Abstract
Major cognitive and emotional faculties are dominantly lateralized in the human cerebral cortex. The mechanism of this lateralization has remained elusive owing to the inaccessibility of human brains to many experimental manipulations. In this study we demonstrate the hemispheric lateralization of observational fear learning in mice. Using unilateral inactivation as well as electrical stimulation of the anterior cingulate cortex (ACC), we show that observational fear learning is controlled by the right but not the left ACC. In contrast to the cortex, inactivation of either left or right thalamic nuclei, both of which are in reciprocal connection to ACC, induced similar impairment of this behavior. The data suggest that lateralization of negative emotions is an evolutionarily conserved trait and mainly involves cortical operations. Lateralization of the observational fear learning behavior in a rodent model will allow detailed analysis of cortical asymmetry in cognitive functions.
Keywords: social fear, anterograde and retrograde tracing
Evidence for hemispheric lateralization exists in various cognitive functions and behaviors in humans (1). In rodents, evidence for cortical lateralization is sparse. Stress-induced neuroendocrine and autonomic responses were shown to be different between left and right medial prefrontal cortex lesions in rats (2, 3). Infantile handling induced increased locomotion in open field tests after right hemisphere lesions (4). In the hippocampus, gene expression profiles (5), receptor expressions (6), and long-term potentiation/long-term depression and innervation patterns (7) displayed certain hemispheric asymmetries. Right and left hemispheric inactivation impaired learning and expression in spatial navigation, respectively (4). Although these data are indicative, evidence for the lateralization of complex emotional behavior is still needed.
The processing and expression of negative emotions such as fear display a right hemispheric dominance in humans, as suggested by neuropsychological studies on stroke patients, EEG, and brain functional MRI (8–10). To date, however, the mechanisms that lead to hemispheric asymmetry are largely unknown owing to the obvious limitation in experimental manipulations that can be carried out in human subjects. For example, it is not known whether hemispheric lateralization is a cortical process or is already in place at the subcortical level, driving the hemispheric differences in complex information processing.
Fear can be vicariously acquired from social observation of a conspecific’s distress (11). This social fear requires the ability to recognize the emotions and feelings of others, suggesting that observational fear is based on empathy (12). In a previous study we had developed a behavioral assay for measuring observational fear learning in mice and found that the anterior cingulate cortex (ACC) is involved in this emotional behavior (13). The ACC has also been implicated in the experience of empathy for pain in humans by brain imaging studies (14). Abnormalities in the structure or function of ACC have been observed in psychiatric illnesses that are characterized by symptoms of cognitive control, such as schizophrenia, attention-deficit/hyperactivity disorder, autism, and bipolar disorders (15–17). In a rare disease, alexithymia patients display an impairment of the ability to identify and communicate one’s emotional state (18). Interestingly, stroke patients developing alexithymia displayed more severe symptoms with a lesion in the right than the left hemisphere (19).
Therefore, we decided to examine the question of whether ACC control of observational fear learning is indeed lateralized in this mouse model. In addition, we tried to define the connections between ACC and thalamic nuclei in control of observational fear using both anatomical and functional tools. We present evidence that control of observational fear is lateralized to the right ACC, whereas it is distributed at the thalamus.
Results
Lateralization of ACC in Observational Fear Learning.
