Abstract
Worldwide, increasing numbers of insects have evolved resistance to a wide range of pesticides, which hampers their control in the field and, therefore, threatens agriculture. Members of the carboxylesterase and cytochrome P450 monooxygenase superfamilies are prominent candidates to confer metabolic resistance to pyrethroid insecticides. Both carboxylesterases and P450 enzymes have been shown to be involved in pyrethroid resistance in Australian Helicoverpa armigera, the noctuid species possessing by far the most reported resistance cases worldwide. However, specific enzymes responsible for pyrethroid resistance in field populations of this species have not yet been identified. Here, we show that the resistance toward fenvalerate in an Australian strain of H. armigera is due to a unique P450 enzyme, CYP337B3, which arose from unequal crossing-over between two parental P450 genes, resulting in a chimeric enzyme. CYP337B3 is capable of metabolizing fenvalerate into 4′-hydroxyfenvalerate, which exhibits no toxic effect on susceptible larvae; enzymes from the parental P450 genes showed no detectable fenvalerate metabolism. Furthermore, a polymorphic H. armigera strain could be bred into a susceptible line possessing the parental genes CYP337B1 and CYP337B2 and a resistant line possessing only CYP337B3. The exclusive presence of CYP337B3 in resistant insects of this strain confers a 42-fold resistance to fenvalerate. Thus, in addition to previously documented genetic mechanisms of resistance, recombination can also generate selectively advantageous variants, such as this chimeric P450 enzyme with an altered substrate specificity leading to a potent resistance mechanism.
Keywords: pest management, cotton bollworm
One of the main threats of agriculture nowadays is the rapid development of resistance of pest insect species to control agents worldwide. The cotton bollworm, Helicoverpa armigera (Hübner), is the noctuid species possessing by far the most reported cases of insecticide resistance worldwide with evolved resistance against pyrethroids, organophosphates, carbamates, organochlorines (www.pesticideresistance.org), and recently against the macrocyclic lactone spinosad (1) and Bacillus thuringiensis-derived toxins (2). This capacity is partly due to its distribution, which is one of the widest for any agricultural insect pest species, covering Africa, the Middle East, southern Europe, India, central and southeastern Asia, eastern and northern Australia, New Zealand, and many eastern Pacific Islands (3). In addition, H. armigera is a significant pest of cotton, the single crop most intensively sprayed with insecticides. Almost 30% of all pesticides used worldwide are directed against this insect pest (4). In Australia, the economic losses due to direct yield reduction and pest management of H. armigera and endemic H. punctigera were estimated to be approximately A$150 million in 1990–1991 (5). In addition, H. armigera is highly polyphagous, feeding on 72 known host plant species distributed in 29 families in Australia (6).
After introduction of pyrethroid insecticides to Australia in 1977 (7), the cotton bollworm first evolved resistance to fenvalerate, a type II pyrethroid ester, in 1983 (8). Since then, different resistance mechanisms to this insecticide have been reported, including reduced penetration through the cuticle (9, 10) and increased target site insensitivity (knockdown resistance; refs. 9, 11, and 12). Furthermore, metabolic-based resistance of H. armigera due to cytochrome P450 monooxygenases (P450s) and carboxylesterases has also been described (9, 13–24), although the specific enzymes involved in the resistance observed in the field have not yet been identified.
Larvae and adults of the H. armigera strain AN02 from eastern Australia exhibit 50-fold resistance to fenvalerate, which is suppressible by piperonyl butoxide, indicating that a P450 enzyme may be involved in the resistance mechanism (13, 25, 26). In 1998, Heckel et al. (15) mapped the gene responsible for fenvalerate resistance to a single locus, subsequently named RFen1. Recently, Wee et al. (24) identified by cDNA-AFLP four candidate genes, the P450s CYP4S1 and CYP337B1, a carboxylesterase-like gene, and a GST, all constitutively up-regulated in resistant individuals of the AN02 strain. Of these four candidates, only the P450 gene CYP337B1 has been found to be tightly linked to the resistance locus RFen1, suggesting that this P450 may be responsible for fenvalerate detoxification (15, 24).
Here, we focus on the Australian H. armigera strain TWB, which also possesses the candidate gene CYP337B1, and on its capability to evolve resistance to fenvalerate. We describe three closely related P450 genes of the CYP337B subfamily and discuss their evolutionary relationships. To study the metabolic capability of these enzymes toward fenvalerate, we cloned and expressed all genes and their corresponding alleles in insect cells. We demonstrate that only one of the seven candidate allozymes is capable of metabolizing fenvalerate. In addition, we bred the TWB strain into a susceptible line possessing only CYP337B1 and CYP337B2 and a resistant line possessing only the resistance gene CYP337B3. In larval toxicity bioassays with fenvalerate, we showed that the resistance level conferred by CYP337B3 is comparable to previous resistance levels in Australia. Finally, in an attempt to explain the exclusive function of CYP337B3, all P450s were modeled and the substrate recognition sites were determined.
Results
Identification and Characterization of the CYP337B Subfamily in H. armigera.
CYP337B1 (GenBank accession no. EF591061) of H. armigera has been described by Wee et al. (24). Using this gene as a probe, we identified and sequenced two clones from a BAC library of the TWB strain of H. armigera (SI Results, BAC Clone Analysis). Clone 18J13 (GenBank accession no. JQ995291) contained CYP337B1 and a similar gene CYP337B2 in a tandem array; clone 33H17 (GenBank accession no. JQ995292) contained a third gene CYP337B3 (gene names assigned by the P450 Nomenclature Committee). The pattern of clone overlap showed extensive similarity interrupted by insertions and deletions (Fig. S1A), including a transposable element Hz-SINE1 previously found within a P450 gene in H. zea (27). Dot-matrix comparisons indicate that a region within clone 18J13 corresponding to the second half of the first exon of CYP337B2 up to the corresponding position in CYP337B1 has been deleted in clone 33H17, resulting in a chimeric gene CYP337B3 (Fig. S1B). This fact could be explained by an unequal crossing-over event (Fig. S2). Comparisons of BAC clones from the same library with genome sequence from two other lepidopteran species has also revealed extensive rearrangements within other P450 clusters (28).
