Abstract
Tropical marine ecosystems are under mounting anthropogenic pressure from overfishing and habitat destruction, leading to declines in their structure and function on a global scale. Although maintaining connectivity among habitats within a seascape is necessary for preserving population resistance and resilience, quantifying movements of individuals within seascapes remains challenging. Traditional methods of identifying and valuing potential coral reef fish nursery habitats are indirect, often relying on visual surveys of abundance and correlations of size and biomass among habitats. We used compound-specific stable isotope analyses to determine movement patterns of commercially important fish populations within a coral reef seascape. This approach allowed us to quantify the relative contributions of individuals from inshore nurseries to reef populations and identify migration corridors among important habitats. Our results provided direct measurements of remarkable migrations by juvenile snapper of over 30 km, between nurseries and reefs. We also found significant plasticity in juvenile nursery residency. Although a majority of individuals on coastal reefs had used seagrass nurseries as juveniles, many adults on oceanic reefs had settled directly into reef habitats. Moreover, seascape configuration played a critical but heretofore unrecognized role in determining connectivity among habitats. Finally, our approach provides key quantitative data necessary to estimate the value of distinctive habitats to ecosystem services provided by seascapes.
Keywords: amino acid, Lutjanus ehrenbergii, mangroves and seagrass, otoliths, Red Sea
The ecological integrity of tropical marine habitats, including mangroves, seagrass beds, and coral reefs, is coming under increasing pressure from human activities (1–3). Habitat destruction and unsustainable exploitation, including mangrove deforestation and overfishing, have led to declines in the function and resilience of these ecosystems on a global scale (4). Efforts to promote ecological integrity and sustainable harvest have traditionally focused on protecting coral reefs. More recently, attention has been directed at the issue of preserving critical seascape functions as well as habitat types, with particular emphasis on seascape connectivity (5). For example, many commercially and ecologically important coral reef fishes, including species of Lutjanidae (snappers), Serranidae (grouper), and Scaridae (parrotfish), use mangroves and seagrass beds as juvenile nursery areas before presumably migrating to coral reef habitats as adults (see reviews in refs. 6–8). Preserving seascape connectivity is therefore likely necessary to maintain coral reef ecosystem function and healthy fisheries (9). However, it has proved remarkably difficult to develop quantitative assessments of habitat use and movements among different habitat types for any reef fish species (10). This lack of quantitative data on seascape connectivity represents a major obstacle to marine spatial management (5) and attempts to value ecosystems services provided by coral reef habitats (11–13).
A number of studies have demonstrated a strong relationship between the presence of coastal wetlands and offshore fish abundance and fisheries yield (14, 15). These studies formed the basis for the nursery hypothesis (6–8), and subsequently, the economic valuation of coastal wetlands (13). The use of coastal wetlands as nursery habitats may, however, be facultative and spatially complex (16). Studies identifying mangroves and seagrass beds as nurseries have noted higher densities of juvenile fishes in those habitats relative to other habitats where juveniles could reside (16, 17), and have documented size-frequency differences among habitats that are consistent with ontogenetic movements of juvenile fishes from mangrove nurseries to adult reef habitats (14, 15). The conclusions of these studies rely, nonetheless, on the assumption that the increased density of juveniles in nursery habitats will result in increased recruitment into adult populations on coral reefs. To accurately parameterize reserve selection models for the development of effective marine reserves (12), we need to identify specific migration corridors between nursery habitats and reef environs.
Determining movement corridors between juvenile and adult habitats requires the ability to either track individuals between habitats or to retrospectively identify juvenile habitat residency of adult fishes. Natural geochemical tags provide an approach that allows for the reconstruction of habitat residency while avoiding the logistic problems inherent with artificial tagging (10). We recently described a unique method for quantifying fish movements in coral reef ecosystems by analyzing amino acid δ13C values in otoliths (ear-bones) (18–20). The technique relies on natural geographic variations in δ13C at the base of food webs among mangrove habitats, coral reefs, and seagrass beds that are permanently recorded by otolith amino acids. Compound-specific stable isotope analysis (SIA) provides more robust tracers of residency bulk SIA and trace element geochemistry, which have met with mixed results in previous attempts to reconstruct nursery use in coral reef fishes (20).
