Abstract
Many bacterial pathogens produce diffusible signal factor (DSF)-type quorum sensing (QS) signals in modulation of virulence and biofilm formation. Previous work on Xanthomonas campestris showed that the RpfC/RpfG two-component system is involved in sensing and responding to DSF signals, but little is known in other microorganisms. Here we show that in Burkholderia cenocepacia the DSF-family signal cis-2-dodecenoic acid (BDSF) negatively controls the intracellular cyclic dimeric guanosine monophosphate (c-di-GMP) level through a receptor protein RpfR, which contains Per/Arnt/Sim (PAS)-GGDEF-EAL domains. RpfR regulates the same phenotypes as BDSF including swarming motility, biofilm formation, and virulence. In addition, the BDSF− mutant phenotypes could be rescued by in trans expression of RpfR, or its EAL domain that functions as a c-di-GMP phosphodiesterase. BDSF is shown to bind to the PAS domain of RpfR with high affinity and stimulates its phosphodiesterase activity through induction of allosteric conformational changes. Our work presents a unique and widely conserved DSF-family signal receptor that directly links the signal perception to c-di-GMP turnover in regulation of bacterial physiology.
Keywords: signal transduction, second messenger, molecular recognition, pathogenesis, cell-cell communication
Quorum sensing (QS) denotes a widely conserved cell-to-cell communication mechanism that coordinates bacterial group behavior and often regulates virulence, biofilm formation, antibiotic production, and plasmid conjugal transfer (1–3). Many bacteria produce, detect, and respond to diffusible QS signal molecules in a cell-density–dependent manner (1, 4), highlighting the critical roles of QS signal and its receptor in bacterial cell–cell communications. The basic principle of QS regulation was originally proposed for systems that use N-acylhomoserine lactone (AHL) signal molecules: they are constitutively produced at a low rate until a critical threshold concentration has been attained, upon which the signal activates its cognate receptor, a LuxR-family protein, by the formation of a protein–ligand complex, which in turn induces or represses expression of target genes (1, 2, 5).
Similar to AHL-type QS systems, the diffusible signal factor (DSF)-dependent QS systems have been recognized as another family of widely conserved QS mechanisms (3, 6). DSF was originally identified in Xanthomonas campestris pv. campestris (Xcc), belonging to the γ subdivision of proteobacteria, and is involved in the regulation of biofilm dispersal and virulence (7, 8). Subsequent studies showed that the Xcc DSF signaling system differs from the AHL-dependent systems in various aspects, including its signal perception mechanism, autoregulation of signal production, and its integration into the global regulatory network of the cell (3, 6, 8–12). The DSF-dependent signaling system in Xcc employs a two-component system consisting of RpfC/RpfG in signal perception and transduction (7, 13, 14). When Xcc reaches a quorate population density, i.e., when the DSF concentration is sufficiently high, autophosphorylation of RpfC triggers a phosphorelay through its His Kinase (HK) domain to receiver (REC) domain to histidine phosphotransfer (HPT) domain, eventually leading to phosphorylation of the response regulator RpfG. Phosphorylation of RpfG is believed to induce a conformational change in the protein that activates its cyclic dimeric guanosine monophosphate (c-di-GMP) phosphodiesterase activity. Degradation of c-di-GMP molecules reduces the intracellular level of this second messenger, resulting in increased intracellular levels of free Clp (12, 15), which regulates the expression of target genes either directly or indirectly via downstream transcriptional factors including Zur and FhrR (14).
DSF-family QS signals have recently been identified in several Burkholderia species (16, 17). In Burkholderia cenocepacia, an opportunistic pathogen of clinical relevance, the major signal has been characterized as cis-2-dodecenoic acid (BDSF) (16). BDSF is structurally similar to DSF of Xcc but differs in a methyl substitution at C11. RpfFBc (encoded by BCAM0581 in strain J2315), which is a bifunctional enzyme with both enoyl-CoA hydratase and thioesterase activities sharing about 37% identity at the protein sequence level with RpfF of Xcc, was found to be the enzyme responsible for BDSF production (16, 18). In contrast to the rpfF gene of Xcc, no rpfC or rpfG homologs could be identified in the vicinity of rpfFBc. Recently, a putative BDSF sensor, BCAM0227, was identified in B. cenocepacia J2315 (19). BCAM0227 shares 35.6% identity with RpfC of Xcc and contains similar domain structures (19). However, BCAM0227 was shown to control only a subset of BDSF-regulated genes and phenotypes (19). In addition, whereas the BDSF synthase RpfFBc is highly conserved among B. cepacia complex (Bcc) species (more than 94% similarity) (17), the putative BDSF sensor BCAM0227 displays only a moderate similarity (about 36%) within Bcc species (3). These findings led us to speculate that another BDSF signaling pathway may exist in B. cenocepacia.
In this study we identified a unique BDSF receptor in strain H111, which is encoded by a BCAM0580 homolog (designated as rpfR) and is located next to the BDSF synthase gene rpfFBc. It is demonstrated that upon interaction with BDSF, this receptor protein becomes a potent c-di-GMP phosphodiesterase. We also present evidence that disruption of rpfR results in similar phenotypic changes as the BDSF-deficient mutant, including attenuated virulence and decreased bacterial motility. Most intriguingly, the rpfR/rpfFBc gene cluster is conserved in diverse Gram-negative bacteria, suggesting that BDSF-type signaling systems are widespread and that RpfR is a member of a unique family of QS signal sensors that combine signal perception and c-di-GMP turnover within one molecule.
Results
BDSF and RpfR Negatively Control the Intracellular C-Di-GMP Level in B. cenocepacia.
