Abstract
Pancreatic adenocarcinoma is the most lethal of the solid tumors and the fourth-leading cause of cancer-related death in North America. Matrix metalloproteinases (MMPs) have long been targeted as a potential anticancer therapy because of their seminal role in angiogenesis and extracellular matrix (ECM) degradation of tumor survival and invasion. However, the inhibition specificity to MMPs and the molecular-level understanding of the inhibition mechanism remain largely unresolved. Here, we found that endohedral metallofullerenol Gd@C82(OH)22 can successfully inhibit the neoplastic activity with experiments at animal, tissue, and cellular levels. Gd@C82(OH)22 effectively blocks tumor growth in human pancreatic cancer xenografts in a nude mouse model. Enzyme activity assays also show Gd@C82(OH)22 not only suppresses the expression of MMPs but also significantly reduces their activities. We then applied large-scale molecular-dynamics simulations to illustrate the molecular mechanism by studying the Gd@C82(OH)22–MMP-9 interactions in atomic detail. Our data demonstrated that Gd@C82(OH)22 inhibits MMP-9 mainly via an exocite interaction, whereas the well-known zinc catalytic site only plays a minimal role. Steered by nonspecific electrostatic, hydrophobic, and specific hydrogen-bonding interactions, Gd@C82(OH)22 exhibits specific binding modes near the ligand-specificity loop S1′, thereby inhibiting MMP-9 activity. Both the suppression of MMP expression and specific binding mode make Gd@C82(OH)22 a potentially more effective nanomedicine for pancreatic cancer than traditional medicines, which usually target the proteolytic sites directly but fail in selective inhibition. Our findings provide insights for de novo design of nanomedicines for fatal diseases such as pancreatic cancer.
Keywords: indirect inhibition mode, tumor metastasis, antiangiogenesis, inhibition of MMPs
Despite extensive studies, metastasis remains to be one of the least-known mechanisms, with typically two distinct outcomes: either relatively easily curable benign tumors or detrimental malignant cancers (1). Before effective metastases, tumor cells must accumulate sufficient genetic mutations and/or epigenetic changes capable of breaking both physical and physiological barriers, with the local domain expended (2). In the process of tumor proliferation beyond a certain size, a cancer tissue suffers from oxygen and nutrient deficiencies, for which new but chaotic blood vessels are formed around the tumor tissue by angiogenesis and vasculogenesis (3). Angiogenesis depends on the balance of several proteins and antiangiogenic factors (3). The matrix metalloproteinases (MMPs) play a central role in these processes of angiogenesis. Proteolysis of the extracellular matrix (ECM) is a prerequisite for angiogenesis, and activated MMPs (specifically, MMP-2 and MMP-9) are present in endothelial cells of blood vessels at sites of angiogenesis. Both MMP-2 and MMP-9 are responsible for degradation of the constituents of basement membranes and the ECM (4). Pancreatic cancer is no exception; MMP-2 and MMP-9 show high levels of gene expression in clinical and experimental models relative to normal pancreas (5). Despite a growing concern about safety of engineered nanomaterials, the endohedral metallofullerenol Gd@C82(OH)22 has recently been successfully demonstrated to have a high efficacy in interfering neoplastic activity, as well as metastasis, in a mouse cancer model, with almost no toxicity to normal cells in vivo and in vitro (6), where the metallofullerenol was originally developed for a high-contrast bioimaging agent such as in magnetic resonance imaging (MRI) by securing a toxic metal in the biocompatibly modified fullerenol cage (7–9).
Results and Discussion
Gd@C82(OH)22 Potently Inhibits Tumor Growth in Human Pancreatic Tumor Xenografts Through Suppression of Angiogenesis.
In our results, Gd@C82(OH)22 can significantly decrease pancreatic tumor growth compared with the control group (Fig. 1 A and B and Table S1), and there is a dose-dependency in the antitumor effect of Gd@C82(OH)22. With the same dose (1 μmol/kg), the antitumor efficiency of Gd@C82(OH)22 was much higher than that of C60(OH)22 (50.1% and 27.8%, respectively). We then directly observed the blood vessels of pancreatic carcinoma using the environmental scanning electron microscope (ESEM). The tumor tissue surface is rough and dense, with a great quantity of disordered blood vessels (Fig. 1C). Much larger, denser, and heavier blood vessels were observed on the surface of tumor in the saline control group under the same magnification of microscope (Fig. 1 Ca and Ce).
