Abstract
Many wildlife species face imminent extinction because of human impacts, and therefore, a prevailing belief is that some wildlife species, particularly large carnivores and ungulates, cannot coexist with people at fine spatial scales (i.e., cannot regularly use the exact same point locations). This belief provides rationale for various conservation programs, such as resettling human communities outside protected areas. However, quantitative information on the capacity and mechanisms for wildlife to coexist with humans at fine spatial scales is scarce. Such information is vital, because the world is becoming increasingly crowded. Here, we provide empirical information about the capacity and mechanisms for tigers (a globally endangered species) to coexist with humans at fine spatial scales inside and outside Nepal’s Chitwan National Park, a flagship protected area for imperiled wildlife. Information obtained from field cameras in 2010 and 2011 indicated that human presence (i.e., people on foot and vehicles) was ubiquitous and abundant throughout the study site; however, tiger density was also high. Surprisingly, even at a fine spatial scale (i.e., camera locations), tigers spatially overlapped with people on foot and vehicles in both years. However, in both years, tigers offset their temporal activity patterns to be much less active during the day when human activity peaked. In addition to temporal displacement, tiger–human coexistence was likely enhanced by abundant tiger prey and low levels of tiger poaching. Incorporating fine-scale spatial and temporal activity patterns into conservation plans can help address a major global challenge—meeting human needs while sustaining wildlife.
Keywords: adaptation, coupled human and natural systems, ecosystem services, overlap, sustainability
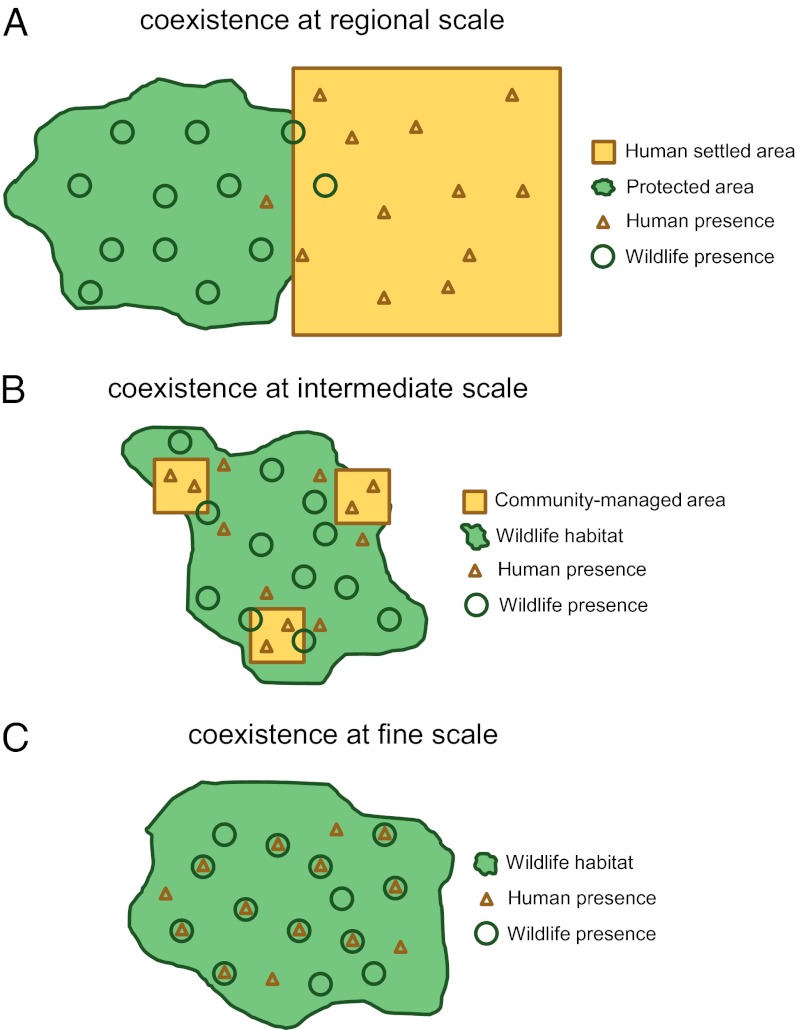
The extent and degree to which threatened wildlife can coexist with humans over a sustained period is a central issue in conservation science and policy (1, 2). Numerous conservation models (e.g., state-managed reserves, community-managed areas, and privately owned sanctuaries) have been proposed and implemented to facilitate coexistence at different spatial scales (3–6). For example, protected areas are designed to facilitate coexistence at a regional scale (Fig. 1A) by conserving wildlife amid a surrounding mosaic of human land uses and activities (4, 5). Alternatively, community-based conservation approaches, which emphasize sustainable natural resource extraction for local consumption and conservation of biodiversity, envision human and wildlife activities being comparatively more interspersed in space and aim to facilitate coexistence at smaller intermediate scales (Fig. 1B) (3, 6). Regardless of the conservation model, however, a rapidly growing world human population and a long history of competition between people and wildlife for limited resources (e.g., food) (1) have led to a general belief among conservation practitioners and policy-makers that some wildlife species, such as large carnivores and ungulates, cannot coexist with humans at fine spatial scales (i.e., regularly use the exact same locations as shown in Fig. 1C) (7–10). This belief motivates conservation policies, including resettlement of human communities (11) away from threatened wildlife populations and expulsion of certain types of nonconsumptive human activities (e.g., research) from protected areas (12). However, empirical and quantitative information on the capacity and mechanisms for wildlife to coexist with humans at fine spatial scales is lacking. Such information is urgently required, because human pressures on protected areas (e.g., livestock grazing, natural resource collection, and hunting), although illicit, have increased enormously (5, 13). In addition, the world is projected to add ∼1.4 billion more people over the next two decades, forcing human and wildlife populations to share the same space (14).