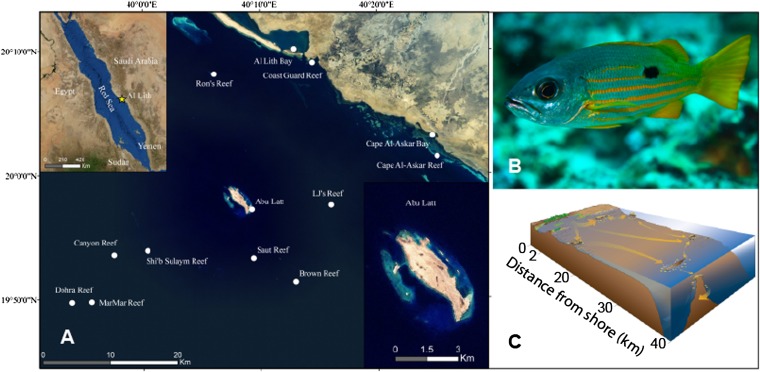
Here we use amino acid δ13C values to quantify seascape connectivity for a commercially important snapper species (Ehrenberg’s snapper, Lutjanus ehrenbergii, Peters 1869) in a coral reef ecosystem from the Red Sea (Fig. 1). Our approach allows for reconstruction of juvenile habitat associations by those fish that have successfully recruited to adult populations on reefs. We characterized unique δ13C signatures from habitats within the study seascape by analyzing five essential amino acid δ13C values from L. ehrenbergii collected from five potential juvenile habitats: coastal wetlands consisting of seagrass bays with fringing mangroves, coastal reefs within 2 km of shore, shelf reefs on the continental shelf, the continental island of Abu Latt at the shelf break, and oceanic reefs surrounded by deep open water (Fig. S1). We then surveyed densities of L. ehrenbergii and collected fish for otolith analysis from two replicate reefs at six distances along a 50-km cross-shelf transect from the coast to oceanic reefs off the continental shelf. Finally, we isolated the juvenile cores from adult L. ehrenbergii otoliths, analyzed their essential amino acid δ13C values, and then classified fish to one of the five potential juvenile habitats based on these multivariate isotope values (see SI Materials and Methods). The multivariate approach allowed us to accurately distinguish residence patterns among source habitats that were not possible using conventional bulk stable isotope analysis (20).
Fig. 1.
Study site and species. (A) Collection sites from coastal wetlands (Al Lith Bay and Cape Al-Askar Bay), coastal reefs (Coast Guard Reef and Cape Al Askar Reef), shelf reefs (Ron’s Reef, LJ’s Reef, Saut Reef, and Brown Reef), a continental island (Abu Latt), and oceanic reefs (Shi’b Sulaym Reef, Canyon Reef, MarMar Reef, and Dohra Reef) near Al Lith, Saudi Arabia in the Red Sea. (B) Ehrenberg’s snapper (Lutjanus ehrenbergii, Peters 1869) is a commercially important reef-associated snapper species in the Indo-West Pacific. (C) Conceptual diagram of habitat configuration and potential seascape connectivity of L. ehrenbergii in the study area.
Results
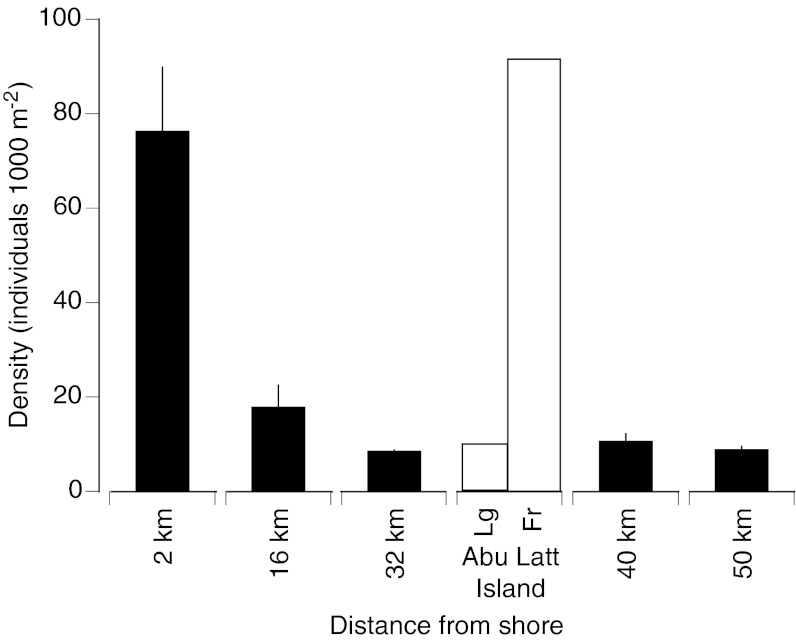
We found significant variability in L. ehrenbergii densities across the continental shelf (Fig. 2). Highest densities were found on nearshore reefs and on the fringing reef surrounding the continental island of Abu Latt. These patterns were consistent with our observations of recently settled juveniles in mangrove and seagrass habitats along the coast and in the lagoon at Abu Latt (SI Materials and Methods). We have, however, never seen juvenile L. ehrenbergii on coastal, shelf, or oceanic reefs despite several years of regular work in this area. Moreover, the sharp drop in densities of adult L. ehrenbergii from nearshore reefs and fringing reefs around Abu Latt Island to shelf and oceanic reefs suggested that the majority of juveniles were moving relatively short distances (∼2 km) from juvenile nursery habitats.