Fig. 1.
Antitumor effect of Gd@C82(OH)22 nanoparticles on a JF305 pancreatic carcinoma model. (A) Tumor growth curves of the nude mice treated with saline, C60(OH)22 nanoparticles (1.0 μmol/kg per day), and Gd@C82(OH)22 nanoparticles (0.2 μmol/kg per day or 1.0 μmol/kg per day). (B) Photographs of representative tumors from mice 26 d after receiving JF305 cell implantation. (C) ESEM microphotograph of the surface of tumors. The saline group (a and e); C60(OH)22 (1.0 μmol/kg per day) group (b and f); Gd@C82(OH)22 (0.2 μmol/kg per day) group (c and g); Gd@C82(OH)22 (1.0 μmol/kg per day) group (d and h). The magnified image shows the detailed form of blood vessels of the surface of tumors in corresponding groups.
To verify the ESEM results, at the same time, we detected the blood vessels by intravital imaging using fluorescent probes (Fig. S1). Tail i.v. injection of fluorescein isothiocyanate (FITC)-labeled dextrans (70 kDa) was used to visualize the local blood vessels around pancreatic tumor xenograft in nude mice. Similarly, a great quantity of disordered blood vessels can be clearly seen in the saline group. The blood vessels in the Gd@C82(OH)22-treated group are far fewer under the same magnification of microscope (Fig. S1).
The microvessels density (MVD) in tumors is closely correlated with the tumor’s prognosis and biological behavior. CD31 is a transmembrane glycoprotein that is highly expressed in endothelium, and it localizes at the endothelial cell junctions, suggesting its important role in transendothelial cellular migration. Anti-CD31 antibody stained the small vessels with immature endothelium, indicating active angiogenesis within the tumor. CD31 antigen is a commonly used endothelial marker for quantifying angiogenesis by calculation of MVD. As expected, there were far fewer blood vessels and lower expression of CD31 in the Gd@C82(OH)22-treated groups (Fig. S2).
Gd@C82(OH)22 Suppressed Angiogenesis via Potently Reducing MMP Expression and Activity.
Our present results strongly suggest that Gd@C82(OH)22 suppresses tumor growth, at least partially, through its inhibitory action to angiogenesis, where the expressions of MMP-2 and MMP-9 are essential for angiogenesis and tumor development. Therefore, the angiogenic growth factors MMP-2 and MMP-9 received further investigation. We measured the expression levels of these proteins in tumor sections through immunohistochemical reactions and found that MMP-2 and MMP-9 expressions in the Gd@C82(OH)22-treated groups were significantly lower than those in control groups (Fig. 2), particularly with MMP-9 mRNA (Fig. 2B and Table S2). Another less critical angiogenic growth factor, VEGF, was also investigated, with similar results (data shown in Fig. S3). These findings suggest that MMP-2/-9 expression is related to the carcinogenesis and prognosis of pancreatic carcinoma; thus, the inhibition of MMPs by Gd@C82(OH)22 has great promise for treating this lethal disease.
Fig. 2.
Influences of Gd@C82(OH)22 treatment on the protein expression and mRNA levels of MMP-2 and MMP-9 in tumor tissues. (A) Immunofluorescence results of MMP-2 and MMP-9 in JF305 pancreatic tumors (200×). (B) Semiquantitative RT-PCR analysis of the genes MMP-2 and MMP-9. Protein levels of MMP-2 (C) and MMP-9 (D) in the tumor of mice treated with saline and C60(OH)22 and Gd@C82(OH)22 nanoparticles quantified by ELISA. Results are expressed as means ± SD. *P < 0.05; **P < 0.01, significantly different from the saline group. (E) The combined activity of MMP-9/MMP-2 was determined by a MMP enzyme-activity assay. Gd@C82(OH)22 effectively inhibits MMP-9/-2 activity, whereas C60(OH)22 has little effect on them compared with control.
