Abstract
The debate about the origins of human prosociality has focused on the presence or absence of similar tendencies in other species, and, recently, attention has turned to the underlying mechanisms. We investigated whether direct reciprocity could promote prosocial behavior in brown capuchin monkeys (Cebus apella). Twelve capuchins tested in pairs could choose between two tokens, with one being “prosocial” in that it rewarded both individuals (i.e., 1/1), and the other being “selfish” in that it rewarded the chooser only (i.e., 1/0). Each monkey’s choices with a familiar partner from their own group was compared with choices when paired with a partner from a different group. Capuchins were spontaneously prosocial, selecting the prosocial option at the same rate regardless of whether they were paired with an in-group or out-group partner. This indicates that interaction outside of the experimental setting played no role. When the paradigm was changed, such that both partners alternated making choices, prosocial preference significantly increased, leading to mutualistic payoffs. As no contingency could be detected between an individual’s choice and their partner’s previous choice, and choices occurred in rapid succession, reciprocity seemed of a relatively vague nature akin to mutualism. Having the partner receive a better reward than the chooser (i.e., 1/2) during the alternating condition increased the payoffs of mutual prosociality, and prosocial choice increased accordingly. The outcome of several controls made it hard to explain these results on the basis of reward distribution or learned preferences, and rather suggested that joint action promotes prosociality, resulting in so-called attitudinal reciprocity.
Keywords: cooperation, tit-for-tat, inequity, mirroring
Repayment of benefits is the most common evolutionary explanation of cooperation among nonrelatives. Such reciprocal altruism (1) would, first of all, require an altruistic impulse (i.e., altruism needs to be present before it can be reciprocated) and, second, a mechanism by which recipients recognize and repay their benefactors. As there is no evidence that animals show altruism while anticipating future repayments—and hence they are unlikely to be motivated by the possibility of repayment—there has been much focus on what drives them to be prosocial in the first place, with prosociality being defined as a motivation to assist others regardless of benefits for the self (2, 3). The main mechanism for human prosociality is assumed to be empathy (4), which has been hypothesized to extend to all mammals (5). After a period during which there was a lack of evidence for nonhuman primate prosociality, including claims of human uniqueness in this regard (6, 7), carefully controlled experiments have demonstrated well developed prosocial tendencies in monkeys (8–10) and apes (11–14). The ecological validity of experimental findings on prosociality is supported by the many naturalistic observations of the same tendencies expressed spontaneously among chimpanzees and other nonhuman primates (3, 15, 16).
To find evidence for social reciprocity in primates, three main strategies have been used. The first is a correlational approach in which services (e.g., grooming, agonistic support) are tabulated across an entire matrix of individuals (e.g., refs. 17, 18). Correlations fail to identify behavioral contingencies, however. If individual A preferentially grooms B and B preferentially supports A, one still wonders what happens on days that A fails to groom B. A sequential analysis, in contrast, explores the contingency between goods and services exchanged across a sequence of interactions. For example, chimpanzees show a contingency between the receipt of grooming from a particular partner and their tendency to subsequently share food with (19) or support that partner (20). The third approach is that researchers seek to experimentally elicit contingent reciprocity, such as in the giving assistance tests (GATs). In the GAT, individuals have the choice between providing instrumental help to another or do nothing. In GAT’s, chimpanzees reciprocally provide favors as long as the experimenter enforces a turn-taking structure (21–23). The chimpanzees do not spontaneously alternate doing favors, however (23). In the prosocial choice test (PCT), in contrast, subjects are offered a choice between a prosocial option that rewards both the actor and their partner (i.e., the 1/1 option) and a selfish option that rewards only the actor (i.e., the 1/0 option). However, when offered a chance to alternating choices in the PCT, both chimpanzees and cotton-top tamarins fail to develop contingent reciprocity (24–26). In these particular PCT studies, too, subjects showed no spontaneous prosocial choice, which may be a necessary precondition to developing reciprocity.