Fig. 2.
Underwater visual census estimates. Adult L. ehrenbergii densities (mean ± SD) on reefs at five distances offshore (n = 2 reefs per distance) and two habitats at Abu Latt Island (24-km offshore). Fr, fringing reef habitat; Lg, lagoon habitat.
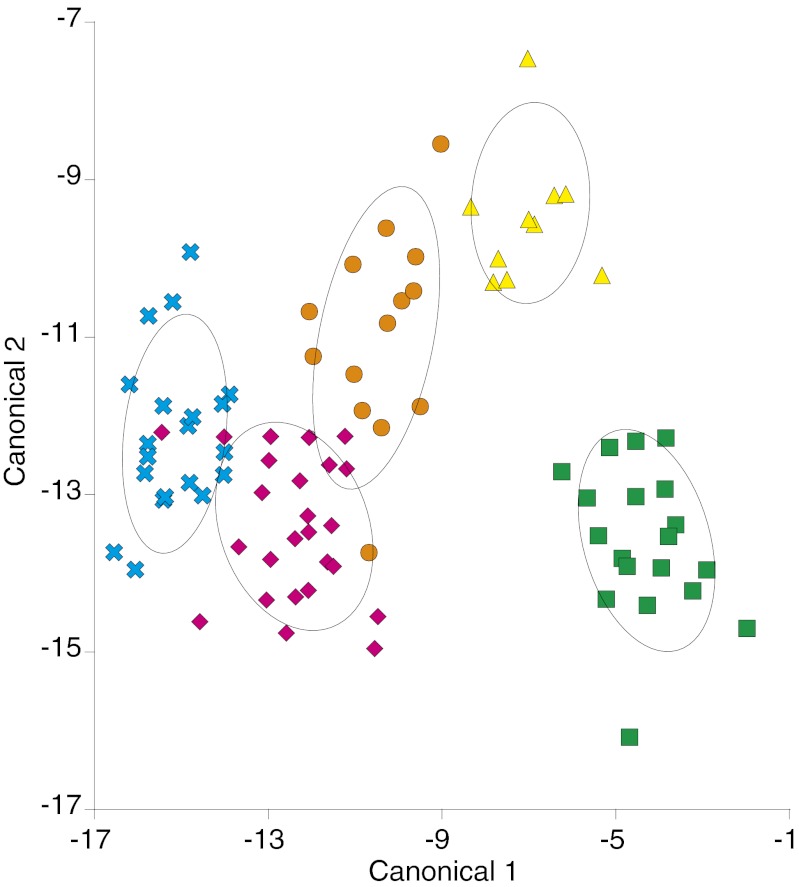
Discriminant function analysis on the muscle essential amino acid δ13C data of L. ehrenbergii showed that each of the five regions was clearly separated in multivariate space (Fig. 3). The first discriminant function identified a gradient from coastal wetlands to oceanic reefs, and the second discriminant function separated coastal wetlands from the shelf island habitat of Abu Latt Island. Moreover, we were able to assign individuals to each of these habitats with a high degree of accuracy based on the multivariate essential amino acid δ13C values. Jackknifed reclassification success rate to each potential juvenile habitat averaged 95% compared with a random reclassification success expectation of 20%.
Fig. 3.