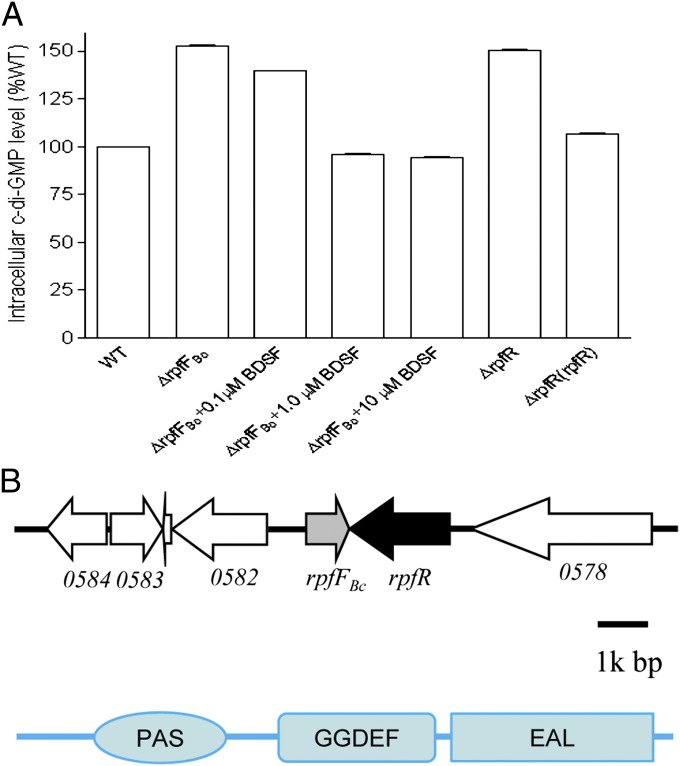
Previous studies in Xcc showed that the response regulator RpfG is a novel c-di-GMP degradation enzyme, which is activated by the DSF sensor RpfC upon perception of DSF signal molecules (9, 14). These findings motivated us to look for a possible relationship between BDSF and intracellular c-di-GMP level in B. cenocepacia. We generated the BDSF− mutant ∆rpfFBc by in-frame deletion of the BCAM0581 homolog (rpfFBc) in B. cenocepacia strain H111. Our previous work has shown that BCAM0581 encodes the BDSF synthase (16). High performance liquid chromatography (HPLC) analysis showed that disruption of rpfFBc caused a substantial increase in the intracellular c-di-GMP concentration, which was restored to the level of the wild type by the exogenous addition of BDSF (Fig. 1A). These data suggest that BDSF plays a role in the regulation of an enzymatic activity associated with c-di-GMP metabolism. We noted that the ORF BCAM0580 (designated as rpfR), which is located next to the BDSF biosynthase gene rpfFBc, encodes a protein containing a PAS, a GGDEF, and an EAL domain (Fig. 1B). The conserved GGDEF and EAL domains are common features of diguanylate cyclases and phosphodiesterases, which are involved in c-di-GMP biosynthesis and degradation, respectively (20). To study whether RpfR is involved in c-di-GMP metabolism in B. cenocepacia, we generated the in-frame deletion mutant ∆rpfR and measured its intracellular c-di-GMP level. This analysis showed that deletion of rpfR increased the intracellular c-di-GMP level by about 50%, resulting in levels similar to that of the rpfFBC mutant. Moreover, in trans expression of rpfR in the mutant ∆rpfR restored c-di-GMP production to a level similar to wild type (Fig. 1A). These data suggest that RpfR has a net c-di-GMP phosphodiesterase activity and that the BDSF signaling system appears to negatively affect the intracellular c-di-GMP level in B. cenocepacia H111.
Fig. 1.
Influence of RpfFBc and RpfR on intracellular c-di-GMP level. (A) Detection of intracellular c-di-GMP level by HPLCy (HPLC) assay. Relative amount of c-di-GMP was calculated on the basis of their peak areas. For the convenience of comparison, c-di-GMP level of B. cenocepacia wild-type strain H111 was arbitrarily defined as 100% and used to normalize the c-di-GMP level ratios of the other strains. Data shown are means of two repeats and error bars indicate SDs. (B) Genomic organization of the region encoding RpfFBc and RpfR in B. cenocepacia H111 and domain structure analysis of RpfR.
To test whether BCAM0227, a putative BDSF sensor previously identified in B. cenocepacia strain J2315 (19), could affect BDSF-mediated degradation of c-di-GMP and thus modulate the activity of RpfR, the BCAM0227 homolog was inactivated in both the wild-type strain H111 and the mutant ∆rpfR. However, mutation of this putative BDSF sensor affected neither the intracellular c-di-GMP level of the wild type nor that of the rpfR mutant (SI Appendix, Fig. S1).
RpfR Is Essential for the Expression of BDSF-Regulated Phenotypes.
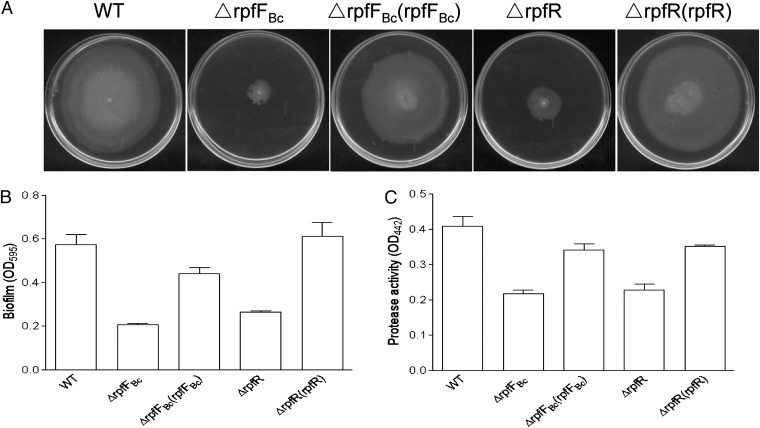
The BDSF signaling system is involved in regulation of swarming motility, biofilm formation, and virulence in B. cenocepacia (19, 21). Given the close genomic association of rpfFBc and rpfR and their similar effect on the intracellular c-di-GMP level (Fig. 1), we hypothesized that RpfR might be functionally related to the BDSF signaling system in B. cenocepacia H111. To test this hypothesis, we investigated whether a rpfR mutant exhibits similar phenotypes as a BDSF− mutant. Intriguingly, deletion of rpfR caused the same phenotypic changes as observed with the BDSF mutant ∆rpfFBc, including reduced bacterial motility (Fig. 2A), compromised biofilm formation (Fig. 2B), and decreased proteolytic activity (Fig. 2C). In contrast, the tested phenotypes of the BCAM0227 mutant were indistinguishable from the wild type (SI Appendix, Fig. S2).