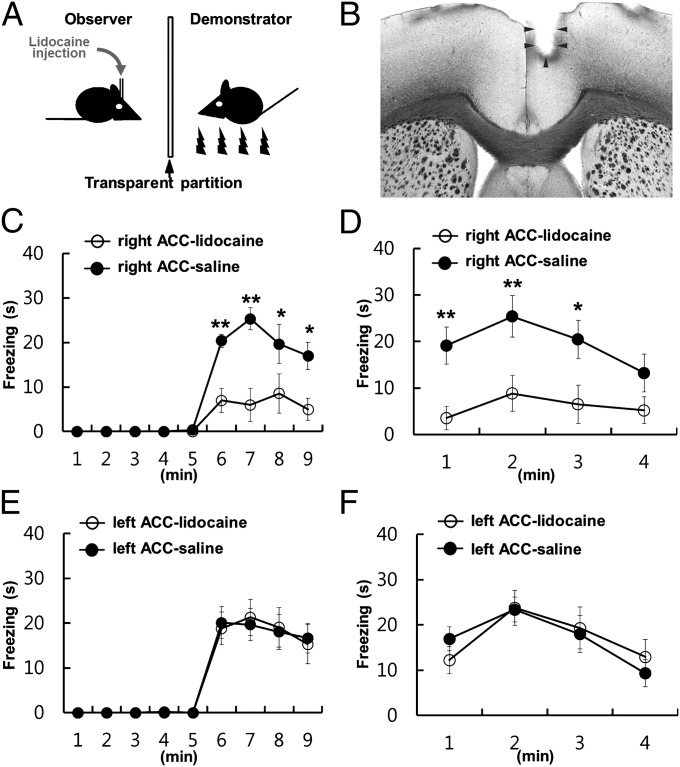
To test the existence of a putative cortical lateralization of observational fear behavior in rodents, we injected lidocaine (4%) through a cannula into either the left or right ACC hemisphere of the observer mice 8 min before observational fear learning (Fig. 1 A and B). Observer mice observed the response of their conspecifics to foot shocks through a Plexiglas partition (9). A lidocaine injection into the right ACC of the observer mice decreased their freezing level compared with the control mice that were similarly treated with saline (Fig. 1C), indicating that the right ACC is involved in observational fear learning. A similar impairment was also observed in the 24-h contextual memory test on the next day (Fig. 1D). In contrast, a similar inactivation of the left ACC did not affect observational fear learning (Fig. 1E) and the 24-h contextual memory recall (Fig. 1F) compared with control mice. These results indicate that the right ACC but not the left is responsible for observational fear learning.
Fig. 1.
Lateralization of ACC in observational fear learning. (A) Diagram of the apparatus and treatment used for observational fear conditioning. (B) Representative photograph indicating the location of the implanted cannula in the right ACC. (C) Mice with lidocaine injections into the right ACC (n = 6) before training failed to acquire fear compared with those receiving saline injections (n = 9). (D) Contextual memory 24 h after the training in C. There were significant differences in the observational training (F1,13 = 13.877, P = 0.003) and the 24-h contextual memory (F1,13 = 9.748, P = 0.008). (E and F) Administration of lidocaine into the left ACC before training had no influence on the acquisition of observational fear (F1,21 = 0.0000000489, P = 1, E) and 24-h contextual memory recall after training (F1,21 = 0.00194, P = 0.965; F) (lidocaine, n = 11; saline, n = 12). Error bars represent SEM. *P < 0.05, **P < 0.01.
Electrical Stimulation of ACC During Observational Fear Learning.
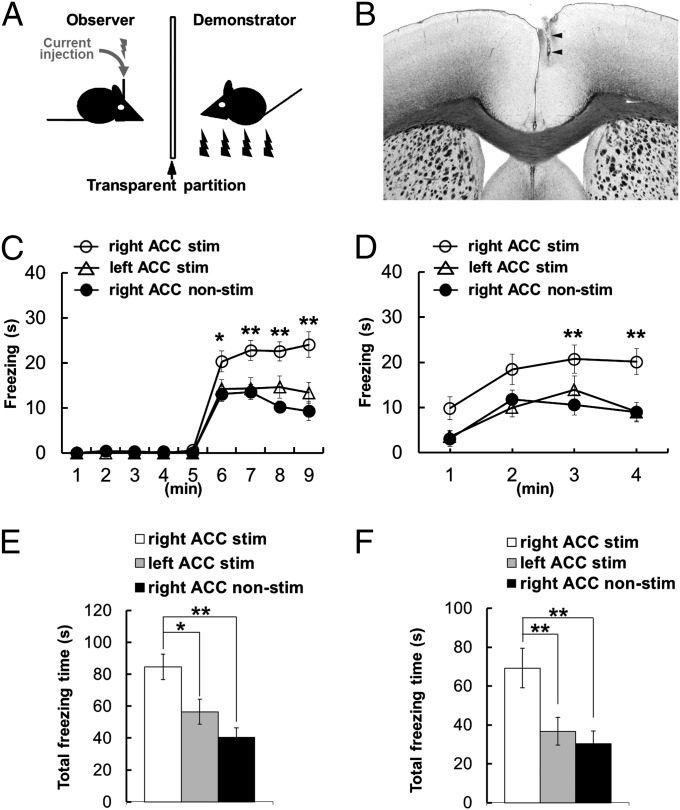
To further examine the ACC asymmetry in social fear learning, we applied unilateral electrical stimulation into the left or right ACC during conditioning. One-hundred-millisecond trains of 100-Hz currents were delivered to the ACC of the observer mice, with 900-ms intertrain intervals, during the 4-min conditioning period when demonstrator mice received foot shocks (Fig. 2 A and B). Stimulation of the right ACC increased observational fear learning of the observer mice (Fig. 2C) and the 24-h contextual memory (Fig. 2D) compared with control mice who had the electrode implanted but no current injection. In contrast, stimulation of the left ACC had no effect on conditioning (Fig. 2C) and the 24-h recall test (Fig. 2D) compared with controls. Furthermore, total freezing time was increased with electrical stimulation into the right ACC during observational fear learning (Fig. 2E) and the 24-h contextual memory recall (Fig. 2F) compared with nonstimulated controls or mice with stimulation into the left ACC. Stimulation of the left or right ACC of observer mice without a demonstrator or with a demonstrator but receiving no foot shock during conditioning evoked no freezing behavior in observer mice in both conditioning and the memory recall test (Fig. S1). These results indicated that electrical stimulation of the right but not the left ACC during conditioning induces facilitation of observational fear learning, and together with the inactivation studies described above suggested that the control of observational fear learning is lateralized to the right ACC.
