gene tomm22 (23). Although the liver defect is the most prominent defect exhibited by supv3l1GtT/GtT mutants, it is not solely responsible for larval lethality, as mutants with rescued supv3l1 still fail to thrive. In addition, supv3l1LKO fish can survive for more than 1 mo. It is formally possible that the prolonged survival of supv3l1LKO fish is due to exceptional stability of Supv3l1 protein made before the inactivation, including maternal supplies, but a more likely scenario is that Supv3l1-deficient mitochondria are sufficient to cope with the metabolic load in young juveniles but insufficient to support the metabolic load of older animals.
There is an increasingly critical need to probe function of developmentally important genes in postdevelopmental processes. For example, mitochondrial dysfunction is thought to contribute to many degenerative or chronic diseases, but mitochondria are also required for development. The ability to experimentally regulate mitochondrial dysfunction in particular tissues or at particular time points in the context of the whole animal may prove a boon to understanding mitochondrial disease. Pharmacological antagonism is also commonly used to affect gene function in a temporally controlled way, but this method is available only for the small number of druggable targets. Pharmacological modulation of protein function is also difficult or impossible to direct to specific cell types or tissues. We show here that intronic insertions of the FT1 cassette allow functional analysis of the host gene in specific tissues and cell types. The methods demonstrated here should also be amenable to analysis of host gene function in postdevelopmental processes using pharmacologically inducible Cre. A large collection of conditional mutations in a significant fraction of the zebrafish genes can be readily generated with this approach. The availability of conditional mutation resources will permit more sophisticated genetic analysis in zebrafish.
Materials and Methods
Detailed descriptions of the fish husbandry, insertion site identification, PCR analysis, and in situ hybridization methods are provided in SI Materials and Methods. All zebrafish experiments have been approved by the Vanderbilt University Institutional Animal Care and Use Committee. To generate gene-trap lines, approximately 1 nL sterile isotonic saline solution containing a mixture of a Tol2-based FT1 plasmid (30 ng/μL) and Tol2 transposase RNA (30 ng/μL) was injected into fertilized eggs of wild-type fish and the eggs were raised to adults. Mosaic adults were crossed with wild-type fish and the progeny were screened for fluorescence at 1, 2, and 5 dpf.
Supplementary Material
Acknowledgments
We thank Pinxun Yu, Amanda Goodrich, and Cory Guthrie for excellent fish care; Emily Kam for help with the pilot screen; Mathew Stephenson for transmission electron microscopy (TEM) analysis; and other members of the W.C. laboratory for discussions. MitoScience (Eugene, OR) provided sample antibodies against mitochondrial proteins. TEM and confocal microscopy were performed in part through the use of the VUMC Cell Imaging Shared Resource. This work is supported by National Institutes of Health Grants R01 EY016092 and DK088686 (to W.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206131109/-/DCSupplemental.
See Commentary on page 15082.
References
- 1.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 2.Skarnes WC, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnütgen F, et al. Genomewide production of multipurpose alleles for the functional analysis of the mouse genome. Proc Natl Acad Sci USA. 2005;102:7221–7226. doi: 10.1073/pnas.0502273102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gossler A, Joyner AL, Rossant J, Skarnes WC. Mouse embryonic stem cells and reporter constructs to detect developmentally regulated genes. Science. 1989;244:463–465. doi: 10.1126/science.2497519. [DOI] [PubMed] [Google Scholar]
- 5.Schnütgen F, et al. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat Biotechnol. 2003;21:562–565. doi: 10.1038/nbt811. [DOI] [PubMed] [Google Scholar]
- 6.Clark KJ, et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat Methods. 2011;8:506–515. doi: 10.1038/nmeth.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami K, et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Petzold AM, et al. Nicotine response genetics in the zebrafish. Proc Natl Acad Sci USA. 2009;106:18662–18667. doi: 10.1073/pnas.0908247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinh A, et al. A versatile gene trap to visualize and interrogate the function of the vertebrate proteome. Genes Dev. 2011;25:2306–2320. doi: 10.1101/gad.174037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boniface EJ, Lu J, Victoroff T, Zhu M, Chen W. FlEx-based transgenic reporter lines for visualization of Cre and Flp activity in live zebrafish. Genesis. 2009;47:484–491. doi: 10.1002/dvg.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddison LA, Lu J, Chen W. Generating conditional mutations in zebrafish using gene-trap mutagenesis. Methods Cell Biol. 2011;104:1–22. doi: 10.1016/B978-0-12-374814-0.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishio S, et al. Adiponectin and adiponectin receptor genes are coexpressed during zebrafish embryogenesis and regulated by food deprivation. Dev Dyn. 2008;237:1682–1690. doi: 10.1002/dvdy.21559. [DOI] [PubMed] [Google Scholar]
- 13.Pereira M, et al. Interaction of human SUV3 RNA/DNA helicase with BLM helicase; loss of the SUV3 gene results in mouse embryonic lethality. Mech Ageing Dev. 2007;128:609–617. doi: 10.1016/j.mad.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Stepien PP, Margossian SP, Landsman D, Butow RA. The yeast nuclear gene suv3 affecting mitochondrial post-transcriptional processes encodes a putative ATP-dependent RNA helicase. Proc Natl Acad Sci USA. 1992;89:6813–6817. doi: 10.1073/pnas.89.15.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korzh S, et al. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev Biol. 2008;8:84. doi: 10.1186/1471-213X-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amsterdam A, et al. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, et al. Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proc Natl Acad Sci USA. 2007;104:12428–12433. doi: 10.1073/pnas.0705502104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dmochowska A, et al. A human putative Suv3-like RNA helicase is conserved between Rhodobacter and all eukaryotes. Acta Biochim Pol. 1999;46:155–162. [PubMed] [Google Scholar]
- 19.Dziembowski A, et al. The yeast mitochondrial degradosome. Its composition, interplay between RNA helicase and RNase activities and the role in mitochondrial RNA metabolism. J Biol Chem. 2003;278:1603–1611. doi: 10.1074/jbc.M208287200. [DOI] [PubMed] [Google Scholar]
- 20.Khidr L, et al. Role of SUV3 helicase in maintaining mitochondrial homeostasis in human cells. J Biol Chem. 2008;283:27064–27073. doi: 10.1074/jbc.M802991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang DD, Shu Z, Lieser SA, Chen PL, Lee WH. Human mitochondrial SUV3 and polynucleotide phosphorylase form a 330-kDa heteropentamer to cooperatively degrade double-stranded RNA with a 3′-to-5′ directionality. J Biol Chem. 2009;284:20812–20821. doi: 10.1074/jbc.M109.009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul E, et al. Disruption of Supv3L1 damages the skin and causes sarcopenia, loss of fat, and death. Mamm Genome. 2009;20:92–108. doi: 10.1007/s00335-008-9168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curado S, et al. The mitochondrial import gene tomm22 is specifically required for hepatocyte survival and provides a liver regeneration model. Dis Model Mech. 2010;3:486–495. doi: 10.1242/dmm.004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.