Abstract
Calcidiol, the major circulating metabolite of vitamin D, supports induction of pleiotropic antimicrobial responses in vitro. Vitamin D supplementation elevates circulating calcidiol concentrations, and thus has a potential role in the prevention and treatment of infection. The immunomodulatory effects of administering vitamin D to humans with an infectious disease have not previously been reported. To characterize these effects, we conducted a detailed longitudinal study of circulating and antigen-stimulated immune responses in ninety-five patients receiving antimicrobial therapy for pulmonary tuberculosis who were randomized to receive adjunctive high-dose vitamin D or placebo in a clinical trial, and who fulfilled criteria for per-protocol analysis. Vitamin D supplementation accelerated sputum smear conversion and enhanced treatment-induced resolution of lymphopaenia, monocytosis, hypercytokinaemia, and hyperchemokinaemia. Administration of vitamin D also suppressed antigen-stimulated proinflammatory cytokine responses, but attenuated the suppressive effect of antimicrobial therapy on antigen-stimulated secretion of IL-4, CC chemokine ligand 5, and IFN-α. We demonstrate a previously unappreciated role for vitamin D supplementation in accelerating resolution of inflammatory responses during tuberculosis treatment. Our findings suggest a potential role for adjunctive vitamin D supplementation in the treatment of pulmonary infections to accelerate resolution of inflammatory responses associated with increased risk of mortality.
Keywords: adjunctive therapy, immunomodulation, antimicrobial peptides, matrix metalloproteinases, steroid hormones
Despite the widespread availability of antimicrobials, bacterial respiratory infections remain a major global cause of death (1). Mortality is associated with infection with antibiotic-resistant organisms (2, 3) and with failure to resolve immunopathological inflammatory responses (4–6). Immunomodulatory agents that augment antimicrobial immune responses and accelerate resolution of pulmonary inflammation could be used as adjuncts to antimicrobial therapy to improve treatment outcomes (7).
Calcitriol, the active metabolite of vitamin D, induces innate antimicrobial responses and suppresses proinflammatory cytokine responses in vitro (8). Calcitriol’s antimicrobial activity is mediated via induction of reactive nitrogen intermediates, reactive oxygen intermediates, antimicrobial peptides, and autophagy (9). Calcitriol also modulates adaptive responses, both indirectly (by suppression of MHC class II expression and IL-12 secretion by antigen-presenting cells) and directly [by suppressing secretion of IFN-γ and IL-2 from CD4+ T-helper type 1 (Th1) cells] (10). Calcitriol is synthesized by the vitamin D 1-α hydroxylase enzyme, the expression of which is up-regulated in leukocytes and pulmonary epithelium following ligation of Toll-like receptors by pathogen-associated ligands (11, 12). Extrarenal generation of calcitriol is dependent on the availability of its precursor calcidiol, the major circulating vitamin D metabolite that supports induction of antimicrobial responses in vitro (11, 13) and the concentrations of which are often low in patients with pulmonary infection (14–16). Vitamin D supplementation elevates circulating calcidiol concentrations, and may therefore enhance response to antimicrobial therapy for respiratory infections. However, the effects of in vivo vitamin D supplementation on immune responses in humans with an infectious disease have not previously been described.
Vitamin D was used to treat tuberculosis in the preantibiotic era (17), and vitamin D supplementation has been shown to enhance healthy tuberculosis contacts’ immunity to mycobacteria (18). These observations prompted us to conduct a randomized controlled trial evaluating the influence of adjunctive high-dose vitamin D on time to bacterial clearance in patients receiving antimicrobial therapy for smear-positive pulmonary tuberculosis (19). We now present results of a detailed analysis of longitudinal changes in the immune response in trial participants during the 8-wk course of intensive-phase antituberculous therapy. Initially we describe the effects of antituberculous therapy alone on circulating and antigen-stimulated immune responses, using samples from patients randomized to the placebo arm of the trial. Subsequently we proceed to characterize the effects of vitamin D supplementation using samples from those randomized to the intervention arm of the study, showing that administration of adjunctive vitamin D exerts pleiotropic immunomodulatory effects in patients with pulmonary tuberculosis.
Results
Effect of Antimicrobials on Circulating Responses.
