Abstract
The Spemann organizer stands out from other signaling centers of the embryo because of its broad patterning effects. It defines development along the anteroposterior and dorsoventral axes of the vertebrate body, mainly by secreting antagonists of growth factors. Qualitative models proposed more than a decade ago explain the organizer’s region-specific inductions (i.e., head and trunk) as the result of different combinations of antagonists. For example, head induction is mediated by extracellular inhibition of Wnt, BMP, and Nodal ligands. However, little is known about how the levels of these antagonists become harmonized with those of their targets and with the factors initially responsible for germ layers and organizer formation, including Nodal itself. Here we show that key ingredients of the head-organizer development, namely Nodal ligands, Nodal antagonists, and ADMP ligands reciprocally adjust each other’s strength and range of activity by a self-regulating network of interlocked feedback and feedforward loops. A key element in this cross-talk is the limited availability of ACVR2a, for which Nodal and ADMP must compete. By trapping Nodal extracellularly, the Nodal antagonists Cerberus and Lefty are permissive for ADMP activity. The system self-regulates because ADMP/ACVR2a/Smad1 signaling in turn represses the expression of the Nodal antagonists, reestablishing the equilibrium. In sum, this work reveals an unprecedented set of interactions operating within the organizer that is critical for embryonic patterning.
Keywords: Xenopus leavis, morphogen gradients
Eighty years ago, the “organizer” experiment by Spemann and Mangold demonstrated that a restricted group of cells of the embryo, the dorsal blastopore lip of the amphibian gastrula, is endowed with extraordinary inducing activities (1–4). Grafted to an ectopic location of a host embryo, this tissue recruits neighboring cells to form a secondary body axis. Naming this tissue an “organizer,” Spemann and Mangold (1924) wrote that “the effect emanating from these preferential regions is not only determinative in a definite restrictive direction, but it possesses all the enigmatic peculiarities which are known to us only from living organisms” (4). After decades, what ultimately defines those “enigmatic peculiarities,” including the mechanisms that ensure the reproducible formation of a body plan that is perfectly patterned, well proportioned, and able to withstand perturbations, remains one of the unsolved mysteries in developmental biology.
Much evidence indicates that the organizer is induced by a dorsal peak of Nodal/Smad signaling (5, 6). In turn, the “inducing” molecules emanating from the organizer are in fact secreted antagonists of the embryonic morphogens, that is, diffusible factors that bind to Nodal, BMP, and Wnts proteins in the extracellular space, confining and dosing their activity along the embryonic axes (5). Regionalization by secreted molecules is refined by transcription factors (7). So far, the best understood of these processes is the establishment of the dorsoventral axis, organized by a gradient of BMP inhibitors (Chordin, Noggin, and Follistatin) (8–13).
The organizer is also required for patterning along the anteroposterior (AP) axis; Spemann himself recognized that the ability to differentially pattern anterior vs. posterior fates relies on the organizer's internal patterning: distinct cell populations within the organizer display different inducing properties, corresponding to the head and trunk organizers. Molecularly, head-organizing activity requires the concomitant repression of Wnt (i.e., by Dkk-1), Nodal [i.e., by Cerberus (Cer) and Lefty], and BMP signaling, whereas the trunk organizer involves repression of BMPs (3, 14–16).
Here we tackled the question of what organizes the organizer: clearly, mechanisms for self-regulation must be embedded within the organizer to ensure the proper and coordinated expression (quantitative, qualitative, and spatiotemporal) of different ligands and of their antagonists. Different domains of the organizer, such as the head and trunk organizers, should talk to each other to mutually regulate each other’s activity and attain robustness when facing fluctuations in individual elements of the organizer’s network. This work aims to shed light on some of these questions. Here we propose a model whereby competition between Nodal and ADMP for a shared receptor, ACVR2a, generates a self-adjusting gene network that regulates head induction by the Spemann organizer.
Results
The Spemann organizer is not only a source of BMP antagonists but, paradoxically, it also expresses a BMP ligand, ADMP (17, 18). Elegant work suggests that this ligand operates in dorsoventral (DV) patterning: ADMP forms a complex with Chordin that transports ADMP to the ventral side of the embryo; there, ADMP turns on BMP expression, thus mediating the communication between the dorsal organizer and the opposite pole of the embryo (8, 12). The biochemical complex between ADMP and Chordin is regulated by other extracellular factors, such as Xolloid-related (Xlr) and Twisted Gastrulation (Tsg) (12). ADMP is also regulating the development of anterior “head” structures (12, 17); however, it remains unclear whether this is secondary to ventralization and thus global inhibition of organizer activity caused by excess of BMP signaling, or alternatively, whether ADMP has also a specific function in anteroposterior (AP) pattering, uncoupled from its DV effects.
