Abstract
Our understanding of the human default mode network derives primarily from neuroimaging data but its electrophysiological correlates remain largely unexplored. To address this limitation, we recorded intracranially from the human posteromedial cortex (PMC), a core structure of the default mode network, during various conditions of internally directed (e.g., autobiographical memory) as opposed to externally directed focus (e.g., arithmetic calculation). We observed late-onset (>400 ms) increases in broad high γ-power (70–180 Hz) within PMC subregions during memory retrieval. High γ-power was significantly reduced or absent when subjects retrieved self-referential semantic memories or responded to self-judgment statements, respectively. Conversely, a significant deactivation of high γ-power was observed during arithmetic calculation, the duration of which correlated with reaction time at the signal-trial level. Strikingly, at each recording site, the magnitude of activation during episodic autobiographical memory retrieval predicted the degree of suppression during arithmetic calculation. These findings provide important anatomical and temporal details—at the neural population level—of PMC engagement during autobiographical memory retrieval and address how the same populations are actively suppressed during tasks, such as numerical processing, which require externally directed attention.
Keywords: electrocorticography, episodic memory, numerical cognition
Our ability to successfully direct and maintain attention to the external world relies on the coordinated activation of several putative attentional brain networks. Over the past decade brain imaging techniques, such as functional MRI (fMRI), have also revealed a select group of brain regions, now well-known as the default mode network (DMN), which deactivate during tasks of externally directed attention (1, 2). Conversely, the DMN shows higher activity during tasks requiring internally focused “self-referential” processing (3–5). Despite the many advances made by neuroimaging studies of DMN function, similar progress in the electrophysiological domain has remained relatively limited. This scarcity of investigation is mostly because the core structures of the DMN are anatomically hidden on the medial surface of the brain, and thus their location and orientation is suboptimal for detection with scalp electroencephalography (EEG). One region of particular interest is the posteromedial cortex (PMC), which is the main hub of the DMN in the human (2, 6) and nonhuman primate (7, 8) brains (Fig. 1), and encompasses the posterior cingulate cortex (PCC), retrosplenial cortex (RSC), precuneus, and Brodmann area 31 (9). Ultimately, more invasive techniques using intracranial electrophysiological recordings from the human brain are methodologically preferable for studying these regions, but obtaining such data has been rare and logistically challenging.
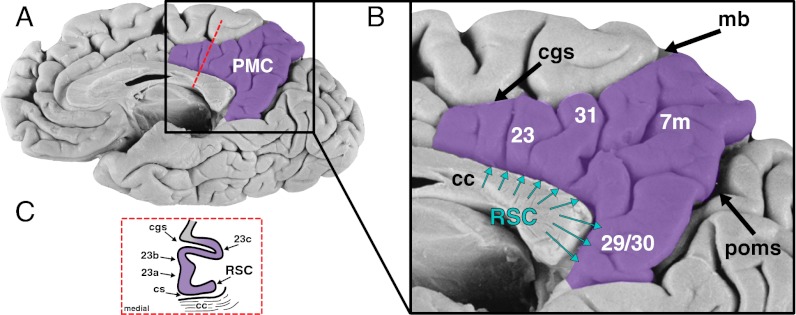
Fig. 1.
Anatomy of the PMC. (A) PMC (highlighted region in purple), which forms a core node of the default mode network, is located on the medial surface of the brain. (B) The PMC is bounded ventrally by the parieto-occipital sulcus (which divides it from the cuneus); dorsally by the cingulate sulcus (cgs) and its marginal branch (mb); and extends anteriorly to approximately mid-cingulate level before it joins the anterior cingulate cortex. The PMC contains the PCC (areas 23a, 23b, and 23c), RSC (areas 29 and 30), medial parietal cortex (area 7m), and a transitional cortical area 31. The RSC is superficially visible as gyral cortex around areas 29 and 30; however, it extends perisplenially around the corpus callosum (cc) hidden within the callosal sulcus (cs; B and C).