Fig. 2.
Influence of RpfR on BDSF-regulated phenotypes. Similar to the BDSF synthase mutant, deletion of rpfR caused a reduction of swarming motility (A), biofilm formation (B), and protease production (C). Data shown are means of three replicates and error bars indicate SDs.
RpfR Is a Key Component of the BDSF Signaling System.
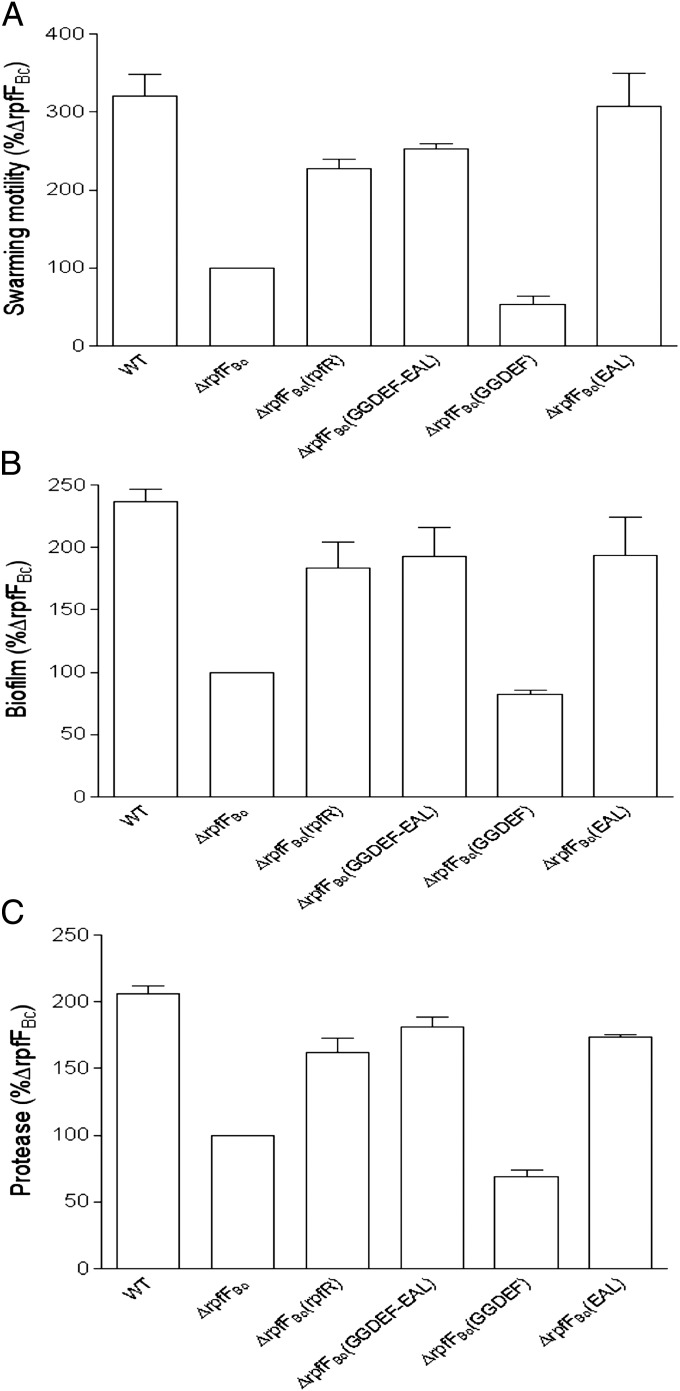
To study the functional relationship between the BDSF synthase and the multidomain protein RpfR, we tested whether expression of rpfR could rescue the rpfFBc mutant phenotypes. Encouragingly, overexpression of RpfR in mutant ∆rpfFBc fully restored the BDSF synthase-deficient mutant phenotypes with respect to motility (Fig. 3A), biofilm formation (Fig. 3B), and protease activity (Fig. 3C). As RpfR contains a PAS signal sensor domain and two additional domains associated with c-di-GMP metabolism, we then determined which of the domains is essential for complementation of the rpfFBc mutant phenotypes. These experiments showed that overexpression of the region containing the GGDEF domain alone in the deletion mutant ∆rpfFBc caused a further slight reduction in motility, biofilm formation, and protease activity compared with the BDSF-deficient mutant (Fig. 3). In marked contrast, overexpression of the region containing either the GGDEF–EAL domains or the EAL domain alone rescued the mutant phenotypes of ∆rpfFBc to levels similar to those obtained after complementation with the full-length rpfR gene (Fig. 3).
Fig. 3.
Effect of in trans protein expression on BDSF-regulated phenotypes. In trans expression of the encoding region of RpfR, the GGDEF, and EAL domains, and the EAL domain only in the BDSF synthase− mutant ∆rpfFBc rescued its phenotype defects in swarming motility (A), biofilm formation (B), and protease production (C). Data shown are means of two replicates and error bars indicate SDs.