If nonhuman primates fail to develop contingent reciprocity in experiments despite their ability to take advantage of iterated paradigms, we should perhaps consider other, simpler mechanisms of reciprocity that do not follow a tit-for-tat structure. The observational literature supports the idea of noncontingent reciprocity in everyday social interactions (27, 28). The purpose of the present study was to examine the effect of different mechanisms of reciprocity on capuchin monkey prosocial behavior by using a token exchange PCT similar to that of de Waal et al. (9). Capuchins are ideal subjects for this type of study given the numerous observations of cooperative and prosocial behavior in the field (29, 30), sensitivity to others’ effort in coordination experiments (31–33), and their robust, spontaneous prosocial behavior in the PCT compared with the chimpanzee, which seems more sensitive to methodological variables (12). Reciprocity may require a prosocial preference to begin with, which was not the case in several previous reciprocity experiments. In capuchins, in contrast, a spontaneous prosocial tendency has been demonstrated in two independent PCT studies (9, 10).
The least cognitively demanding reciprocity mechanism is symmetry-based. Here, reciprocity is tied to mutual social preferences. If symmetrical characteristics of the dyadic relationship (e.g., mutual association) induce prosocial tendencies, the resulting behavior will automatically be reciprocally distributed without a need for mental scorekeeping (34, 35). However, as such symmetry-based reciprocity requires an established social relationship, this mechanism is unlikely between individuals that do not live together. For this reason, our study tests capuchin monkeys in in-group pairs as well as with out-group members with whom they have no interaction outside the experimental setting.
Like most nonhuman primates, capuchin monkeys are hostile toward out-group members in the wild (29, 30) and in captivity (36–38), even though intergroup hostility tends to be limited to the adult males and alpha female in brown capuchins (30). In the present study, we speak of out-group pairs rather than strangers because although members of different groups lack visual contact, they do have auditory contact and had seen each other a couple of times during two past experiments (9, 37). It is important to note, however, that the same situation occurs between groups of wild capuchins, which also have frequent auditory and occasional visual contact with neighboring groups (30). Previous research with the same capuchins tested in the present study has demonstrated successful discrimination between in-group and out-group members’ facial images without any additional cues by using a touchscreen face-recognition task (39).
Social exchanges between partners outside the experimental setting can be ruled out for the out-group condition in the present study. This condition is relevant in relation to claims that only humans cooperate with strangers, i.e., individuals with whom they do not interact on a regular basis (6, 7). We would not go so far as to claim that our study will shed light on “strong reciprocity,” the evolutionary account proposed to explain cooperation with strangers (6, 7) that has been countered by alternative explanations (40–42), but take the more modest position that, by testing monkeys with out-group members, we may illuminate the role of overall social relationships on prosocial tendencies. Inclusion of out-group members allows us to distinguish reciprocity within established relationships from reciprocity restricted to occasional brief encounters.
Current Experiment
A range of experimental conditions was used to elucidate the role of reciprocity in prosocial choice. The conditions are listed as follows in the order in which they were presented to in-group and out-group pairs:
i) The unilateral condition, which tested for prosocial choice if only one individual was choosing. This condition lacked opportunities for direct reciprocity. In the in-group/out-group comparison, it allowed a test for symmetry-based reciprocity, which should be absent in out-group pairs lacking in interaction opportunities outside the testing environment.
ii) The alternating condition, in which both partners alternately made choices. This iterated paradigm allowed us to test sensitivity to the enhanced payoffs resulting from mutual prosociality and/or temporal contingency between the choices by both partners.
iii) The yoked control condition, in which the actor makes choices every other trial; on alternate trials, the experimenter reproduces the choice made by the partner on that trial during the corresponding alternating session. The partner is still present, but does not participate. This condition allowed us to determine whether any change from the unilateral to the alternating condition was a result of either (a) the partner’s actual behavior and choice, or (b) the rewards produced by the partner. The yoked control reproduces (b), but eliminates (a).
iv) Two partner absent tests, in which one individual chooses without a partner. During the open-panel partner-absent test, understanding of the token values was tested by allowing the actor to access both compartments of the test chamber and get both rewards if a prosocial choice was made. The closed-panel partner-absent test assessed whether the capuchins had formed a lasting preference for either token.