Recent invasive electrophysiological studies of the DMN have highlighted its well-known deactivation during conditions of externally directed attention. For example, consistent with the neuroimaging literature in humans, work in the macaque has shown that single-unit firing rate is suppressed during tasks of directed attention in the PCC (10). Furthermore, this suppression in firing rate is correlated with behavioral performance, where lower levels of PCC firing suppression are associated with longer reaction times (RTs) and higher error rate, a correlation also previously reported with fMRI in humans (11, 12). Similarly, direct cortical recordings using electrocorticography (ECoG) from human DMN (13–16) have shown suppression of broadband γ-power [a correlate of population firing rate (17, 18)] during attentional effort. More recently, this broadband suppression across human DMN regions has also been shown to correlate with behavioral performance (16). Taken together, these findings have provided clear electrophysiological evidence for DMN suppression.
What remains to be explored electrophysiologically is the study of task activation within the DMN. Recently, we reported the putative activation of the PMC to nontask periods of cued-rest, observing a remarkable heterogeneity of response in functional, temporal, and spatial domains (15). Anecdotally, in two single electrodes (from two separate subjects), we observed a selective response to autobiographical memory judgments in locations that did not overlap with sites showing the cued-rest activation (15).
In the present study, we explore further the PMC responses during memory conditions using a larger number of subjects and with more comprehensive electrode coverage to gain direct insight to the memory contexts in which there is a significant electrophysiological activation within the PMC, as the core structure of the DMN. Toward this end, we designed the present study to address the following three questions: (i) Can we establish an increased electrophysiological response within the PMC during tasks of autobiographical episodic memory processing and, if so, do we observe a similar response across different probes of self-referential memory processing? (ii) What is the temporal profile and relative magnitude of electrophysiological response in the PMC during internal memory processing, and how do these properties relate to the degree of deactivation during external attention within the same neural population? (iii) Do these responses preferentially involve a specific subregion or are they seen across a larger extent of the PMC?
To address these questions we obtained direct cortical recordings (ECoG) from eight subjects (all fluent in English) who were implanted with grids or strips of intracranial electrodes covering the PMC. Our sample of eight subjects (mean age ± SD = 33.5 ± 9.1 y), was balanced not only for sex (male/female: 4/4) and hemisphere (left/right: 4/4), but also for sex within hemisphere (left: 2 male/2 female; right: 2 male/2 female) (see Table S1 for subject information). All subjects were implanted for clinical purposes related to the surgical treatment of refractory epilepsy. Of final note, none of the included subjects had a clinically identified seizure focus proximal to or within the PMC, and we excluded any electrodes that contained artifacts or pathological activity.
Consistent with our previous work (15, 19), we used a simple task (Fig. S1) that effectively probes default network function. This task involves basic true/false judgments of memory sentences (three subtypes) or complete mathematical equations via a keypad press (“1” = true; “2” = false). Three memory sentence types were presented with differing self-referential content: Self-episodic statements (e.g., “I used a computer today”); Self-semantic statements (e.g., “I use a computer often”); and Self-judgment statements (e.g., “I am smart”). The rationale for using self-semantic and self-judgment trials was to determine whether the predicted response of the PMC during the self-episodic trials is because of the self-referential or episodic nature of these memory probes. As a condition devoid of self-referential content, but still requiring attentional focus, we used basic arithmetic equations (e.g., “2 + 52 = 56”; Math condition). These four conditions were all self-paced in duration. Interleaved across trials was a fixed 5-s cued-rest period, where only a centered cross sign was displayed and the subjects were instructed to fixate and rest (Rest condition). See SI Materials and Methods for all task stimuli and details of subject task performance.
Results
Using the anatomical criteria described in Fig. 1, a total of 33 intracranial electrodes were located within the PMC across all subjects (15 left hemisphere; 18 right hemisphere) and were the focus of data analysis reported below (see SI Materials and Methods and Fig. S2 for details regarding electrode localization). Event-related changes in spectral power were initially screened for a wide range of frequencies (1–200 Hz) and then more specifically for band-limited changes. For functional electrode selection and the bulk of analysis reported, we focused on the event-related changes in broad-frequency high γ-power (HG; 70–180 Hz), as used by previous investigators (13, 15, 16). Changes in power across this frequency range reflect one of the most robust measures of local population activity as recorded by ECoG, particularly given that power in this range is functionally selective, spatially restricted (20), and well-correlated with both population firing rate (17) and fMRI responses (21). Given the RT-based variability of trial duration, we studied both stimulus onset-locked and response-locked data, but focused our quantitative comparisons upon response-locked data, as detailed below (SI Materials and Methods). Changes in HG power were normalized to z-score values relative to a surrogate distribution as previously used (SI Materials and Methods and Fig. S3). These normalized HG response values were used to initially survey the PMC for any condition-based changes in activity (SI Materials and Methods).