Discrimination of juvenile Lutjanus ehrenbergii habitats based on δ13C values of essential amino acids. Multivariate separation of habitats visualized after discriminant function analysis of five essential amino acid δ13C values from L. ehrenbergii collected from five potential juvenile habitats: coastal wetlands (green squares: n = 19 fish), coastal reefs (orange circles: n = 15), shelf reefs (magenta diamonds: n = 25), Abu Latt Island lagoon and fringing reefs (yellow triangles: n = 10), and oceanic reefs (cyan crosses: n = 20). Colored symbols represent individual fish surrounded by 95% confidence ellipses.
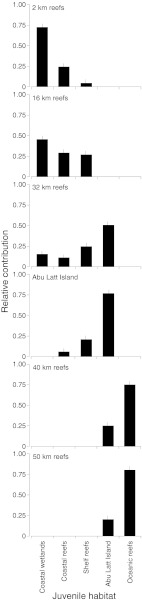
Essential amino acid δ13C values in otoliths revealed a complex pattern of habitat use by juvenile L. ehrenbergii (Fig. 4). Our data also showed that many L. ehrenbergii larvae had apparently settled directly into adult reef habitats. Although we never saw juvenile L. ehrenbergii on offshore reefs, as much as 50% of the adults on coastal and shelf reefs and nearly 80% of adults on oceanic reefs had resided in these habitats for their entire postsettlement lives. These juveniles were likely either highly cryptic, residing inside the reef matrix during daylight hours, or inhabiting depths that were beyond the limits of open-circuit SCUBA equipment. Regardless of their whereabouts, the otolith amino acid technique allowed us to definitively quantify the proportion of each adult population that had resided in different nursery habitats as juveniles.
Fig. 4.
Relative contribution (mean ± SD) of L. ehrenbergii from five potential juvenile habitats to adult populations on offshore coral reefs. Adult L. ehrenbergii were collected from reefs at six distances from the coast along a 50-km cross-shelf transect from Al Lith, Saudi Arabia in the Red Sea (2-km reefs, n = 25 fish; 16-km reefs, n = 20; 32-km reefs n = 20; Abu Latt Island n = 20; 40-km reefs n = 20; and 50-km reefs n = 20) and classified to one of five potential juvenile nursery habitats by otolith essential amino acid δ13C values.
Our results confirmed the importance of mangrove and seagrass systems to inshore fish populations. Over 70% and 45% of adult L. ehrenbergii at the 2-km and 16-km reefs, respectively, had migrated from these coastal wetland habitats as juveniles. A number of individuals had also moved at least 30 km from inshore nurseries to reefs on the edge of the continental shelf. The shelf break did, however, act as a barrier for inshore juveniles because no adults on oceanic reefs beyond the continental shelf had resided in mangrove or seagrass environments.
Discussion
Our results provided direct measurements of remarkable movements by juvenile snapper from coastal wetlands to coral reefs at least 30 km from the coast, and from a shelf island to oceanic reefs across deep open water. Although connectivity was high among coastal wetland and reef environs on the shallow continental shelf, we found no evidence of wetland use in adults from oceanic reefs. Juveniles from near shore areas were apparently reluctant to move beyond the continental shelf. However, juveniles that settled around Abu Latt Island, on the shelf edge, were able to swim across deep open water to the oceanic reefs. These results reveal complex patterns of ontogenetic movement that we were unable to detect using conventional SCUBA-based surveys. We were able to quantify the relative contributions from each nursery habitat to adult populations and to identify specific corridors used by juvenile fish to migrate across the shelf to reef environments. These data are, in turn, critical to parameterize reserve selection algorithms for the development of effective networked marine reserves (12, 21).
Compound-specific SIA data revealed a high degree of plasticity in nursery habitat use. These findings have important implications, both for understanding coral reef fish population biology as well as designing well-informed management strategies. Coastal and shelf reefs appeared to have greater functional connectivity within the seascape than the oceanic reefs. At least three different juvenile source habitats contributed to adult L. ehrenbergii populations on coastal and shelf reefs. Conversely, the oceanic reefs were primarily locally recruiting. Coastal and shelf-reef habitats may, therefore, have a greater source redundancy and thus be less vulnerable to fluctuations in juvenile supply from individual habitats. It appears likely that the shallow continental shelf, typically less than 50-m deep, facilitated enhanced interreef movement compared with the deep open water between oceanic reefs. The shelf break was not a hard barrier, however, because juveniles from Abu Latt Island, located on the edge of the continental shelf, were able to move across open waters to oceanic reefs.