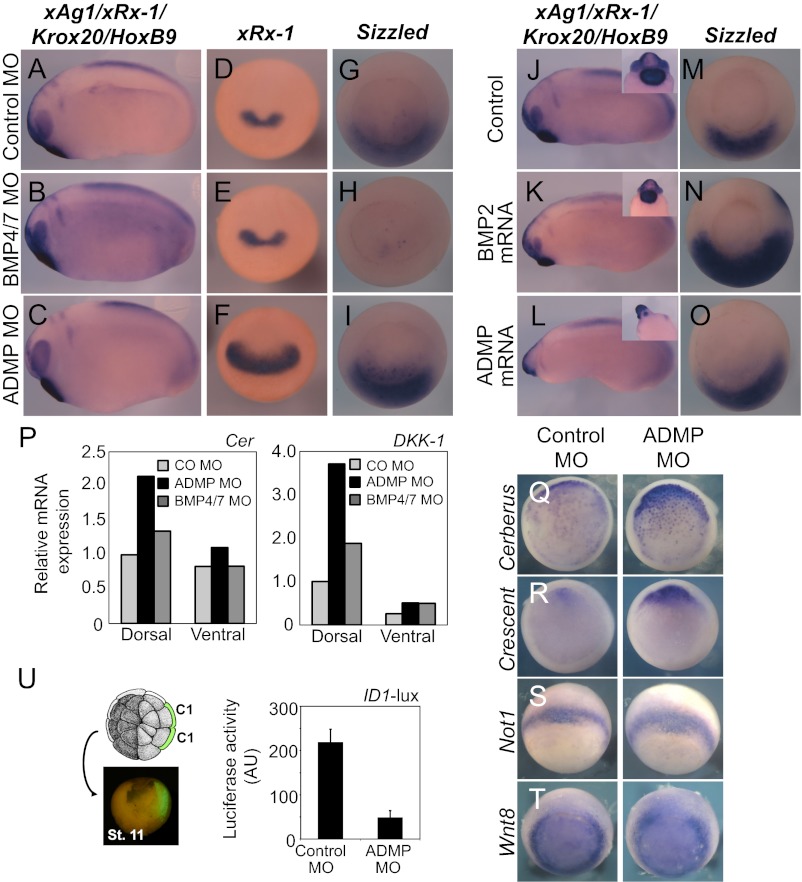
To untangle this issue, we compared the role of ADMP and BMPs in DV and AP patterning by gain- and loss-of-function assays in Xenopus embryos. As shown in Fig. 1 A–C, ADMP knockdown by means of injected ADMP morpholinos (MOs) results in embryos with enlarged heads, whereas injection of BMP4/7 morpholinos had limited effects on head size. This was confirmed by the expression of xRx-1 and BF-1 that mark anterior neural tissue (Fig. 1 D–F and Fig. S1 A–C). Intriguingly, when embryos were analyzed for expression of Sizzled, a ventrally expressed Smad1 target, we observed a different requirement: BMP4/7 were critical for Sizzled expression, whereas loss of ADMP had no effect (Fig. 1 G–I). Consistently, it is BMP4/7, rather than ADMP, that limits Chordin expression on the dorsal side of gastrula-stage embryos (Fig. S1 D–F).
Fig. 1.
ADMP inhibits head formation through repression of head inducers. (A–I) Embryos were radially injected in the marginal zone at the four-cell stage with morpholino antisense oligo (MO): Control MO (80 ng/embryo), BMP4 and BMP7 MOs (mix of 25 ng each/embryo) or ADMP MO (80 ng/embryo). Embryos were stained by in situ hybridization for neural markers (mix of xAg1, xRx-1, Krox20, and HoxB9) at late neurula stage (A–C), for xRx-1 at early neurula stage (D–F; anterior view), or for Sizzled at gastrula stage (G–I; ventral view, dorsal up; Nieuwkoop and Faber stage 10.5). Number of embryos (n) was ≥18, derived from two independent experiments; for each class the frequency of the shown phenotype was >80%. (J–O) Similar in situ hybridization to A–I of embryos injected with ADMP mRNA (15 pg/embryo) or BMP2 mRNA (15 pg/embryo). Insets in J–L show the close-up of head/anterior region in ventral view. Number of embryos (n) was ≥20, derived from two independent experiments; for each class the frequency of the shown phenotype was >80%. (P) Cerberus (Cer) and DKK-1 expression was monitored by qPCR on dorsal and ventral halves of embryos injected with ADMP, BMP4/7, or control morpholino (CoMO). Expression levels are normalized to EF1a mRNA. (Q–T) Expression of head-organizer markers in anterior endodermal cells is inhibited by ADMP. Four-cell stage embryos were injected with ADMP MO (80 ng) or control MO (CoMO, 80 ng). At stage 11, embryos were fixed, removed of the blastocele roof to reveal the underlying endoderm (for Q and R), and processed for in situ hybridization for the indicated markers. Number of embryos (n) was ≥20, derived from two independent experiments; for each marker the frequency of the shown expression was >70%. For additional markers, refer to Fig. S1 G–I. (U, Left) Thirty-two–cell-stage embryos shown from animal pole. Embryos were injected with the Smad1-responsive reporter ID1-lux in dorsal “C1” blastomeres. Targeting to anterior endoderm was confirmed at gastrula by coinjected GFP mRNA (Lower Left picture). (Right) Luciferase was determined on extracts from stage 10.5 embryos coinjected with control or ADMP MO.
In a complementary gain-of-function experimental set-up, we also found that injection of titrated amounts of ADMP mRNA reduces the size of the head at doses unable to trigger overt changes in Sizzled expression domains (Fig. 1 L–O). In contrast, BMP2 was clearly effective at regulating DV patterning at doses of injected mRNA displaying limited effects on AP patterning (Fig. 1 K–N). Taken together, these data suggest that ADMP is a relevant inhibitor of the head-inducing property of the Spemann organizer, and that this can be experimentally uncoupled from its well-established functions in DV patterning.