Fig. 2.
Electrical stimulation of ACC during observational fear learning. (A) Diagram of the apparatus and treatment used for observational fear conditioning. (B) Representative photograph indicating the location of the electrode used for electrical stimulation in the right ACC. (C and D) Electrical stimulation of the right ACC (n = 18) increased both the observational freezing behavior (F1,29 = 25.122, P < 0.001; C) and the 24-h memory recall (F1,29 = 7.385, P = 0.011; D) compared with the nonstimulated controls (n = 16). Electrical stimulation into the left ACC (n = 21) had no effect on observational fear learning (F1,33 = 1.008, P = 0.323; C) and 24-h contextual memory recall (F1,33 = 0.041, P = 0.841; D) compared with nonstimulated controls. (E and F) Total freezing time showed an enhanced effect on right electrical stimulation into the right ACC in observational fear learning (E) and 24-h contextual memory recall (F) compared with nonstimulated controls or mice with stimulation into the left ACC. Trains were delivered every 1 s for 4 min during training in observational fear conditioning. Error bars represent SEM. *P < 0.05, **P < 0.01.
Lesion of the Thalamic Regions in Reciprocal Connection with ACC.
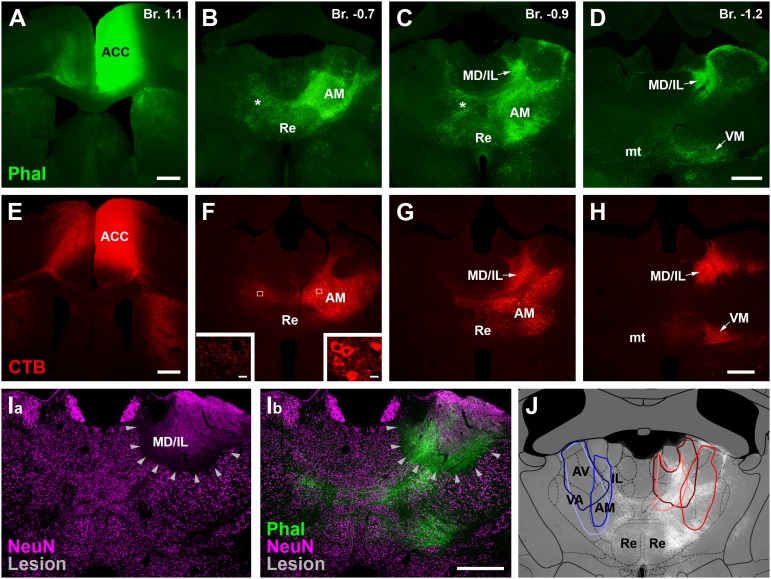
All cortical regions receive thalamic afferents and depend on the integrity of this subcortical input. Disruptions of thalamic activities result in phenotypes resembling the disturbance of corresponding cortical regions (20). Thus, we asked whether the lateralized process observed at the cortical level in social fear is already present at the level of the thalamus. To examine the behavioral consequences of unilateral thalamic lesions, first we characterized the thalamic territories that are in connection with the ACC in mice. We injected the anterograde tracer Phaseolus vulgaris leucoagglutinin (Phal) into the ACC, using the same coordinates as in the lidocaine experiments above (Fig. 3A and Fig. S2). ACC fibers innervated two thalamic regions, the anteromedial thalamic nucleus (AM) (Fig. 3 B and C and Fig. S2) and the lateral part of the mediodorsal thalamus together with the adjacent intralaminar nuclei (MD/IL) (Fig. 3 B and C and Fig. S2). A weaker contralateral projection could be observed in case of the ACC–AM pathway. No overt difference was found between the left and right ACC–thalamic connections (Fig. S2). Injection of the retrograde tracer cholera toxin B subunit (CTB) into ACC labeled thalamic cell bodies in the same termination fields, demonstrating the classic, reciprocal connectivity between a cortical and a thalamic region (Fig. 3 E and H) (21). In contrast to the ACC–thalamic connection, however, the thalamo–ACC pathway was strictly unilateral.