We detected transcripts for all three genes in a cDNA library from the TWB strain. Sequence comparison (Fig. 1A) suggests that CYP337B1 and CYP337B2 evolved by gene duplication and diverged by approximately 25% at the amino acid level since their origin. CYP337B3 appears to be a chimeric gene between CYP337B2 and CYP337B1 produced by crossing-over between nucleotides 531 and 532 in the coding sequence (Fig. 1A and Fig. S2). Therefore, CYP337B3 is almost identical to CYP337B2 in its first 177 amino acids and to CYP337B1 in its last 315 amino acids. All three P450 genes possess only one intron located close to the end of the conserved heme-binding site starting with the nucleotide 1319 in the coding sequence. Direct sequencing of each of the three genes amplified from a cDNA pool showed double peaks revealing the presence of different alleles for CYP337B1 and CYP337B2, whereas no double peaks were seen for CYP337B3 indicating the presence of only one allele in our population (GenBank accession no. JQ284029). After cloning, 113 clones of CYP337B2 were sequenced to find all three alleles assumed by the pattern of differing nucleotides (CYP337B2v1, CYP337B2v2, and CYP337B2v3; GenBank accession nos. JQ284026, JQ284027, and JQ284028, respectively). Sequencing 23 clones of CYP337B1 yielded three alleles (CYP337B1v1, CYP337B1v3, and CYP337B1v4; GenBank accession nos. JQ284023, JQ284024, and JQ284025, respectively). Additional SNPs were unique to a single clone and not characterized further except for a CYP337B3 clone with a SNP changing aa 462 from valine to alanine, which was heterologously expressed and tested. Alleles of CYP337B1 differ by 10–29 synonymous and 5–8 nonsynonymous nucleotide substitutions and CYP337B2 alleles by 2–20 synonymous and 2–7 nonsynonymous nucleotide substitutions. Nonsynonymous substitutions of CYP337B3 compared with the regions of the corresponding parent gene fall within the variation of the parent allozymes except for aa 4 with a valine in CYP337B3 and an isoleucine in CYP337B2 allozymes (Fig. 1A).
Fig. 1.
Gene duplication and functional diversification in the CYP337B subfamily. (A) Comparative scheme of the protein sequences of CYP337B1, CYP337B3, and CYP337B2. Chimeric origin of CYP337B3 is indicated by a gray box for the N-terminal part (aa 1–177) corresponding to CYP337B2 and a white box for the C-terminal part (aa 178–492) corresponding to CYP337B1. Altered amino acids between CYP337B3 and the corresponding parent allozymes are indicated. Five conserved regions of P450s are marked as black boxes: W-x-x-x-R motif in the C-helix, I-helix groove, E-x-x-R motif in the K-helix, “meander” located after the K′-helix, and heme-binding loop preceding the L-helix (from left to right; ref. 29). (B) Comparison of the substrate recognition site 1 (SRS1) between the CYP337B1 allozymes, CYP337B3, and CYP337B2 allozymes. Altered amino acids between CYP337B1 allozymes and CYP337B3 are marked in bold.
Of the five conserved regions of P450s (29), the first is identical for all seven allozymes (Fig. 1A); the remaining four conserved regions, and five of six substrate recognition sites (SRSs; Fig. S3A) of CYP337B3 are consistent with the corresponding regions of CYP337B1 (especially CYP337B1v1). However, SRS1 of CYP337B3, most likely ranging from aa 96 to aa 120 (Fig. 1B), exactly matches that of CYP337B2 allozymes and differs by 9–10 amino acids (10–11 nonsynonymous nucleotide substitutions) from CYP337B1 allozymes (Fig. 1B). The model of CYP337B3 (Fig. S3B) reveals that the altered amino acids in SRS1 lie within a distance of 12.9 Å to 24.0 Å to the heme-bound oxygen.
Metabolic Capability of CYP337B1, CYP337B2, and CYP337B3 Allozymes.
Before P450 expression in a H. armigera-derived cell line, Ha2302, the cells were tested for the presence of CYP337B1, CYP337B2, and CYP337B3. Only CYP337B1 could be detected by PCR using genomic DNA as a template (Fig. S4A). Messenger RNA of CYP337B1 could also be detected by RT-PCR (Fig. S4A), but Ha2302 cells showed only minor and negligible activities toward two model substrates (0.006 pmol product per mg of microsomal protein per min for 7-methoxy- and 7-benzyloxyresorufin). Subsequently, the seven P450 enzymes were successfully expressed in similar amounts in Ha2302 cells (Fig. S4B). To test the metabolic activities of the P450s, we performed activity assays with known P450 model substrates, which reveals activities for all P450s toward at least one substrate (Table S1) and demonstrates that all seven enzymes possessed catalytic activity. For all P450s except CYP337B3, the activity is reduced when the V5 epitope and the His-tag are present. As expected by sequence similarity, the metabolic activity of CYP337B3 toward the model substrates is more similar to CYP337B1 allozymes than to CYP337B2 allozymes. We then tested the abilities of all enzymes to metabolize fenvalerate. No metabolism of fenvalerate was detected for six of the P450s tested (Fig. 2); however, CYP337B3 (three constructs, with or without epitope tag) metabolized fenvalerate (Rt of 43.8 min) at an average of 25% in 4 h into one determined metabolite (Rt of 39.8 min; Fig. 2D and Fig. S5Aa) possessing a UV spectrum very similar to fenvalerate. Recovery was 89% on average. No turnover of fenvalerate by microsomal enzymes of nontransfected Ha2302 cells was detected (Fig. S5Ad). The metabolism of fenvalerate by CYP337B3 was inhibited by adding the known P450 inhibitor piperonyl butoxide and by omitting NADPH necessary for the function of most P450 enzymes (Fig. S5 Ab and Ac, respectively).