Determination of the immunomodulatory effects of vitamin D supplementation necessitated an initial, comprehensive characterization of the changes in immune responses induced by antituberculous therapy alone. To this end, 42 soluble factors and 14 hematological parameters, detailed in Materials and Methods, were measured in samples of serum, plasma, and whole blood taken from 51 patients randomized to the placebo arm of the trial at 0, 2, 4, 6, and 8 wk of treatment (for trial profile, see Fig. S1; for baseline characteristics, see Table S1). Parameters were selected on the basis that they played a role in host defense against Mycobacterium tuberculosis (MTB) (20) or that they were biomarkers of treatment response (21). Median serum concentrations of seven soluble factors [IL-2, IL-5, IL-13, IL-17, TNF, FGF-β, and matrix metalloproteinase-7 (MMP-7)] were below the limit of detection at baseline, and these were excluded from statistical analyses. The remaining 49 parameters were assessed using principal component analysis (PCA), a well-established mathematical technique for reducing the dimensionality of complex datasets by transforming the data to a new coordinate system. The first three coordinates (principal components) are represented as a 3D plot. The first principal component accounts for as much of the variability in the data as possible, and each succeeding component has the highest variance possible under the constraint that it is uncorrelated with preceding components. This method allows visualization of the differences between patient samples and analytes within complex datasets (22). The resultant PCA plot (Movie S1A) showed that baseline samples were less tightly clustered than follow-up samples. Comparison of the median sum of Euclidean distances between points in the PCA plot at baseline vs. 8 wk confirmed that this convergence was statistically significant (P < 0.0001) (Fig. S2A), indicating that patients had a relatively heterogeneous circulating immunological profile at baseline that became more homogenous as treatment progressed.
Rank-regression analysis (23) was applied to PCA-transformed data to identify parameters whose concentration changed significantly over time. Table S2 presents details of the 42 circulating immunological parameters so identified. Of the hematological parameters investigated, platelet count, neutrophil count, and monocyte count decreased during the course of treatment (P ≤ 0.0018), but lymphocyte count and eosinophil count both increased (P ≤ 0.0019). Increases were also seen in hemoglobin concentration and red blood cell parameters (P ≤ 0.015), reflecting resolution of microcytic anemia as treatment progressed. Decreases in erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) (P ≤ 1.24 × 10−12) and an increase in serum albumin concentration (P = 5.17 × 10−21) were also seen, indicating resolution of the acute-phase response. These changes were accompanied by a decrease in circulating concentrations of all cytokines, chemokines, antimicrobial peptides (AMP), MMP, and angiogenic factors identified (P ≤ 0.0240), except for CC chemokine ligand 2 (CCL2) and MMP-2, the concentrations of which increased during the course of treatment (P ≤ 0.0035).
To investigate the relationship between changes in cell counts and circulating concentrations of inflammatory mediators observed during treatment, a PCA network was created for the parameters listed in Table S2. This network connected each analyte to one other analyte with which it shared the most similar pattern of change over time; the distance between analytes in the network represents their Pearson correlation coefficients. Seven distinct clusters were identified (Movie S2A). For parameters whose values fell during treatment, the tightest cluster incorporated three MMP (MMP-1, MMP-8, and MMP-9) with three AMP [human neutrophil peptides (HNP) 1–3, neutrophil gelatinase-associated lipocalin (NGAL), and cathelicidin (LL-37)]; this cluster was close to neutrophils, CXC chemokine ligand 8 (CXCL8), and prostaglandin E2 (PGE2). Neutrophils were linked to monocytes and CRP, which in turn was linked to IL-6 and ESR. Platelet count and CCL5 formed a distinct grouping, and the other IFN-γ–stimulated chemokines, CXCL9 and CXCL10, were linked to each other and to IFN-γ, which was linked to IL-6. The angiogenic factors, EGF, hepatocyte growth factor (HGF), and VEGF were linked to each other and to IL-7, IL-10, IL-15, and soluble IL-2 receptor (IL-2R). For parameters increasing during treatment, one network incorporated red blood cell parameters, albumin, and MMP-2, and another linked lymphocyte and eosinophil counts.
Effect of Antimicrobials on Antigen-Stimulated Responses.