MMP-9 Can Interact with Gd@C82(OH)22 Clusters While Maintaining Its Native Fold.
Then, we focused on the interaction of Gd@C82(OH)22 [or control fullerenol C82(OH)22] with MMP-9 using all-atom, molecular-dynamics simulations, where MMP-9 was chosen because of its stronger effect than MMP-2 and also because of its major proangiogenic role in the tumor metastasis (4, 10–15). [Our experiments were done with C60(OH)22 as control, because C82(OH)22 is not stable; however, in our simulations, we chose C82(OH)22 as control to better capture the effect of the metal ion Gd3+ when comparing with Gd@C82(OH)22 (C60(OH)22 control runs were also performed and results are similar.] Four Gd@C82(OH22) [or C82(OH)22] nanoparticles were mixed with the MMP-9 protein in our simulation system (see SI Methods for more details) and allowed to move freely around MMP-9 while searching for possible binding sites or interacting by themselves. In one representative trajectory (Fig. 3), Gd@C82(OH)22 was shown to form a dimer even before interaction with MMP-9 at ∼21.7 ns. The aggregation of Gd@C82(OH)22 continued to make a trimer by about 40.0 ns, whereas the remaining one started to interact near the loops L34 and L45 of MMP-9. After forming a trimer, intriguingly, one end of the aggregate of Gd@C82(OH)22 was attracted by charged residues (i.e., K214 and E427) in SC and S1′ loops of MMP-9 (61.0 ns). After about 20 ns of “tailgating” interaction to MMP-9, the aggregate, as a whole, ended up binding at the bottom of the lower subdomain of MMP-9, composed of part of helix hA and hB, as well as part of SC and S1′ loops. Upon the binding, however, the MMP-9 seemed stable. The root mean square deviation (rmsd) of MMP-9 showed no obvious differences among the final structures from simulations along with Gd@C82(OH)22, C82(OH)22, or water only (the latter two as controls) (Fig. S4). The rmsds fluctuate between 1.5 and 2.5 Å from the native X-ray crystal structure. We have doubled the simulation length to 500 ns for some representative trajectories, and similar rmsd fluctuations were found (Fig. S4), indicating that the overall protein structure of MMP-9 is very stable even under the “attack” of Gd@C82(OH)22. To the contrary, the local flexibility pattern measured by the root mean square fluctuation (rmsf) clearly differentiated the effect of the metallofullerenol Gd@C82(OH)22 and the normal fullerenol C82(OH)22 on MMP-9; the loop connecting the sheet sI (residues H119 to Q126) and the helix hA (residues N127 to L132) was shown to be more flexible with Gd@C82(OH)22, and the loop L34 became less flexible with C82(OH)22 (Fig. S5; also see below).
Fig. 3.
Molecular dynamics with MMP-9 and Gd@C82(OH)22. (A) X-ray crystal structure of the catalytic domain of MMP-9: two Zn2+ and three Ca2+ are depicted with orange and pink balls, respectively. (B) Endohedral metallofullerenol Gd@C82(OH)22, where the Gd atom is presented inside the fullerenol cage with a pink ball. (C) Molecular dynamics setup with a central MMP-9 surrounded by four Gd@C82(OH)22 at the tetrahedral corners solvated with about 22,000 water molecules in a 90 Å × 90 Å × 90 Å cubic box. (D) Characteristic temporal snapshots of Gd@C82(OH)22 binding onto MMP-9. The metallofullerenol Gd@C82(OH)22 can form an aggregate, followed by binding to MMP-9 on the hydrophobic patch near the ligand specificity loop S1′.
The clustering of Gd@C82(OH)22 in the aqueous solution was also observed previously by using the synchrotron radiation small-angle X-ray scattering (SR-SAXS) and atomic force microscopy (AFM), where Gd@C82(OH)22 existed as a ∼22-nm diameter nanoparticle (6). This implies that the hydrophobic character of the aromatic cage still plays an important role in determining its solvation dynamics, despite significant hydroxyl functionalization and the net negative charge on fullerenol cage of Gd3+@[C82(OH)22]3−. Contrary to the traditional “molecular” form (one drug molecule only), the clustered binding to MMP-9 imposes the significance of the so-called “particulate” form (multiple molecules [Gd@C82(OH)22]n). A large surface area of the aggregate was proposed to attribute a wide range of inhibition of angiogenic factors [i.e., MMP-2/-9, VEGF, CXC chemokine ligand (Cxcl)1, and FGF6] at the mRNA level (16), as well as a more efficient blockage of tumor blood vessels of chaotic and disordered structures rather than the normal blood vessels under tight regulatory control (6).