Finally, we tested whether equity or inequity of rewards impacted prosocial choices (i.e., equal vs. unequal rewards) given that capuchin monkeys show sensitivity to inequity (43, 44). In the unilateral PCT, capuchins tend to behave prosocially, unless their partner receives a higher-quality reward, in which case they choose the prosocial token at chance levels (9). Note that inequity to the advantage of the partner makes the exchange of benefits more advantageous in an alternating design, as every 1/2 outcome is followed by a 2/1 outcome if the partner reciprocates.
Results
Prosocial Choice.
Brown capuchin monkeys (n = 8 adult females and n = 4 juvenile males) were tested in same-sex pairs by using a PCT similar to de Waal et al. (ref. 9; Fig. 1). Following 30 forced choice trials to familiarize the prosocial or selfish consequences of each token, monkeys were tested in two unilateral sessions (n = 30 trials each), in which one individual made the token choices for the entire test session (i.e., the actor) and the other individual was passive (i.e., the partner).
Fig. 1.

Experimental setup. A capuchin actor selects a token from the jumble of six tokens (n = 3 of each type). Her partner watches through the clear Lexan panel separating the two individuals. Following a token choice, the experimenter holds her hand up in a begging gesture, and the capuchin returns the token to her hand.
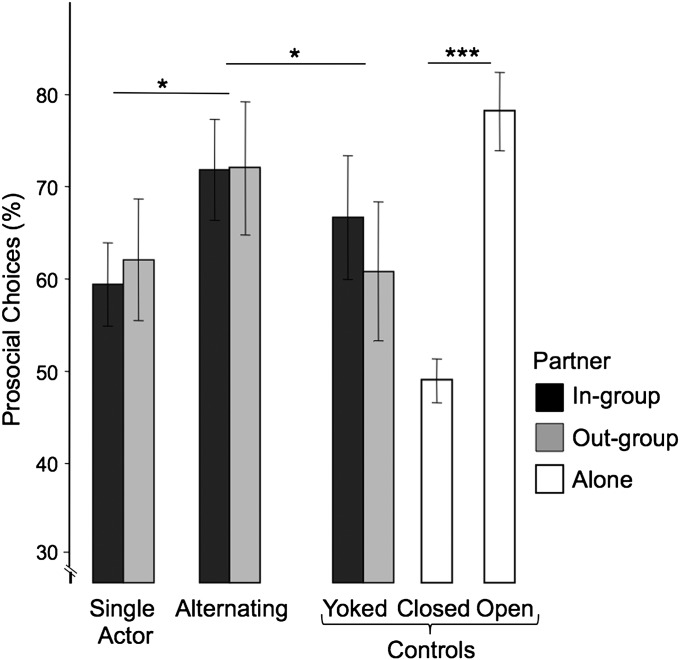
Across the six 10-trial blocks of the unilateral sessions, we found no significant change in the percent of prosocial choice (repeated-measures ANOVA, F5,55 = 0.70, P = 0.63), suggesting the monkeys had learned the token values during familiarization. There was also no significant difference in the percent of prosocial choices for in-group vs. out-group partners (mixed-measures ANOVA, F1,11 = 0.07, P = 0.80).