Fig. 1.
Schematic diagram of human–wildlife coexistence at different scales. Protected areas aim to facilitate coexistence between wildlife and humans at regional scales (A) by spatially segregating them into distinct zones. Community-managed areas, in which people can extract natural resources on a limited basis, such as pro-wildlife cattle ranches and community forests, encourage coexistence at comparatively smaller intermediate scales (B). Most conservation models, however, are based on the belief that some wildlife species, like large carnivores, cannot coexist with humans at fine spatial scales (C) because of a fundamental conflict over limited resources (e.g., food). We empirically test this prevailing belief using data from camera traps to quantify the capacity and mechanisms of tigers, a notoriously elusive carnivore, to coexist with humans at a fine spatial scale (i.e., exact same point locations) in Chitwan, Nepal.
To help fill this critical information gap, we investigated the spatiotemporal patterns of tigers (Panthera tigris) and human activities inside and outside Chitwan National Park in Nepal (27°30′ N to 27°43′ N, 84°9′ E to 84°29′ E) (Fig. S1). We focused on the globally endangered tiger, because the conventional belief is that they cannot persist in areas with high human densities (e.g., >10 people/km2) (7, 8). We chose Chitwan for four main reasons. First, Chitwan National Park, established in 1973, covers ∼1,000 km2 and is 1 of 28 reserves in the world that can support >25 breeding female tigers (15, 16). Second, human activities inside and outside the park are diverse (and are likely to affect tiger behavior differently) (17): local residents collect forest products (e.g., fodder for livestock and fuel wood) to support their resource-dependent livelihoods (18), a growing number of tourists from around the world visit the area each year (19), Nepal Army personnel patrol the park to deter illegal activities (e.g., wildlife hunting and logging), and motorized vehicles frequently transport people throughout the area. Third, the park and multiple-use forests outside the park are crucial parts of a landscape-level initiative to connect tiger reserves in India and Nepal through habitat corridors (20). Fourth, the park is a flagship reserve and has received exceptional financial and technical support from the Nepal government and many international organizations, such as the World Wildlife Fund (15). To a large degree, the fate of tigers along the base of the Himalayas, a globally important region for tigers, depends on the success or failure of conservation efforts in Chitwan (21, 22).
In this study, we tested three specific hypotheses: (i) tiger density is higher inside the Chitwan National Park than in the multiple-use forest outside the park; (ii) tigers avoid locations visited by people and/or vehicles; and (iii) tigers are more active at night to avoid human disturbance. To test these hypotheses empirically, we used data from motion-detecting field cameras set inside and outside the park in 2010 and 2011 (Materials and Methods).
Results
We recorded relatively high tiger densities, abundant prey, and ubiquitous human presence inside and outside of the park in 2010 and 2011 (Tables 1 and 2). Specifically, tiger density across the study site was 4.44/100 km2 [95% confidence interval (CI) = 3.19–5.67] in 2010 and 6.35/100 km2 (95% CI = 4.08–7.09) in 2011 (Table 1). Contrary to expectation, tiger density did not significantly differ between the inside and outside of the park in either year, leading us to reject hypothesis 1. However, tiger density significantly increased inside the park from 2010 (3.51/100 km2, 95% CI = 2.5–4.8) to 2011 (8.7/100 km2, 95% CI = 5.57–12.1) (Table 1). In both years, mean prey detection frequency inside the park, which is considered to have some of the highest ungulate densities in South Asia (23), did not significantly differ from outside the park (Table 2). High numbers of tigers and prey animals were recorded during the 2-y period, despite humans triggering 85% of the cameras and accounting for 75% of all detections. Local residents, typically collecting forest resources, accounted for 96% of all human foot traffic outside the park (Figs. S2 and S3), and they were approximately three times as prevalent outside the park as inside in both years (Table 2). However, the detection frequency of total people on foot, local residents, and army personnel inside the park significantly increased from 2010 to 2011 (Table 2).
Table 1.
Tiger population size and density (animals per 100 km2) calculated from spatially explicit capture–recapture models
| 2010 |
2011 |
|||||
| Parameter | Mean | SD | 95% CI | Mean | SD | 95% CI |
| Inside the park | ||||||
| Population size | 18.29 | 3.5 | 13–25 | 45.27 | 8.88 | 28–62 |
| Density | 3.51 | 0.67 | 2.5–4.8 | 8.7 | 1.71 | 5.57–12.10 |
| Outside the park | ||||||
| Population size | 16.84 | 5.45 | 7–27 | 13.46 | 4.90 | 4–22 |
| Density | 5.89 | 1.91 | 2.45–9.44 | 4.82 | 1.71 | 2.1–8.04 |
| Entire study site | ||||||
| Population size | 25.02 | 3.75 | 18–32 | 35.79 | 5.52 | 25–46 |
| Density | 4.44 | 0.66 | 3.19–5.67 | 6.35 | 0.98 | 4.61–8.33 |
Estimates of tiger density inside and outside of the park are not independent from one another for two reasons: the model sampling regions overlap, and one tiger was present in both regions.
Table 2.