Head induction requires the activity of antagonists of Nodal and Wnt ligands, such as Cerberus, Lefty, and DKK-1, whose expression marks a distinct territory of the organizer corresponding to the “leading edge” endoderm. Together with the prechordal plate mesoderm, such anterior endoderm defines a “head-organizing” center located anteriorly to the trunk organizer expressing ADMP itself (3). Importantly, loss of ADMP, but not loss of BMP4/7, enhanced expression of head-inducing and anterior endoderm markers, such as Cer, DKK-1, Crescent, and Frzb as assayed by qPCR and in situ hybridization (Figs. 1 P–R and Fig. S1G). Notably, this effect was not secondary to global perturbation of the entire organizer (see in situ hybridization for FoxA4, Lim1, and Not1) or to expression of the posteriorizing factor Wnt8 (Fig. 1 S and T and Fig. S1 H and I). This suggests that ADMP limits head development, by opposing head-organizer function. We next asked whether ADMP activates Smad1 signaling in this territory. As shown in Fig. 1U, ADMP-dependent transcriptional activity in anterior endoderm could be revealed by microinjecting the Smad1 reporter plasmid ID1-luciferase (lux) specifically in the C1 blastomeres, from which the anterior endoderm derives (19). In agreement with a role of ADMP/Smad1 signaling as inhibitor of head-organizer function, injection of Dominant Negative Smad5 mRNA in dorsal, but not ventral blastomeres causes enlargement of head structures (Fig. S2). We propose that ADMP is a limiting factor for the activity of the head organizer by signaling through Smad1/5 within the anterior endoderm.
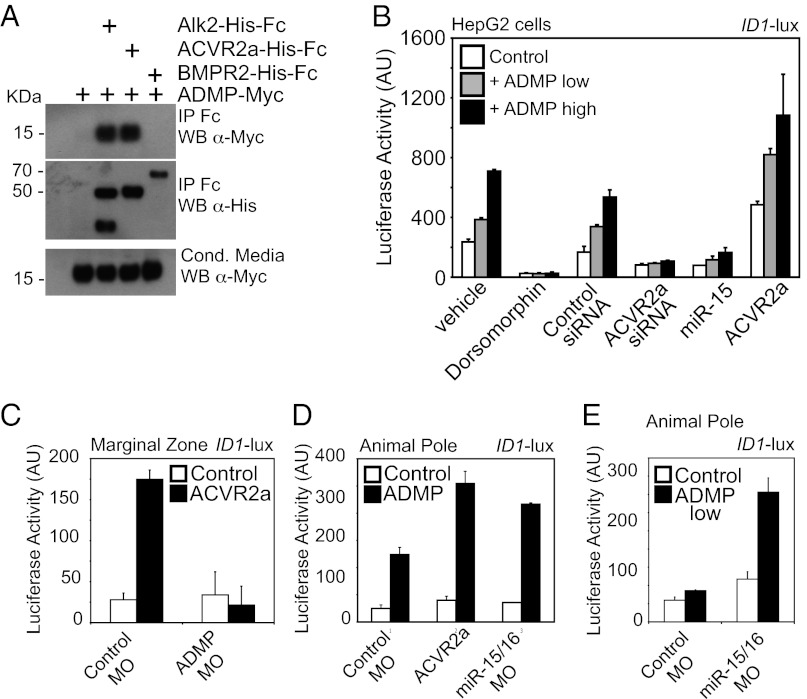
TGFβ and BMP ligands signal through binding to type II receptors that form heteromeric complexes with type I receptors, which in turn phosphorylate R-Smads. Whereas these ligands could share the same type II receptor, they signal through different type I receptor and this accounts for the specific activation of different classes of R-Smads (20). Previous work had shown that ADMP binds to the type I BMP receptor ALK2 (12), prompting us to focus on the still unknown identity of the type II ADMP receptor. For this, we assayed the binding of ADMP protein to BMPR2 and ACVR2a. The latter serves as receptor for Nodal and Activin ligands, but it has been shown to be capable of regulating the activity of BMP6/7 ligands in some biological contexts (21, 22). To assay for direct biochemical interaction, mature Myc-tagged ADMP was produced in the conditioned media of transfected 293T cells and incubated with recombinant Fc-tagged soluble versions of AVCR2a or BMPR2. ALK2 was used as positive control. As shown in Fig. 2A, ADMP protein could be copurified with ACVR2a and ALK2, but not with BMPR2.
Fig. 2.
ADMP signals through ACVR2a. (A) ADMP copurifies with ACVR2a and ALK2 receptors, but not with BMPR2. Conditioned media from HEK293T cells expressing mature Myc-tagged cADMP were incubated with the extracellular domains of the indicated receptors fused to Fc and immobilized on protein A sepharose beads. Copurifying mature ADMP was visualized by anti-Myc immunoblotting. (B) ADMP activates Smad1/5-dependent transcription through ACVR2a. Human HepG2 cells were transfected with Smad1/5-responsive ID1-luciferase reporter alone (control) or with increasing doses of xADMP expression plasmid (10 ng/cm2, low; 40 ng/cm2, high). Where indicated, cells were treated overnight with the type I BMP receptor kinase inhibitor Dorsomorphin (10 μM) or cotransfected with control or ACVR2a-siRNA (50 pmol/cm2 each), mature miR-15 (700 pg/cm2), or an ACVR2a expression plasmid (20 ng/cm2). Data are given as mean and SD. (C and D) Overexpression of ACVR2a fosters endogenous (C) and ectopic (D) ADMP signaling in Xenopus embryo. In C, embryos were injected in the marginal zone with ID1-luciferase reporter alone (Control) or in combination with ACVR2a mRNA (250 pg/embryo). Where indicated, embryos were coinjected with control or ADMP morpholinos. In D, embryos were injected in the animal pole with ID1-luciferase reporter alone (Control) or in combination with ADMP mRNA (100 pg/embryo). Where indicated, embryos were coinjected with control-MO, ACVR2a mRNA, or miR-15/16 MO. (E) Embryos were injected in the animal pole with ID1-luciferase reporter alone (Control) or in combination with a suboptimal dose of ADMP mRNA (10 pg/embryo). Where indicated, embryos were coinjected with control or miR-15/16 MOs.