When both individuals took turns during the alternating sessions, there was a significant increase in prosocial choice (F1,11 = 26.11, P < 0.001; Fig. 2). This increase occurred in in-group and out-group pairs, which again did not significantly differ (F1,11 = 0.04, P = 0.86), and there was no correlation between one partner’s increase in prosociality vs. the other partner’s increase (in-group, r = −0.53, n = 6, P = 0.28; out-group, r = −0.06, n = 6, P = 0.93). Prosocial choices increased slightly but nonsignificantly across the six 10-trial blocks of the alternating sessions (F5,55 = 2.58, P = 0.09). There was no evidence that this increase resulted from a contingency between a monkey’s own choice vs. its partner’s choice in the previous trial. Pooling the data for all pairs, the overall 2 × 2 χ2 value for both partners’ choices was 0.88 (df = 1, P = 0.34; Table 1). Analyzing the same data by type of pair, by individual, or by condition did not reveal any significant contingencies (e.g., pooled data on in-group pairs, χ2 = 2.84, P = 0.09; out-group pairs, χ2 = 0.13, P = 0.72). By using logistic regression, it was tested whether an individual’s choice in trial n could be predicted by their partner’s choice in trial n − 1, or in trials n − 1 and n − 2, or in trials n − 1, n − 2, and n − 3 (26). There was no significant effect of the partner’s previous choice(s) on the actor’s choice for out-group pairs (Wald χ2 = 0.366, df = 1, P = 0.55). In-group pairs actually made slightly more prosocial choices following their partner’s selfish choice (Wald χ2 = 4.46, df = 1, P = 0.04). In sum, there was no demonstrable tit-for-tat behavior for any pairing.
Fig. 2.
Comparison of prosocial choices across sessions. The mean (±SEM) percent prosocial choices across conditions for in-group and out-group pairs and partner-absent control sessions (*P < 0.05 and ***P < 0.001).
Table 1.
Contingency table (2 × 2) illustrating the number of choices made by all individuals across all alternating sessions as a function of their partner’s choice in the previous trial
| Partner’s choice at trial n − 1 (%) |
|||
| Actor’s choice at trial n | Selfish | Prosocial | Total |
| Selfish | 77 (23.2) | 255 (76.8) | 332 (100) |
| Prosocial | 268 (26.0) | 764 (74.0) | 1,032 (100) |
To test whether the increase observed during the alternating session resulted from simple reinforcement learning resulting in an association of the prosocial token with rewards, we conducted a yoked control session in which only the actor produced choices, which alternated with the reward that the partner had produced in a previous alternating sessions. Actors picked the prosocial token significantly less frequently during the yoked control test than the corresponding alternating condition session (F1,11 = 6.93, P = 0.02; Fig. 2). Again, there was no significant effect of in-group vs. out-group pairing (F1,11 = 0.063, P = 0.81).
Finally, all subjects were tested without a partner to assess their understanding of the tokens after completion of the testing series. In the open-panel test, the monkeys could produce two rewards for themselves, and they did so enthusiastically, i.e., 78.25 ± 8.20% (mean ± SD). There was no change in the percent of prosocial choices across the three 10-trial blocks (F2,22 = 0.95, P = 0.40), which suggests they understood how the token “worked.” There was a significant decrease in prosocial choice, however, from the open-panel to the closed-panel condition (F1,11 = 86.71, P < 0.001). During the closed-panel tests, prosocial choice became indistinguishable from the 50% chance level (one-sample t test, t11 = −0.22, P = 0.083) and was significantly lower than in the corresponding unilateral condition (F1,11 = 11.824, P = 0.006), suggesting no preference for either token in the absence of a partner. Again, there was no change over time during the closed-panel tests (divided into three 10-trial blocks, F2,22 = 0.12, P = 0.89).
Inequity.
To test if capuchins could overcome inequity aversion through reciprocity, the unilateral, alternating, and yoked control conditions were compared under two reward conditions. During equal rewards, a selfish choice produced a piece of apple for the actor only and a prosocial choice produced a piece of apple for both partners. For unequal rewards, the selfish choice remained one apple reward for the actor only, whereas a prosocial choice rewarded the actor with apple and the partner with a grape. So, actors always received an apple and the only consequence that varied was the partner’s reward quality. A food preference test before the experiment demonstrated that all individuals preferred grape over apple (choosing the grape on average 91.25 ± 12.99% of the time; paired t test, t11 = 11.00, P < 0.001).