There is little movement data on juvenile coral reef fishes to compare with our results because of the difficulties associated with tagging small fish (10). Mumby (21) constrained the maximum distance fish migrate between mangroves and reefs in their reserve selection algorithm to 10 km based upon the maximum distance between offshore mangrove cays and reef sites in Belize. Acoustic tracking of adult coral reef fishes has revealed within-reef migrations to spawning aggregation sites over distances of up to 20 km (22), and interreef movements of up to 16 km (23). The fact that significant numbers of juvenile L. ehrenbergii were migrating up to 30 km among reefs on the continental shelf and across oceanic waters beyond the shelf break highlights how little we know about seascape connectivity of tropical marine fishes (24).
We used a direct method to identify juvenile nurseries that retrospectively determined habitat use during juvenile stages of adult fish on reefs. The approach allowed us to quantify relative contributions of individuals from nursery habitats to reef populations, and to categorize additional important juvenile habitats that we had been unable to adequately identify using conventional techniques. For example, individuals that settled directly onto reefs contributed at least 70% to L. ehrenbergii populations on oceanic reefs. However, reefs with the highest connectivity to coastal wetlands also had the highest adult L. ehrenbergii densities. Densities of adult L. ehrenbergii on coastal reefs were fourfold higher than those on the outer shelf and oceanic reefs. This correlation supports previous studies showing higher adult abundance of fishes on reefs closer to nursery sources (14, 15, 25). However, we were able to demonstrate that a higher proportion of individuals on coastal reefs had indeed resided in mangrove and seagrass nurseries before moving out to adult habitats compared with populations on reefs further offshore.
Our description of juvenile coral reef fish movements represents a unique direct estimation of seascape connectivity for any reef fish species. The functioning and resilience of coral reefs and the fisheries they support are directly linked to connectivity, both by dispersal and ontogenetic movement, within tropical seascapes (26). The ability to quantify the contributions of different nurseries to reef fish populations and identify important migration corridors is critical to identify management priorities (5) and parameterize models of habitat value (11, 12) and metapopulation persistence (27). Our results are particularly timely given the increasing use of spatial management approaches, including networks of marine protected areas in coral reef ecosystems (21, 28, 29). Although at least some of these efforts, including the recent rezoning of the Great Barrier Reef Marine Park, have explicitly recognized the importance of maintaining links among habitats (30), zoning decisions have necessarily been based on imprecise rules of thumb rather than empirical data on seascape connectivity (5). More time is needed before the effectiveness of these rules can be evaluated. Nonetheless, the lack of a mechanistic understanding of the role that seascape configuration plays in determining connectivity significantly hinders the ability to predict the influence of extrinsic factors, including climate change on reef fish populations (31). It is clear, however, that to effectively maintain functioning ecosystems and sustainable fisheries in structurally complex ocean ecosystems, management plans must conserve the functional integrity of ecosystems at the seascape level rather than focusing solely on individual habitat types. More generally, our approach provides a quantitative method for estimating the value of ecosystem services provided by distinctive habitats to fisheries yields within a seascape (11–13). This method will, in turn, allow for more accurate accounting of these services, including the assessment of suitable remediation requirements when these habitats are removed during tourism or aquaculture developments.
Materials and Methods
Ehrenberg’s snapper, Lutjanus ehrenbergii (Peters 1869), were collected from five distinct habitats: (i) coastal wetlands (n = 2 sites), (ii) coastal reefs (n = 2), (iii) shelf reefs (n = 4), (iv) offshore island patch reefs (n = 1), and (v) oceanic reefs (n = 4), along a 50-km cross-shelf transect from coastal Saudi Arabia in the Red Sea in November 2008, March 2009, and June 2010 (Fig. 1 and Fig. S2). Densities of L. ehrenbergii were estimated by visual survey on SCUBA. Individual fish were counted along four replicate 100-m by 10-m transects at 5- and 15-m depth from each reef and then averaged per distance. We visualized the separation of potential juvenile habitats using a quadratic discriminant function analysis (32) on the muscle essential amino acid δ13C data of L. ehrenbergii grouped into five regions according to their collection location across the continental shelf. See Table S1 for variance and loadings of quadratic discriminant function analysis on juvenile snapper habitat signatures. Briefly, total free amino acids were isolated by acid hydrolysis and then converted to isopropyl-TFAA derivatives (19), before individual isotopic analysis on an Agilent 6890N gas chromatograph coupled via continuous flow interface to a Thermo Finnigan Mat 253 isotope ratio monitoring-mass spectrometer (see SI Materials and Methods).