Whole-blood samples taken from 28 patients randomized to the placebo arm of the trial were stimulated ex vivo with a panel of mycobacterial antigens, and the concentration of IFN-γ in supernatants of baseline samples was compared between stimuli. Of the MTB-specific antigens tested, recombinant early-secreted antigenic target 6 kDa (rESAT-6) and recombinant culture filtrate protein 10 kDa (rCFP-10) induced the greatest IFN-γ responses (Fig. S3). We therefore proceeded to assay concentrations of the 39 soluble factors listed in Materials and Methods in supernatants of whole blood stimulated with these two antigens at 0, 2, 4, 6, and 8 wk of treatment. Median concentrations of six soluble factors (IL-2, IL-5, IL-13, EGF, FGF-β, and MMP-7) were below the limit of detection in these samples, and these parameters were therefore excluded from statistical analyses. The remaining 33 parameters underwent PCA transformation and rank regression analysis. The resultant PCA plots for rESAT-6–and rCFP-10–stimulated responses (Movie S1 B and C) were similar to each other: samples converged from a loosely clustered pattern at baseline toward a more tightly clustered pattern at 4 wk, and then back to a more loosely clustered pattern at 8 wk, changes confirmed as being statistically significant by analysis of the sums of Euclidean distances at these time points (P < 0.0001) (Fig. S2 B and C). Twenty-seven antigen-stimulated parameters contributed to the pattern of response to antituberculous therapy (Table S2). All analytes whose concentration changed significantly during the course of intensive-phase antituberculous therapy showed a decrease in secretion over time. Of note, IFN-γ was among the analytes whose antigen-stimulated concentration did not change significantly during the course of antituberculous therapy, even when corrected for changes in lymphocyte count (Fig. S4).
To determine whether changes in antigen-stimulated immune responses corresponded to changes in whole-blood cellular composition during antituberculous therapy, network PCA was applied to the antigen-stimulated analytes listed in Table S2 together with cell-count data obtained for the relevant samples before antigenic stimulation. Similar PCA networks were identified for rCFP-10– and rESAT-6–stimulated responses (Movie S2 B and C). Platelets, CCL5, IL-4, G-CSF, and CCL11 were connected in both plots, replicating the platelet–CCL5 connection observed in the analysis of circulating parameters (Movie S2A). Neutrophils occupied a similar space to neutrophil granule-associated proteins MMP-9 and NGAL. IL-7 clustered with another angiogenic factor, VEGF, and Th1 cytokines. Lymphocytes were not connected directly to any cytokines stimulated by rESAT-6 or rCFP-10.
Effects of Vitamin D on Circulating Responses.
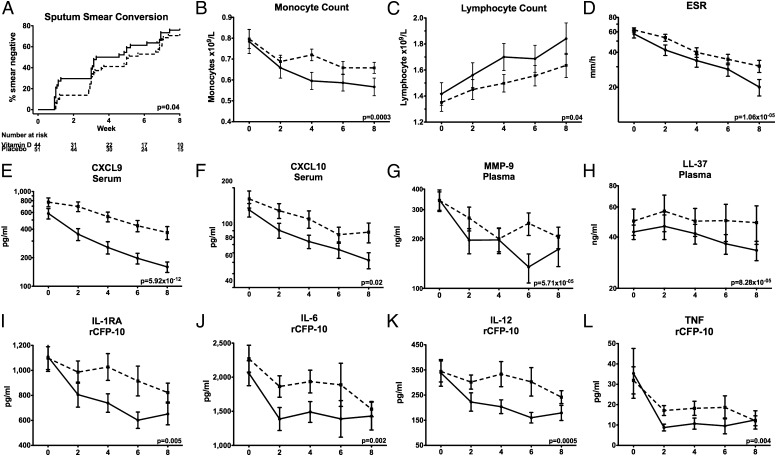
We have previously reported results of the intention-to-treat analysis of study data, indicating that administration of adjunctive vitamin D was associated with a trend toward faster sputum culture conversion (P = 0.14) (19). We repeated this analysis in the subgroup of 95 participants fulfilling per-protocol analysis criteria, adjusting for factors previously shown to influence time-to-sputum conversion in this dataset (19) (age, ethnicity, baseline sputum smear, neutrophil count, and presence or absence of cavitation on baseline chest radiograph). Median time to sputum culture conversion in this subset of patients was 35 d in the intervention group and 46.5 d in the control group (hazard ratio 1.27, 95% confidence interval 0.76–2.13, P = 0.36), and median time to sputum smear conversion in the intervention arm was significantly shorter than that in the control arm (23 vs. 36 d; hazard ratio 1.69, 95% confidence interval 1.02–2.79, P = 0.04) (Fig. 1A).
Fig. 1.
Kinetics of circulating and antigen-stimulated immune responses during the course of antituberculous therapy in the presence vs. the absence of adjunctive vitamin D. Vitamin D accelerated sputum smear conversion in patients fulfilling per-protocol analysis criteria (A). Monocyte counts fell more quickly (B) and lymphocyte counts rose more quickly (C) among patients in the intervention arm of the trial. Vitamin D also accelerated treatment-induced decreases in ESR (D), circulating concentrations of CXCL9 (E), CXCL10 (F), MMP-9 (G), and LL-37 (H) and rCFP-10–stimulated supernatant concentrations of IL-1RA (I), IL-6 (J), IL-12p40/p70 (K), and TNF (L). Means ± SEM at 0, 2, 4, 6, and 8 wk of treatment are presented. Dotted lines, placebo arm; solid lines, vitamin D arm.