Detection frequencies (mean ± SE) of tigers, human presence types, and tiger prey species
| 2010 |
2011 |
|||
| Category | Inside park | Outside park | Inside park | Outside park |
| Tiger | 10 ± 1.8 | 6.7 ± 1* | 13.9 ± 2.5 | 2.3 ± 0.6* |
| Total people on foot | 456.8 ± 89.2* | 716.7 ± 152.3 | 745.4 ± 136.9* | 1,041.3 ± 207.2 |
| Local residents | 218.9 ± 73.9* | 688.5 ± 151 | 381.6 ± 99* | 1,003.8 ± 202.6 |
| Tourists | 101.3 ± 27.2 | 24.3 ± 11.1 | 109.3 ± 36.3 | 13.8 ± 7.1 |
| Army personnel | 136.6 ± 45.2* | 3.8 ± 2.1 | 254.5 ± 70.9* | 23.7 ± 14 |
| Vehicles | 339.7 ± 88.2 | 286.8 ± 193.9 | 455.4 ± 124.7 | 378 ± 252.67 |
| Total prey animals | 214.2 ± 37.8 | 142.5 ± 26.3 | 199.6 ± 28 | 187.3 ± 30 |
| Spotted deer | 163.6 ± 36.7 | 103.5 ± 25.4 | 164.6 ± 27.7 | 145.2 ± 27 |
| Barking deer | 18 ± 5.4 | 20.2 ± 4.4 | 7.4 ± 1.3 | 12.4 ± 1.9 |
| Wild boar | 17.7 ± 3.1 | 10.2 ± 2.2 | 14.9 ± 3.1 | 15.7 ± 3.4 |
| Sambar | 11.8 ± 4.1 | 8.7 ± 2.4 | 6.8 ± 2.2 | 13.9 ± 2.5 |
| Hog deer | 2.3 ± 0.9 | — | 3.7 ± 1.2 | — |
| Gaur | 0.8 ± 0.5 | — | 2.1 ± 1.7 | — |
Values in bold indicate within-year samples that were significantly different from one another (Mann–Whitney u test, P < 0.05). Hog deer and gaur were not detected outside the park in both years. Unlike detection frequency, estimates of tiger density are based on identified individuals and take into account imperfect detection. Consequently, in our study, tiger detection frequencies and density estimates inside and outside of the park differed relative to each other in 2010.
*Between-year samples within the same row were significantly different (Mann–Whitney u test, P < 0.05).
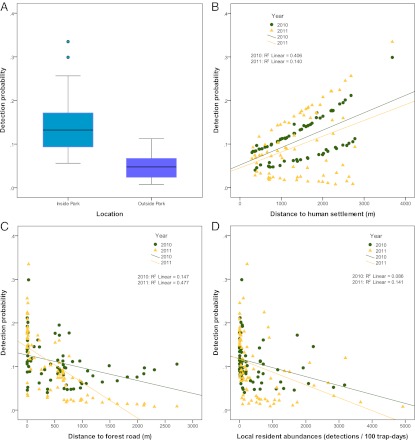
Surprisingly, even at a fine spatial scale (i.e., camera trap locations), abundances of total prey, people on foot, and vehicles had no significant effects on the probability of tiger occupancy across both years (Table 3), leading us to reject hypothesis 2. Tigers occupied ∼80% of the camera trap locations during the 2-y period (ψ = 0.82, SE = 0.04), with no significant difference between the 2 y. However, human-related covariates did influence the probability of detecting tigers (Table 3). The probability of detecting tigers in 2010 and 2011 was higher at locations farther from human settlement (β = 0.35, 95% CI = 0.15–0.54) and inside the park (β = 0.96, 95% CI = 0.51–1.41) (Fig. 2). Being inside the park had the strongest effect on tiger detection probability. The positive relationships between tiger detection probability and being inside the park and distance to settlement did not change significantly between 2010 and 2011. In 2011, however, tigers were more likely to be detected at locations closer to forest roads (β = −0.55, 95% CI = −0.99 to −0.12) and less likely to be detected at locations with higher abundances of local residents (β = −0.41, 95% CI = −0.81 to −0.01) than in 2010 (Fig. 2 and Table 3). With all covariates set to their mean, the model-averaged detection probability was higher in 2010 (p = 0.1, SE = 0.01) than 2011 (p = 0.07, SE = 0.01).
Table 3.
Summary of top-ranked tiger occupancy models
| Model | K | AICc | ΔAICc | wi | LL |
| ψ(·) p(road + year + road × year + settlement + location + location × year) | 8 | 1,720.31 | — | 0.46 | −851.64 |
| ψ(·) p(road + year + road × year + settlement + location) | 7 | 1,721.59 | 1.28 | 0.24 | −853.40 |
| ψ(·) p(road + year + road × year + settlement + location + local + local × year) | 9 | 1,722.28 | 1.97 | 0.17 | −851.50 |
| ψ(·) p(road + year + road × year + settlement + location + settlement × location + local + local × year) | 10 | 1,722.81 | 2.5 | 0.13 | −850.61 |
Interaction terms are shown (e.g., road × year). Covariate coefficient estimates were averaged from these four top-ranked models. The AICc of the intercept-only model [i.e., ψ(·) p(·)] was 1,797.4. AICc, second-order Akaike’s information criterion; ΔAICc, difference in AICc values between each model and the model with the lowest AICc value; K, number of model parameters (includes intercepts and covariates); LL, logarithm of the likelihood; location, location of the camera trap (i.e., inside or outside Chitwan National Park); local, abundance of local residents; p, detection probability; road, distance to nearest forest road; settlement, distance to nearest human settlement; year, year data collected (i.e., 2010 or 2011); wi, AICc model weight; ψ, occupancy; (·), parameter held constant (i.e., intercept only).
Fig. 2.
Tiger detection probability with respect to human-related covariates. Predictions of tiger detection probability are based on model-averaged covariate coefficient estimates with respect to (A) location (i.e., inside or outside of the park), (B) distance to human settlement, (C) distance to forest road, and (D) local resident abundances (detections per 100 trap-d). Boxes in A represent the 25th and 75th percentiles, whiskers represent the 95% confidence limits, black lines within boxes represent medians, and circles outside the whiskers represent outlier values. B–D display detection probabilities by year (2010 values are indicated by circles and 2011 values are indicated by triangles), and they include linear regression lines (2010 linear regression line in green and 2011 linear regression line in yellow) with R2 values shown inside.