To retrospectively identify where each adult L. ehrenbergii spent its juvenile period, we isolated the juvenile core of adult L. ehrenbergii otoliths (see SI Materials and Methods and Fig. S3) from fish collected on reefs at six distances offshore along a 50-km cross-shelf transect (2 km, 16 km, 32 km, Abu Latt Island, 40 km, and 50 km). We analyzed the δ13C values of the same five essential amino acids as used to develop the nursery habitat signatures described above (Fig. S4). We used a maximum-likelihood estimator (33) to classify the juvenile cores of adult otoliths to one of the five potential nursery habitats to calculate the relative contribution of each of the five potential juvenile habitat regions to the adult populations on coral reefs at six distances along the 50-km cross-shelf transect from Al Lith, Saudi Arabia. For more details on the amino acid δ13C analyses and data processing, please see the SI Materials and Methods. Raw data are available in McMahon (34).
Supplementary Material
Acknowledgments
We thank L. Houghton for laboratory assistance; C. Braun for creating the site map; E. P. Oberlander for generating the seascape connectivity diagram; Dream Divers, Jeddah, Saudi Arabia for boat and dive operation assistance; and two anonymous reviewers for comments on the manuscript. This research was based on work supported by Awards USA 00002 and KSA 00011 from King Abdullah University of Science and Technology; additional funding was provided by the Woods Hole Oceanographic Institution and an International Society for Reef Studies-Ocean Conservancy Coral Reef Fellowship. K.W.M. received support from the National Science Foundation Graduate Research Fellowship Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206378109/-/DCSupplemental.
References
- 1.Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 3.Cote IM, Gill JA, Gardner TA, Watkinson AR. Measuring coral reef decline through meta-analysis. Philos Tran R Soc B. 2005;360:385–395. doi: 10.1098/rstb.2004.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 5.McCook LJ, et al. Management under uncertainty: Guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs. 2009;28:353–366. [Google Scholar]
- 6.Beck MW, et al. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience. 2001;51:633–641. [Google Scholar]
- 7.Adams AJ, et al. Nursery function of tropical back-reef systems. Mar Ecol Prog Ser. 2006;318:287–301. [Google Scholar]
- 8.Nagelkerken I, et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat Bot. 2008;89(2):155–185. [Google Scholar]
- 9.Mumby PJ, Hastings A. The impact of ecosystem connectivity on coral reef resilience. J Appl Ecol. 2008;45:854–862. [Google Scholar]
- 10.Thorrold SR, et al. Quantifying larval retention and connectivity in marine populations with artificial and natural markers. Bull Mar Sci. 2002;70:291–308. [Google Scholar]
- 11.Sanchirico JN, Mumby PJ. Mapping ecosystem functions to the valuation of ecosystem services: Implications of species-habitat associations for coastal land-use decisions. Theor Ecol. 2009;2:67–77. [Google Scholar]
- 12.Edwards HJ, Elliott IA, Pressey RL, Mumby PJ. Incorporating ontogenetic dispersal, ecological processes and conservation zoning into reserve design. Biol Conserv. 2010;143:457–470. [Google Scholar]
- 13.Barbier EB, et al. The value of estuarine and coastal ecosystem services. Ecol Monogr. 2011;81:169–193. [Google Scholar]
- 14.Mumby PJ, et al. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature. 2004;427:533–536. doi: 10.1038/nature02286. [DOI] [PubMed] [Google Scholar]
- 15.Aburto-Oropeza O, et al. Mangroves in the Gulf of California increase fishery yields. Proc Natl Acad Sci USA. 2008;105:10456–10459. doi: 10.1073/pnas.0804601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagelkerken I, Dorenbosch M, Verberk WCEP, Cocheret de la Moriniere E, van der Velde G. Importance of shallow-water biotopes of a Caribbean bay for juvenile coral reef fishes: Patterns in biotope association, community structure and spatial distribution. Mar Ecol Prog Ser. 2000;202:175–192. [Google Scholar]