To determine whether the effect of vitamin D on time to sputum clearance in the per-protocol subgroup was associated with immunomodulatory activity, we compared the effect of antituberculous therapy on the circulating immunological parameters investigated above in 51 patients randomized to the placebo arm of the trial vs. 44 patients randomized to receive adjunctive vitamin D using PCA and rank regression on the interaction term “treatment duration*allocation.” The PCA plot is shown in Movie S3A. The 17 circulating parameters identified as being significantly affected by vitamin D are detailed in Table S3. All of these parameters were also significantly affected by intensive-phase antituberculous therapy: in every case, vitamin D accelerated the effect of antituberculous therapy. The analyte most affected by vitamin D was the chemokine CXCL9, whose serum concentration decreased significantly faster in patients randomized to receive vitamin D vs. placebo (P = 5.92 × 10−12). Serum concentrations of three other chemokines (CXCL10, CCL3, and CCL5) also fell more rapidly in patients randomized to vitamin D vs. placebo (P ≤ 0.0164), as did IFN-γ (P = 0.0012). Monocyte counts fell more rapidly (P = 0.0003) and lymphocyte counts rose more rapidly (P = 0.0364) among patients receiving vitamin D. Neutrophil counts were not significantly affected by allocation, but plasma concentrations of neutrophil-associated AMP (LL-37, HNP1–3, and NGAL) and MMP-9 fell more quickly in patients receiving adjunctive vitamin D (P ≤ 0.0112). Administration of vitamin D also induced a more rapid drop in ESR and serum CRP concentration (P ≤ 0.0072), indicating accelerated resolution of the acute-phase response.
Network PCA indicated that the accelerated fall in monocyte count in patients receiving vitamin D was linked to a rise in lymphocyte count and a decrease in ESR and serum CRP concentration (Fig. S5A). The IFN-γ–inducible chemokines CXCL9 and CXCL10 were linked to IFN-γ, IL-2R, IL-10, and CCL3. NGAL, HNP1–3, and MMP-9 formed another cluster, which was linked to LL-37 and PGE2. The kinetics of change in a representative group of circulating immunological parameters over the course of intensive-phase therapy in vitamin D vs. placebo arms are presented in Fig. 1 B–H.
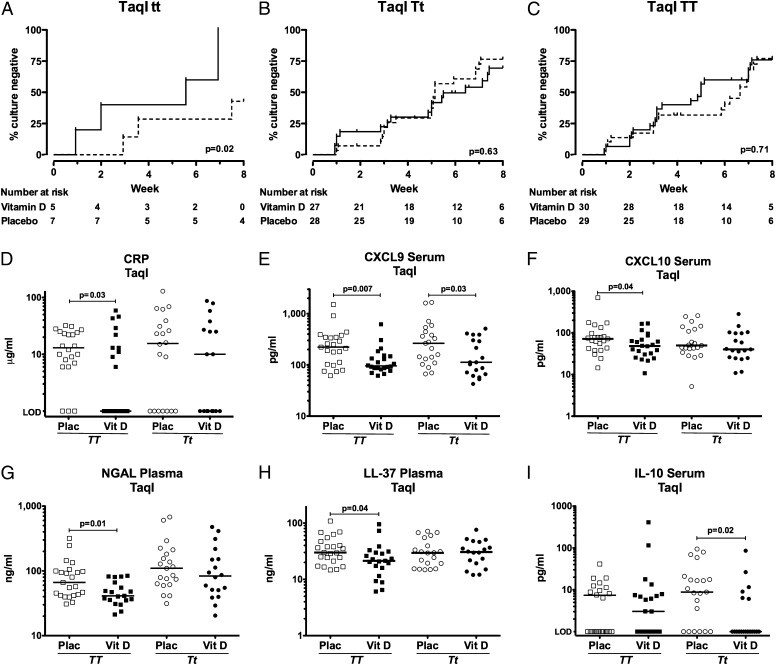
We have previously reported that the effect of vitamin D on time to sputum culture conversion in the intention-to-treat analysis was modified by the TaqI genotype of the vitamin D receptor (VDR), such that vitamin D hastened sputum culture conversion in patients with the tt genotype, but not in those with Tt or TT genotypes (Fig. 2 A–C) (19). To investigate whether the effects of vitamin D on circulating immune responses were also restricted to patients with this genotype, we stratified analysis of the effects of vitamin D on 8-wk values of analytes listed in Table S3 according to TaqI genotype; patients with the tt genotype were not included in this analysis because of small numbers with immunological data available (n = 7). In patients with the TT genotype, vitamin D supplementation significantly reduced 8-wk circulating concentrations of CRP, CXCL9, CXCL10, NGAL, and LL-37, and in those with the Tt genotype, vitamin D significantly reduced 8-wk circulating concentrations of CXCL9 and IL-10 (Fig. 2 D–I) (P ≤ 0.04). Our finding that vitamin D supplementation modulated immune responses in patients with the Tt and TT genotypes indicates that immunomodulatory effects of vitamin D are not restricted to individuals with the tt genotype of the TaqI VDR polymorphism.