The nondestructive binding mode of Gd@C82(OH)22 seems contrary to the recent studies about nanotoxicity of the single-wall carbon nanotube (SWCNT), where the SWCNT was shown to severely destroy the protein hydrophobic core and tertiary structure via strong hydrophobic and aromatic π−π stacking interactions (17, 18). Our simulation shows that Gd@C82(OH)22 can have interaction with various types of surface residues, including polar and charged ones. The effective number of contacting amino acid types was about 11 for Gd@C82(OH)22, whereas C82(OH)22 interacts more broadly with 14 different types (Fig. S6). This suggests that although the lack of strong hydrophobicity might not empower Gd@C82(OH)22 to destroy the MMP-9 core and, thus, lose its protease function, it possibly inhibits MMP-9 through a specific binding, by which it could either directly block the Zn2+-coordinated reaction site or, rather, indirectly interfere incoming substrate by interacting with critical regions, such as the ligand-specificity loop S1′, as shown Fig. 3.
Gd@C82(OH)22 Indirectly Inhibits MMP-9 Through a Specific Binding Mode.
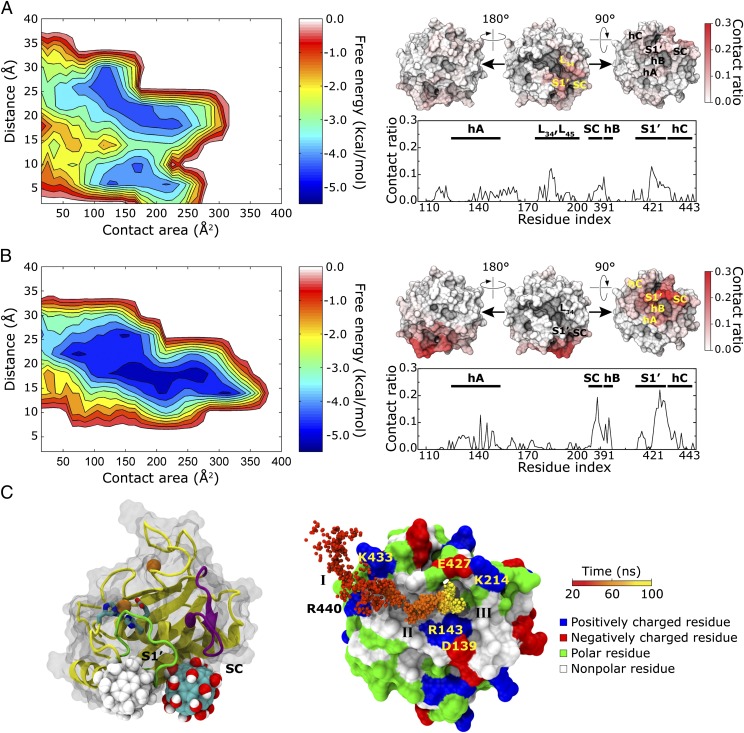
The binding specificity was analyzed by the free energy landscapes in Fig. 4 along two reaction coordinates; one is the minimum distance (xdist) between Gd@C82(OH)22 [or C82(OH)22] and the catalytic zinc ion of MMP-9, and the other is the contacting surface area (yarea) between them. By counting the probability of events pe in a bin (x′dist, y′area), the potential of mean force was calculated by using W(x′dist, y′area) = −RT ln pe(x′dist, y′area) in the unit of kcal/mol. Fig. 4 A and B clearly reveals different binding modes for Gd@C82(OH)22 and C82(OH)22 toward MMP-9. The normal fullerenol C82(OH)22 is characterized to have either multiple binding modes as depicted in multiple energy minima in the short distance, or a nonspecific binding, as shown in a low-energy surface diffused over the long-distance region of the free-energy landscape. On the other hand, the metallofullerenol Gd@C82(OH)22 binds to MMP-9 in a more specific manner, with a narrower distance distribution, a relatively larger contact area, and a more simplified pathway toward the global free-energy minimum. To some extent, the binding of Gd@C82(OH)22 seems to be guided even before the actual contact with MMP-9 takes place (because of its long-range electrostatic interactions; more discussion below). Before the actual contact, for example, C82(OH)22 starts to interact with MMP-9 with relatively more surface sites (i.e., large distance distribution), implying that the fullerenol approaches from solvent to MMP-9 in various directions, whereas Gd@C82(OH)22 has a limited window to approach MMP-9, as indicated in a relatively narrower distance distribution. Despite the restricted contact with MMP-9, Gd@C82(OH)22 binds more tightly, with ∼0.4 kcal/mol stronger binding affinity than that of C82(OH)22.