PMC Contains Functional Subregions Activated During Memory Retrieval and Suppressed During Arithmetic Calculation.
To initially survey the response of all PMC electrodes across conditions, we performed counts of responsive electrodes for each condition based on a magnitude threshold (see SI Materials and Methods for details regarding time-frequency selection).
From a larger group of electrodes within the PMC (n = 33), we found differing numbers of electrodes being responsive to the presented conditions. The majority of sites within the anatomical boundary of the PMC did not respond above threshold to any of the memory, math, or cued-rest conditions. However, of those PMC sites that did respond, the largest number reflected increased HG activity during the self-episodic condition (11 electrodes; 33% overall PMC), followed by the self-semantic condition (eight electrodes; 24% overall PMC). Interestingly, no electrodes responded to the self-judgment condition and only a few electrodes responded during the cued-rest condition (four electrodes; 12% overall PMC). All electrodes that were responsive during the math condition (eight electrodes; 24% overall PMC) showed a decreased HG response.
To see if these conditional responses reflected separate or overlapping functional groups, we cross-tabulated electrode counts (either increase or decrease) for all responsive conditions. Although there was overlap between sites responsive to self-episodic and other conditions, such as self-semantic (overlap 4) and math (overlap 6), not all detected changes were reflective of a singular subgroup of electrodes. For example, only one electrode showed an increase to both the self-episodic and rest conditions. However, a follow up analysis showed that all electrodes deactivated during the math condition responded positively to either the self-episodic condition, self-semantic condition, or both. Consistent with our prior observations, the electrophysiology of PMC shows a functional heterogeneity, which distinguishes sites responsive to self-referential processing with those responsive to cued-rest periods, yet all sharing suppression during arithmetic calculation (15).
PMC Sites Responsive During Episodic Memory Are Not Active During Self-Judgment or Rest-Fixation.
After characterizing discrete counts of PMC sites responding to various experimental conditions, we focused on the specific magnitude and latency changes of HG responses within these identified electrodes for each individual subject. We focused our analysis on electrodes responsive to the self-episodic condition, as one the most common conditions of DMN engagement (22), and for which all subjects showed one or more responsive sites (see Figs. S4 and S5 for detailed single subject responses). In general, the greatest increase in HG power was seen for the self-episodic condition. The onset of the HG response was typically late (>400 ms) and began its offset before the subject responded on the keypad (latency properties discussed below). In the sites that were responsive to self-episodic statements, the self-semantic condition produced lower HG activity (Fig. S4). The HG responses to self-judgment and cued-rest trials were either negative or distributed around zero. Moreover, a clear decrease in HG response magnitude was consistently seen for the math condition.