Fig. 2.
Immunomodulatory actions of vitamin D are not restricted to individuals with the tt genotype of the TaqI vitamin D receptor polymorphism. Vitamin D supplementation accelerates sputum culture conversion in patients with the tt genotype of the TaqI vitamin D receptor polymorphism (A), but not in those with the Tt (B) or TT (C) genotypes (Pinteraction = 0.03). Vitamin D, solid line; placebo, dotted line. In contrast, the immunomodulatory actions of vitamin D are not restricted to those with the tt genotype of the TaqI polymorphism. In patients with the TT genotype, vitamin D supplementation significantly reduced 8-wk circulating concentrations of CRP (D), CXCL9 (E), CXCL10 (F), NGAL (G), and LL-37 (H); in patients with the Tt genotype, statistically significant reductions in 8-wk serum concentrations of CXCL9 (E) and IL-10 (I) were also seen in patients randomized to vitamin D (Vit D) vs. Placebo (Plac). Data for patients with the tt genotype are not presented because of small numbers entering per-protocol analysis. Line at median; TT placebo (□) n = 23, TT vitamin D (■) n = 22, Tt placebo (○) n = 21, Tt vitamin D (●) n = 19. LOD, limit of detection.
Effects of Vitamin D on Antigen-Stimulated Responses.
We next investigated the effect of vitamin D supplementation on antigen-stimulated responses using PCA and rank regression interaction analysis on 33 analytes detailed above for 19 patients allocated to vitamin D vs. 28 patients allocated to placebo. The PCA plots generated for responses to rESAT-6 and rCFP-10 were similar to each other, and samples from patients allocated to vitamin D vs. placebo were clearly separated (Movie S3 B and C). Vitamin D supplementation influenced supernatant concentrations of seven analytes in both rESAT-6– and rCFP-10–stimulated whole blood (Table S3); notably, IFN-γ was not among them (Fig. S4). Vitamin D enhanced the suppressive effect of antimicrobial therapy on secretion of IL-1 receptor antagonist (IL-1RA), IL-6, IL-12, and TNF (P ≤ 0.0437), and attenuated treatment-induced reductions in secretion of IL-4, CCL5, and IFN-α (P ≤ 0.0323). Network PCA showed that IL-12, TNF, and IL-1RA were linked, but IL-6 was connected to monocytes directly or via VEGF, and CCL5 and IL-4 were tightly clustered and linked to IFN-α for both antigens (Fig. S5 B and C). Vitamin D significantly reduced antigen-stimulated secretion of IL-12, TNF, IL-1RA, IL-6, CXCL10, CCL3, CCL4, and VEGF (Fig. 1 I–L) and enhanced CCL5, CCL11, IL-4, and IFN-α secretion.
Discussion
Our study represents the most detailed characterization of the effects of antituberculous therapy on the immune response conducted to date, and is unique in being a clinical investigation into the immunomodulatory actions of in vivo vitamin D supplementation during treatment of an infectious disease. In patients taking antimicrobial therapy for smear-positive pulmonary tuberculosis, adjunctive vitamin D accelerated sputum smear conversion, augmented treatment-induced increases in lymphocyte count, and enhanced the suppressive effect of treatment on monocyte count, inflammatory markers, and circulating concentrations of chemokines, AMP, and MMP-9. Administration of vitamin D also enhanced treatment-induced suppression of antigen-stimulated Th1 cytokine responses, but attenuated treatment-induced suppression of antigen-stimulated IL-4, CCL5, and IFN-α secretion.