Fig. 2.
HPLC chromatograms of in vitro metabolism studies with 4 nmol fenvalerate per 0.8 mg of microsomal proteins and 4 h of incubation at 30 °C. All studies were performed by using the NADPH regeneration system. Relevant parts of the chromatograms derived from detection by 230 nm are displayed. Baselines were corrected by subtraction of a blank run. 1, fenvalerate; 2, 4′-hydroxyfenvalerate. Studies performed with microsomes containing CYP337B1v1 (A), CYP337B1v4 (B), CYP337B1v3 (C), CYP337B3 (D), CYP337B2v1 (E), CYP337B2v3 (F), and CYP337B2v2 (G).
Isolation and Identification of the Fenvalerate Metabolite.
The metabolite was isolated from scaled-up in vitro assays performed with esfenvalerate as the parent compound. Esfenvalerate, the (2S,αS) isomer of racemic fenvalerate, was chosen to simplify the following metabolite identification. CYP337B3 metabolized 25% of esfenvalerate in 24 h with a recovery of 85%. In total, approximately 7.3 μg of metabolite (in esfenvalerate equivalents), with a purity of 93.4% determined at 210 nm by using the first HPLC system, could be isolated. The metabolite was further analyzed by GC-MS (Fig. S5B) and 1H-NMR (Table S2) to be identified as 4′-hydroxyfenvalerate (SI Results, Identification of the Fenvalerate Metabolite).
Presence of CYP337B3 Confers Resistance to Fenvalerate in TWB Larvae.
Gene-specific PCR primers flanking the crossover point at nucleotide 531 were used to detect the three genes in genomic DNA of individuals used in single-pair matings. Within progeny of parents with all three genes, genetic segregation between CYP337B3 and the combination of CYP337B1 and CYP337B2 was observed, as expected if BAC clones 18J13 and 33H17 represent alternative haplotypes for the same homologous chromosome. This fact enabled us to breed the TWB strain into one line possessing only CYP337B1 and CYP337B2 and another line possessing only CYP337B3, by screening several generations of single-pair crosses by PCR. The resulting lines were homozygous for alternative haplotypes at the CYP337B locus; i.e., one haplotype containing one copy each of CYP337B1 and CYP337B2, and the other haplotype containing one copy of CYP337B3. The resistance levels toward fenvalerate of third-instar larvae of both lines and heterozygous larvae derived from crosses between both lines were determined by toxicity bioassays (Fig. 3 and Table S3). Larvae of the homozygous CYP337B3 line, with a LD50 of 1.864 μg per larva, were 42.4-fold more resistant than larvae of the homozygous CYP337B1-CYP337B2 line, with a LD50 of 0.044 μg per larva. Heterozygous larvae exhibited an intermediate LD50 of 0.546 μg per larva and a resistance factor (RF) of 12.4. Toxicity bioassays with the metabolite 4′-hydroxyfenvalerate [55% of (2S,αR)/(2R,αS) enantiomers and 45% of (2S,αS)/(2R,αR) enantiomers determined by GC-MS] performed with CYP337B1-CYP337B2 third-instar larvae resulted in a mortality of one among the 17 larvae (5.88%) treated with the lower dose, and no mortality among the three larvae treated with the higher dose.
Fig. 3.
Dose–response curves of selected lines of Australian H. armigera toward fenvalerate. For each of the six doses and a control per line, 40 third-instar larvae were tested by topical application. Received mortalities (without control) and regression lines from probit transformed mortalities were plotted at probit scale against logarithmic doses of fenvalerate. Mortalities of 0% were changed to 0.01% and of 100% were changed to 99.99% to be able to plot them. Filled squares, homozygous CYP337B1-CYP337B2 line; open circles, heterozygous line; filled circles, homozygous CYP337B3 line.
Discussion
Pesticide resistance of insect pests has a great impact on their control and threatens agriculture. Diverse molecular mechanisms underlie metabolic (30) and target site (31) insecticide resistance. Different resistance mechanisms to pyrethroids have been reported in Australian H. armigera, including target site insensitivity (7, 9, 12), metabolism by carboxylesterases (19–23), and P450s (13–18, 24).
Up to now, 13 heterologously expressed insect P450s have been shown to be capable of metabolizing one or more pyrethroids (29, 30, 32–34). These P450s belong to three families, CYP6, CYP9, and CYP321 (32, 33, 35–42); our results add CYP337 to the list. Yang et al. (43) demonstrated that CYP9A12 and CYP9A14 from the Chinese laboratory-selected pyrethroid-resistant YGF strain of H. armigera are capable of metabolizing esfenvalerate after heterologous expression in yeast. Comparable to the majority of the investigations cited above, Yang et al. (43) concluded that metabolism occurred by observing the disappearance of the substrate, without attempting to identify putative metabolites.
Further P450s have been described to be potentially involved in fenvalerate metabolism in H. armigera. Ranasinghe et al. (16) observed an increased expression level of CYP6B7 mRNA in fenvalerate-resistant Australian H. armigera. However, in 2007, Grubor et al. (25) mapped CYP6B7 to AFLP linkage group 14, thus rejecting it as a candidate for the semidominant resistance locus RFen1 mapped to AFLP linkage group 13 (15). Therefore, it seems unlikely that CYP6B7 is responsible for resistance, at least in the AN02 strain of Australian H. armigera. Also, Zhang et al. (44) reported on an increased expression level and several amino acid changes of CYP6B7 in the fenvalerate-resistant Chinese HDFR strain of H. armigera. It is also known that strains of the same species from different geographical origins could evolve differing resistance mechanisms (45).