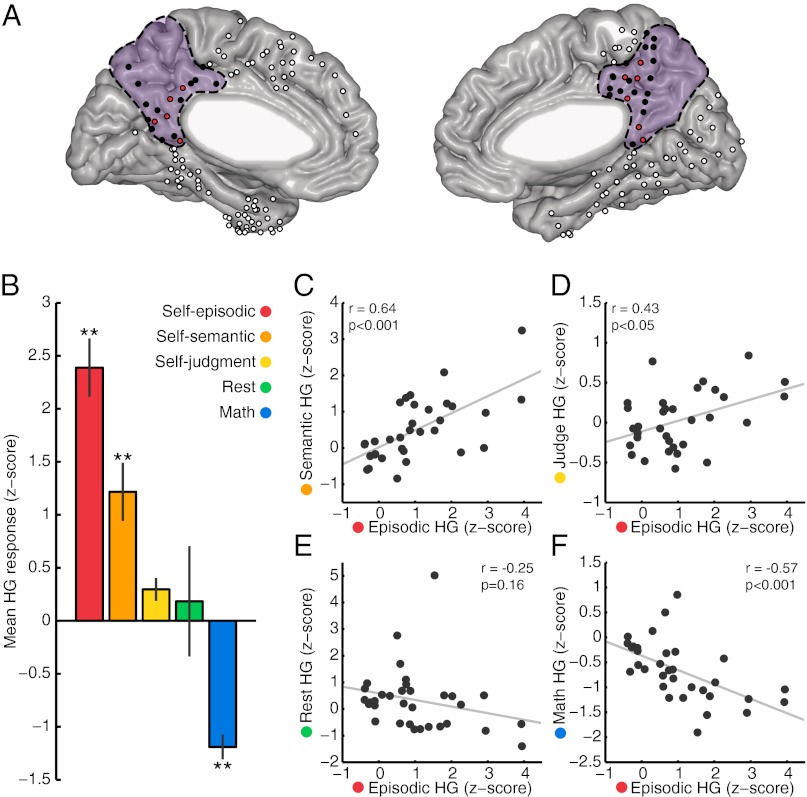
Fig. 2 shows the electrode locations for all subjects on a normalized brain and their mean HG response magnitude across conditions. Consistent with the single-subject trends (Fig. S4), the mean HG response magnitude across all of the self-episodic selective sites showed a clear conditional difference (Fig. 2B;) [one-way repeated-measures ANOVA F(4,40) = 17.43, P < 0.001]. This main effect of task condition reflected a positive-negative scaling of HG responses across conditions (episodic** >semantic** >judge >rest >math**; **significantly different from all other conditions P < 0.01 corrected). As emphasized below, this main effect of condition upon HG response was not a consequence of electrode selection, being preserved when including all PMC electrodes [F(4,128) = 15.21, P < 0.001]. Furthermore, these differences across conditions were only observed for response-locked activity (discussed below) (Fig. S6).
Fig. 2.
Response magnitude across conditions. (A) Electrode locations from all subjects on a standardized Montreal Neurological Institute brain, with the PMC marked in purple. PMC electrodes responsive to the self-episodic condition have red fill and other PMC sites have black fill. Electrodes falling outside the anatomical boundaries of the PMC are filled in white. (B) Mean HG response across conditions for all electrodes that were identified as significantly responsive during the self-episodic condition (11, marked red in A). Mean HG response for these electrodes was significantly different across conditions [one-way ANOVA; F(4,40) = 17.43, P < 0.001], with post hoc test showing self-episodic, self-semantic, and math to be significantly different from all other conditions (**P < 0.01, corrected). Scatter correlation plots of mean HG response for all PMC electrodes (n = 33, marked black in A) comparing the self-episodic condition with self-semantic (C), self-judgment (D), rest (E), and math (F) conditions, respectively.
Although HG power was significantly different across conditions, this difference did not uniquely change as a function of the subject’s response (i.e., “true” or “false”). A two × four repeated-measures ANOVA [within factors: response (two) and task conditions (four); rest excluded] of HG power across all PMC electrodes showed a significant main effect of response on HG power [F(1,31) = 10.14, P < 0.03], but no significant response-condition interaction [F(3,93) = 1.52, P = 0.22]. Furthermore, post hoc paired t tests showed no significant true/false differences in mean HG across conditions (P > 0.05 for all conditions). In light of previous reports suggesting left hemisphere dominance for episodic retrieval in DMN regions (23), we also performed a two × five mixed-model ANOVA [between factor: hemisphere (two); within factor: task conditions (five)] of HG power across all PMC electrodes. This analysis showed no significant difference in HG response magnitude between the two hemispheres [F(1,32) = 1.18, P = 0.29], nor an interaction effect of condition-hemisphere [F(4,124) = 0.45, P = 0.78]. Consequently, “task response” and “hemisphere” were not included as independent variables in subsequent analysis.
PMC Response During Episodic Memory Predicts Deactivation Level During Arithmetic Calculation.