- 17.Cocheret de la Moriniere E, Ollux BJA, Nagelkerken I, van der Velde G. Post-settlement life cycle migration patterns and habitat preference of coral reef fish that use seagrass and mangrove habitats as nurseries. Estuar Coast Shelf Sci. 2002;55:309–321. [Google Scholar]
- 18.McMahon KW, Fogel ML, Elsdon TS, Thorrold SR. Carbon isotope fractionation of amino acids in fish muscle reflects biosynthesis and isotopic routing from dietary protein. J Anim Ecol. 2010;79:1132–1141. doi: 10.1111/j.1365-2656.2010.01722.x. [DOI] [PubMed] [Google Scholar]
- 19.McMahon KW, Fogel ML, Johnson BJ, Houghton LA, Thorrold SR. A new method to reconstruct fish diet and movement patterns from δ13C values in otolith amino acids. Can J Fish Aquat Sci. 2011;68:1330–1340. [Google Scholar]
- 20.McMahon KW, Berumen ML, Mateo I, Elsdon TS, Thorrold SR. Carbon isotopes in otolith amino acids identify residency of juvenile snapper (Family: Lutjanidae) in coastal nurseries. Coral Reefs. 2011;30:1135–1145. [Google Scholar]
- 21.Mumby MJ. Connectivity of reef fishes between mangroves and coral reefs: Algorithms for the design of marine reserves at seascape scales. Biol Conserv. 2006;128:215–222. [Google Scholar]
- 22.Starr RM, Sala E, Ballesteros E, Zabala M. Spatial dynamics of the Nassau grouper, Epinephelus striatus, in a Caribbean atoll. Mar Ecol Prog Ser. 2007;343:239–249. [Google Scholar]
- 23.Chateau O, Wantiez L. Movement patterns of four coral reef fish species in a fragmented habitat in New Caledonia: Implications for the design of marine protected area networks. ICES J Mar Sci. 2009;66:50–55. [Google Scholar]
- 24.Turgeon K, Robillard A, Grégoire J, Duclos V, Kramer DL. Functional connectivity from a reef fish perspective: Behavioral tactics for moving in a fragmented landscape. Ecology. 2010;91:3332–3342. doi: 10.1890/09-2015.1. [DOI] [PubMed] [Google Scholar]
- 25.Dorenbosch M, Grol MGG, Nagelkerken I, van der Velde G. Seagrass and mangroves as potential nurseries for the threatened Indo-Pacific humphead wrasse, Cheilinus undulates, and Caribbean rainbow parrotfish, Scarus guacamaia. Biol Conserv. 2006;129:277–282. [Google Scholar]
- 26.Gaines SD, Gaylord B, Gerber LR, Hastings A, Kinlan BP. Connecting places: The ecological consequences of dispersal in the sea. Oceanography (Wash DC) 2007;20(3):90–99. [Google Scholar]
- 27.Hastings A, Botsford LW. Persistence of spatial populations depends on returning home. Proc Natl Acad Sci USA. 2006;103:6067–6072. doi: 10.1073/pnas.0506651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sale PF, et al. Critical science gaps impede use of no-take fishery reserves. Trends Ecol Evol. 2005;20:74–80. doi: 10.1016/j.tree.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Mora C, et al. Ecology. Coral reefs and the global network of Marine Protected Areas. Science. 2006;312:1750–1751. doi: 10.1126/science.1125295. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes L, et al. Establishing representative no-take areas in the Great Barrier Reef: Large scale implementation of theory on marine protected areas. Conserv Biol. 2005;19:1733–1744. [Google Scholar]
- 31.Wilson SK, et al. Crucial knowledge gaps in current understanding of climate change impacts on coral reef fishes. J Exp Biol. 2010;213:894–900. doi: 10.1242/jeb.037895. [DOI] [PubMed] [Google Scholar]
- 32.White JW, Ruttenberg BI. Discriminant function analysis in marine ecology: Oversights and their solutions. Mar Ecol Prog Ser. 2007;329:301–305. [Google Scholar]
- 33.Millar RB. A versatile computer program of mixed stock fishery composition estimation. Can Tech Rep Fish Aquat Sci. 1990;1753:29–38. [Google Scholar]
- 34.McMahon KW. 2011. Functional connectivity of coral reef fishes in a tropical seascape assessed by compound-specific stable isotope analyses. PhD thesis (Massachusetts Institute of Technology-Woods Hole Oceanographic Institution Joint Program in Oceanography: Applied Ocean Science and Engineering, Woods Hole, MA)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.