To investigate the functions of the GGDEF and EAL domains in detail, critical catalytic residues of either the GGDEF or the EAL domain were mutagenized. In trans expression of RpfR harboring a mutation in the GGDEF motif (changed to GGAAF) complemented the swarming motility, biofilm formation, and protease activity defects of the rpfR mutant (SI Appendix, Fig. S3). In contrast, mutation of the EAL motif (changed to AAL) not only failed to complement the rpfR mutant, but further reduced swarming motility, biofilm, and protease activity (SI Appendix, Fig. S3). Furthermore, in trans expression of RocR (PA3947) from Pseudomonas aeruginosa, which encodes a well-characterized phosphodiesterase (22), rescued the rpfR mutant phenotypes (SI Appendix, Fig. S4). Taken together, these data strongly indicate that RpfR is a key component of the BDSF signaling system and that its functionality in vivo is related to the c-di-GMP phosphodiesterase activity conferred by the EAL domain.
BDSF Is a Signal Ligand of RpfR.
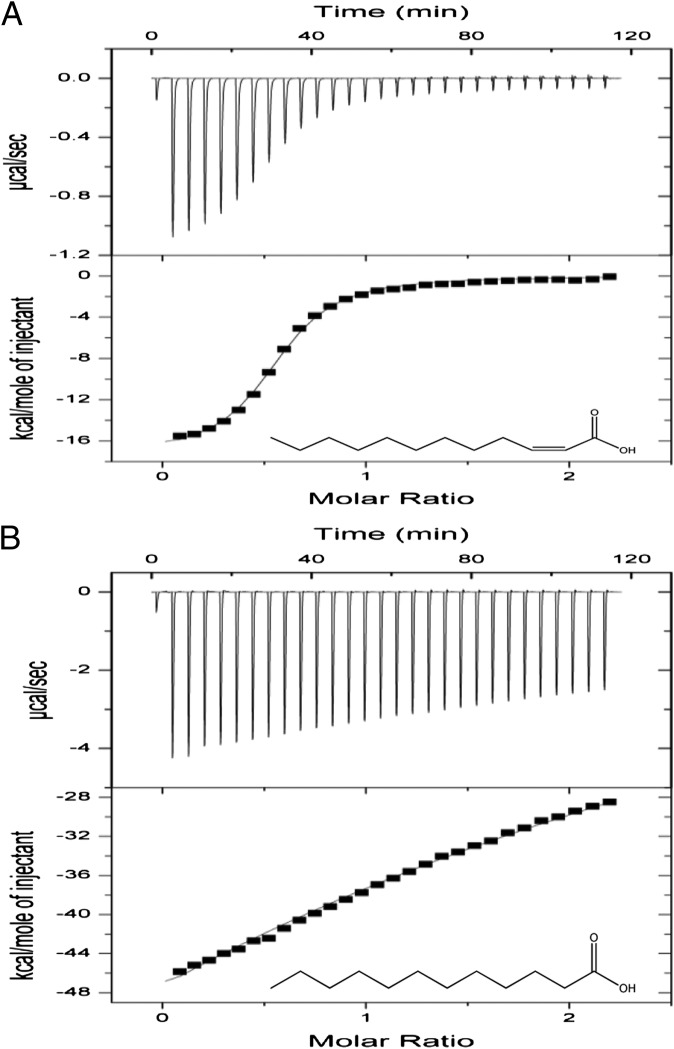
Disruption of the BDSF synthase gene rpfFBc did not affect transcription of rpfR (19), suggesting that BDSF may influence the activity of RpfR through ligand–protein interaction. This possibility was investigated by the aid of isothermal titration calorimetry (ITC) analysis. RpfR, which contains 667 aa with a calculated molecular weight of 73.4 kDa, was purified to homogeneity using affinity chromatography (SI Appendix, Fig. S5) and examined for its interaction with BDSF. These experiments showed that BDSF binds strongly to the purified RpfR protein (Fig. 4A). Calculation of the binding isotherm data showed that RpfR binds to BDSF in 1:1 stoichiometry with an estimated dissociation constant (Kd) of 8.77 × 10−7 M. We also tested the molecular interaction between RpfR and DSF, which is a BDSF analog that was previously shown to restore the defects of the rpfFBc mutant, including biofilm formation and virulence gene expression (17). DSF was found to bind strongly to RpfR with an affinity (Kd =1.37 × 10−6 M) close to that of BDSF (SI Appendix, Fig. S6). In contrast, the saturated BDSF isomer lauric acid or the trans-isomer of BDSF binds to RpfR with a much lower affinity (Kd = 8.0 × 10−4 M and 1.5 × 10−4 M, respectively) (Fig. 4B and SI Appendix, Fig. S6). This finding is in good agreement with previous results showing that the configuration of the double bond of DSF is a critical structural feature for the biological activity of the signal molecule (8).
Fig. 4.
ITC analysis of the molecular interaction between BDSF and RpfR. (A) ITC titration of 20 μM RpfR with 200 μM BDSF in PBS buffer at 21 °C. (B) ITC titration of 20 μM RpfR with 200 μM of the saturated isomer of BDSF in PBS buffer at 21 °C.
Consistent with these results we found that supplementing the media with DSF was enough to rescue the mutant phenotypes of the rpfFBc mutant, whereas addition of lauric acid or the trans-isomer of BDSF had no or only a minor effect (SI Appendix, Fig. S7). In addition, we purified the polypeptides encompassing only the PAS or GGDEF–EAL domain of RpfR and tested them for BDSF binding. ITC analyses revealed that only the PAS domain is required for BDSF binding (SI Appendix, Fig. S8).