Here, we report on a newly identified P450 enzyme that confers resistance to fenvalerate in the Australian H. armigera strain TWB. CYP337B3 and its parent genes were cloned and characterized from the TWB strain. CYP337B3 is a chimeric P450 between CYP337B2 and CYP337B1 produced by an unequal crossing-over event. With its C-terminal part including four of five conserved regions and five of six substrate recognition sites identical to CYP337B1, CYP337B3 would presumably exhibit substrate specificity comparable to CYP337B1, whereas CYP337B2 should clearly differ in its substrate specificity compared with both CYP337B1 and CYP337B3. We demonstrated that only the chimeric P450 CYP337B3 is capable of metabolizing fenvalerate to 4′-hydroxyfenvalerate. Hydroxylation in the 2′- or 4′-position of the phenoxybenzyl alcohol moiety of fenvalerate is known from plants and mammals (46). A 4′-hydroxy metabolite is also formed from trans-cypermethrin by H. armigera (47); from deltamethrin by CYP6BQ9 in Tribolium castaneum (41) and CYP6M2 in Anopheles gambiae (42); and from τ-fluvalinate by CYP9Q1, CYP9Q2, and CYP9Q3 in Apis mellifera (33). All four pyrethroids possess the same phenoxybenzyl alcohol moiety. Furthermore, we detected a potential minor metabolite, with an even higher hydrophilicity than 4′-hydroxyfenvalerate. Because of the low amount, we were not able to isolate and identify this metabolite.
To test the capability of CYP337B3 to confer resistance toward fenvalerate in vivo, we bred the TWB strain into two lines. The susceptible line possesses only CYP337B1 and CYP337B2, whereas the homozygous resistant line possesses only CYP337B3. Homozygous resistant larvae exhibited an RF of 42 compared with the homozygous susceptible line. Heterozygous resistant larvae originating from crosses between both lines exhibited a RF of 12, indicating a semidominant resistance. Forrester et al. (48) tested the toxicity of various pyrethroids on fourth-instar larvae from a susceptible and a pyrethroid-resistant Australian H. armigera strain homozygous for a metabolic detoxification mechanism that was fully suppressible by piperonyl butoxide, indicating a P450-based resistance mechanism with an estimated RF of 49. This value is very similar to ours, indicating that the resistance of the TWB strain and the Forrester strain most likely could be explained exclusively by the activity of CYP337B3. Furthermore, we could demonstrate in a bioassay with susceptible larvae that 4′-hydroxyfenvalerate is either not intrinsically toxic, or that it could be easily further metabolized to a nontoxic compound even in susceptible larvae. Therefore, the hydroxylation of fenvalerate in 4′-position is the detoxification mechanism in vivo. The hydroxylated metabolite may be further glycosylated in the insects increasing its hydrophilicity even more compared with its highly lipophilic (log KOW of 6.2) (46, 49) and almost water insoluble (2–85 μg/L at 20 °C) (49) parent compound, greatly facilitating its excretion.
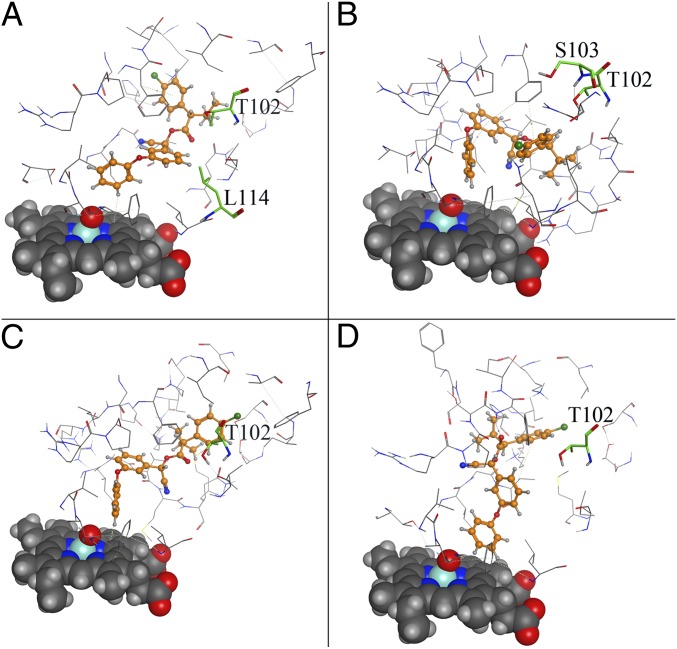
To explain the differences in substrate specificity between CYP337B3 and its parent enzymes, especially the more similar CYP337B1, we modeled all allozymes and determined the SRSs of CYP337B3 by comparison with known SRSs of CYP6Z1 of Anopheles gambiae (50). We found that CYP337B3 differs from CYP337B1 in SRS1 by 9–10 amino acids. All four fenvalerate isomers could be separately docked inside the active center of CYP337B3 with the 4′-carbon of the phenoxybenzyl alcohol moiety in a distance of 3.1–3.4 Å to the heme-bound oxygen, and the aa threonine 102 (SRS1) lies 3.9–5.9 Å from the ligand (Fig. 4).
Fig. 4.
Docking models of CYP337B3 with all four fenvalerate isomers. The heme group is shown in space filling mode, and fenvalerate isomers are shown in ball and stick mode with the carbon atoms in orange. Amino acids of the SRS1 region that are different between CYP337B3 and CYP337B1 allozymes and that are in a distance of 4.5 Å from the fenvalerate isomer are labeled and shown in stick mode with their carbon atoms in green. (A) Esfenvalerate: distance between C4′ and O coordinated to Fe: 3.403 Å, interaction energy: −57.107 kcal/mol. (B) (2R,αR)-fenvalerate: distance between C4′ and O coordinated to Fe: 3.367 Å, interaction energy: −46.519 kcal/mol. (C) (2R,αS)-fenvalerate: distance between C4′ and O coordinated to Fe: 3.212 Å, interaction energy: −52.776 kcal/mol. (D) (2S,αR)-fenvalerate: distance between C4′ and O coordinated to Fe: 3.078 Å, interaction energy: −46.560 kcal/mol.