Fig. 3.
Lesion of the thalamic regions in reciprocal connection with ACC. Tracer injection of the anterograde Phal (green; A–D) or retrograde CTB (red; E–H) tracers into the ACC (A and E, respectively) revealed strong bidirectional projections with the AM (B, C, F, and G) and MD/IL (C, D, G, and H) thalamic nuclei. Note bilateral corticothalamic (stars in B and C) but unilateral thalamocortical projection. Insets: White frames, enlarged in F, show that only the ispilateral AM (Right) contains CTB-positive cell bodies. The contralateral side possesses only axonal staining (Left) (for details, see Materials and Methods and Fig. S2). (I, a) Neurochemical lesion in the thalamus (arrowheads) visualized with NeuN immunostaining (magenta). (I, b) Combination of anterograde tracing with Phal from ACC (green) and lesion in the same animal demonstrates that the thalamic territory in connection with ACC was targeted. (J) Schematic drawings of three left (blue) and three right (red) thalamic lesions at a single AP level. A grid from the Paxinos mouse brain atlas was used to identify the location (32). Phal-labeled corticothalamic fibers (white) in the background show that the lesions targeted the appropriate nuclei. Fig. S3 shows the total extent of the lesions. mt, mammillothalamic tract; Re, reuniens thalamic nucleus; VM, ventromedial thalamic nucleus. (Scale bars, 500 μm.)
Control of Observational Fear Is Not Lateralized at Thalamus.
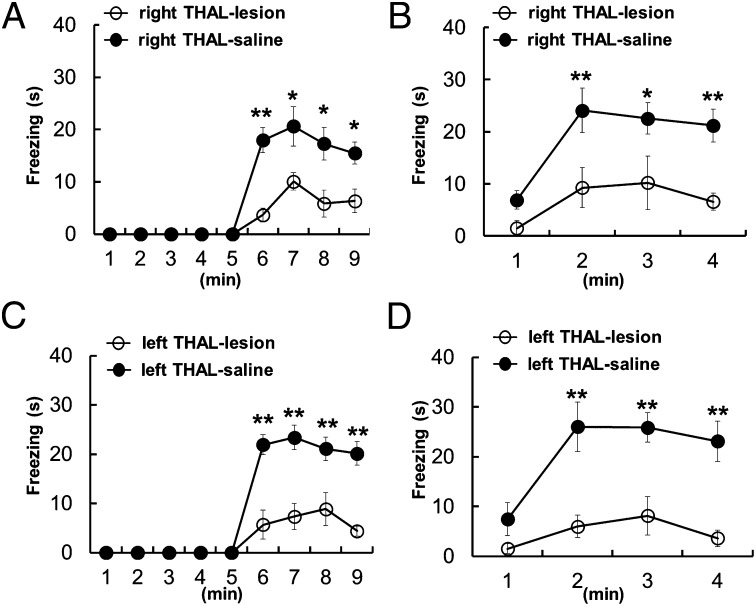
On the basis of these anatomical findings, we lesioned the left or right thalamic regions connected with the ACC and then compared their effect on the expression of social fear. For precise post hoc identification of the lesion sites we injected ibotenic acid, which can be visualized by the lack of NeuN staining in the injection site (Fig. 3I). In some cases, to confirm that the lesioned thalamic region was indeed the part that is in connection with the ACC, we used a combined experimental paradigm in which tract tracing of ACC fibers (with Phal) was combined with a neurochemical lesion of the thalamus (Fig. 3 I and J). The average size of a lesion was 0.3–0.4 mm in diameter, with a maximal extent of 0.5 mm × 0.5 mm [anteroposterior (AP) × mediolateral] and 1.0 mm [dorsoventral (DV)] (Fig. S3). Only results from those animals in which the lesion was unilateral and its size was larger than 0.1 mm were included in the analysis (11 of 30 animals). Most of these selected lesions targeted either left or right AM and spread into MD/IL to varying extents (Fig. S3). Observer mice with ibotenic acid injections in the left or right hemisphere showed significantly decreased freezing behavior compare with those with a saline injection, both in observational fear conditioning (Fig. 4 A and C) and the 24-h memory recall test (Fig. 4 B and D). The decreased level of freezing cannot be due to a change in the anxiety level or locomotion because the thalamic-lesioned mice showed behavior similar to control mice in the open-field test and the elevated plus maze (Fig. S4). However, no difference was found between the left and right thalamic-lesioned groups in the freezing level, both in learning (Fig. 4 A and C) and the 24-h memory recall test (Fig. 4 B and D), indicating that the control of observational fear is not lateralized at the level of the thalamus.