To explore the relationship of response magnitude across conditions, and in part assess the overlap of functional engagement, we correlated all conditions against the self-episodic condition for all PMC electrodes (Fig. 2 C–F). This comparison showed significant positive and negative correlations between conditions. Magnitude of HG response during the self-episodic condition was significantly correlated positively with the magnitude of HG response during self-semantic (Fig. 2C) and, to a lesser extent, the self-judgment conditions (Fig. 2D). Conversely, the self-episodic condition was significantly correlated negatively with the math condition (Fig. 2F). By extension, a linear regression model showed that as a predictor the self-episodic condition significantly explained 32% of the variance in HG response to the math condition [r2 = 0.32; model F(1,31) =14.62; coefficients t = −3.82, P < 0.001]. Follow-up analyses confirmed that these correlations were only seen for the “late” response-locked window used (Fig. S7). Given the relevance of this anticorrelation to default-mode function, we further explored the response properties between self-episodic and math conditions. We found a significant difference in the response duration of HG increases during the self-episodic condition (37% of trial > upper threshold) compared with the duration of HG suppression during the math condition [59% of trial < lower threshold, t(20) = 5.3, P < 0.0001] for the same electrodes (responsive to the self-episodic condition) (SI Materials and Methods).
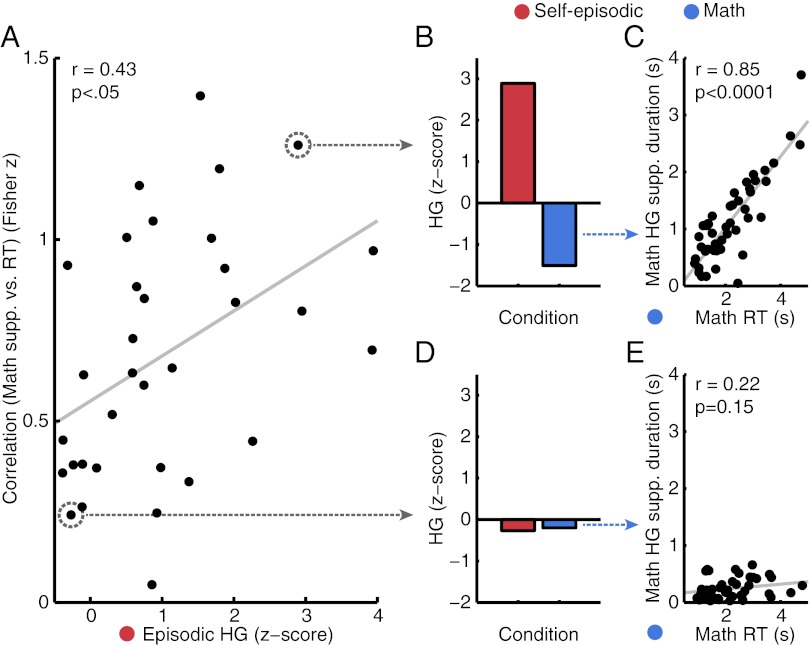
Consistent with the magnitude correlations reported above, the duration of suppression during the math condition was also positively correlated with the magnitude of response to the self-episodic condition (r = −0.59, P < 0.001). To probe the degree of this functional anticorrelation, more detailed analysis showed that on a trial-by-trial basis, the duration of suppression for the math condition positively correlated with RT across all PMC electrodes (r = 0.68, P < 0.0001; correlations averaged after Fisher’s z transform). Interestingly, this correlation was even more pronounced for electrodes with a significant response during the self-episodic condition (r = 0.89, P < 0.0001). Given this finding, we then sought to more directly examine the relationship between magnitude of activation during episodic memory retrieval and the trial-based sustainability of activity suppression during the math condition across each electrode independently. For the measure of sustainability, we chose the trial-based correlation value between suppression duration and RT of each electrode. Remarkably, as shown in Fig. 3, the sustained suppression of activity during the math condition was itself positively correlated with the magnitude of response for the self-episodic condition (r = 0.43, P = 0.013). Therefore, electrodes with greater HG response magnitude during the self-episodic condition showed higher suppression sustainability during the math condition. In combination these results clearly establish electrophysiologically that the PMC sites most responsive to episodic-memory retrieval were the most suppressed during the math condition both in magnitude (Fig. 2) and duration at the single trial level (Fig. 3).
Fig. 3.