BDSF Acts as an Allosteric Activator of RpfR.
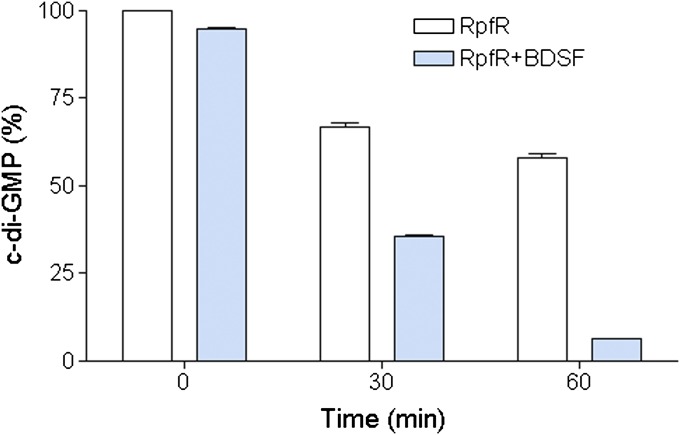
The above findings suggest that BDSF may influence RpfR activity through ligand–protein interaction. Therefore, we tested the c-di-GMP phosphodiesterase activity of RpfR in the presence or absence of BDSF. The results showed that purified RpfR protein degraded c-di-GMP, generating pGpG (SI Appendix, Fig. S9). Moreover, the enzymatic activity of RpfR was substantially boosted in the presence of BDSF (SI Appendix, Fig. S9). Quantitative analysis revealed that addition of BDSF to RpfR increased the degradation of c-di-GMP by 31.1% at 30 min and 51.7% at 60 min after initiation of the reaction, respectively, in comparison with the enzyme control without BDSF (Fig. 5). The c-di-GMP degradation activity of RpfR was also stimulated by DSF, whereas negligible activation was observed with lauric acid or the trans-isomer of BDSF (SI Appendix, Fig. S10). These in vitro data are highly consistent with the in vivo results that deletion of either rpfFBc or rpfR led to an elevated intracellular c-di-GMP level (Fig. 1A).
Fig. 5.
Impact of BDSF on RpfR enzyme activity. Exogenous addition of BDSF to a RpfR protein solution increased its c-di-GMP phosphodiesterase activity. For the convenience of comparison, peak of c-di-GMP in the RpfR solution without BDSF at 0 min was defined as 100% and used to normalize the c-di-GMP level ratios at different time points. Data shown are means of two replicates and error bars indicate SDs.
To determine how BDSF could influence the c-di-GMP phosphodiesterase activity of RpfR, we used circular dichroism (CD) spectroscopy to study BDSF–RpfR interaction. The RpfR protein exhibited an intense negative ellipticity from 208 nm to 234 nm (SI Appendix, Fig. S11), suggesting a large unordered contribution and a small, but detectable contribution of the α-helical structure (23). In contrast, addition of BDSF resulted in a notable conformational change in RpfR, including a decreased intensity of the negative ellipticity from 205 nm to 219 nm, a slightly increased intensity of the negative ellipticity after 220 nm, and a smoothed peak of positive ellipticity (SI Appendix, Fig. S11), indicating changes in α-helical structure and β-sheet content, respectively (23). Taken together, our results suggest that binding of BDSF to the PAS domain induces an allosteric conformational change in RpfR such that the c-di-GMP phosphodiesterase activity, which is conferred by the EAL domain of the protein, is stimulated.
BDSF Signaling System Contributes to the Pathogenicity of B. cenocepacia.
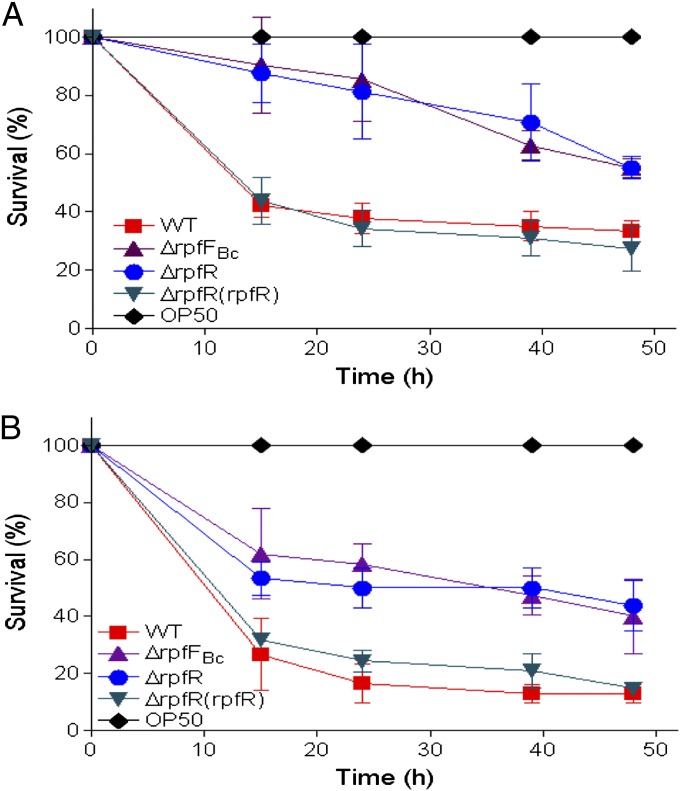
Previous studies have shown that BDSF synthase-negative mutants of B. cenocepacia are attenuated in their virulence (21, 24). To investigate whether RpfR is required for virulence, we tested both the rpfR and rpfFBc mutants in the Caenorhabditis elegans infection model (25). As expected, deletion of either rpfFBc or rpfR led to a reduction of bacterial virulence in both the slow killing and the fast killing assays (Fig. 6). More than 67% of the C. elegans worms incubated with the wild-type strain H111 were killed within 48 h postinoculation in the slow killing assay (Fig. 6A), whereas only 45 and 44.8% of the worms were killed when the rpfFBc and rpfR mutant strains were used as food sources, respectively (Fig. 6A). The fast killing assays showed similar results; the mortality of the worms fed with the H111 wild type and the rpfFBc and rpfR mutants were 87, 60, and 56%, respectively, at 48 h postinfection (Fig. 6B). Importantly, the pathogenicity of the rpfR mutant could be restored to the level of the wild type by complementing in trans with rpfR.