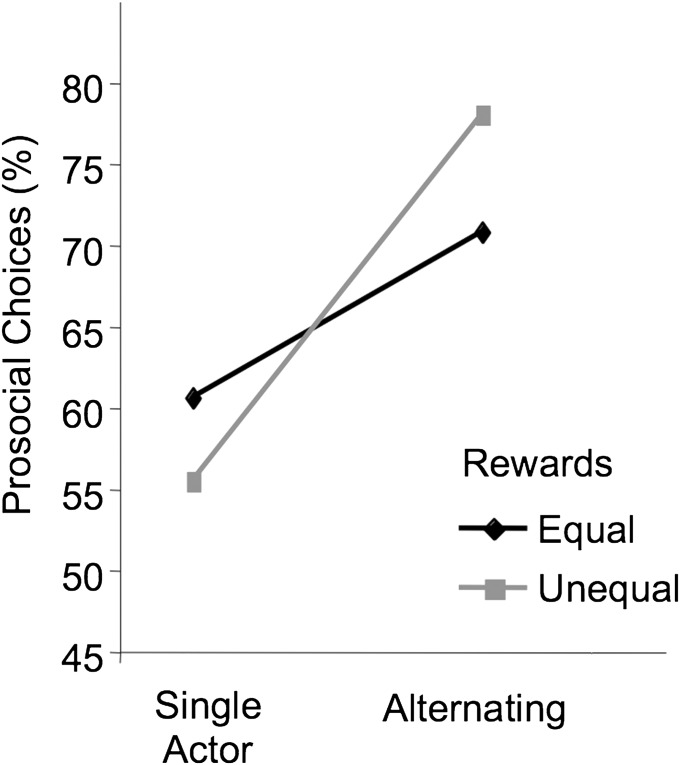
During the unilateral condition, there was no significant difference in the percentage of prosocial choices between equal and unequal rewards, but there was a significant interaction between alternating condition and reward (F1,11 = 5.20, P = 0.043). During unilateral sessions, actors chose the prosocial token at a higher rate when rewards were equal than when rewards were unequal, whereas the opposite was true for alternating sessions (Fig. 3). Similar to the equal reward conditions, we found no contingency when rewards were unequal (in-group, χ2 = 0.04, df = 1, P = 0.84; out-group, χ2 = 0.24, df = 1, P = 0.62). During the yoked control, there was a slight but nonsignificant decrease from the corresponding alternating session when rewards were unequal (F1,11 = 3.10, P = 0.10).
Fig. 3.
The effect of alternating and reward condition on prosocial choices. Although there was no significant difference between equal and unequal rewards, there was a significant interaction between equity and alternating choices (P < 0.05).
Discussion
This study confirms a spontaneous tendency in capuchin monkeys to benefit a partner (9, 10), a tendency that increases if they have the opportunity to alternate choices with their partner, especially if the rewards are unequal. This ability to maximize valuable rewards suggests sensitivity to the payoff distribution. Like humans, capuchins may understand the advantages of reciprocity. However, they seem to do so without any evidence for a tit-for-tat strategy. In fact, in-group pairs were actually slightly more likely to make a prosocial choice than a selfish choice following a selfish choice by their partner. This suggests that a simpler mechanism may be allowing the monkeys to maximize benefits without the cognitive demands of memory-based contingent reciprocity (45).
Reciprocity can be achieved in several ways without mental scorekeeping (27). Given the short time delay between trials (∼15 s), the monkeys may have perceived that they were “acting together” or mirroring each other’s behavior as in behavioral coordination (46). Previous work has demonstrated that slightly asynchronous actions in capuchin monkeys can facilitate behavior similarity in a way that is equivalent to simultaneous action, a phenomenon known as isomorphic coordination (46). Capuchin monkeys are more affiliative and willing to interact with humans who have recently mimicked their actions (47), and mimicry increases prosocial behavior, affiliation, and a feeling of interconnectedness in humans (48–50). Mimicry, behavioral coordination, and synchronization may facilitate positive emotions and bonding, inducing state-matching between individuals, such as in emotional contagion (16). The same mechanism may underlie “attitudinal reciprocity” (51, 52), defined as a short-term mirroring of attitudes that results in an exchange of benefits (51). In this experiment, the “mirroring” was not of the particular choices made, but resulted from shared task participation while performing identical actions. That capuchins rewarded their partners more when both individuals were participating fits previous indications of increased food sharing if both partners invested in its acquisition (31–33).