Among 51 patients randomized to receive antituberculous therapy plus placebo, we observed an increase in circulating lymphocyte counts and a reduction in circulating neutrophil counts, monocyte counts, and concentrations of IFN-inducible parameters following initiation of antituberculous therapy, consistent with previous reports (24–26). These changes were associated with decreases in circulating concentrations of lymphocyte chemoattractants CXCL9 and CXCL10, and an increase in the monocyte chemoattractant CCL2; they may therefore reflect reduced recruitment of lymphocytes and increased recruitment of monocytes to the lung. In keeping with this hypothesis, the proportion of macrophages in sputum of tuberculosis patients has been reported to increase as treatment progresses (27). Interestingly, increases in circulating lymphocyte count following initiation of antituberculous therapy were not associated with any change in antigen-stimulated production of IFN-γ as treatment progressed. In contrast, antigen-stimulated production of CCL5, IL-4, G-CSF, IFN-α, and CXCL10 were greatly decreased over the course of intensive-phase therapy. Antigen-stimulated CXCL10 responses have been reported to be more sensitive than IFN-γ for the diagnosis of active tuberculosis (28), and these data suggest that this panel of analytes may also hold promise as antigen-stimulated biomarkers of treatment response. Resolution of thrombocytosis is another well-recognized phenomenon associated with tuberculosis treatment (29), and network analysis revealed this to be linked to a decrease in circulating CCL5 and antigen-stimulated CCL5 and IL-4 among patients in our study. Although best known for their role in hemostasis, platelets are also recognized to secrete CCL5 (30), which can enhance production of IL-4 by CD4+ T cells (31). The role of platelets in the antimycobacterial response warrants further investigation.
Having characterized the immune response to antituberculous therapy, we proceeded to investigate how this was affected by administration of adjunctive vitamin D. In contrast to studies investigating immunomodulatory actions of vitamin D supplementation in healthy people and in those with noncommunicable diseases (32–35), we report pleiotropic immunomodulatory actions of vitamin D in tuberculosis patients. This difference may reflect the very high prevalence of profound deficiency at baseline among participants in our study; the relatively high dose of vitamin D administered; or the fact that MTB can up-regulate expression of the vitamin D 1-α hydroxylase CYP27B1 to generate immunomodulatory concentrations of calcitriol at sites of infection (11). Among patients fulfilling criteria for per-protocol analysis (n = 96), vitamin D accelerated sputum smear conversion (P = 0.04). This finding contrasts with results of our previously published intention-to-treat analysis (n = 126), in which a trend toward faster conversion in vitamin D-supplemented patients did not attain statistical significance (19). This difference may reflect the superior compliance of participants included in the per-protocol analysis, which excluded patients who did not take a full course of study medication. Vitamin D also suppressed circulating concentrations of IFN-γ and IFN-γ-inducible chemokines CXCL9 and CXCL10, MMP-9, and antigen-stimulated Th1 responses. These in vivo findings are consistent with reported immunomodulatory actions of calcitriol in vitro (8, 36, 37). In contrast to these suppressive actions, vitamin D also attenuated treatment-induced falls in antigen-stimulated CCL5, IL-4, and IFN-α. IL-4 has recently been reported to induce expression of CYP24A, the principal catabolic enzyme of both calcidiol and calcitriol (38); the increase in antigen-stimulated IL-4 secretion observed in the intervention arm of the study may therefore represent part of a negative-feedback loop via which calcitriol regulates its own concentration at the site of disease. The finding that administration of vitamin D enhanced antigen-stimulated IFN-α responses is of particular interest, given the pivotal role of type 1 interferons in antiviral responses (39), and the clinical observation of a sixfold reduction in upper respiratory tract infections among patients in the intervention arm of the trial (19). Modulation of antigen-stimulated responses by vitamin D supplementation may represent changes in numbers of circulating lymphocyte subpopulations or direct effects of vitamin D on lymphocyte function. More detailed characterization of the effects of vitamin D supplementation on numbers and cytokine profiles of lymphocyte subsets is warranted.
Although many of the immunomodulatory effects of in vivo vitamin D supplementation that we observed were in keeping with the in vitro actions of calcitriol, there were two exceptions: calcitriol has been reported to induce IL-10 (36) and the antimicrobial peptides LL-37 and NGAL (40, 41) in vitro, but we found that in vivo vitamin D supplementation suppressed circulating concentrations of IL-10, LL-37, and NGAL. All three of these markers are suppressed by antituberculous therapy alone (Table S2), and the fact their concentration fell more quickly among patients in the intervention arm of the study may arise as an indirect consequence of enhanced microbial killing in patients receiving vitamin D. Alternatively, this observation may represent a direct suppressive effect of vitamin D on release of these mediators into the circulation from neutrophil granules.