Fig. 4.
Binding free-energy landscapes and residue-specific contacts on MMP-9, as well as representative binding modes and pathway of Gd@C82(OH)22 on MMP-9. (A) Binding free-energy surface for fullerenol C82(OH)22 on MMP-9 shows a nonspecific binding mode (Left), and almost all surface residues of MMP-9 contribute to contact with C82(OH)22 (Right). (B) Metallofullerenol Gd@C82(OH)22 interacts with MMP-9 along a specified binding mode (Left) and contacts with only a specific set of residues near the ligand-specificity S1′ loop and SC loop (Right). A residue was assigned to be in a contact when any atom in the residue was within 5.0 Å of any atom of Gd@C82(OH)22 [or C82(OH)22]. The site participation is presented by the total number of frames of each residue in contact normalized by all frames and trajectories. (C, Left) Representative binding mode (a solid ball) showing that Gd@C82(OH)22 binds between the S1′ ligand-specificity loop (green ribbon) and the SC loop (purple ribbon), leading to the ligand binding groove. An alternative mode with a gray ball is shown that Gd@C82(OH)22 can bind at the back entrance of the S1′ cavity leading into the active site (ball and stick for active sites and orange ball for the catalytic Zn2+). (Right) Possible binding pathway: depending on major driving forces and duration time (only the first 100 ns is shown), the binding dynamics is characterized with three different phases. Phase I: a diffusion-controlled nonspecific electrostatic interaction; phase II: a transient nonspecific hydrophobic interaction; and phase III: a specific hydrophobic and hydrogen-bonded stable binding.
Binding modes between Gd@C82(OH)22 and C82(OH)22 were more explicitly compared by a site-specific contact analysis in Fig. 4 A and B. As implied in the specific binding mode of the free energy analysis, Gd@C82(OH)22 intensively binds with residues near the S1′ ligand-specificity loop and SC loop encompassing the hydrophobic patch under the bottom of the lower subdomain of MMP-9. In contrast, the normal fullerenol C82(OH)22 exhibits a rather broad contact surface including both the upper and lower rims of MMP-9, consistent with the nonspecific binding modes, as shown in the free-energy landscape. The difference lies in L34 and L45 loops of the upper subdomain of MMP-9, where Gd@C82(OH)22 rarely interacts but where C82(OH)22 frequently binds. This partly explains the rmsf decrease of these loops with the presence of C82(OH)22 (Fig. S5).
With regard to the potential inhibitory mechanism, no clear evidence has been found that either Gd@C82(OH)22 or C82(OH)22 could directly bind into the zinc-coordinated catalytic sites involving H401, E402, H405, and H411, to which no contact has been observed (Fig. 4), thereby discarding the direct inhibitory mechanism. Interestingly, recent studies of the MMP inhibitors are focusing more on the indirect inhibition mechanism (19). Because the zinc-binding sites are highly homologous over various MMPs (20, 21), a small-molecular antagonist directly targeting the catalytic site often fails to selectively inhibit a target MMP, causing various side effects (22). For specificity enhancement, the indirect approach attempts to inhibit the exodomain–substrate interaction by designing selective inhibitors for the unique exocites (23) or allosteric sites (24). The specificity loop has often been focused as a hotspot for selective MMP inhibitors because of its high sequence and length variability in different MMPs. Pyrimidine dicarboxamide derivatives are notable examples with low-nanomolar affinity for MMP-13, interacting only with the unique S1′ pocket but not touching the catalytic zinc at all (25).