Fig. 6.
Influence of RpfFBc and RpfR on the virulence of B. cenocepacia against C. elegans. (A) Mutants ∆rpfFBc(▲) and ∆rpfR(●) showed reduced virulence compared with their parental wild-type strain H111 (■) and ∆rpfR complemented strain (▼) in slow killing (A) and fast killing (B) assays. Data presented are the mean of triplicate experiments and error bars represent SDs.
Discussion
In this study we identified the unique BDSF receptor RpfR, a multidomain protein containing a PAS, a GGDEF, and an EAL domain (Fig. 1B). This sensor protein is unique in that it uses the QS signal BDSF to alter the levels of the intracellular second-messenger c-di-GMP. An rpfR mutant was phenotypically similar to the BDSF synthase mutant ∆rpfFBc. Both mutants showed increased intracellular c-di-GMP levels (Fig. 1A), reduced bacterial motility (Fig. 2A), decreased biofilm formation (Fig. 2B), reduced proteolytic activity (Fig. 2C), and attenuated virulence (Fig. 6). These results suggest that RpfR is a key component in the BDSF signaling pathway. Interestingly, the EAL but not the GGDEF domain was required to rescue the rpfR mutant phenotypes in complementation experiments (Fig. 3), suggesting that BDSF stimulates the c-di-GMP phosphodiesterase activity of the protein. This hypothesis was further supported by the finding that the intracellular c-di-GMP level of the rpfFBc mutant was increased relative to the wild type, but could be reduced to the level of the wild type when exogenous BDSF was added to the medium (Fig. 1A). Furthermore, biochemical analyses showed that binding of BDSF to RpfR not only caused allosteric conformational changes in the protein (SI Appendix, Fig. S11), but also stimulated c-di-GMP phosphodiesterase activity (Figs. 1A and 5). To our knowledge, RpfR represents a unique example of a c-di-GMP metabolic enzyme that is directly activated by cell–cell communication signals via protein–ligand interaction.
We have shown that BDSF binds to the PAS domain of RpfR (SI Appendix, Fig. S8). Although a range of PAS domains characterized so far are able to bind molecules like flavin mononucleotide, flavin adenine dinucleotide, di/tricabolic acids, or heme groups (26), there is only one study showing direct binding of fatty acids to a PAS domain (27). The multidomain regulatory protein Rv1364c from Mycobacterium tuberculosis, which contains a PAS domain, a phosphate domain, a kinase domain, and an anti-σF antagonist-like domain, is involved in regulation of the stress-dependent σ factor σF. The PAS domain of Rv1364v purified from Escherichia coli was shown to contain palmitic acid (27), which is a saturated long chain fatty acid. Further in vitro binding assay confirmed that the purified PAS domain can bind palmitic acid and other fatty acids including palmtoleic acid and oleic acid (27), but the biological significance of Rv1364v and fatty acid interaction in M. tuberculosis remains unknown.
In Xcc, binding of DSF to its cognate receptor RpfC leads to the activation of c-di-GMP phosphodiesterase activity of RpfG and concomitantly to a lowered intracellular c-di-GMP level (9–11). Likewise, binding of BDSF to RpfR stimulates c-di-GMP degradation and thus reduces the intracellular c-di-GMP concentration in B. cenocepacia. However, the signal transduction mechanisms and the proteins used in the two regulatory cascades are utterly different. The DSF receptor RpfC is a hybrid sensor kinase that phosphorylates its cognate response regulator RpfG. In addition to a REC domain RpfG contains a HD–GYP domain, which was demonstrated to be responsible for the c-di-GMP phosphodiesterase activity (9). By contrast, the BDSF receptor RpfR does not trigger a phosphorelay but is directly modulating c-di-GMP levels via its GGDEF and EAL domains and thus represents a one-component signal transduction system. The coupling of signal perception and second messenger turnover within one molecule may allow B. cenocepacia to very quickly adapt to changing environmental conditions. Another remarkable difference between the two systems is that RpfC is a membrane protein (6, 14), whereas RpfR lacks obvious transmembrane domains (Fig. 1B), suggesting that it interacts with BDSF in the cytosol. There is no significant homology between the two signal receptor proteins. Intriguingly, the PAS domain of RpfR, although capable of binding DSF (SI Appendix, Fig. S8), shows no similarity with the putative DSF binding domain of RpfC, suggesting that the two proteins have evolved independently and represent two different families of fatty acid signal receptors. In summary, these findings suggest that the general role of fatty acid signaling systems in the regulation of virulence is conserved in various microorganisms, but that the regulatory and signaling mechanisms differ among taxa.