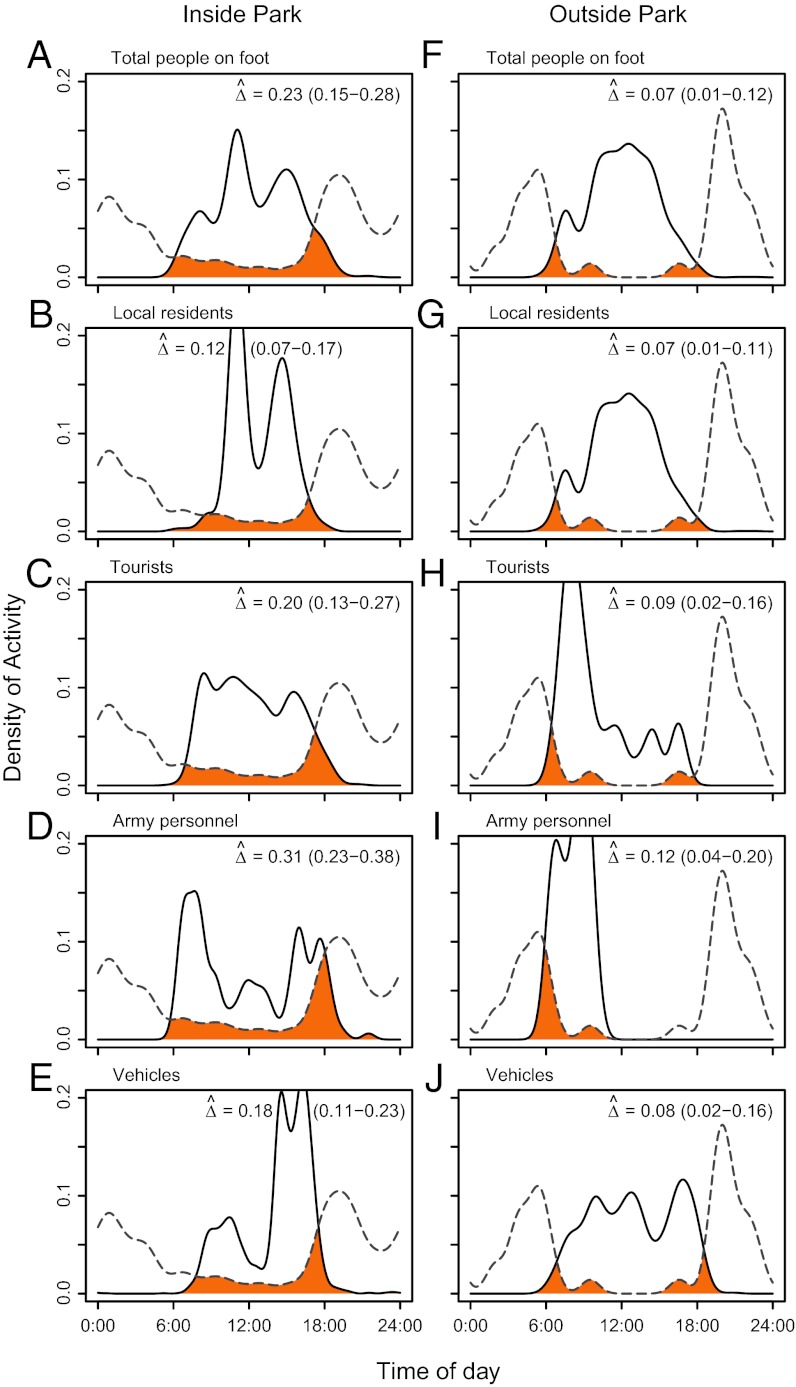
In both years, tigers offset their temporal activities, especially outside the park, by being less active during the day when human activity peaked (2010 data are shown in Fig. 3 and 2011 data are shown in Fig. S4), which supports hypothesis 3. Over the 2-y period, on average, only 20% of all tiger detections in the park occurred during the day between 0600 and 1800 hours (i.e., average times of sunrise and sunset during study), whereas only 5% of tiger activity outside the park occurred during the day. Tiger temporal activity across both years overlapped the most with army personnel and the least with local residents.
Fig. 3.
Temporal overlap of tiger and human activity patterns in 2010. Activity patterns of tiger (dashed lines) and human (solid lines) presence types inside (A–E) and outside (F–J) Chitwan National Park, Nepal in 2010. (A and F) Total people on foot. (B and G) Local residents. (C and H) Tourists. (D and I) Army personnel. (E and J) Vehicles. The estimate of temporal overlap, 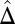 (from zero [no overlap] to one [complete overlap]), is indicated by the orange area, and it is shown in each panel. Overlap was defined as the area under the curve formed by taking the minimum of the two activity patterns at each point in time. Approximate 95% bootstrap CIs of overlap estimates are indicated in parentheses. Average time of sunrise was 0600 hours, and average time of sunset was 1800 hours during the study.
(from zero [no overlap] to one [complete overlap]), is indicated by the orange area, and it is shown in each panel. Overlap was defined as the area under the curve formed by taking the minimum of the two activity patterns at each point in time. Approximate 95% bootstrap CIs of overlap estimates are indicated in parentheses. Average time of sunrise was 0600 hours, and average time of sunset was 1800 hours during the study.
Discussion
In contrast to the general belief, we found that tigers and people frequently co-occurred at fine scales both inside and outside the park in both years. The estimates of tiger density across our study site in Chitwan were higher than numerous sites in Central and North India (24) and several times higher than sites in Laos, Indonesia, Malaysia, and Bhutan (25–28). In addition, tiger occupancy was 12–30% greater than sites in Indonesia and India (29, 30). Human foot traffic across the study site was also orders of magnitude greater than traffic reported for other areas of the tigers’ range (using similar methodology) (25, 26). Over the last decade, tigers have maintained high densities in Chitwan (15, 31), although human density in settled areas surrounding the park has increased 20% (212–255 people/km2) (32), approximately two times the average human density (127 people/km2) among 12 of 13 tiger range countries (except Bangladesh) in 2010 (14).