Interestingly, and in contrast to the effects of vitamin D supplementation on sputum clearance that we have previously demonstrated (19), we found that immunomodulatory effects of vitamin D were observed in patients having the TT and Tt genotypes of the TaqI VDR polymorphism. This observation suggests that if these responses can be augmented—by administering vitamin D at higher doses, for example—then tuberculosis patients might derive a clinical benefit from vitamin D supplementation irrespective of TaqI genotype. More broadly, the ability of vitamin D to accelerate resolution of potentially immunopathological inflammatory responses without compromising bacterial killing raises the possibility that supplementation might also have benefits in patients receiving antimicrobial therapy for pneumonia and sepsis, in whom failure to resolve hypercytokinaemia is associated with increased mortality (5, 6).
Materials and Methods
Details of the trial protocol have previously been reported; participants were randomized to receive four fortnightly doses of 2.5 mg vitamin D3 vs. placebo in addition to standard antituberculous therapy (19). Antigen-stimulated whole-blood assays were performed as previously described (42). Concentrations of IL-1β, IL-1RA, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 (p40/p70), IL-13, IL-15, IL-17, G-CSF, GM-CSF, IFN-α, IFN-γ, TNF, CXCL8, CXCL9, CXCL10, CCL2, CCL3, CCL4, CCL5, CCL11, EGF, FGF-β, HGF, and VEGF were quantified using a human 30-plex bead immunoassay panel (Invitrogen). Serum CRP and albumin concentrations were assayed using an Architect ci8200 analyzer (Abbott Diagnostics). Serum PGE2 concentration was analyzed by high-sensitivity competitive enzyme immunoassay (Assay Designs). Concentrations of LL-37, HNP1-3, and NGAL were analyzed by ELISA (Hycult Biotechnology). Concentrations of MMP-1, -2, -3, -7, and -8 were determined by Fluorokine MAP multianlalyte profiling (R&D Systems); concentration of MMP-9 was determined by DuoSet ELISA (R&D Systems). Antigen-stimulated AMP and MMP concentrations were corrected by subtraction of unstimulated values. Full blood counts were performed using a LH750 hematology analyzer (Beckman Coulter). ESR was measured by the Wintrobe method using a s2000 analyzer (Desaga). DNA extraction and genotyping were performed as previously described (19).
PCA was conducted using Qlucore Omics Explorer 2.2 (Qlucore). Analyte concentrations were log2-converted and normalized to the mean for each analyte with variance −1 to +1. Rank-regression analysis was applied to PCA-transformed data to identify parameters whose concentration was affected by antituberculous therapy (by making within-patient comparison of samples at different time points among patients allocated to placebo) and vitamin D (by making between-patient comparison of samples from patients allocated to placebo vs. vitamin D at each time point). This analysis yielded t statistics (calculated as the regression coefficient for each parameter divided by its SD) representing the magnitude of difference in concentration of a given parameter between groups being compared; P values, representing the probability that such differences could have arisen by chance alone; and q values, representing the lowest false discovery rate for which differences would be accepted as statistically significant under the Benjamini–Hochberg procedure for multiple-testing correction (43). The effects of allocation on circulating immune responses at 8 wk within genetically defined subgroups were analyzed using Mann–Whitney U tests. The effect of allocation on time to sputum clearance was analyzed by Cox regression analysis, adjusting for age, ethnicity, baseline sputum smear, neutrophil count, and presence or absence of cavitation on baseline chest radiograph as previously described (19).
Further details are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Delmiro Fernandez-Reyes (Medical Research Council National Institute of Medical Research) for advice on statistical analysis; Dr. Alleyna Claxton (Homerton University National Health Service Foundation Trust) for assistance with development of microbiological outcome measures; Drs. John C. Moore-Gillon (Barts and The London National Health Service Trust), Thomas C. Stokes (Queen Elizabeth Hospital), and Stefan Lozewicz (North Middlesex Hospital) for acting as Principal Investigators; the members of our Data Monitoring Committee, Dr. Guy E. Thwaites (Chair), Dr. Brenda E. Jones, and Dr. Tuan Q. Phung; all the tuberculosis nurses and administrative staff who referred patients to the study; and all patients who participated in the trial. The study was funded by the British Lung Foundation (TB05/11) and the United Kingdom Medical Research Council (U1175 22141). Merck Serono donated study medication.