To determine whether AVCR2a is also a functionally relevant ADMP receptor, we depleted endogenous ACVR2a from mammary HepG2 cells and monitored ADMP responsiveness using the synthetic ID1-lux Smad1/5 luciferase reporter. As shown in Fig. 2B, siRNA-mediated knockdown of ACVR2a abolishes ADMP responsiveness, an effect phenocopied by transfection of mature miR-15, a microRNA that we previously showed to down-regulate ACVR2a expression in human cells and Xenopus embryos (6). Conversely, ADMP responsiveness was increased after raising ACVR2a levels. A complementary set of experiments was carried out in Xenopus embryos: ACVR2a overexpression—or depletion of miR-15/16—fosters endogenous and overexpressed ADMP signaling (Fig. 2 C and D). Moreover, loss of miR-15/16 empowers the effects of suboptimal doses of microinjected ADMP mRNA (Fig. 2E).
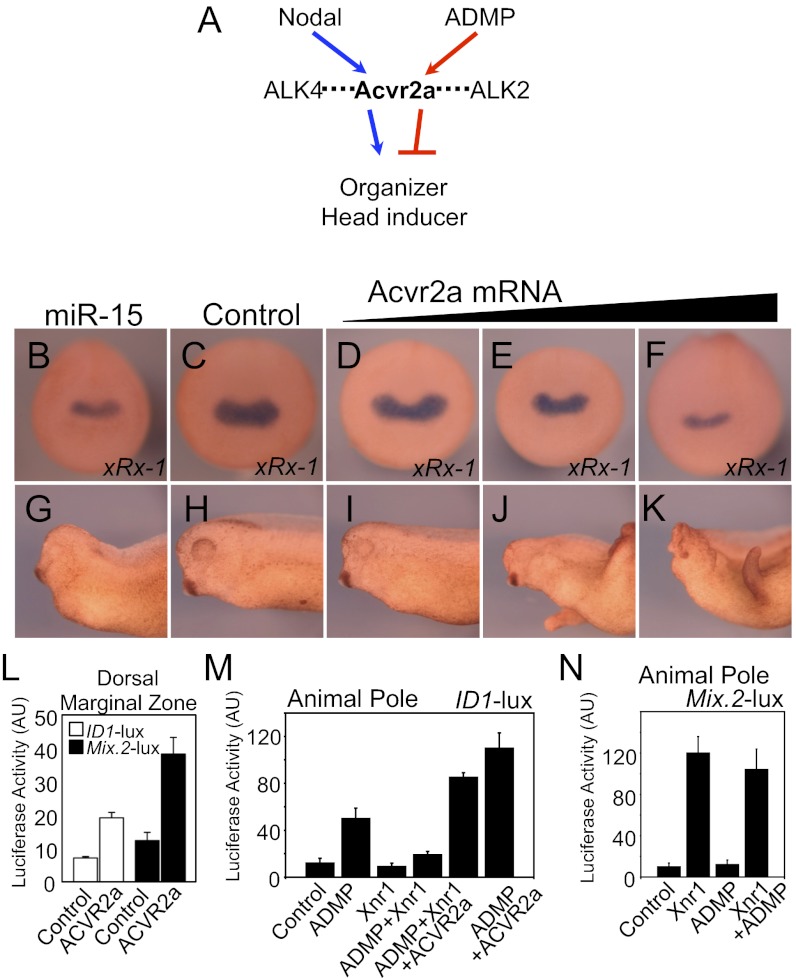
Genetic and embryological evidence indicate that the organizer is induced by Nodal/ACVR2/Smad2 signaling (23–26), whereas our data imply that ACVR2a may also carry an opposite function, that is, repression of head-organizer gene expression by the ADMP/ACVR2a/Smad1 pathway (Fig. 3A). We thus sought to determine whether in fact ACVR2a is able to carry such a dual function. Consistently with the well-established requirement of ACVR2a as Nodal receptor, attenuation of endogenous ACVR2a by microinjected miR-15 leads to microcephaly (Fig. 3 B and G). However, further raising ACVR2a levels by microinjecting increasing doses of ACVR2a mRNA also caused a progressive reduction of head structures and reduction of head-specific markers (Fig. 3 D–F and I–K and Fig. S3 A–E). Interestingly, this phenotype is anticipated at gastrula stage by the reduction of the head-organizer marker Crescent without overt effects on more posterior organizer markers (Chordin and FoxA4), or expression of ventralizing/posteriorizing factors Wnt8 and Vent1 (Fig. S3 F–J). Critically, the microcephaly phenotypes were dependent on enhanced activity of endogenous ADMP (Fig. S4 A–D). In other words, unleashing ACVR2a levels reveals a potentially dominant role for the head-repressing signal delivered by ADMP. To control that the phenotype of ACVR2a overexpression was not secondary to differentiation of the ectoderm into mesoderm, we coinjected ACVR2a mRNA together with mRNA of the head inducer gene DKK-1, and obtained embryos with normal anterior structures (Fig. S4 E–G). Thus, gain of ACVR2a relies upon the ADMP-dependent inhibition of head-organizer genes, rather than on the loss of responsive capacity of the ectoderm.
Fig. 3.