Fig. 4.
Control of observational fear is not lateralized at thalamus. (A and B) Mice with ibotenic acid injection into right thalamic nuclei (n = 6) 1 wk before training exhibited impaired acquisition compared with those receiving saline injections into right thalamus (n = 11) in observational fear (F1,15 = 12.307, P = 0.003, A) and 24-h contextual memory test (F1,15 = 11.295, P = 0.004, B). (C and D) Unilateral injection of ibotenic acid into left thalamic nuclei (n = 5) also caused impaired observational fear learning during training (F1,14 = 27.979, P < 0.001; C) and 24-h contextual memory (F1,14 = 16.245, P = 0.001; D) compared with those receiving saline injection into left thalamus (n = 11). There was no difference between the left and right thalamic lesioned groups in the freezing level in the learning (A and C) (F1,9 = 0.000995, P = 0.976) and the 24-h memory recall test (B and D) (F1,9 = 0.488, P = 0.502). Error bars represent SEM. *P < 0.05, **P < 0.01.
Discussion
Manipulations, both inactivation and stimulation, of the right but not the left ACC modulate observational fear behavior, indicating asymmetrical emotional processing in the mouse cerebral cortex. These results are reminiscent of the right hemispheric dominance in negative emotional processing and expression in humans (8–10). This demonstrates that hemispheric lateralization of negative emotions is not unique to humans but is an evolutionarily conserved feature of the mammalian lineage to which both rodents and primates belong.
Unlike in the cortex, however, we did not see evidence for lateralization in the thalamic nuclei that are connected to the ACC in observational fear learning. The sensitivity of social fear to the manipulation of the left thalamus but not the left ACC suggests a possibility that left cortical targets other than the ACC may be involved in this form of emotional learning. Recently it has been demonstrated that the anteromedial thalamic nucleus of mice is one of the few thalamic regions that have quite widespread cortical outputs (22). This suggests a possibility that the anteromedial thalamus has the potential to influence many cortical regions. Accordingly, besides the involvement of the right ACC, other cortical processing on the left side may be involved in the control of social fear.
Besides the thalamus, the ACC has prominent reciprocal synaptic connections with the amygdala (23). Our previous results demonstrated that the ACC neuronal activities were synchronized with those of the lateral amygdala at the θ rhythm frequency during observational fear (13). The existence of functionally lateralized ACC–amygdala connectivity is not known in rodents. However, in adult humans fearful face presentation causes event-related activation in the right amygdala and ACC (24). A number of other studies also indicated specific involvement of the right amygdala in negative emotional signaling (25–27), which suggests—along with the previously mentioned observations—the relevance of a functional asymmetry in the amygdala–ACC connection. Further studies with unilateral amygdala lesions and hemispheric difference in ACC–amygdala synchronization may provide valuable information regarding whether this brain circuit is working in social fear learning unilaterally.
The exact effect of 100-Hz stimulation in the brain is a controversy. High-frequency stimulation in the human subthalamic nucleus (STN) decreased the neuronal firing activity of STN neurons (28, 29). Recent data, however, suggest that interruption of function may not take place because in the human STN 100-Hz simulation did not silence the activity of STN neurons (30). In our previous study (31), high-frequency stimulation in the thalamus did not cause interruption of the function, further suggesting the validity of this approach. Irrespective of the exact mechanisms the electrical stimulation showed a clear lateralized effect, which was in the opposite direction than the inactivation. In addition, there is a possibility that 100-Hz stimulation may induce plastic changes in the ACC, which certainly deserves further attention. However, because the effect of 100-Hz stimulation on acquisition was immediate, long-term plasticity may not explain at least the acquisition of observational fear learning.