These findings suggest that possibly T102, or others of the altered amino acids in SRS1 found in CYP337B3, may be essential for fenvalerate recognition and binding. Therefore, the fusion of the N-terminal part of CYP337B2 including SRS1 with the C-terminal part of CYP337B1 resulted in the creation of an enzyme with altered substrate specificity. Point mutations in insecticide targets are a common cause of resistant variants, but convincing evidence of insecticide resistance-conferring P450 sequences arising by point mutations is lacking (see, however, ref. 51 for an example of point mutations in CYP51, the target of sterol 14α-demethylase inhibiting fungicides). So far, all other described P450s associated with insecticide resistance are expressed in susceptible insects and overexpressed in resistant insects (16, 17, 29, 30, 39, 41, 42, 44, 45, 52–57). Overexpression can be caused by trans- (52) or cis-acting factors, the latter often associated with transposable element insertion in the promoter region (53, 58, 59). In a few cases, the expression is enhanced by an additional duplication of the P450 gene (58–62).
An analogous situation where recombination within a P450 cluster affects detoxicative capabilities occurs with human CYP2D6, which is responsible for metabolizing approximately 20% of all drugs used in medicine (SI Discussion). Some nonfunctional CYP2D6 alleles are produced by unequal crossing-over with neighboring CYP2D pseudogenes which harbor inactivating mutations. CYP337B3, however, is a functional product of unequal crossing-over of two other functional genes. We remain ignorant of any possible fitness cost due to the loss of any “normal” functions of CYP337B1 and CYP337B2; but the ability to retain all three enzymatic activities in heterozygotes for the two haplotypes could enable the maintenance of a balanced polymorphism in a population exposed to pyrethroid selection pressure. The case of CYP337B3 shows that recombination is an additional genetic mechanism capable of generating resistant variants; a mechanism that is enhanced by the occurrence of genes in tandem arrays such as P450s.
Materials and Methods
See SI Materials and Methods for details.
Insects.
The TWB strain of H. armigera (Hübner) (Lepidoptera: Noctuidae) was collected from the vicinity of Toowoomba, Queensland, Australia, in January 2003 and maintained in the laboratory in Jena since August 2004. Larvae were reared on Bio-Serv diet (General Purpose Lepidoptera) at 26 °C and 55% humidity with a 16:8 (light:dark) photoperiod. The strain has been exclusively propagated by single-pair crosses to reduce the effects of inbreeding.
Ha2302 Cell Culture.
Ha2302 cells (63) were a kind donation of Barbara Möckel (Technical University Darmstadt, Darmstadt, Germany) via Marcel Westenberg (Laboratory of Virology, Wageningen University, Wageningen, The Netherlands) in 2006. The cell line was developed in 1995 from hemolymph of second- and third-instar larvae from Spanish H. armigera supplied by the University of Cordoba (Córdoba, Spain) through continuous subculturing. Cells were adapted to Sf-900 II serum-free medium (Invitrogen) in the laboratory in Jena in November 2009. Cells were cultivated as adherent cells at 27 °C.
Heterologous Expression in Ha2302 Cells and Isolation of Microsomes.
CYP337B1, CYP337B2, and CYP337B3 were amplified from cDNA of TWB by PCR using gene-specific primers, including a 5′-Kozak sequence in the forward primer. Two reverse primers were designed for each gene with one of them lacking the stop codon for V5 epitope and His-tag fusion expression after ligation into the vector pIB/V5-His TOPO TA (Invitrogen). The other primer included the stop codon resulting in untagged proteins (for primers, see Table S4). Correct orientation of the insert was checked by colony PCR after transformation of TOP10 Escherichia coli cells (Invitrogen), and the presence of different alleles was determined after sequencing. Ha2302 cells were transfected with 400 ng of plasmid by using Insect GeneJuice Transfection Reagent (Novagen) following the manufacturer’s protocol. After 48 h, stable transfected cell lines were established by selecting with 60 μg⋅ml−1 blasticidin. Microsomes were prepared by lysing cells in a hypotonic buffer following by differential centrifugation of the cell homogenate. Protein content was determined by using the Quick Start Bradford Protein Assay (Bio-Rad).
In Vitro Metabolism Assays with Fenvalerate.
Microsomal proteins (0.8 mg) were incubated with 4 nmol fenvalerate (Sigma-Aldrich; dissolved in 4 μL of DMSO) by using a NADPH regeneration system in a total volume of 800 μL of 0.1 M potassium phosphate buffer at pH 7.4, with 0.05 g/L of sodium cholate at 30 °C for 4 h. Piperonyl butoxide (Fluka) was used in an inhibition assay with 40 nmol per assay. Inhibition study with the lack of NADPH was performed by omitting the NADPH regeneration system in the reaction. Samples were extracted with ethyl acetate. After concentration of combined ethyl acetate fractions, residues were redissolved in 25 μL of methanol and 375 μL of acetonitrile and filtrated for HPLC. HPLC analysis was performed on a Hewlett-Packard (Agilent) HPLC 1100 Series equipped with a C18 column (Nucleodur Sphinx RP; Macherey-Nagel) by using a gradient program. Fenvalerate and its metabolites were detected at 210, 230, and 275 nm. The main metabolite was isolated in multiple runs by preparative HPLC from a scaled-up assay with esfenvalerate (Sigma-Aldrich) using two HPLC systems and analyzed by gas chromatography with electron impact mass spectrometry and 1H-NMR.