Our contact-site analysis suggests an alternative way to inhibit MMP-9. We present two representative binding modes from the free-energy minima in MMP-9 and Gd@82(OH)22 (Fig. 4C). In one mode, Gd@C82(OH)22 can bind at the end gate between the S1′ ligand-specificity loop and the SC loop, which lead to the ligand binding groove (Fig. 4C). Similarly, but at the opposite side of S1′ loop, an alternative mode shows that Gd@C82(OH)22 tightly binds into the back entrance of the S1′ cavity made of the Met-turn Ala417-Tyr420, the S1′ wall Pro420-Arg424, the specificity loop Arg424-Leu431 wrapping the S1′ pocket around, and the part of hexic hC (Fig. 4C, gray ball). Both binding modes exhibited that Gd@C82(OH)22 could be favorably stabilized by interaction with both hydrophobic and hydrophilic residues.
In addition to previous results proposing the indirect inhibition mechanism, our results further propose that Gd@C82(OH)22 might destabilize or interfere with the incoming substrate by allosterically modulating the S1′ ligand-specificity loop, thereby inhibiting the enzymatic activity of MMP-9 in an “indirect” way, even with no direct touch with the catalytic Zn2+.
Possible Gd@C82(OH)22−MMP-9 Binding Pathway.
Understanding possible inhibitor-binding sites and relevant pathways is critical in designing new drug molecules of specific inhibition. We then examined the possible binding pathway of Gd@C82(OH)22 onto MMP-9 and corresponding driving forces. In Fig. 4C, we presented a possible binding dynamics obtained from a representative trajectory. Depending on the major interaction types, the binding dynamics may be divided into three phases (phases I, II, and III): phase I as a long-range electrostatic interaction, phase II as a nonspecific hydrophobic interaction, and phase III as a combination of both specific electrostatic and hydrophobic interactions.
In phase I, Gd@C82(OH)22 diffuses closely to MMP-9 by nonspecific electrostatic interaction, especially via mostly interacting with two positively charged Lys433 and Arg440. The electrostatic guidance can be seen by calculating isoelectric potential around the MMP-9 (Fig. S7). Given the net negative charge (−3.0 e in our calculations) of MMP-9, the negative electric field around MMP-9 prevails over the positive one, reaching much deeper into the solvent. Furthermore, toward the zinc-binding site, the large lobes of negative electric fields are formed, with which the negatively induced fullerenol cage of Gd@C82(OH)22 partly explains why Gd@C82(OH)22 approaches MMP-9 along a more specified direction and why the direct association into the catalytic site is prohibited, even though the negative charges are dispersed over the fullerenol cage. That is, the long-range electrostatic interaction steers the diffusion of Gd@C82(OH)22 toward MMP-9’s Lys433 and Arg440 residues (26, 27), henceforth limiting the initial contact modes, as discussed in the previous section. On the other hand, with a neutral surface C82(OH)22 could interact with MMP-9 at much broader sites. This initial contact is important because it can hold the incoming Gd@C82(OH)22 and then guide the nanoparticle to the next phase of more stable binding. The two basic residues Lys433 and Arg440 play an ideal role as the initial contact, which imposes a long range electrostatic attraction to Gd@C82(OH)22, followed by short-distance hydrogen bonds with available hydroxyl groups in the fullerenol cage.
The nanoparticle Gd@C82(OH)22 is then transferred into the nearby hydrophobic surface patch, where it nonspecifically binds with surface exposed hydrophobic residues (named phase II). It would be thermodynamically beneficial for Gd@C82(OH)22 to interact with hydrophobic residues because the metallofullerenol is still largely hydrophobic despite its negative surface charges and multiple hydroxyl groups. This will reduce the desolvation penalty at the interface between the MMP-9 and metallofullerenol, thereby facilitating their interaction.