ADMP and Nodal compete on ACVR2a. (A) Schematic drawing depicting Nodal and ADMP sharing ACVR2a. (B–K) Embryos were radially injected in the marginal zone at the four-cell stage with mature miR-15 (15 ng/embryo) or ACVR2a mRNA (10, 100, or 300 pg/embryo). (B–F) Neurula-stage embryos were stained for xRx-1. (G–K) Close up picture of anterior/head region of sibling embryos of B–F, grown until tadpole stage (stage 37). Pictures are the representatives of >20 embryos from two independent experiments. Frequency of the shown phenotype was >80%. (L) Dorsal overexpression of ACVR2a increases both ADMP- and Nodal-dependent transcriptional activity. Embryos were injected in the dorsal marginal zone with either ID1-luciferase reporter or Mix.2-luciferase reporter with or without AVCR2a (300 pg) mRNA. Targeting to dorsal mesoderm was confirmed at gastrula by coinjected GFP mRNA. Data are given as mean and SD. (M) Overexpression of Xnr1 outcompetes ADMP if ACVR2a levels are limiting. Embryos were injected in the animal pole with the ADMP-responsive ID1-luciferase reporter alone (control) or with combinations of ADMP (100 pg/embryo), Xnr1 (100 pg/embryo), and ACVR2a (300 pg) mRNAs. Data are given as mean and SD. (N) ADMP does not outcompete Xnr1. Embryos were injected in the animal pole with the Nodal-responsive Mix.2-luciferase reporter alone (control) or with combinations of the indicated mRNAs (same doses as in L).
Loss of miR-15 has been shown to enhance Nodal-dependent signaling, phenocopying gain of ACVR2a (6). This suggests that ACVR2a may be in limiting amounts for the available Nodal ligands. Given that ADMP limits the size of the head organizer also by binding to ACVR2a (Fig. 2), we hypothesized the following model: occupancy by Nodal saturates a limited pool of ACVR2a receptors limiting ADMP accessibility to ACVR2a; however, ADMP ultimately manages to outcompete Nodal ligands in some contexts, switching ACVR2a signaling from prohead to head suppressor. If so, experimentally raising ACVR2a to a nonlimiting concentration should increase both ADMP and Nodal-dependent transcription. This was indeed verified by coinjecting in the dorsal marginal zone the ID1-lux and Mix.2-lux reporter plasmids together with ACVR2a mRNA (Fig. 3L). To test for extracellular competition between Nodal and ADMP ligands, we used Xenopus animal cap assays to monitor Nodal/Smad2 and ADMP/Smad1 activities with the Mix.2-lux or ID1-lux reporters, respectively. As shown in Fig. 3M, Xnr1 potently antagonized ADMP activity and this was dependent on ACVR2a availability. In the converse experiment, overexpression of ADMP, over a range of mRNA doses, had no effects on the induction of the Mix.2 promoter by Xnr1 (Fig. 3N). Coinjection of Cerberus-short mRNA (coding for a secreted fragment of Cer corresponding to the sole Nodal binding domain) (16), but not of Smad4, opposed Xnr1 inhibition on ADMP signaling, corroborating the notion that Nodal antagonizes ADMP signaling at the extracellular level (Fig. S5).
These data suggest that ADMP ligands are by themselves unable to squelch Nodal ligands from ACVR2a. We thus reasoned that additional factor(s) should exist to facilitate ADMP signaling by inhibiting Nodal extracellularly. The Nodal antagonists Cerberus and Lefty, so far here considered only as read-outs of head-organizer size, stood up as the most likely candidates to fulfill this function.
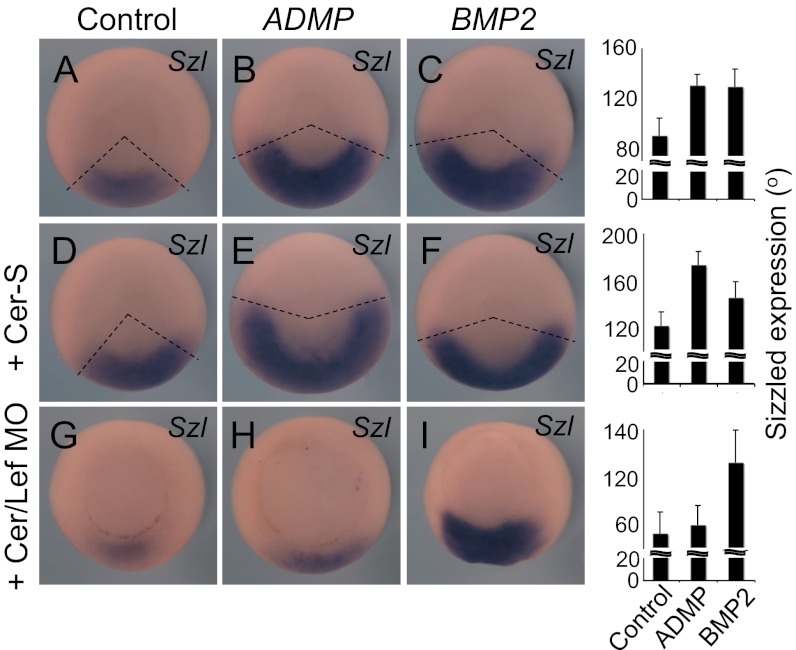
To test whether Cerberus and Lefty are relevant factors to allow ADMP signaling, we first monitored ADMP responsiveness in an heterologous assay, that is, by assaying Sizzled expression on the ventral side of the embryo. Strikingly, injection of ADMP mRNA strongly cooperates with CerS mRNA (Fig. 4 A, B, D, and E). Conversely, in Cerberus/Lefty double morphants, excess of endogenous Nodal ligands leaves little room for ADMP activity (Figs. 4 G and H). As critical specificity control for these assays, we also tested BMP2 mRNA, which up-regulates Sizzled similarly to ADMP (Fig. 4C); because BMP only marginally relies on ACVR2a for signaling (21), the prediction would be that BMP should be relatively insensitive to Cerberus/Lefty levels. In line, as shown in Fig. 4 C, F, and I, gain- or loss-of-Nodal antagonists had minimal effect on BMP responsiveness. These results reinforce the notion that ADMP activity is opposed by increased availability of endogenous Nodal ligands and that, viceversa, ADMP activity is enhanced by Nodal antagonism.