Toxicity Bioassay with Fenvalerate.
The TWB strain was screened by PCR (for primers, see Table S4) and bred over six generations into one line possessing CYP337B1 and CYP337B2 and another line possessing only CYP337B3. Both lines were considered to be homozygous. Different doses of fenvalerate (0.0000; 0.0100; 0.0316; 0.1000; 0.3162; 1.0000; 3.1623 μg; Sigma-Aldrich; dissolved in 1 μL of acetone) were applied topically on the dorsal part of third-instar larvae (40 individuals per dose) of both lines and on heterozygous larvae derived from crossings between both lines (in both directions). Larvae were kept on Bio-Serv diet under normal rearing conditions. After 48 h, larvae were scored as alive (healthy, able to turn from dorsal to ventral position) or dead (unable to turn from dorsal to ventral position, dead). Observed mortalities were transformed by probit analysis using SPSS Statistics version 17.0 to determine regression lines including slope and LD50 values.
Supplementary Material
Acknowledgments
We thank Dr. Heiko Vogel for providing cDNA of H. armigera; Domenica Schnabelrauch for sequencing; Dr. Burkhard Schmidt (Rheinisch-Westfälische Technische Hochschule Aachen) and Dr. Michael Reichelt for support in analytical challenges; Stephanie Fuhr, Kristina Voigt, Markus Garlipp, Ulrike Wokoun, and Anneke Purz for technical assistance in breeding of TWB lines; and Dr. Yannick Pauchet for support and constructive comments on the original manuscript. Financial support was provided by Max-Planck-Gesellschaft.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JQ284023–JQ284029, JQ995291, and JQ995292).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202047109/-/DCSupplemental.
References
- 1.Aheer GM, Aziz MA, Hameed A, Ali A. Evaluation of resistance to different insecticides in field strains of Helicoverpa armigera (Lepidoptera: Noctuidae) in Punjab, Pakistan. Entomol Res. 2009;39:159–167. [Google Scholar]
- 2.Zhang H, et al. Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS ONE. 2011;6:e22874. doi: 10.1371/journal.pone.0022874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitt GP. The ecology of Heliothis species in relation to agroecosystems. Annu Rev Entomol. 1989;34:17–52. [Google Scholar]
- 4.Ahmad M. Insecticide resistance mechanisms and their management in Helicoverpa armigera (Hübner) – A review. J Agric Res. 2007;45:319–335. [Google Scholar]
- 5.Fitt GP. Cotton pest management: Part 3. An Australian perspective. Annu Rev Entomol. 1994;39:543–562. [Google Scholar]
- 6.Zalucki MP, Daglish G, Firempong S, Twine P. The biology and ecology of Heliothis armigera (Hübner) and H. punctigera Wallengren (Lepidoptera: Noctuidae) in Australia – What do we know? Aust J Zool. 1986;34:779–814. [Google Scholar]
- 7.McCaffery AR. Resistance to insecticides in heliothine Lepidoptera: A global view. Philos Trans R Soc Lond B Biol Sci. 1998;353:1735–1750. [Google Scholar]
- 8.Gunning RV, Easton CS, Greenup LR, Edge VE. Pyrethroid resistance in Heliothis armiger (Hübner) (Lepidoptera: Noctuidae) in Australia. J Econ Entomol. 1984;77:1283–1287. [Google Scholar]
- 9.Gunning RV, Easton CS, Balfe ME, Ferris IG. Pyrethroid resistance mechanisms in Australian Helicoverpa armigera. Pestic Sci. 1991;33:473–490. [Google Scholar]
- 10.Gunning RV, Devonshire AL, Moores GD. Metabolism of esfenvalerate by pyrethroid-susceptible and -resistant Australian Helicoverpa armigera (Lepidoptera: Noctuidae) Pestic Biochem Physiol. 1995;51:205–213. [Google Scholar]
- 11.Daly JC. Ecology and genetics of insecticide resistance in Helicoverpa armigera: Interactions between selection and gene flow. Genetica. 1993;90:217–226. [Google Scholar]
- 12.Gunning RV. Bioassay for detecting pyrethroid nerve insensitivity in Australian Helicoverpa armigera (Lepidoptera: Noctuidae) J Econ Entomol. 1996;89:816–819. [Google Scholar]
- 13.Daly JC, Fisk JH. Inheritance of metabolic resistance to the synthetic pyrethroids in Australian Helicoverpa armigera (Lepidoptera: Noctuidae) Bull Entomol Res. 1992;82:5–12. [Google Scholar]
- 14.Pittendrigh B, et al. Cytochrome P450 genes from Helicoverpa armigera: Expression in a pyrethroid-susceptible and -resistant strain. Insect Biochem Mol Biol. 1997;27:507–512. doi: 10.1016/s0965-1748(97)00025-8. [DOI] [PubMed] [Google Scholar]
- 15.Heckel DG, Gahan LJ, Daly JC, Trowell S. A genomic approach to understanding Heliothis and Helicoverpa resistance to chemical and biological insecticides. Philos Trans R Soc Lond B Biol Sci. 1998;353:1713–1722. [Google Scholar]
- 16.Ranasinghe C, Campbell B, Hobbs AA. Over-expression of cytochrome P450 CYP6B7 mRNA and pyrethroid resistance in Australian populations of Helicoverpa armigera (Hübner) Pestic Sci. 1998;54:195–202. [Google Scholar]
- 17.Ranasinghe C, Hobbs AA. Isolation and characterization of two cytochrome P450 cDNA clones for CYP6B6 and CYP6B7 from Helicoverpa armigera (Hubner): Possible involvement of CYP6B7 in pyrethroid resistance. Insect Biochem Mol Biol. 1998;28:571–580. doi: 10.1016/s0965-1748(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 18.Ranasinghe C, Hobbs AA. Induction of cytochrome P450 CYP6B7 and cytochrome b5 mRNAs from Helicoverpa armigera (Hubner) by pyrethroid insecticides in organ culture. Insect Mol Biol. 1999;8:443–447. doi: 10.1046/j.1365-2583.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 19.Gunning RV, Moores GD, Devonshire AL. Esterases and esfenvalerate resistance in Australian Helicoverpa armigera (Hübner) Lepidoptera:Noctuidae. Pestic Biochem Physiol. 1996;54:12–23. doi: 10.1006/pest.1996.0031. [DOI] [PubMed] [Google Scholar]