In conclusion, the involvement of the right but not the left ACC in observational social fear, but no lateralization at the thalamus, may provide insight into the asymmetrical hemispheric mechanisms underlying psychopathic and mental disorders in humans.
Materials and Methods
Animals were housed with a 12-h light/12-h dark cycle and ad libitum access to food and water. In all experiments, we used adult male C57BL/6J mice, 12 to 15 wk old, and followed the ethical guidelines of the Institutional Animal Care and Use Committee (Key-Sun Kim) in the Korea Institute of Science and Technology and the ethical boards (Emilía Madarász) in the Institute of Experimental Medicine, Hungarian Academy of Sciences.
Observational Fear Conditioning.
Observational fear conditioning was carried out according to the procedures described previously (13). Briefly, the apparatus for observational fear conditioning consisted of two identical chambers (each, 18 × 17.5 × 38 cm) containing a transparent Plexiglas partition in the middle and a stainless steel rod floor (5-mm-diameter rods, spaced 1 cm apart) (which are modified passive avoidance cages; Coulbourn Instruments). Sounds and smells could be transmitted between the chambers under the rod floor. For observational fear conditioning, mice (observer and demonstrator) were individually placed in apparatus chambers for 5 min, and then a 2-s foot shock (1 mA) was delivered every 10 s for 4 min to one of the mice (demonstrator) via a computer-controlled animal shocker (WinLinc; Coulbourn Instruments). To assess contextual memory, we placed the observers back into the training context 24 h after training and observed freezing behavior for 4 min. Fear response was video-recorded and quantified by an experimenter blind to the condition and genotype by measuring the length of the time during which a mouse showed freezing behavior by hand, defined as lack of movement (except for respiratory movements) for longer than 2 s.
Cannula Implantation and Microinjection.
For microinjection, a cannula (PlasticsOne) was unilaterally implanted in the right or left hemisphere at AP +1.0 mm, lateral (L) +0.3 mm or −0.3 mm, and DV 1.2 mm for the ACC from bregma using a stereotaxic apparatus (Kopf Instruments). Experiments began 14 d after surgery. Lidocaine [4% (vol/vol), 0.3 μL] or saline [0.9% NaCl (vol/vol), 0.3 μL] was infused into each brain area via an inner cannula (33 gauge) connected to a 25-μL Hamilton syringe; the flow rate (0.1 μL min−1) was regulated by a syringe pump (SP100i; WPI). Experiments began 8 min after a single microinjection. The position of the cannula was verified histologically after experiments.
ACC Electrical Stimulation.
All mice were implanted with monopolar tungsten stimulating electrodes (0.125 mm in diameter; A-M Systems) placed unilaterally within the right or left ACC region (AP +1.0 mm, L +0.2 mm or −0.2 mm, DV 1.2 mm) using a stereotaxic device (Kopf Instruments) under 2% (wt/vol) avertine anesthesia. Mice were allowed to recover for at least 7 d. We delivered cathodal current through the electrode and allowed the charge to dissipate through a grounding electrode (stainless steel) implanted in the skull. Mice received 100-ms trains of square pulses (−100 μA, 100-μs stimulus duration, 100 Hz) through a current stimulator (Model 2100; A-M Systems). Trains were delivered every 1 s for 4 min during training in observational fear conditioning. Stimulation positions were confirmed by postmortem histology.
Thalamic Lesions.
For ibotenic acid lesions, a fine glass pipette (diameter, 50 μm) glued to a Hamilton syringe was lowered to the thalamus (AP −0.7 mm, ML 0.6 mm, DV 3.5 mm from the brain surface). Unilateral lesions were performed using ibotenic acid (0.2 μL of 10 μg ibotenic acid/μL in 0.01 M PBS) or saline [0.9% NaCl (vol/vol), 0.2 μL]. (Control right, n = 9; lesioned right, n = 22; control left, n = 6; lesioned left, n = 8.)