Response magnitude to the self-episodic condition predicts trial-by-trial suppression during math condition. (A) Scatter correlation plot of HG response magnitude to the self-episodic condition with correlation value between trial-based HG math suppression and math RT (Fisher z) for all PMC channels. Those electrodes that showed higher magnitude responses to the self-episodic condition (x axis) also showed the strongest correlation between HG math suppression and RT (y axis). (B) For example, electrodes with strong HG response magnitudes to the self-episodic conditions also showed strong suppression of HG magnitude during the math condition (as noted above in Fig. 2). Additionally, these channels also displayed a stronger trial-by-trial–based correlation of suppression duration and trial duration (RT) for the math condition (C). (D and E) Consequently, those sites with lower response magnitudes during the self-episodic condition showed much weaker (nonsignificant) suppression duration vs. RT correlation for the math condition.
Episodic Memory Processing Engages Ventral and Dorsal Subregions of the PMC at Different Latencies.
Anatomically, self-episodic responsive sites showed an interesting spatial distribution. Although these electrodes differed in the relative superior-inferior (dorsal-ventral) location, they were typically close to the splenium of the corpus callosum and the ventral posterior aspect of the PMC (i.e., a perisplenial region).
Initial observations across single subjects indicated that the onset of HG response to the self-episodic condition differed across ventral and dorsal electrodes within the PMC (Fig. S4). To quantify this observation, we plotted the HG onset and offset in rank order, marking electrodes as either dorsal or ventral PMC (Fig. 4). This process revealed an interesting trend for more ventral electrodes to respond to the self-episodic condition earlier than the more dorsal sites (Fig. 4A). No electrode responded earlier than 400 ms (onset range 407–751 ms, m = 627 ms; time to peak m = 1.5 s). To control for the confound that dorsal-ventral differences were not simply a result of one group being psychophysiologically slower, we correlated HG onset and offset times with RT across all subjects and sites and found no significant relationship (Fig. 4 D and E).
Fig. 4.
HG response latency to self-episodic condition across the PMC. (A) HG onset (Left, relative to stimulus onset; Stim) and offset (Right, relative to response; Resp) latency for the self-episodic condition sorted in ascending order and colored relative to ventral or dorsal location indicated in C for all responsive electrodes (n = 11). (B) Mean duration of HG response (above threshold) for self-episodic condition for ventral and dorsal electrodes (n.s., no significant difference). (C) Schematic of the PMC and the ventral/dorsal division used for electrode classification. Latencies show a trend for greater delays in response for dorsal sites compared with more ventral sites. Scatter correlation of HG onset (D) and offset (E) with RT, shown for all PMC electrodes (n = 33). As there was no significant correlation with onset/offset and RT, the differences in latencies shown in A are more likely related to anatomical differences rather than consistent behavioral differences between ventral and dorsal groups.
We then compared how this difference in latency onset influenced the duration of response to the self-episodic condition by comparing the response duration (percentage of trial HG > threshold) for dorsal and ventral sites (Fig. 4B). Although not significantly different, there was a consistent trend that dorsal sites had a later onset and shorter duration of activation, whereas the ventral sites had earlier onset and longer duration of activation during episodic memory retrieval.
Functional Responses Recorded During Episodic Memory Retrieval Are from Neural Populations Within the PMC.
One might perhaps question whether the responses captured in the most ventral electrodes are caused by electrophysiological signals emanating from the nearby parahippocampal formation, or more generally question the veracity of the anatomical boundaries used. We specifically addressed this issue by comparing the task-relevant activation of electrodes located in the ventral boundaries of the PMC. We found that the responsiveness of electrodes during episodic memory retrieval does not progressively continue as one moves out of the PMC toward the parahippocampal gyrus. Indeed, the sulcal boundaries we used for defining the ventral limit of PMC (i.e., parieto-occipital sulcus) proved to be a robust landmark for the functional responses reported above. Fig. S8 shows the loss of response to the episodic memory condition within four subjects (two left hemisphere, two right hemisphere). In these subjects, the interhemispheric ECoG electrodes continued ventrally out of the PMC.