Fig. 4.
Cerberus facilitates ADMP signaling by relieving Nodal competition for ACVR2a. (A–I) In situ hybridization of Sizzled (Szl) indicates the activities of ADMP and BMP2. Embryos were radially injected in the marginal zone at the four-cell stage with the indicated combinations of ADMP mRNA (30 pg/embryo) BMP2 mRNA (20 pg/embryo), CerS mRNA (30 pg/embryo), and Cerberus + Lefty MOs (60 ng/embryo). Representative embryos are shown dorsal side up and Sizzled expression domains are highlighted with dotted lines. To quantify expansions or reduction, the angle of the Sizzled ventral crescent was measured in >20 embryos (from two independent experiments) as quantifications are shown in the Right columns with SD. Note that full-length Cerberus does not affect ADMP activity, whereas it inhibits BMP responsiveness (Fig. S6).
Next, we tested whether, similarly to ACVR2a overexpression, extracellular antagonism of Nodal ligands facilitates head repression by ADMP. Injection of suboptimal doses of ADMP and CerS mRNAs strongly cooperated in head repression, whereas no synergy could be observed between CerS and BMP2 mRNAs (Fig. S7 A–F). In line, Nodal antagonism is required for restraining the expression of the head inducers Cer and DKK-1 in anterior endoderm, as revealed by in situ hybridization in Cerberus/Lefty morphants (Fig. S7 G–N). Together, the data indicate ADMP signaling in the anterior endoderm serves as feedback inhibitor to attenuate expression of the head-inducing program (Fig. 5A).
Fig. 5.
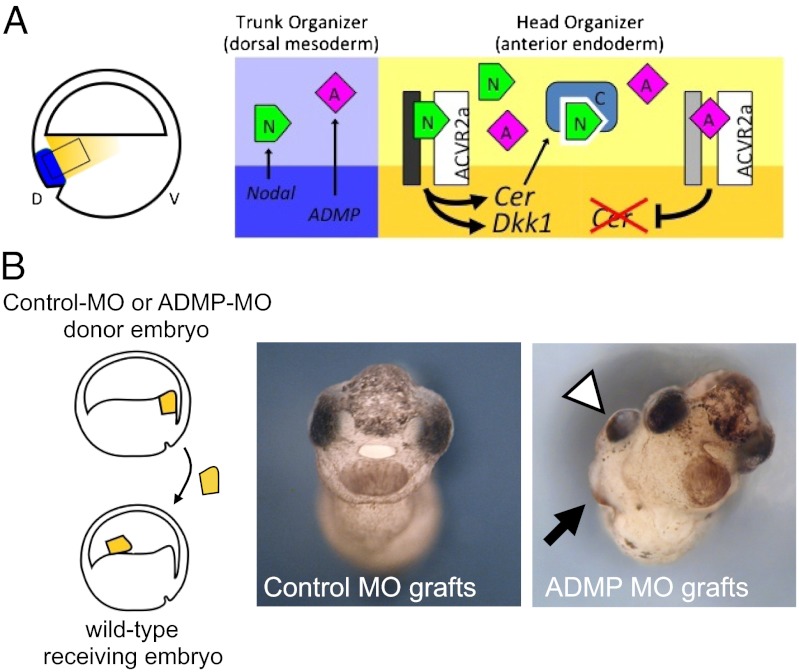
ADMP inhibition promotes head-organizer activity in explanted anterior endoderm. (A) Simplified scheme depicting the molecular interactions between Nodal, ADMP, and ACVR2a. (Left) In a Xenopus gastrula, the organizer is formed of dorsal mesodermal cells (blue), and of the abutting anterior endoderm (yellow expressing head-inducing molecules. D, dorsal; V, ventral. The expression pattern of ADMP, Cerberus, Dkk, Nodals, and ACVR2a has been previously described (6, 14, 17, 22, 27). Right, corresponding to the boxed region on the Left, depicts a model for the interplay between Nodal, ADMP, and Nodal antagonists. Darker and lighter backgrounds correspond to the intracellular and extracellular space, respectively. Nodal signals through ACVR2a and activates the expression of head inducers, such as Cerberus and Dkk-1. Cerberus secretion limits Nodal diffusion, enabling ADMP signaling, that, in turn, feedbacks on the expression of head inducers, including Cerberus itself. N, Nodal; A, ADMP; C, Cerberus. Italicized names indicate gene transcription. (B) Anterior endoderm explants dissected from early gastrula embryos (stage 10.5) injected with control or ADMP morpholinos were transplanted into the blastocele of wild-type recipient embryos (Einsteck grafts). Recipient embryos were allowed to develop until tadpole stage, when they were scored for the presence of ectopic head structures (white arrowheads, cement glands; black arrows, eyes). Percentage of induced heads: control, MO-injected grafts, 0/40; ADMP, MO-injected grafts, 5/30.