Passing through the hydrophobic surface, Gd@C82(OH)22 finally ends up in a stable binding site near the S1′ ligand-specificity loop and SC loop (phase III) (Fig. 4C). Although Gd@C82(OH)22 is swiftly crossing over the hydrophobic surface because of the lack of specific interactions, it is stabilized over about 60 ns using specific hydrogen bondings with hydrophilic and charged residues such as Arg143, Lys214, Thr426, and Glu427, as well as the backbone amide and carbonyl groups. It even has a larger contact with hydrophobic residues eventually as well, which are provided by S1′ loop (Pro429 and Pro430), SC loop (Leu212), and hB (Phe396 and Leu397). In contrast to phase I (mainly electrostatic interaction) or phase II (mainly hydrophobic interaction), phase III exhibits an optimal balance between electrostatic and hydrophobic interaction forces in effectively stabilizing a relatively large nanomolecule on a protein surface.
Conclusion
In summary, we investigated the inhibition capability and underlying molecular mechanism of Gd@C82(OH)22 using a combination of in vivo, in vitro, and in silico approaches. Our nude mouse model clearly shows that Gd@C82(OH)22 effectively blocks tumor growth in human pancreatic cancer xenografts. Our in vitro assays have shown that Gd@C82(OH)22 not only depresses expressions of MMPs but also reduces their activities. Meanwhile, our molecular-dynamics simulations have revealed detailed inhibition dynamics and molecular mechanism behind the Gd@C82(OH)22–MMP-9 interaction. It has been validated that Gd@C82(OH)22 can form a cluster in aqueous solution and interact with MMP-9 as a cluster, as observed in the experiment. We have shown that Gd@C82(OH)22 inhibits MMP-9 mainly via an indirect inhibition mechanism by interacting an exocite far from the zinc catalytic site, but not by directly blocking the active site or destroying the tertiary structure. Energetically and spatially, the ligand-specificity S1′ loop of MMP-9 is characterized as the potential target for Gd@C82(OH)22, henceforth proposing an allosteric control mechanism that Gd@C82(OH)22 inhibits MMP-9 by interfering binding of the incoming ligands as remotely modulating the S1′ loop. The specific binding of Gd@C82(OH)22 is attributed to the amphiphilic-surface characteristic: the hydrophobic fullerene carbon, as well as hydrophilic fullerenol cage by either surface hydroxyl groups or the induced charges by the encaged Gd metal ion. Both the suppression of MMPs expression and specific binding mode make Gd@C82(OH)22 a potentially more effective nanomedicine for pancreatic cancer than the traditional medicines, which usually target the proteolytic sites directly but fail in selective inhibition. These findings provide insights for de novo design of nanomedicine for fatal diseases such as pancreatic cancer. Our findings also imply that the pharmacokinetic action of nanoparticles could be markedly different from the traditional target-based molecular drugs. The non-zinc active site binding naturally avoids the difficulty of using complicated quantum mechanics–based energy functions in computational MMP inhibitor development, which has drawn tremendous effort (19).
Methods
See SI Methods for experimental information about preparation of Gd@C82(OH)22 nanoparticles, human pancreatic tumor xenograft in the nude model, ESEM observation of tumor surface and blood vessels, ELISA, quantitative real-time reverse transcriptase–PCR (RT-PCR), intravital fluorescent imaging of the blood vessels on tumor surface, immunohistochemistry assay, and cellular MMP enzyme activity assays. We have also provided computational protocols on molecular dynamics simulation and force-field parameterization of Gd@C82(OH)22 using DFT-based quantum mechanics (Figs. S8 and S9; see other material in Supporting Information for further validation of our parametrization). The large scale molecular dynamics simulations were carried out with IBM Blue Gene supercomputer.
Supplementary Material
Acknowledgments
We thank Dong Han and Jiayi Xie for help with the use of the ESEM. We also thank Bruce Berne and Sailing He for helpful discussions. This work was financially supported by Ministry of Science and Technology 973 Program Grants 2010CB934004, 2012CB934003, and 2011CB933401 and National Natural Science Foundation of China (NSFC) Grants 21001034 and 10975040. R.Z. acknowledges the support from the IBM Blue Gene Science Program. P.Y. acknowledges partial support from the Environmental Molecular Sciences Laboratory (EMSL) through its intramural program. Part of the quantum mechanics calculations was performed using EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research, located at Pacific Northwest National Laboratory.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204600109/-/DCSupplemental.