The hypothetical connection between joint action and prosociality is particularly well supported by the yoked control test. The significant drop in prosocial choice if outcomes were not produced by the partner, but merely mimicked in a yoked control, demonstrates that the monkeys were not just responding to rewards or partner presence, but to their partner’s actual behavior, at least in the equal rewards condition. So, even though no contingency was found between the choices by both individuals, “acting together” may have stimulated prosocial choice compared with tests in which one partner made all choices whereas the other was passive. Although there was a similar decrease in the unequal rewards condition, it was not significant, suggesting that the presence of high-quality rewards may have overridden the effects of joint action.
The capuchins were tested with both in-group and out-group partners to determine if another simpler form of reciprocity, symmetry-based, played a role in the unilateral condition. As there was no difference between in-group and out-group pairs, the possibility that reciprocity was a byproduct of relationships outside the experimental context could be excluded. This finding was particularly surprising given the hostile tendency of capuchins toward out-group members in the wild and a previous study in which this species chose the prosocial token at chance levels when paired with out-group partners (9). The present study used a full within-subjects design to directly compare each individual’s behavior with in-group and out-group partners, as well as a broader mixed-sex subject pool. That in-group and out-group pairs followed the same patterns throughout the different conditions suggests that capuchins are using the same cognitive mechanisms regardless of their relationship with the partner outside of the experiment. Finally, the dramatic decrease from the open panel to the closed-panel partner-absent conditions suggests that the capuchins understood the token values, had neither developed a preference nor a lack of interest in either token, and could flexibly respond to changing conditions throughout the test sessions.
Capuchins demonstrated a great deal of behavioral flexibility in this prosocial choice task. Taken together, these results demonstrate that noncontingent mechanisms can produce outcomes similar to the often assumed (but rarely demonstrated) tit-for-tat strategy, thus arguing against the notion that effective reciprocity implies mental scorekeeping.
Methods
Subjects and Housing.
Subjects were eight adult female (age 6–39 y) and four adolescent male (age 4–6 y) brown capuchin monkeys (C. apella) housed in two separate social groups at the Yerkes National Primate Research Center. One group consisted of 15 monkeys housed in 25 m2, and the other of 11 monkeys in 31 m2. Both groups had access to indoor and outdoor areas and were visually, but not acoustically, isolated from each other. The monkeys received Purina monkey chow and water ad libitum, and trays containing fresh produce every evening. Monkeys were never food- or water-deprived, and all procedures were approved by the Emory University Institutional Animal Care and Use Committee before the commencement of the study.
Only monkeys who had been trained to exchange tokens were selected to participate in this study. Each subject was paired with a maternal nonkin, familiar group member in the in-group condition, and an unfamiliar partner from the other group in the out-group condition, for a within-subjects design. Partners were of the same sex and selected to be as closely matched in age and rank as possible. For each test, the subject designated as the actor interacted with the experimenter and the other subject served as a passive partner.
Token Exchange.
This experiment used the token exchange paradigm of de Waal et al. (9). Tokens for this study were 3.5 × 5-cm PVC pipes. Each condition used two different types of token, which were physically identical, but painted with visually distinct colors and patterns. Six tokens (three of each type) were presented jumbled together in a 13 × 23-cm shallow plastic bin (Fig. 1). In addition to minimizing any location biases for the monkeys (as a dichotomous choice might incur), using “jumble” of tokens minimized experimenter biases by making it difficult to cue the subjects toward a particular token.
Token preferences were tested before the commencement of the study to make sure subjects did not have an inherent preference for either token as in de Waal et al. (9). In the present study, the average initial preference for any token was 53.13 ± 4.58% (mean ± SD), and no tokens failed the initial preference test.