This prompted us to reinvestigate a long-standing issue in embryology: although the anterior endoderm expresses head-inducing factors, it hardly induces ectopic heads after transplantation in recipient embryos (27–29). We noticed that expression of Cerberus in explanted anterior endoderm rapidly decays (Fig. S8 A and B). Our model suggests that activity of ADMP ligands, already diffused in the anterior endoderm from the adjoining trunk organizer, may play a role in this event. Indeed, in anterior endoderm explanted from ADMP-MO–injected embryos, Cerberus expression remained stable for a longer period (Fig. S8 C and D). Remarkably, anterior endoderm explants from ADMP morphants, but not from control embryos, were able to induce ectopic heads (without trunk) once grafted in recipient embryos (Fig. 5B). These findings support the model shown in Fig. 5 and reveal the potential for head-organizing properties of the Xenopus anterior endoderm.
Discussion
For ectodermal cells, Nodal attenuation is critical to prevent the spreading of the trunk territory into the anterior part of the embryo, thus allowing head development (3, 16, 23). This is mediated by the anti-Nodal functions of Cerberus and Lefty emanating from the head organizer. Beyond these well-known effects, the present work sheds unique light on the role of Nodal antagonism as an intimate regulator of the organizer itself. As Nodal levels increase, anterior endoderm levels of Cerberus and Lefty would also rise; in part, this feedback limits the potentially explosive Nodal autoinduction loop, but also serves as permissive element for ADMP signaling (Fig. 5 and Fig. S9). The system “self-regulates” because ADMP, in turn, tends to quench back head-organizer gene expression, including that of Nodal antagonists, reestablishing the equilibrium between Nodal and ADMP activity (Fig. 5 and Fig. S9). Thus, we propose that the function of Nodal antagonists within the organizer is to limit their own expression and that of DKK; of note, this is an antihead function, opposite to their prohead function in the ectoderm germ layer (i.e., outside of the organizer).
A critical element of the network is the TGFβ receptor ACVR2a. This receptor is shared between Nodal and ADMP but, due to its post-transcriptional regulation by an abundant miRNA of the embryo, miR-15/16, its expression levels are limited (6). Thus, we propose that ACVR2a cannot “handle” all of the available Nodal and ADMP ligands: in the presence of Nodal (as visualized in embryos lacking Nodal antagonism), Nodal/ACVR2a receptor complexes dominate ADMP/ACVR2a complexes, thereby impeding ADMP signaling. Such competition is biologically meaningful because ADMP remains a potential fatal threat for the head organizer, inhibiting expression of DKK-1 and Cer (ref. 17 and this study).
The vertebrate genome encodes many TGFβ-related ligands but many fewer type I and type II receptors and only a handful of Smads; promiscuity in receptor use has been so far only hypothesized as a main determinant for context-dependent responses (30). Moreover, a wealth of research indicates that Smads and the receptors can be limited in vivo by multiple mechanisms including miRNA regulation, sequestration, phosphorylation, and ubiquitination (31). The present work suggests that ligand competition for a shared receptor might serve as a remarkably simple tool to regulate signal intensity, mutual antagonism, and dynamics between different TGFβ family members.
As development proceeds, the head- and trunk-inducing regions of the Spemann organizer become physically separated. Are the loops here described relevant only for reciprocal quantitative blending of the different elements of the head organizer or may these also be related to spatial patterning? We speculate that the dorsal mesoderm fulfils two distinct functions: serving as a “dorsal center” through Chordin/Noggin/Follistatin function, but also as a “posterior pole” defined by transcription of Nodal and ADMP. The anteriormost tip of the leading-edge endoderm only weakly expresses Cer or DKK-1 and thus may represent a territory where ADMP/ACVR2 dominates Nodal signaling (Fig. S7 G and K and Fig. 5A). Supporting this argument, head formation could be observed in ADMP-depleted leading-edge explants. Intriguingly, Cer and DKK-1 expression expands to the whole anterior endoderm in ADMP-depleted embryos, and this is phenocopied by Cerberus/Lefty knockdowns, revealing that Nodal antagonism is essential to set the anterior border of the head organizer (Fig. S7 G–N). It is interesting to compare this “border” with the orthogonal “ventral center” located at the far ventral end of the embryo (12): both are defined by ADMP signaling but, whereas the ventral center can sustain its own identity by the BMP autoregulatory loop, the anterior border relies entirely on the “posterior pole” for ADMP production and Chordin-mediated transport (8). Perhaps, this explains why anteroposterior development, despite the self-regulatory loops here described, remains less robust than dorsoventral polarity, as evident by the invariable “overposteriorized” phenotype of embryos with deficient dorsal development (3, 32).
In sum, this study provides a molecular framework to understand anteroposterior patterning and self-regulation within the Spemann organizer.
Experimental Procedures
Embryological Manipulations and Microinjection.
Xenopus embryo manipulations, in situ hybridization, and capped mRNA preparation were as previously described (6). All morpholinos were purchased from Gene Tools. MOs were resuspended in Hepes 0.5 mM, pH 7.6 (25 mg/mL stock) and heated to 70 °C for 5 min before microinjection. Embryos at the four-cell stage were microinjected radially with a volume of 4 μl for each blastomere.
Protein Interactions and Western Blotting.