Footnotes
Conflict of interest statement: Merck Serono donated €7,000 to Queen Mary University of London in 2010 to support an academic meeting entitled “Vitamin D: Mechanisms of Action in Health and Disease”; this meeting was convened by C.J.G. and A.R.M.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200072109/-/DCSupplemental.
References
- 1.WHO . Global Burden of Disease: 2004 Update. Geneva: WHO; 2008. [Google Scholar]
- 2.Tleyjeh IM, Tlaygeh HM, Hejal R, Montori VM, Baddour LM. The impact of penicillin resistance on short-term mortality in hospitalized adults with pneumococcal pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2006;42:788–797. doi: 10.1086/500140. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi NR, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PF, et al. Predictors of short-term prognosis in patients with pulmonary tuberculosis. J Infect Dis. 1988;158:366–371. doi: 10.1093/infdis/158.2.366. [DOI] [PubMed] [Google Scholar]
- 5.Kellum JA, et al. GenIMS Investigators Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yende S, et al. GenIMS Investigators Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Adjunctive therapies for community-acquired pneumonia: a systematic review. J Antimicrob Chemother. 2008;62:661–668. doi: 10.1093/jac/dkn283. [DOI] [PubMed] [Google Scholar]
- 8.Martineau AR, et al. IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: The role of cathelicidin LL-37. J Immunol. 2007;178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 9.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7:337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 10.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: Modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 12.Hansdottir S, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabri M, et al. Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 15.Martineau AR, et al. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J. 2010;35:1106–1112. doi: 10.1183/09031936.00087009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martineau AR, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci USA. 2011;108:19013–19017. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793–798. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 18.Martineau AR, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 19.Martineau AR, et al. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet. 2011;377:242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrin FM, Lipman MC, McHugh TD, Gillespie SH. Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect Dis. 2007;7:481–490. doi: 10.1016/S1473-3099(07)70112-3. [DOI] [PubMed] [Google Scholar]
- 22.Jolliffe IT. Principal Component Analysis. 2nd Ed. New York: Springer; 2002. [Google Scholar]
- 23.Cuzick J. Rank Regression. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. 2nd Ed. New York: John Wiley & Sons; 2005. [Google Scholar]
- 24.Jones BE, et al. CD4 cell counts in human immunodeficiency virus-negative patients with tuberculosis. Clin Infect Dis. 1997;24:988–991. doi: 10.1093/clinids/24.5.988. [DOI] [PubMed] [Google Scholar]
- 25.Brahmbhatt S, et al. Immune markers measured before treatment predict outcome of intensive phase tuberculosis therapy. Clin Exp Immunol. 2006;146:243–252. doi: 10.1111/j.1365-2249.2006.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry MP, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro-Rodrigues R, et al. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol. 2002;9:818–823. doi: 10.1128/CDLI.9.4.818-823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruhwald M, et al. CXCL10/IP-10 release is induced by incubation of whole blood from tuberculosis patients with ESAT-6, CFP10 and TB7.7. Microbes Infect. 2007;9:806–812. doi: 10.1016/j.micinf.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Tozkoparan E, Deniz O, Ucar E, Bilgic H, Ekiz K. Changes in platelet count and indices in pulmonary tuberculosis. Clin Chem Lab Med. 2007;45:1009–1013. doi: 10.1515/CCLM.2007.194. [DOI] [PubMed] [Google Scholar]
- 30.Schober A, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 31.Saito S, Yamaguchi E, Nakayama H, Miyamoto K, Kawakami Y. Modulatory roles of RANTES in IL-4 production by human blood CD4(+)T cells. Cytokine. 2000;12:1380–1384. doi: 10.1006/cyto.2000.0736. [DOI] [PubMed] [Google Scholar]
- 32.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134:128–132. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 33.Schleithoff SS, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 34.Jorde R, et al. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010;50:175–180. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Yusupov E, et al. Vitamin D and serum cytokines in a randomized clinical trial. Int J Endocrinol. 2010;2010:2010. doi: 10.1155/2010/305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coussens A, et al. 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127:539–548. doi: 10.1111/j.1365-2567.2008.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helming L, et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 38.Edfeldt K, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA. 2010;107:22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaoka A, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 40.Wang TT, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 41.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 42.Schölvinck E, et al. Gamma interferon-based immunodiagnosis of tuberculosis: Comparison between whole-blood and enzyme-linked immunospot methods. J Clin Microbiol. 2004;42:829–831. doi: 10.1128/JCM.42.2.829-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.