Tiger density has remained high in Chitwan despite an increasing human population size, likely because tigers are adjusting their activity in space and time according to the type and magnitude of human presence in the forest. Although more wary near human settlement (i.e., lower detection probability), tigers spatially overlapped with people on foot and vehicles at a fine spatial scale in both years, perhaps by using the night to avoid human disturbance associated with local resource collection. The time spent, noise made, and physical impact on the forest during resource collection likely disturbs animal behavior more than nonconsumptive human activities (e.g., wildlife viewing by tourists). For instance, the collection of woody biomass, which is a frequent activity in Chitwan’s forests (18, 33), requires repeated and relatively loud chopping in a given area for an extended period. Tigers across the study site in Chitwan were consequently one-sixth less active during the day than at sites in Malaysia and Indonesia, where human activity was considerably less (26, 34). In particular, the much greater prevalence of local resource collection outside the park than inside the park may have caused tigers there to become almost completely inactive during the day (Fig. 3 and Fig. S4).
The 55% increase in the presence of local residents across the study site from 2010 to 2011 may have caused tigers to alter their space use by being more wary in areas with higher local resident foot traffic. In 2011, increased detection of tigers near forest roads, which are energetically efficient means of traversing the landscape (35), may indicate that tigers were also avoiding the smaller trails typically used by local residents when on foot. Moreover, the increase in tiger density inside the park in 2011 was concurrent with greater numbers of local residents entering the forests across the study site, which suggests that the park is an important refuge from high levels of disturbance for tigers, whereas the forest outside the park, despite supporting several tigers, does not seem to serve that function to the same extent. Increased presence of local residents across the study site may reflect their greater reliance on Chitwan’s forests for fuel wood. Possible explanations for increasing demand for fuel wood include an (i) unexpected increase in the price of kerosene and liquefied petroleum gas and (ii) curbed illegal use of electrical services (e.g., unauthorized connections between households and main electrical lines) because of stricter enforcement. The 2 y of data that we collected are insufficient, however, to conclusively test the abovementioned arguments. Collecting information over a longer time frame than 2 y will enable stronger inferences about spatiotemporal interactions between humans and tigers and capacity for long-term coexistence in human-dominated regions.
Co-occurring high densities of tigers and people inside and outside of the park at fine scales may have been enhanced by two other factors. First, tiger prey numbers have increased in forests directly outside the park after the implementation of conservation-oriented policies in 1996, such as removal of livestock and participatory forest management (36, 37). For instance, forest biomass outside the park increased after livestock were prohibited from grazing there, enabling these forests to support a higher density of wild ungulates (i.e., tiger prey) (36). Moreover, local communities are reforesting many areas outside the park (37), thus improving wild ungulate habitat conditions. With high numbers of prey inside and outside the park, other factors, such as tiger social structure (e.g., female philopatry) and territorial behavior, may influence tiger space use more so than fine-scale spatial heterogeneity of prey abundance (38, 39). Second, human exploitation of tigers, such as poaching, has been relatively controlled since the end of the civil war in Nepal in 2006 (15). Exploitation is a key determinant of tiger abundance, because it can increase mortality rates and lead tigers to avoid areas with people (40).
Our findings affirm the notion that effective management policies, such as those policies that improve habitat conditions and lower exploitation, are more important to tiger conservation than human density per se (41). Unique socioeconomic and institutional factors in Chitwan, such as park management intensity, tourism infrastructure, initiatives to include local communities in ecodevelopment, massive efforts to reduce and control poaching, and social tolerance to tigers (19, 42, 43), likely increased the capacity for tigers and people to coexist at fine spatial scales. As such, the spatial and temporal interactions between people and tigers observed in Chitwan may differ in other human-dominated regions that have different socioeconomic and institutional characteristics. Similar research conducted in other human-dominated regions would be extremely useful in expanding and clarifying our understanding of how tigers behaviorally respond to humans at fine spatiotemporal scales. For instance, it would be important to address some questions. What energetic costs to tigers are associated with temporal displacement (e.g., decreased hunting success at night)? Are there disturbance thresholds (thresholds are given in ref. 44) beyond which tigers dramatically alter their spatial and temporal behavior? What effects do behavioral changes in space and time have on tiger population persistence?
Here, we have shown that tigers can adapt and thrive in a human-dominated landscape by displacing their spatial and temporal activity from humans. Our study shows the need for and feasibility of incorporating temporal activity patterns into conservation planning, which typically focuses on spatial relationships (zoning information in ref. 45). For example, reducing the abundance of livestock left unattended at night when carnivores are typically active will reduce livestock predation (46). Vehicular activity may also be restricted during certain times (e.g., dusk and dawn) to facilitate crepuscular and nocturnal dispersal across and use of human-dominated landscapes.
Whether illicit or authorized, human activities within natural ecosystems around the world, such as hunting bushmeat, herding livestock, and collecting forest products, are pervasive and in many cases, increasing (13, 47–49). The reaction of threatened wildlife to these activities will vary according to context-specific conditions, including region, type, and frequency of human activities, behavioral ecology of the wildlife species, and management policies. Similarly, conservation actions intended to modify the ways people and wildlife interact in space and time must fully consider the context-specific social and political implications (e.g., altering access to land for different groups of people) (50) in addition to ecological effects. Regardless of context, however, conservation plans informed by fine-scale spatial and temporal insights can help address a major global challenge—meeting human needs while sustaining wildlife in an increasingly crowded world.