- 20.Gunning RV, Moores GD, Devonshire AL. Esterase inhibitors synergise the toxicity of pyrethroids in Australian Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) Pestic Biochem Physiol. 1999;63:50–62. doi: 10.1006/pest.1996.0031. [DOI] [PubMed] [Google Scholar]
- 21.Young SJ, Gunning RV, Moores GD. The effect of piperonyl butoxide on pyrethroid-resistance-associated esterases in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) Pest Manag Sci. 2005;61:397–401. doi: 10.1002/ps.996. [DOI] [PubMed] [Google Scholar]
- 22.Young SJ, Gunning RV, Moores GD. Effect of pretreatment with piperonyl butoxide on pyrethroid efficacy against insecticide-resistant Helicoverpa armigera (Lepidoptera: Noctuidae) and Bemisia tabaci (Sternorrhyncha: Aleyrodidae) Pest Manag Sci. 2006;62:114–119. doi: 10.1002/ps.1127. [DOI] [PubMed] [Google Scholar]
- 23.Gunning RV, et al. Use of pyrethroid analogues to identify key structural features for enhanced esterase resistance in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) Pest Manag Sci. 2007;63:569–575. doi: 10.1002/ps.1377. [DOI] [PubMed] [Google Scholar]
- 24.Wee CW, Lee SF, Robin C, Heckel DG. Identification of candidate genes for fenvalerate resistance in Helicoverpa armigera using cDNA-AFLP. Insect Mol Biol. 2008;17:351–360. doi: 10.1111/j.1365-2583.2008.00809.x. [DOI] [PubMed] [Google Scholar]
- 25.Grubor VD, Heckel DG. Evaluation of the role of CYP6B cytochrome P450s in pyrethroid resistant Australian Helicoverpa armigera. Insect Mol Biol. 2007;16:15–23. doi: 10.1111/j.1365-2583.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 26.Daly JC, Fisk JH. Expression of pyrethroid resistance in adult Helicoverpa armigera (Lepidoptera: Noctuidae) and selective mortality in field populations. Bull Entomol Res. 1993;83:23–28. [Google Scholar]
- 27.Chen S, Li X. Transposable elements are enriched within or in close proximity to xenobiotic-metabolizing cytochrome P450 genes. BMC Evol Biol. 2007;7:46. doi: 10.1186/1471-2148-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.d’Alençon E, et al. Extensive synteny conservation of holocentric chromosomes in Lepidoptera despite high rates of local genome rearrangements. Proc Natl Acad Sci USA. 2010;107:7680–7685. doi: 10.1073/pnas.0910413107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feyereisen R. 2012. Insect CYP genes and P450 enzymes. Insect Molecular Biology and Biochemistry, ed Gilbert LI (Elsevier, London), pp 236–316.
- 30.Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- 31.Heckel DG. Molecular genetics of insecticide resistance in Lepidoptera. In: Goldsmith MR, Marec F, editors. Molecular Biology and Genetics of the Lepidoptera. Boca Raton, FL: CRC; 2010. pp. 239–269. [Google Scholar]
- 32.Duangkaew P, et al. Characterization of mosquito CYP6P7 and CYP6AA3: Differences in substrate preference and kinetic properties. Arch Insect Biochem Physiol. 2011;76:236–248. doi: 10.1002/arch.20413. [DOI] [PubMed] [Google Scholar]
- 33.Mao W, Schuler MA, Berenbaum MR. CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera) Proc Natl Acad Sci USA. 2011;108:12657–12662. doi: 10.1073/pnas.1109535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feyereisen R. Arthropod CYPomes illustrate the tempo and mode in P450 evolution. Biochim Biophys Acta. 2011;1814:19–28. doi: 10.1016/j.bbapap.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Sasabe M, Wen Z, Berenbaum MR, Schuler MA. Molecular analysis of CYP321A1, a novel cytochrome P450 involved in metabolism of plant allelochemicals (furanocoumarins) and insecticides (cypermethrin) in Helicoverpa zea. Gene. 2004;338:163–175. doi: 10.1016/j.gene.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 36.Rupasinghe SG, Wen Z, Chiu T-L, Schuler MA. Helicoverpa zea CYP6B8 and CYP321A1: Different molecular solutions to the problem of metabolizing plant toxins and insecticides. Protein Eng Des Sel. 2007;20:615–624. doi: 10.1093/protein/gzm063. [DOI] [PubMed] [Google Scholar]
- 37.Kaewpa D, Boonsuepsakul S, Rongnoparut P. Functional expression of mosquito NADPH-cytochrome P450 reductase in Escherichia coli. J Econ Entomol. 2007;100:946–953. doi: 10.1603/0022-0493(2007)100[946:feomnp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Boonsuepsakul S, Luepromchai E, Rongnoparut P. Characterization of Anopheles minimus CYP6AA3 expressed in a recombinant baculovirus system. Arch Insect Biochem Physiol. 2008;69:13–21. doi: 10.1002/arch.20248. [DOI] [PubMed] [Google Scholar]
- 39.Müller P, et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008;4:e1000286. doi: 10.1371/journal.pgen.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen Z, Zeng RS, Niu G, Berenbaum MR, Schuler MA. Ecological significance of induction of broad-substrate cytochrome P450s by natural and synthetic inducers in Helicoverpa zea. J Chem Ecol. 2009;35:183–189. doi: 10.1007/s10886-009-9598-4. [DOI] [PubMed] [Google Scholar]