Protein interaction assay was as described (12), using proteins harvested from the conditioned medium of HEK293T cells. Briefly, HEK293T cells were transfected with chicken ADMPmyc-pCS2, and cultivated in DMEM/2% serum (vol/vol). After 48 h, conditioned medium was harvested and filtered in a 0.45-mm filter (Millipore). Receptor binding assay was carried out using human recombinant chimeric Fc-Alk-2-HIS, Fc-ACVR2a-HIS, and Fc-BMPR2-HIS (R&D Systems). Recombinant receptors (1 μg/sample) were incubated for 2 h at 4 °C with the conditioned medium containing ADMP-myc protein. Ligand-receptor complexes were purified for 3 h at room temperature (RT) using protein A sepharose beads (GE Healthcare). Beads were preblocked with a solution of 2% nonfat milk (wt/vol) in PBS, 0.05% CHAPS for 16 h at 4 °C, incubated with nonspecific rabbit Igg (2 mg/sample) diluted in KMC buffer (50 mM Hepes, pH 7.4, 128 mM NaCl, 5 mM KCl, 5 mM MgCl2, 1.2 mM CaCl2, 0.2% BSA) for 2 h at RT, and then washed three times with KMC buffer. After three washes in wash buffer [50 mM Hepes, pH 7.4, 150 mM NaCl, 1.8 mM MgCl2, 5% glycerol (vol/vol), 0.1% Np40], samples were boiled in protein sample buffer [50 mM Tris·HCl, pH 7.8, 2% SDS (wt/vol), 10% glycerol (vol/vol), 2% b-MeSH (vol/vol)] and loaded in precast SDS-page gels (Invitrogen). The coprecipitated complexes were revealed by Western blotting as described previously (33) using anti-myc (Covance, 1:1,000) or anti-HIS (1:2,000) antibodies.
Supplementary Material
Acknowledgments
We thank O. Wessely for comments on the manuscript. D.B.-Z. is supported by the Adams Fellowship Program of the Israel Academy of Sciences and Humanities and M.I. has been supported by TOYOBO Biotechnology Foundation, Uehara Memorial Foundation, and the Seventh Framework Programme Marie Curie International Incoming Fellowship. E.E is a recipient of a Cariparo PhD Fellowship. This work is supported by Istituto Superiore di Sanità Nazionale “Giovani Ricercatori” (to G.M.), Associazione Italiana per la Ricerca sul Cancro - My first AIRC grant (AIRC-MFAG) (to M.C.), AIRC grants (to S.D.) and by University of Padua “Strategic-Grant,” Cariparo Foundation “Excellence-Grant”, AIRC, and Comitato Promotore Telethon Grants (to S.P.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203000109/-/DCSupplemental.
References
- 1.De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 3.Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–434. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- 4.Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int J Dev Biol. 2001;45:13–38. [PubMed] [Google Scholar]
- 5.De Robertis EM, Larraín J, Oelgeschläger M, Wessely O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martello G, et al. MicroRNA control of Nodal signalling. Nature. 2007;449:183–188. doi: 10.1038/nature06100. [DOI] [PubMed] [Google Scholar]
- 7.Sudou N, Yamamoto S, Ogino H, Taira M. Dynamic in vivo binding of transcription factors to cis-regulatory modules of cer and gsc in the stepwise formation of the Spemann-Mangold organizer. Development. 2012;139:1651–1661. doi: 10.1242/dev.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- 9.Inomata H, Haraguchi T, Sasai Y. Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell. 2008;134:854–865. doi: 10.1016/j.cell.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 14.Glinka A, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 15.Glinka A, Wu W, Onichtchouk D, Blumenstock C, Niehrs C. Head induction by simultaneous repression of Bmp and Wnt signalling in Xenopus. Nature. 1997;389:517–519. doi: 10.1038/39092. [DOI] [PubMed] [Google Scholar]
- 16.Piccolo S, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosch R, Niehrs C. Requirement for anti-dorsalizing morphogenetic protein in organizer patterning. Mech Dev. 2000;90:195–203. doi: 10.1016/s0925-4773(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 18.Moos M, Jr, Wang S, Krinks M. Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development. 1995;121:4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- 19.Vodicka MA, Gerhart JC. Blastomere derivation and domains of gene expression in the Spemann Organizer of Xenopus laevis. Development. 1995;121:3505–3518. doi: 10.1242/dev.121.11.3505. [DOI] [PubMed] [Google Scholar]
- 20.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 21.Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–20958. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi M, et al. Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation. J Endocrinol. 2008;199:445–455. doi: 10.1677/JOE-08-0226. [DOI] [PubMed] [Google Scholar]
- 23.Brennan J, et al. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- 24.Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 25.Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev Biol. 1999;209:282–297. doi: 10.1006/dbio.1999.9257. [DOI] [PubMed] [Google Scholar]
- 26.Song J, et al. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev Biol. 1999;213:157–169. doi: 10.1006/dbio.1999.9370. [DOI] [PubMed] [Google Scholar]
- 27.Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- 28.Schneider VA, Mercola M. Spatially distinct head and heart inducers within the Xenopus organizer region. Curr Biol. 1999;9:800–809. doi: 10.1016/s0960-9822(99)80363-7. [DOI] [PubMed] [Google Scholar]
- 29.Tam PP, Steiner KA. Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development. 1999;126:5171–5179. doi: 10.1242/dev.126.22.5171. [DOI] [PubMed] [Google Scholar]
- 30.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 31.Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176–184. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Gerhart J. Evolution of the organizer and the chordate body plan. Int J Dev Biol. 2001;45:133–153. [PubMed] [Google Scholar]
- 33.Dupont S, et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell. 2009;136:123–135. doi: 10.1016/j.cell.2008.10.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.