Materials and Methods
From January to May (i.e., the dry season before monsoon) in 2010 and 2011, we used state of the art camera trap technology (51) to collect field data on tigers, their main prey species [spotted deer (Axis axis), barking deer (Muntiacus muntjak), wild boar (Sus scrofa), sambar (Rusa unicolor), hog deer (A. porcinus), and gaur (Bos gaurus)] (52), and human presence (local residents, tourists, army personnel, and vehicles). In both years, we sampled the exact same locations inside and outside the Chitwan National Park [both regions dominated by Sal (Shorea robusta) forest] in four successive blocks, each sampled for ∼20 d at ∼20 locations. In 2011, we also sampled one additional location in each block; thus, we placed traps in a total of 76 locations in 2010 and 79 locations in 2011 (one trap was stolen in 2011) (Table S1). Traps were placed ∼1 km apart across the study site, and their spatial coordinates were recorded using a global positioning system receiver. Cameras were set to operate 24 h/d with no more than mechanical minimum delay between sequential photographs. For each picture, we recorded entity (i.e., tiger, prey species, or human presence type), location (based on trap identification), date, and time. We summed the number of detections for each entity for each camera trap. Detections were defined as (i) consecutive pictures of different individuals or vehicles, (ii) consecutive pictures of individuals or vehicles >0.5 h apart, and (iii) nonconsecutive pictures of individuals or vehicles (25). If the number of detections varied between cameras in a pair, we used the larger number. We calculated detection frequency (number of detections per 100 trap-d) of each species and human presence type at each camera trap (25).
The Mann–Whitney u statistic was used to test for significant differences in detection frequencies of each entity inside and outside the park within and between years. Data on individually identified adult tigers (not possible for prey animals and people) enabled us to also estimate tiger density. Tiger density was estimated using a spatially explicit capture–recapture model, which accounts for imperfect detection (53). The model integrates individual animal capture histories and spatial locations of camera traps using a statistical point process model. This approach avoids having to use an ad hoc effective sample area (e.g., minimum convex polygon), which often inflates density estimates (54), and instead, it calculates density as the number of animal activity centers that fall within some region encompassing the trap array. We ran three capture–recapture models for each year using data from different groups of camera traps: (i) inside the park, (ii) outside the park, and (iii) entire study site. The models were specified with a Bernoulli encounter process, in which an individual tiger may be captured in each trap only one time during each sampling occasion (i.e., 1-d interval from 1200 to 1200 hours). The Bernoulli encounter process was related to spatial animal movements using a half-normal detection function, similar to the function commonly used in distance sampling (55). We added all zero encounter histories (5 × number of identified tigers) to augment each model dataset. After a burn in of 1,000 iterations, parameter posterior distributions computed from a single chain of 49,000 Markov chain Monte Carlo iterations were used to determine parameter mean, SD, and 95% CI values (53).
We used occupancy models to estimate the relative effect of prey and human covariates on the spatial variability in tiger presence (56) across the entire study site over the 2-y period. Occupancy models are ideal for camera trap data, because they formally account for imperfect detection and allow the probability of an animal occupying and being detected at a location to vary in response to covariates. We evaluated the effects of prey, human presence types (i.e., total number of detections of people on foot, local residents, tourists, army personnel, and vehicles at each camera trap), location (i.e., a binary variable indicating whether the data were from inside or outside the park), distance to settlement (i.e., straight-line distance from camera trap to nearest human settlement abutting forests inside and outside park) and forest road (i.e., roads in the forests inside and outside the park), and year (i.e., a binary variable indicating whether the data were from 2010 or 2011) on tiger occupancy and detection. We combined tiger detection and covariate data from 2010 and 2011 using data from camera traps that were placed in the exact same locations in both years (i.e., 75 locations in 2010 and 2011 for a total of 150 locations). We ran models where the prey covariate was included as a single variable, summing the total number of detections for all six primary prey species at each camera trap (29). We used this method rather than including the total number of detections from each of the six prey species as covariates, because two prey species (i.e., gaur and hog deer) were not detected outside the park. Because spotted deer comprised 75% of all prey detections (Figs. S2 and S3), we also ran each model just using the total number of spotted deer detections at each camera trap as the prey covariate. This method produced similar results to the results produced using the combined prey covariate; therefore, we only report models using the combined prey covariate. Models were ranked according to their second-order Akaike’s information criterion (AICc), with higher-ranked models having lower AICc values. Because several models with different combinations of covariates performed comparatively well (i.e., ΔAICc < 4), we averaged model results (i.e., covariate coefficients, detection probability, and occupancy) from the top-ranked models using standard methods (i.e., multimodel inference) (57). Model-averaged coefficient estimates were considered significant if their unconditional 95% CIs did not include zero. We used kernel density estimation to estimate the probability density function of the activity patterns (i.e., density of activity) of tigers and types of human presence. Then, we used the procedures described in ref. 34 to measure the extent of overlap between them.
Supplementary Material
Acknowledgments
Thanks to B. Axinn, D. Ghimire, M. Cotton, B. Gurung, D. B. Tamang, D. Miquelle, B. Pandav, and the International Trust for Nature Conservation (UK) for their support throughout this project; R. Kumal, B. Mahato, I. B. Kumal, and the Tiger Tops Jungle Lodge for field support; J. A. Royle, M. Ridout, M. Linkie, D. Linden, and R. Malcolm for their assistance with analyses; the Nepal Department of National Parks and Wildlife Conservation for permission to collect data inside Chitwan National Park; and A. Viña, W. McConnell, J. Millington, and two anonymous reviewers for helpful comments on earlier drafts of this paper. Funding was provided by the United States Fish and Wildlife Service Rhinoceros and Tiger Conservation Fund, National Science Foundation (Partnerships in International Research and Education, Dynamics of Coupled Natural and Human Systems Program), Michigan State University, and National Aeronautics and Space Administration’s Earth and Space Science Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210490109/-/DCSupplemental.