Discussion
Using intracranial recordings from human subjects, we were able to characterize the electrophysiological response properties of the PMC during conditions of memory retrieval that contained specific self-referential episodic items (self-episodic), generic semantic self-referential items (self-semantic), or items of self-referential psychological attributes (self-judgments). In contrast, we also used equations of arithmetic calculation that required focused attention with no self-referential content along with random periods of cued-rest fixation. Our findings confirm a consistent electrophysiological increase of activity within the PMC during autobiographical episodic memory retrieval. Interestingly, the same degree of response is not seen for other memory conditions that have significant self-referential content (i.e., self-semantic and self-judgment conditions). Our findings also confirm that the PMC sites engaged by memory retrieval show a pronounced suppression of activity during basic arithmetic calculations, the magnitude and duration of which are highly correlated with the magnitude of response during episodic memory retrieval. Overall, the engagement of the PMC during episodic memory retrieval was initiated no earlier than 400 ms, localized to a perisplenial region of the PMC, and within this anatomical distribution, the response latencies increased from ventral to dorsal sites.
Anticorrelated Task Responses of PMC Neural Populations.
We observed that the level of increased activation by neural populations in the PMC during episodic memory retrieval predicted their degree of suppression during arithmetic calculation (see SI Discussion for discussion of neural population response). This finding suggests that a population of neurons recruited during a task of internal focus (e.g., episodic memory retrieval) becomes actively suppressed during externally directed attention in a sustained manner for the duration of the subject’s engagement with the task. Such a finding is in line with the available evidence that the degree of DMN suppression correlates with behavioral performance during externally guided attention-demanding tasks (12, 16). Our findings are consistent with this view but add important electrophysiological evidence that those DMN regions suppressed during external attention are indeed the same neural populations activated during more internally focused processes, which when insufficiently suppressed may contribute to a “cognitive interference” that diminishes the quality of attentional engagement (see SI Discussion for discussion of anticorrelated networks).
Anatomical Considerations of PMC Activation and Response Latency.
The high temporal resolution of ECoG also provided another intriguing finding, namely the latency of PMC response to episodic memory conditions. The fact that these responses were not observed to begin until after ∼400 ms, and peak around ∼1.5 s, fits within the expected temporal structure of integrative associative cortical function. This finding suggests that the PMC is only engaged after visual transduction (primary visual cortex), feature detection (ventro-temporal cortex), and initiation of processes in the medial temporal lobe (hippocampus/entorhinal cortex). This latency is more closely related to later potentials observed with scalp EEG at parietal locations for “old/new” recognition judgments (positive going potential onset ∼400 ms) (24). In line with this observation, ventral sites in the PMC responded relatively earlier than those located more dorsally. This result is consistent with the anatomical data suggesting a significantly closer and stronger anatomical connections between ventral PMC structures (RSC) and hippocampal (subiculum, presubiculum, and parasubiculum), parahippocampal (entorhinal cortex and areas TH and TF), and thalamic (anterior and lateral dorsal nuclei) structures known to be important for memory processing (25, 26). Consistent with this anatomical relationship, θ-oscillations, a canonical signature of the medial temporal lobe, have also been shown to be a predominate rhythmic motif of the human PMC (27). Although we did not observe similar responses to episodic memory probes from adjacent parahippocampal electrodes, it is possible that other regions within the parahippocampal gyrus (that were not covered by any of our electrodes) might have shown a similarly strong response. In future cases with simultaneous recordings from parahippocampal and hippocampal structures along with PMC subregions, one might be able to compare the temporal profile of activation across this anatomically connected network (see SI Discussion regarding limitations in PMC coverage for the present study). In light of our findings it is reasonable to predict that responses in the hippocampal and parahippocampal regions will precede those reported here in the PMC.
Functional Role of the PMC in Cognition.
The refined temporal and anatomical resolution of intracranial electrophysiology enabled us to measure the activity of specific populations of neurons within the PMC during different conditions of memory retrieval. Although the response to episodic memory items was more prevalent and stronger in magnitude, there was no significant response to conditions of self-judgment and only significantly lesser response to self-semantic items. This finding suggests that the PMC, as the hub of the DMN, may not function within a generic “self” processing system (28). In line with other views of DMN function (29, 30), this profile of PMC engagement may be explained by a specific role in the construction of episodic memories rather than self-referential processing.