References
- 1.Klein CA. Cancer. The metastasis cascade. Science. 2008;321:1785–1787. doi: 10.1126/science.1164853. [DOI] [PubMed] [Google Scholar]
- 2.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: Contrasting, overlapping and compensatory functions. Biochim Biophys Acta. 2010;1803:103–120. doi: 10.1016/j.bbamcr.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stathis A, Moore MJ. Advanced pancreatic carcinoma: Current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, et al. Multihydroxylated [Gd@C82(OH)22]n nanoparticles: Antineoplastic activity of high efficiency and low toxicity. Nano Lett. 2005;5:2050–2057. doi: 10.1021/nl051624b. [DOI] [PubMed] [Google Scholar]
- 7.Bolskar RD, et al. First soluble M@C60 derivatives provide enhanced access to metallofullerenes and permit in vivo evaluation of Gd@C60[C(COOH)2]10 as a MRI contrast agent. J Am Chem Soc. 2003;125:5471–5478. doi: 10.1021/ja0340984. [DOI] [PubMed] [Google Scholar]
- 8.Cagle DW, Kennel SJ, Mirzadeh S, Alford JM, Wilson LJ. In vivo studies of fullerene-based materials using endohedral metallofullerene radiotracers. Proc Natl Acad Sci USA. 1999;96:5182–5187. doi: 10.1073/pnas.96.9.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato H, et al. Lanthanoid endohedral metallofullerenols for MRI contrast agents. J Am Chem Soc. 2003;125:4391–4397. doi: 10.1021/ja027555+. [DOI] [PubMed] [Google Scholar]
- 10.Vu TH, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergers G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 13.Jodele S, et al. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- 14.Heissig B, et al. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–750. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chantrain CF, et al. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- 16.Meng H, et al. Potent angiogenesis inhibition by the particulate form of fullerene derivatives. ACS Nano. 2010;4:2773–2783. doi: 10.1021/nn100448z. [DOI] [PubMed] [Google Scholar]
- 17.Zuo G, Huang Q, Wei G, Zhou R, Fang H. Plugging into proteins: Poisoning protein function by a hydrophobic nanoparticle. ACS Nano. 2010;4:7508–7514. doi: 10.1021/nn101762b. [DOI] [PubMed] [Google Scholar]
- 18.Ge C, et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci USA. 2011;108:16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen JA, Major Jourden JL, Miller MT, Cohen SM. To bind zinc or not to bind zinc: An examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta. 2010;1803:72–94. doi: 10.1016/j.bbamcr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer. 2006;94:941–946. doi: 10.1038/sj.bjc.6603043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tallant C, Marrero A, Gomis-Rüth FX. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim Biophys Acta. 2010;1803:20–28. doi: 10.1016/j.bbamcr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 23.Martens E, et al. A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochim Biophys Acta. 2007;1770:178–186. doi: 10.1016/j.bbagen.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Chen Z, Wang Y, Bonewald L, Steffensen B. Inhibition of MMP-2 gelatinolysis by targeting exodomain-substrate interactions. Biochem J. 2007;406:147–155. doi: 10.1042/BJ20070591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engel CK, et al. Structural basis for the highly selective inhibition of MMP-13. Chem Biol. 2005;12:181–189. doi: 10.1016/j.chembiol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Tan RC, Truong TN, McCammon JA, Sussman JL. Acetylcholinesterase: Electrostatic steering increases the rate of ligand binding. Biochemistry. 1993;32:401–403. doi: 10.1021/bi00053a003. [DOI] [PubMed] [Google Scholar]
- 27.Wade RC, Gabdoulline RR, Lüdemann SK, Lounnas V. Electrostatic steering and ionic tethering in enzyme-ligand binding: Insights from simulations. Proc Natl Acad Sci USA. 1998;95:5942–5949. doi: 10.1073/pnas.95.11.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.