After 1 wk of recovery, the behavioral experiments (observational fear conditioning, 24-h memory recall, locomotor and anxiety tests) were performed on the control and lesioned animals. Then all animals were anesthetized and perfused (see above). The brains were sectioned and extensively washed. Sections were treated first with a blocking solution containing 5% (vol/vol) normal donkey serum (NDS) and 0.5% (vol/vol) Triton-X for 30 min at room temperature, then with a primary antibody against NeuN (mouse 1:3,000) in phosphate buffer (PB) containing 0.1% NDS and 0.1% Triton-X at room temperature for overnight. Next day the sections were extensively washed and incubated in Cy3-conjugated donkey anti-mouse for 2 h at room temperature. After further PB washes, sections were mounted in Vectashield and imaged using an epifluorescent microscope.
Photos were taken using a 5× objective from the thalamus at every 100 μm. Animals were selected for both anatomical and behavioral analysis, in which the lesions were restricted to unilateral AM and/or MD/IL and were larger than 100 μm in diameter. In control groups, one animal was also discarded because of a mechanical lesion caused by the pipette insertion. In the final analysis, the following animals were included: control right, n = 14; lesioned right, n = 6; control left, n = 14; lesioned left, n = 5.
Then the images were placed over the corresponding mouse brain maps (32) in Adobe Photoshop CS4, and the lesioned areas were outlined.
Anterograde and Retrograde Tracing.
An anterograde tracer, Phal, and a retrograde tracer, CTB, were iontophoretically injected (7-7 s on/off duty cycle; 3 μA, for 10 min) separately into either right or left ACC (AP 1.1 mm, ML 0.3–0.6 mm, DV 0.5–1.0 mm) of adult male mice under ketamine (75 mg/kg)/xylazine (5 mg/kg) anesthesia. After 1 wk, the animals were perfused first with saline (0.9%) and then with 4% paraformaldehyde solutions. Then brains were removed and cut into 50-μm-thick coronal sections. Sections were intensively washed with PB and then treated with a blocking solution containing 5% NDS and 0.5% Triton-X for 30 min at room temperature. The primary antibody against Phal (rabbit 1:10,000–20,000) and CTB (goat 1:20,000) was diluted in PB containing 0.1% NDS and 0.1% Triton-X. After primary antibody incubation (1 d at room temperature or 2 to 3 d at 4 °C), sections were treated with either Alexa 488-conjugated goat anti-rabbit IgG or Cy3-conjugated donkey anti-goat (respectively) for 2 h at room temperature.
After further PB washes, sections were mounted in Vectashield and imaged using an epifluorescent or confocal microscope.
In these experiments Phal was used as an anterograde and CTB as a retrograde tracer. CTB is known to label not only the cell bodies of the retrogradely labeled cells but also their axon arbors. When the thalamocortical projection was examined obviously only the retrograde (cell body) labeling was considered. This demonstrated clear unilateral thalamocortical projection (Fig. 3). Interestingly, however, CTB labeled axons in the thalamus contralateral to the cortical injection side (Fig. 3F). Because thalamic neurons do not have contralateral axons, our interpretation is that these fibers represent the collaterals of corticothalamic cells labeled by the cortical injection. Indeed, the anterograde Phal demonstrated clear contralateral labeling.
Anxiety and Locomotor Activity Tasks.
The elevated plus maze was made of plastic and consisted of two white open arms (25 × 8 cm), two black enclosed arms (25 × 8 × 20 cm), and a central platform (8 × 8 × 8 cm) in the form of a cross. The maze was placed 50 cm above the floor. Mice were individually placed in the center with their heads directed toward one of the closed arms. The total time spent in each arm or center and total number of entries into each arm were analyzed by video monitoring for 5 min.
The open-field box was made of white plastic (40 × 40 × 40 cm), and the open field was divided into a central field (center, 20 × 20 cm) and an outer field (periphery). Individual mice were placed in the periphery of the field, and the paths of the animals were recorded by a video camera. The total distance traveled was analyzed using EthoVision XT (Noldus).
Statistics.
Statistical analyses were conducted using SigmaStat 3.5. Two-way repeated ANOVA, one-way ANOVA, and Student’s t test were used for behavioral analyses. P < 0.05 was considered statistically significant. Tukey’s post hoc comparison was then used to detect significant differences between groups. All data are shown as means ± SEM.
Supplementary Material
Acknowledgments
This work was supported by the Korean-Hungarian Joint Laboratory Program, the National Honor Scientist Program of Korea, and Hungarian Scientific Research Fund 75676. F.M. is a János Bolyai Research Fellow.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213903109/-/DCSupplemental.