References
- 1.Woodroffe R, Thirgood S, Rabinowitz A. People and Wildlife: Conflict or Coexistence? New York: Cambridge Univ Press; 2005. [Google Scholar]
- 2.Dickman AJ, Macdonald EA, Macdonald DW. A review of financial instruments to pay for predator conservation and encourage human-carnivore coexistence. Proc Natl Acad Sci USA. 2011;108:13937–13944. doi: 10.1073/pnas.1012972108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkes F. Community-based conservation in a globalized world. Proc Natl Acad Sci USA. 2007;104:15188–15193. doi: 10.1073/pnas.0702098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley N. Guidelines for Applying Protected Area Management Categories. Gland, Switzerland: International Union for Conservation of Nature; 2008. [Google Scholar]
- 5.Western D, Russell S, Cuthill I. The status of wildlife in protected areas compared to non-protected areas of Kenya. PLoS One. 2009;4:e6140. doi: 10.1371/journal.pone.0006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Western D, Strum SC, Wright RM. Natural Connections: Perspectives in Community-Based Conservation. Washington, DC: Island Press; 1994. [Google Scholar]
- 7.Cardillo M, et al. Human population density and extinction risk in the world’s carnivores. PLoS Biol. 2004;2:E197. doi: 10.1371/journal.pbio.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karanth KK, Nichols JD, Karanth KU, Hines JE, Christensen NL., Jr The shrinking ark: Patterns of large mammal extinctions in India. Proc Biol Sci. 2010;277:1971–1979. doi: 10.1098/rspb.2010.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brashares JS, Arcese P, Sam MK. Human demography and reserve size predict wildlife extinction in West Africa. Proc Biol Sci. 2001;268:2473–2478. doi: 10.1098/rspb.2001.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks S, Harcourt A. Reserve size, local human density, and mammalian extinctions in US protected areas. Conserv Biol. 2002;16:800–808. [Google Scholar]
- 11.Agrawal A, Redford K. Conservation and displacement: An overview. Conserv Soc. 2009;7:1–10. [Google Scholar]
- 12.Bagla P. India. Field biologists cry foul over ban. Science. 2012;335:1429. doi: 10.1126/science.335.6075.1429. [DOI] [PubMed] [Google Scholar]
- 13.Wittemyer G, Elsen P, Bean WT, Burton ACO, Brashares JS. Accelerated human population growth at protected area edges. Science. 2008;321:123–126. doi: 10.1126/science.1158900. [DOI] [PubMed] [Google Scholar]
- 14.United Nations, Department of Economic and Social Affairs, Population Division . World Population Prospects: The 2010 Revision. United Nations, NY; 2010. [Google Scholar]
- 15.Department of National Parks and Wildlife Conservation . Tiger Conservation Action Plan for Nepal 2008–2012. Kathmandu, Nepal: Government of Nepal; 2007. [Google Scholar]
- 16.Wikramanayake E, et al. A landscape based conservation strategy to double the wild tiger population. Conserv Lett. 2011;4:219–227. [Google Scholar]
- 17.George SL, Crooks KR. Recreation and large mammal activity in an urban nature reserve. Biol Conserv. 2006;133:107–117. [Google Scholar]
- 18.Straede S, Treue T. Beyond buffer zone protection: A comparative study of park and buffer zone products’ importance to villagers living inside Royal Chitwan National Park and to villagers living in its buffer zone. J Environ Manage. 2006;78:251–267. doi: 10.1016/j.jenvman.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Bookbinder MP, Dinerstein E, Rijal A, Cauley H, Rajouria A. Ecotourism’s support of biodiversity conservation. Conserv Biol. 1998;12:1399–1404. [Google Scholar]
- 20.Ministry of Forests and Soil Conservation . Terai Arc Landscape—Nepal Strategic Plan (2004–2014) Kathmandu, Nepal: Government of Nepal; 2004. [Google Scholar]
- 21.Sanderson E, et al. Setting Priorities for the Conservation and Recovery of Wild Tigers: 2005–2015. New York: WCS, WWF, Smithsonian, and NFWF-STF; 2006. [Google Scholar]
- 22.Walston J, et al. Bringing the tiger back from the brink-the six percent solution. PLoS Biol. 2010;8:493–506. doi: 10.1371/journal.pbio.1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenberg JF, Seidensticker J. Ungulates in southern Asia: A consideration of biomass estimates for selected habitats. Biol Conserv. 1976;10:293–308. [Google Scholar]
- 24.Jhala Y, Qureshi Q, Gopal R. Can the abundance of tigers be assessed from their signs? J Appl Ecol. 2011;48:14–24. [Google Scholar]
- 25.Johnson A, Vongkhamheng C, Hedemark M, Saithongdam T. Effects of human-carnivore conflict on tiger (Panthera tigris) and prey populations in Lao PDR. Anim Conserv. 2006;9:421–430. [Google Scholar]
- 26.Kawanishi K, Sunquist ME. Conservation status of tigers in a primary rainforest of Peninsular Malaysia. Biol Conserv. 2004;120:329–344. [Google Scholar]
- 27.O'Brien TG, Kinnaird MF, Wibisono HT. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv. 2003;6:131–139. [Google Scholar]
- 28.Wang SW, Macdonald DW. Livestock predation by carnivores in Jigme Singye Wangchuck National Park, Bhutan. Biol Conserv. 2006;129:558–565. [Google Scholar]
- 29.Karanth KU, et al. Monitoring carnivore populations at the landscape scale: Occupancy modelling of tigers from sign surveys. J Appl Ecol. 2011;48:1048–1056. [Google Scholar]
- 30.Wibisono HT, et al. Population status of a cryptic top predator: An island-wide assessment of tigers in Sumatran rainforests. PLoS One. 2011;6:e25931. doi: 10.1371/journal.pone.0025931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The World Bank—Global Tiger Initiative Secreteriat 2011. Global Tiger Recovery Program 2010–2022. Available at http://www.globaltigerinitiative.org. Accessed November 1, 2011.