Taking these functional, temporal, and anatomical data together, what might be the unique role for PMC in episodic memory and its role in cognition more broadly? Our data point toward an associative/integrative function not simply of primary sensory information, but as a higher-order convergence zone for information processing that supports the construction of putative episodic scenes (29, 31) and their possible semantic conceptual associations (32–34). Given the equal response of the PMC to both true and false episodic memory statements, it is reasonable to believe that activity in the PMC does not necessarily signal that an event has in fact happened and been stored in memory. Rather, the PMC is engaged not only during the retrieval of real events from memory but also in the process of determining that an event has not taken place in reality. This finding is in line with the neuroimaging findings of increased activity in the PMC while envisioning future events (30). In contrast to previous neuroimaging studies, we found limited overlap between episodic responses and cued-rest periods (22, 35); however, differences in task structure and trial duration make it difficult to fully exclude the occurrence of autobiographical recollection during resting states.
Our findings provide a clear electrophysiological basis for the canonical antagonistic function of the PMC between tasks of internal memory and external attention. Although our study provides important electrophysiological data about the temporal profile and anatomical localization of response within the core of the human DMN, we are mindful that it leaves many open questions that require more specific experimental paradigms and simultaneous recordings from additional nodes of the default mode and dorsal attention networks. Such a system-wise approach will help us elucidate the temporal hierarchy of information exchange between the PMC and brain structures crucial for the rich internal elaboration of our past and future experiences.
Materials and Methods
This study was carried out at the Laboratory of Behavioral and Cognitive Neurology at Stanford University Medical Center (http://lbcn.stanford.edu). All subjects provided voluntary written consent to participate in research recordings as part of a protocol approved by the Stanford Institutional Review Board office. Subject details are summarized in Table S1. Information describing electrode localization, data recording, and analysis are detailed in SI Materials and Methods and SI Discussion.
Supplementary Material
Acknowledgments
We thank the patients and their families for their involvement in the study; the Stanford Epilepsy Monitoring Unit and Vinitha Rangarajan for help with patient data; Dora Hermes for assistance with electrode localization; Anthony Wagner and his laboratory for their continuous feedback throughout the study; and the laboratory of Brian Wandell for brain image processing tools (mrVISTA; http://white.stanford.edu/mrvista). The study was funded by Stanford University NeuroVentures Program, Stanford Institute for NeuroInnovation and Translational Neuroscience, and by National Institutes of Health Grant 1R01 NS0783961. B.L.F. was supported by a Dean’s Fellowship from Stanford University School of Medicine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206580109/-/DCSupplemental.
References
- 1.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 4.Harrison BJ, et al. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margulies DS, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantini D, et al. Default mode of brain function in monkeys. J Neurosci. 2011;31:12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA. 2009;106:5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden BY, Smith DV, Platt ML. Cognitive control signals in posterior cingulate cortex. Front Hum Neurosci. 2010;4:223. doi: 10.3389/fnhum.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 13.Miller KJ, Weaver KE, Ojemann JG. Direct electrophysiological measurement of human default network areas. Proc Natl Acad Sci USA. 2009;106:12174–12177. doi: 10.1073/pnas.0902071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerbi K, et al. Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Front Syst Neurosci. 2010;4:27. doi: 10.3389/fnsys.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dastjerdi M, et al. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc Natl Acad Sci USA. 2011;108:3023–3028. doi: 10.1073/pnas.1017098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ossandón T, et al. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J Neurosci. 2011;31:14521–14530. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller KJ. Broadband spectral change: Evidence for a macroscale correlate of population firing rate? J Neurosci. 2010;30:6477–6479. doi: 10.1523/JNEUROSCI.6401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008;3:177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KJ, et al. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27:2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermes D, et al. Neurophysiologic correlates of fMRI in human motor cortex. Hum Brain Mapp. 2012;33:1689–1699. doi: 10.1002/hbm.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 23.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: I. Three-dimensional and cytoarchitectonic organization. J Comp Neurol. 2000;426:339–365. doi: 10.1002/1096-9861(20001023)426:3<339::aid-cne1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 27.Foster BL, Parvizi J. Resting oscillations and cross-frequency coupling in the human posteromedial cortex. Neuroimage. 2012;60:384–391. doi: 10.1016/j.neuroimage.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 31.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 32.Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar M, Aminoff E, Mason M, Fenske M. The units of thought. Hippocampus. 2007;17:420–428. doi: 10.1002/hipo.20287. [DOI] [PubMed] [Google Scholar]
- 34.Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen NC, et al. Remembering the past: Two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.