- 41.Zhu F, et al. A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc Natl Acad Sci USA. 2010;107:8557–8562. doi: 10.1073/pnas.1000059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson BJ, et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem Mol Biol. 2011;41:492–502. doi: 10.1016/j.ibmb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Yue L, Chen S, Wu Y. Functional expression of Helicoverpa armigera CYP9A12 and CYP9A14 in Saccharomyces cerevisiae. Pestic Biochem Physiol. 2008;92:101–105. [Google Scholar]
- 44.Zhang H, et al. Cloning and expression of cytochrome P450 CYP6B7 in fenvalerate-resistant and susceptible Helicoverpa armigera (Hübner) from China. J Appl Entomol. 2010;134:754–761. [Google Scholar]
- 45.Brun-Barale A, et al. Multiple P450 genes overexpressed in deltamethrin-resistant strains of Helicoverpa armigera. Pest Manag Sci. 2010;66:900–909. doi: 10.1002/ps.1960. [DOI] [PubMed] [Google Scholar]
- 46.Kaneko H. Fenvalerate. In: Roberts T, Hutson D, editors. Metabolic pathways of agrochemicals – Part two: Insecticides and fungicides. Cambridge: R Soc Chem; 1999. pp. 659–666. [Google Scholar]
- 47.Lee K-S, Walker CH, McCaffery A, Ahmad M, Little E. Metabolism of trans-cypermethrin by Heliothis armigera and H. virescens. Pestic Biochem Physiol. 1989;34:49–57. [Google Scholar]
- 48.Forrester NW, Cahill M, Bird LJ, Layland JK. Management of pyrethroid and endosulfan resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. Bull Entomol Res. 1993;(Suppl 1):1–132. [Google Scholar]
- 49.Eisler R. 2000. Handbook of Chemical Risk Assessment – Health Hazards to Human, Plants, and Animals, Volume 2, Organics (Lewis Publishers, Boca Raton, FL), pp 1089–1131.
- 50.Chiu T-L, Wen Z, Rupasinghe SG, Schuler MA. Comparative molecular modeling of Anopheles gambiae CYP6Z1, a mosquito P450 capable of metabolizing DDT. Proc Natl Acad Sci USA. 2008;105:8855–8860. doi: 10.1073/pnas.0709249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyand RA, Brown JKM. Sequence variation in the CYP51 gene of Blumeria graminis associated with resistance to sterol demethylase inhibiting fungicides. Fungal Genet Biol. 2005;42:726–735. doi: 10.1016/j.fgb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Cariño FA, Koener JF, Plapp FW, Jr, Feyereisen R. Constitutive overexpression of the cytochrome P450 gene CYP6A1 in a house fly strain with metabolic resistance to insecticides. Insect Biochem Mol Biol. 1994;24:411–418. doi: 10.1016/0965-1748(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 53.Daborn PJ, et al. A single p450 allele associated with insecticide resistance in Drosophila. Science. 2002;297:2253–2256. doi: 10.1126/science.1074170. [DOI] [PubMed] [Google Scholar]
- 54.Daborn P, Boundy S, Yen J, Pittendrigh B, ffrench-Constant R. DDT resistance in Drosophila correlates with Cyp6g1 over-expression and confers cross-resistance to the neonicotinoid imidacloprid. Mol Genet Genomics. 2001;266:556–563. doi: 10.1007/s004380100531. [DOI] [PubMed] [Google Scholar]
- 55.Brandt A, et al. Differential expression and induction of two Drosophila cytochrome P450 genes near the Rst(2)DDT locus. Insect Mol Biol. 2002;11:337–341. doi: 10.1046/j.1365-2583.2002.00344.x. [DOI] [PubMed] [Google Scholar]
- 56.Rongnoparut P, Boonsuepsakul S, Chareonviriyaphap T, Thanomsing N. Cloning of cytochrome P450, CYP6P5, and CYP6AA2 from Anopheles minimus resistant to deltamethrin. J Vector Ecol. 2003;28:150–158. [PubMed] [Google Scholar]
- 57.Yang Y, Chen S, Wu S, Yue L, Wu Y. Constitutive overexpression of multiple cytochrome P450 genes associated with pyrethroid resistance in Helicoverpa armigera. J Econ Entomol. 2006;99:1784–1789. doi: 10.1603/0022-0493-99.5.1784. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt JM, et al. Copy number variation and transposable elements feature in recent, ongoing adaptation at the Cyp6g1 locus. PLoS Genet. 2010;6:e1000998. doi: 10.1371/journal.pgen.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Itokawa K, Komagata O, Kasai S, Masada M, Tomita T. Cis-acting mutation and duplication: History of molecular evolution in a P450 haplotype responsible for insecticide resistance in Culex quinquefasciatus. Insect Biochem Mol Biol. 2011;41:503–512. doi: 10.1016/j.ibmb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Emerson JJ, Cardoso-Moreira M, Borevitz JO, Long M. Natural selection shapes genome-wide patterns of copy-number polymorphism in Drosophila melanogaster. Science. 2008;320:1629–1631. doi: 10.1126/science.1158078. [DOI] [PubMed] [Google Scholar]
- 61.Puinean AM, et al. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010;6:e1000999. doi: 10.1371/journal.pgen.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wondji CS, et al. Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res. 2009;19:452–459. doi: 10.1101/gr.087916.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marteijn RCL, Oude-Elferink MMA, Martens DE, de Gooijer CD, Tramper J. Effect of low inoculation density in the scale-up of insect cell cultures. Biotechnol Prog. 2000;16:795–799. doi: 10.1021/bp000104d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.