References
- 1.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan RM, Szechtman H. Asymmetrical influence of mesocortical dopamine depletion on stress ulcer development and subcortical dopamine systems in rats: Implications for psychopathology. Neuroscience. 1995;65:757–766. doi: 10.1016/0306-4522(94)00531-9. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denenberg VH, Garbanati J, Sherman DA, Yutzey DA, Kaplan R. Infantile stimulation induces brain lateralization in rats. Science. 1978;201:1150–1152. doi: 10.1126/science.684436. [DOI] [PubMed] [Google Scholar]
- 5.Klur S, et al. Hippocampal-dependent spatial memory functions might be lateralized in rats: An approach combining gene expression profiling and reversible inactivation. Hippocampus. 2009;19:800–816. doi: 10.1002/hipo.20562. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami R, et al. Asymmetrical allocation of NMDA receptor epsilon2 subunits in hippocampal circuitry. Science. 2003;300:990–994. doi: 10.1126/science.1082609. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara Y, et al. Hippocampal CA3 and CA2 have distinct bilateral innervation patterns to CA1 in rodents. Eur J Neurosci. 2012;35:702–710. doi: 10.1111/j.1460-9568.2012.07993.x. [DOI] [PubMed] [Google Scholar]
- 8.Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16:7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahern GL, Schwartz GE. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia. 1985;23:745–755. doi: 10.1016/0028-3932(85)90081-8. [DOI] [PubMed] [Google Scholar]
- 10.Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport. 1998;9:3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- 11.Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- 12.Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: The neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon D, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 15.Fornito A, et al. Morphology of the paracingulate sulcus and executive cognition in schizophrenia. Schizophr Res. 2006;88:192–197. doi: 10.1016/j.schres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Bush G, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 17.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 18.Sifneos PE. The prevalence of ‘alexithymic’ characteristics in psychosomatic patients. Psychother Psychosom. 1973;22:255–262. doi: 10.1159/000286529. [DOI] [PubMed] [Google Scholar]
- 19.Spalletta G, et al. Alexithymic features in stroke: Effects of laterality and gender. Psychosom Med. 2001;63:944–950. doi: 10.1097/00006842-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Steriade M, Jones EG, McCormick DA. Thalamus, Volume II. Experimental and Clinical Aspects. Amsterdam: Elsevier Science; 1997. [Google Scholar]
- 21.Deschênes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: Reciprocity versus parity. Brain Res Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 22.Rubio-Garrido P, Pérez-de-Manzo F, Porrero C, Galazo MJ, Clascá F. Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb Cortex. 2009;19:2380–2395. doi: 10.1093/cercor/bhn259. [DOI] [PubMed] [Google Scholar]
- 23.Gabbott PLA, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 24.Hung Y, Smith ML, Taylor MJ. Development of ACC-amygdala activations in processing unattended fear. Neuroimage. 2012;60:545–552. doi: 10.1016/j.neuroimage.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: Evidence for greater involvement of the right amygdala. Behav Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Scicli AP, Petrovich GD, Swanson LW, Thompson RF. Contextual fear conditioning is associated with lateralized expression of the immediate early gene c-fos in the central and basolateral amygdalar nuclei. Behav Neurosci. 2004;118:5–14. doi: 10.1037/0735-7044.118.1.5. [DOI] [PubMed] [Google Scholar]
- 27.Kolber BJ, et al. Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J Neurosci. 2010;30:8203–8213. doi: 10.1523/JNEUROSCI.1216-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res. 2004;156:274–281. doi: 10.1007/s00221-003-1784-y. [DOI] [PubMed] [Google Scholar]
- 29.Welter ML, et al. Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Arch Neurol. 2004;61:89–96. doi: 10.1001/archneur.61.1.89. [DOI] [PubMed] [Google Scholar]
- 30.Carlson JD, Cleary DR, Cetas JS, Heinricher MM, Burchiel KJ. Deep brain stimulation does not silence neurons in subthalamic nucleus in Parkinson’s patients. J Neurophysiol. 2010;103:962–967. doi: 10.1152/jn.00363.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, et al. Bidirectional modulation of fear extinction by mediodorsal thalamic firing in mice. Nat Neurosci. 2012;15:308–314. doi: 10.1038/nn.2999. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 3rd Ed. Amsterdam: Elsevier; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.