- 32.Central Bureau of Statistics 2012. Nepal Census Information Site. Available at https://sites.google.com/site/nepalcensus/. Accessed March 1, 2012.
- 33.Stræde S, Helles F. Park-people conflict resolution in Royal Chitwan National Park, Nepal: Buying time at high cost? Environ Conserv. 2000;27:368–381. [Google Scholar]
- 34.Linkie M, Ridout MS. Assessing tiger-prey interactions in Sumatran rainforests. J Zool. 2011;284:224–229. [Google Scholar]
- 35.Karanth KU. Estimating tiger Panthera tigris populations from camera-trap data using capture–recapture models. Biol Conserv. 1995;71:333–338. [Google Scholar]
- 36.Gurung B, Smith JLD, McDougal C, Karki JB, Barlow A. Factors associated with human-killing tigers in Chitwan National Park, Nepal. Biol Conserv. 2008;141:3069–3078. [Google Scholar]
- 37.Nagendra H, Pareeth S, Sharma B, Schweik CM, Adhikari KR. Forest fragmentation and regrowth in an institutional mosaic of community, government and private ownership in Nepal. Landsc Ecol. 2008;23:41–54. [Google Scholar]
- 38.Smith JLD, McDougal C, Sunquist ME. Female land tenure system in tigers. In: Tilson R, Seal US, editors. Tigers of the World: The Biology, Biopolitics, Management and Conservation of an Endangered Species. Noyes Publications, NJ; 1987. pp. 97–108. [Google Scholar]
- 39.Smith JLD. The role of dispersal in structuring the Chitwan tiger population. Behaviour. 1993;124:165–195. [Google Scholar]
- 40.Frank LG, Woodroffe R. Behaviour of carnivores in exploited and controlled populations. In: Gittleman JL, Funk SM, Macdonald D, Wayne RK, editors. Carnivore Conservation. Cambridge, UK: Cambridge Univ Press; 2001. pp. 419–442. [Google Scholar]
- 41.Linnell JDC, Swenson JE, Anderson R. Predators and people: Conservation of large carnivores is possible at high human densities if management policy is favourable. Anim Conserv. 2001;4:345–349. [Google Scholar]
- 42.Carter NH, Riley SJ, Liu J. Utility of a psychological framework for carnivore conservation. Oryx. 2012 doi: 10.1017/S0030605312000245. [DOI] [Google Scholar]
- 43.Dinerstein E, et al. In: Riding the Tiger: Tiger Conservation in Human-Dominated Landscapes. Seidensticker J, Christie S, Jackson P, editors. Cambridge, UK: Cambridge University Press; 1999. pp. 316–333. [Google Scholar]
- 44.Liu J, et al. Complexity of coupled human and natural systems. Science. 2007;317:1513–1516. doi: 10.1126/science.1144004. [DOI] [PubMed] [Google Scholar]
- 45.Hull V, et al. Evaluating the efficacy of zoning designations for protected area management. Biol Conserv. 2011;144:3028–3037. [Google Scholar]
- 46.Valeix M, Hemson G, Loveridge AJ, Mills G, Macdonald DW. Behavioural adjustments of a large carnivore to access secondary prey in a human-dominated landscape. J Appl Ecol. 2012;49:73–81. [Google Scholar]
- 47.DeFries R, Hansen A, Newton AC, Hansen MC. Increasing isolation of protected areas in tropical forests over the past twenty years. Ecol Appl. 2005;15:19–26. [Google Scholar]
- 48.Georgiadis NJ, Ihwagi F, Olwero JG, Romañach SS. Savanna herbivore dynamics in a livestock-dominated landscape. II: Ecological, conservation, and management implications of predator restoration. Biol Conserv. 2007;137:473–483. [Google Scholar]
- 49.Liu J, et al. Ecological degradation in protected areas: The case of Wolong Nature Reserve for giant pandas. Science. 2001;292:98–101. doi: 10.1126/science.1058104. [DOI] [PubMed] [Google Scholar]
- 50.Ramnath M. Surviving the forest rights act: Between Scylla and Charybdis. Econ Polit Wkly. 2008;43:37–42. [Google Scholar]
- 51.O'Connell AF, Nichols JD, Karanth KU. Camera Traps in Animal Ecology: Methods and Analyses. New York: Springer; 2010. [Google Scholar]
- 52.Seidensticker J, McDougal C. Tiger predatory behaviour, ecology and conservation. Symp Zool Soc Lond. 1993;65:105–125. [Google Scholar]
- 53.Royle JA, Nichols JD, Karanth KU, Gopalaswamy AM. A hierarchical model for estimating density in camera-trap studies. J Appl Ecol. 2009;46:118–127. [Google Scholar]
- 54.Obbard ME, Howe EJ, Kyle CJ. Empirical comparison of density estimators for large carnivores. J Appl Ecol. 2010;47:76–84. [Google Scholar]
- 55.Royle JA, Gardner B. In: Camera Traps in Animal Ecology: Methods and Analyses. O'Connell AF, Nichols JD, Karanth KU, editors. Berlin: Springer; 2011. pp. 163–190. [Google Scholar]
- 56.MacKenzie DI, et al. Estimating site occupancy rates when detection probabilities are less than one. Ecology. 2002;83:2248–2255. [Google Scholar]
- 57.Burnham KP, Anderson DR. Multimodel inference. Sociol Methods Res. 2004;33:261–304. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.