RpfR appears to be not the only BDSF sensor/response regulator in B. cenocepacia. In a recent study McCarthy et al. (2010) identified BCAM0227 as a BDSF sensor of B. cenocepacia J2315 (19). Several lines of evidence, however, indicate that the BCAM0227 signaling system plays only a subordinate role in responding to accumulated BDSF signals. Disruption of BCAM0227 only affected the expression of a subset of genes of the BDSF regulon as evidenced by transcriptomic profiling and phenotypic assays (19). Specifically, whereas mutation of rpfFBc drastically reduced motility and adherence to porcine mucin, inactivation of BCAM0227 had no effect (19). In this study we have shown that disruption of rpfR results in very similar phenotypic changes as inactivation of rpfFBc, including reduced motility (Fig. 2A), impaired biofilm formation (Fig. 2B), and lowered proteolytic activity (Fig. 2C). In contrast, none of these BDSF-regulated phenotypes was altered in the BCAM0227 mutants of B. cenocepacia H111 and ΔrpfR (SI Appendix, Fig. S2). There is no obvious similarity between BCAM0227 and RpfR, neither in peptide sequence nor in domain structure. Whereas BCAM0227 contains two transmembrane domains, followed by a HisKA, HATPase-c and a REC domain (19), RpfR consists of PAS–GGDEF–EAL domains (Fig. 1B). An analysis of the currently available Burkholderia genome sequences (28) revealed that homologs of BCAM0227 are only found in five B. cenocepacia strains (J2315, PC184, MC0-3, CCGE1002, and H111), whereas at least 16 strains belonging to diverse Burkholderia species contain not only rpfF but also the rpfR gene adjacent to it, suggesting that BCAM0227 is encoding an alternative BDSF receptor that is used by a few Burkholderia strains as an independent parallel signaling system.
The rpfF/rpfC/RpfG gene cluster is conserved in a range of bacterial species belonging to the genera Xanthomonas, Xylella, Stenotrophomonas, Methylobacillus, Thiobacillus, and Leptospirillum (6). Likewise, a BLAST search with the rpfFBc/rpfR gene cluster revealed that this sensor/signal gene pair is highly conserved in diverse bacterial species including members of the genera Burkholderia, Achromobacter, Yersinia, Serratia, Enterobacter, Pantoea, and Cronobacter (SI Appendix, Table S1). In conclusion, our data suggest that the RpfFBc/RpfR system is a unique QS signaling mechanism that is present in various bacterial pathogens. Our findings may trigger further studies to investigate roles and signaling mechanisms of this QS system in diverse bacterial genera.
Materials and Methods
Bacterial Growth Conditions and Virulence Assays.
Bacterial strains used in this work are listed in SI Appendix, Table S2. B. cenocepacia H111 strains were cultured at 37 °C in NYG medium [5 g peptone (Difco), 3 g yeast extract (Difco), and 20 g glycerol per liter] (29) or LB Lennox. The following antibiotics were supplemented when necessary: tetracycline, 100 μg ml−1; trimethoprim, 25 μg ml−1; and gentamicin, 10 μg ml−1. Killing assays were performed using C. elegans strain Bristol N2. Nematodes were maintained on nematode growth medium at 23 °C (30). Slow killing assays were performed on NGM medium and fast killing assays on high-osmolarity peptone-glucose-sorbitol medium (25). BDSF signal molecules were added to the medium as indicated.
Mutagenesis and Phenotype Analysis.
B. cenocepacia H111 was used as the parental strain to generate the in-frame deletion mutants of rpfFBc and rpfR, respectively, following the method described previously (16). Point mutations in pBBR-rpfR were generated on the basis of the QuikChange site-directed mutagenesis system (Agilent). B. cenocepacia strain H111 and its derivatives were grown in 1 L of NYG medium at 37 °C for 24 h with shaking at 200 rpm. Intracellular c-di-GMP molecules were isolated as described previously (31). Biofilm formation and protease assay were performed following the previously described methods (32, 33). Detailed descriptions of the above methods are provided in SI Appendix.
Protein Purification and Analysis.
Detailed descriptions are given in SI Appendix. Briefly, the coding region of rpfR was amplified with the primers listed in SI Appendix, Table S3 and fused to the expression vector pGEX-6p-1 (Amersham). The fusion gene construct was transformed into the E. coli strain BL21. Affinity purification of GST–RpfR fusion proteins and determination of the enzyme activity of RpfR were performed following the method described previously (34). Far-UV CD analysis of RpfR was carried out on a JASCO J-810 spectropolarimeter as previously described (35).
Supplementary Material
Acknowledgments
We thank Aurélien Carlier and Ulrich Omasits for bioinformatic support, Urs Jenal for valuable discussions, and Amy Lim and Alexander Grunau for excellent technical assistance. This work was supported by the Biomedical Research Council; the Agency of Science, Technology and Research (A*Star), Singapore (L.H.Z.); Swiss National Science Foundation Project 31003A_122013 (to L.E.); and Swiss SystemsX.ch initiative Grant IPP 2011/121 (to C.H.A. and L.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205037109/-/DCSupplemental.