One token was designated as the selfish token, which conferred a reward upon the actor only. The other token, the prosocial token, rewarded both the actor and the partner. For the equal reward condition, all rewards were a 1-cm3 piece of apple. For the unequal reward condition, the selfish token conferred a piece of apple on the actor and the prosocial token rewarded the actor with a piece of apple and the partner with a highly preferred grape. Thus, the actor always received an apple reward in both reward conditions, regardless of which token was chosen.
Each trial consisted of the experimenter presenting the actor with the tokens in a dish. The actor was given 30 s to select a token. When the actor had selected a token and took it into the test chamber, the experimenter held her hand in a begging gesture in front of the actor. The actor had 30 s to return the token to the experimenter by placing it in her hand. The experimenter then placed the token in a clearly visible spot between both monkeys to remind them of the choice and distributed the rewards according to the token choice. Before delivering the rewards, the experimenter held up both rewards so that both monkeys could see their own and their partner’s rewards. After a 15-s intertrial interval, the next trial began. If the actor failed to choose or return the token within the allotted time, or the actor did not place the token in the experimenter’s hand (i.e., threw it on the floor or at the experimenter), the trial was cancelled, no reward was given, and the intertrial interval commenced. Failures occurred in fewer than 1% of the trials. Each test session consisted of 30 trials.
Token Familiarization.
The purpose of token familiarization session was to familiarize the actor with the token values. This session consisted of 30 forced choice trials: 15 in which only the selfish token was available and 15 in which only the prosocial token was available. The order of the trials was randomly determined by using an online random number generator (www.random.org). Exchanges were be rewarded differently by token, as described earlier.
Test Phases.
Subjects completed four different test phases as described in the subsequent sections. After the first subject completed the familiarization and phase 1 in the role of actor, the roles were switched on a subsequent day and the second subject completed familiarization and phase 1. When both subjects had completed phase 1, they moved on to phase 2. All test phases were repeated for equal and unequal reward conditions for in-group and out-group pairs, and the order in which each pair completed the conditions was randomized. All statistics were tested by using SPSS software (version 17.0), and, unless otherwise specified, all comparisons were done by using mixed-measures ANOVAs.
Phase 1: Unilateral sessions.
Unilateral sessions were designed to test the level of prosociality individuals demonstrated without immediate, direct reciprocity involved. Procedure for these sessions was similar to that of de Waal et al. (9). One individual served as an actor for the entire session, the other as a passive partner. The actor was given 30 free-choice trials following the token exchange procedure described earlier. All unilateral sessions were repeated on a second day to ensure that any learning effects had attenuated before moving on.
Phase 2: Alternating sessions.
Alternating sessions consisted of 30 trials as described earlier. These sessions differed from single-actor sessions in that from trial to trial the role of actor and partner switched between both subjects. In other words, the monkeys alternated choosing tokens. Alternating sessions were repeated for a second day to allow the monkeys to learn about their partner’s behavior.
Phase 3: Control conditions.
Condition A.
This control (yoked control) was designed to test whether the monkeys were responding to their partner’s choices in the alternating sessions rather than just the rewards they received. In these sessions, both individuals were present, but only one individual served as the actor, making a choice every other trial (just as in the alternating sessions). Instead of letting the second individual make a choice in the remaining trials (as they would have in the alternating sessions), the experimenter selected the token that corresponded to choices made by the second individual during a previous alternating session between the same two monkeys (Table 2). Thus, the second individual’s choices were precisely replicated by us (i.e., “yoked”), but not made by the monkey itself. This means that the first individual (the actor) attained exactly the same number of rewards as in the corresponding alternating session, but these rewards were not a result of the second individual’s behavior.
Table 2.