References
- 1.Fuqua C, Greenberg EP. Listening in on bacteria: Acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 2.Zhang LH, Dong YH. Quorum sensing and signal interference: Diverse implications. Mol Microbiol. 2004;53:1563–1571. doi: 10.1111/j.1365-2958.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 3.Deng Y, Wu J, Tao F, Zhang LH. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem Rev. 2011;111:160–173. doi: 10.1021/cr100354f. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology. 2007;153:3923–3938. doi: 10.1099/mic.0.2007/012856-0. [DOI] [PubMed] [Google Scholar]
- 6.He YW, Zhang LH. Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol Rev. 2008;32:842–857. doi: 10.1111/j.1574-6976.2008.00120.x. [DOI] [PubMed] [Google Scholar]
- 7.Barber CE, et al. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol. 1997;24:555–566. doi: 10.1046/j.1365-2958.1997.3721736.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang LH, et al. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004;51:903–912. doi: 10.1046/j.1365-2958.2003.03883.x. [DOI] [PubMed] [Google Scholar]
- 9.Ryan RP, et al. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc Natl Acad Sci USA. 2006;103:6712–6717. doi: 10.1073/pnas.0600345103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.He YW, et al. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J Biol Chem. 2006;281:33414–33421. doi: 10.1074/jbc.M606571200. [DOI] [PubMed] [Google Scholar]
- 11.He YW, et al. Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: Identification of novel cell-cell communication-dependent genes and functions. Mol Microbiol. 2006;59:610–622. doi: 10.1111/j.1365-2958.2005.04961.x. [DOI] [PubMed] [Google Scholar]
- 12.Tao F, He YW, Wu DH, Swarup S, Zhang LH. The cyclic nucleotide monophosphate domain of Xanthomonas campestris global regulator Clp defines a new class of cyclic di-GMP effectors. J Bacteriol. 2010;192:1020–1029. doi: 10.1128/JB.01253-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol Microbiol. 2000;38:986–1003. doi: 10.1046/j.1365-2958.2000.02196.x. [DOI] [PubMed] [Google Scholar]
- 14.He YW, et al. Xanthomonas campestris cell-cell communication involves a putative nucleotide receptor protein Clp and a hierarchical signalling network. Mol Microbiol. 2007;64:281–292. doi: 10.1111/j.1365-2958.2007.05670.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LH. A novel C-di-GMP effector linking intracellular virulence regulon to quorum sensing and hypoxia sensing. Virulence. 2010;1:391–394. doi: 10.4161/viru.1.5.12487. [DOI] [PubMed] [Google Scholar]
- 16.Boon C, et al. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2008;2:27–36. doi: 10.1038/ismej.2007.76. [DOI] [PubMed] [Google Scholar]
- 17.Deng Y, Wu J, Eberl L, Zhang LH. Structural and functional characterization of diffusible signal factor family quorum-sensing signals produced by members of the Burkholderia cepacia complex. Appl Environ Microbiol. 2010;76:4675–4683. doi: 10.1128/AEM.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi H, Christensen QH, Feng Y, Wang H, Cronan JE. The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Mol Microbiol. 2012;83:840–855. doi: 10.1111/j.1365-2958.2012.07968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy Y, et al. A sensor kinase recognizing the cell-cell signal BDSF (cis-2-dodecenoic acid) regulates virulence in Burkholderia cenocepacia. Mol Microbiol. 2010;77:1220–1236. doi: 10.1111/j.1365-2958.2010.07285.x. [DOI] [PubMed] [Google Scholar]
- 20.Römling U, Gomelsky M, Galperin MY. C-di-GMP: The dawning of a novel bacterial signalling system. Mol Microbiol. 2005;57:629–639. doi: 10.1111/j.1365-2958.2005.04697.x. [DOI] [PubMed] [Google Scholar]
- 21.Deng Y, Boon C, Eberl L, Zhang LH. Differential modulation of Burkholderia cenocepacia virulence and energy metabolism by the quorum-sensing signal BDSF and its synthase. J Bacteriol. 2009;191:7270–7278. doi: 10.1128/JB.00681-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao F, Yang Y, Qi Y, Liang ZX. Catalytic mechanism of cyclic di-GMP-specific phosphodiesterase: A study of the EAL domain-containing RocR from Pseudomonas aeruginosa. J Bacteriol. 2008;190:3622–3631. doi: 10.1128/JB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Won HS, Lee TW, Park SH, Lee BJ. Stoichiometry and structural effect of the cyclic nucleotide binding to cyclic AMP receptor protein. J Biol Chem. 2002;277:11450–11455. doi: 10.1074/jbc.M112411200. [DOI] [PubMed] [Google Scholar]
- 24.Ryan RP, McCarthy Y, Watt SA, Niehaus K, Dow JM. Intraspecies signaling involving the diffusible signal factor BDSF (cis-2-dodecenoic acid) influences virulence in Burkholderia cenocepacia. J Bacteriol. 2009;191:5013–5019. doi: 10.1128/JB.00473-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Köthe M, et al. Killing of Caenorhabditis elegans by Burkholderia cepacia is controlled by the cep quorum-sensing system. Cell Microbiol. 2003;5:343–351. doi: 10.1046/j.1462-5822.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 26.Henry JT, Crosson S. Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu Rev Microbiol. 2011;65:261–286. doi: 10.1146/annurev-micro-121809-151631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King-Scott J, et al. Structural characterization of the multidomain regulatory protein Rv1364c from Mycobacterium tuberculosis. Structure. 2011;19:56–69. doi: 10.1016/j.str.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Winsor GL, et al. The Burkholderia Genome Database: Facilitating flexible queries and comparative analyses. Bioinformatics. 2008;24:2803–2804. doi: 10.1093/bioinformatics/btn524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels MJ, Barber CE, Turner PC, Cleary WG, Sawczyc MK. Isolation of mutants of Xanthomonas campestris pathovar campestris showing altered pathogenicity. J Gen Microbiol. 1984;130:2447–2455. [Google Scholar]
- 30.Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda A, Wood TK. Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885) PLoS Pathog. 2009;5:e1000483. doi: 10.1371/journal.ppat.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber B, et al. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology. 2001;147:2517–2528. doi: 10.1099/00221287-147-9-2517. [DOI] [PubMed] [Google Scholar]
- 33.Safarík I. Thermally modified azocasein—a new insoluble substrate for the determination of proteolytic activity. Biotechnol Appl Biochem. 1987;9:323–324. [PubMed] [Google Scholar]
- 34.He YW, Boon C, Zhou L, Zhang LH. Co-regulation of Xanthomonas campestris virulence by quorum sensing and a novel two-component regulatory system RavS/RavR. Mol Microbiol. 2009;71:1464–1476. doi: 10.1111/j.1365-2958.2009.06617.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang LH, Weng LX, Dong YH, Zhang LH. Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase) J Biol Chem. 2004;279:13645–13651. doi: 10.1074/jbc.M311194200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.