Comparison of the yoked control and alternating sessions
| Trial | Individual exchanging | Token choice | Reward for individual 1 |
| Example from unequal, alternating session | |||
| 1 | 1 | A | Apple |
| 2 | 2 | A | No reward |
| 3 | 1 | B | Apple |
| 4 | 2 | B | Grape |
| 5 | 1 | B | Apple |
| 6 | 2 | A | No reward |
| Corresponding yoked control session | |||
| 1 | 1 | A or B | Apple |
| 2 | No exchange | — | No reward |
| 3 | 1 | A or B | Apple |
| 4 | No exchange | — | Grape |
| 5 | 1 | A or B | Apple |
| 6 | No exchange | — | No reward |
The only difference between the two sessions is that, during alternating trials, individual 2 was exchanging, but, during the yoked control trials, individual 2 did not exchange. The rewards distributed on those trials corresponded to the choices individual 2 made during the alternating session.
Condition B.
This control (partner-absent tests) was designed to assess the subjects’ understanding of the tokens at the end of each partner condition and to rule out the possibility that individuals have simply developed a large preference for the prosocial token. Procedures followed those of the unilateral sessions except that there was no partner present. The order of the two tests was counterbalanced across individuals. During the open-panel test, the Lexan panel that divided the test chamber was opened halfway to allow individuals to access both rewards following a prosocial choice. This test confirmed an understanding that the prosocial token delivers a reward to each test compartment. It also tested for a potential loss of interest in the prosocial token over sessions. For the closed-panel test, the Lexan panel was closed so that, regardless of token choice, the monkeys received only one piece of apple.
Acknowledgments
The authors thank T. McKenney for assistance with testing and data collection, S. F. Brosnan and P. Rochat for helpful discussions, S. Yamamoto and S. Perry for helpful comments on the manuscript, and the animal care and veterinary staff at the Yerkes National Primate Research Center (YNPRC) for maintaining the health of our study subjects. This work was supported by National Science Foundation Grant IOS-0718010 (to F.B.M.d.W.), National Center for Research Resources Grant P51RR165, and Office of Research Infrastructure Programs/Office of the Director Grant P51OD11132 (to YNPRC). The YNPRC is fully accredited by the American Association for Accreditation for Laboratory Animal Care.
Footnotes
The authors declare no conflict of interest.
References
- 1.Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- 2.Burkart JM, Hrdy SB, Van Schaik CP. Cooperative breeding and human cognitive evolution. Evol Anthropol. 2009;18:175–186. [Google Scholar]
- 3.de Waal FBM, Suchak M. Prosocial primates: Selfish and unselfish motivations. Philos Trans R Soc Lond B Biol Sci. 2010;365:2711–2722. doi: 10.1098/rstb.2010.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batson CD, Moran T. Empathy-induced altruism in a prisoner’s dilemma. Eur J Soc Psychol. 1999;29:909–924. [Google Scholar]
- 5.de Waal FBM. Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 6.Bowles S, Gintis H. Origins of human cooperation. In: Hammerstein P, editor. The Genetic and Cultural Evolution of Cooperation. Cambridge, MA: MIT Press; 2003. pp. 429–443. [Google Scholar]
- 7.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425:785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 8.Burkart JM, Fehr E, Efferson C, van Schaik CP. Other-regarding preferences in a non-human primate: Common marmosets provision food altruistically. Proc Natl Acad Sci USA. 2007;104:19762–19766. doi: 10.1073/pnas.0710310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal FBM, Leimgruber K, Greenberg AR. Giving is self-rewarding for monkeys. Proc Natl Acad Sci USA. 2008;105:13685–13689. doi: 10.1073/pnas.0807060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshminarayanan VR, Santos LR. Capuchin monkeys are sensitive to others’ welfare. Curr Biol. 2008;18:R999–R1000. doi: 10.1016/j.cub.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 11.Hare B, Kwetuenda S. Bonobos voluntarily share their own food with others. Curr Biol. 2010;20:R1–R2. doi: 10.1016/j.cub.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 12.Horner V, Carter JD, Suchak M, de Waal FBM. Spontaneous prosocial choice by chimpanzees. Proc Natl Acad Sci USA. 2011;108:13847–13851. doi: 10.1073/pnas.1111088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warneken F, Hare B, Melis AP, Hanus D, Tomasello M. Spontaneous altruism by chimpanzees and young children. PLoS Biol. 2007;5:e184. doi: 10.1371/journal.pbio.0050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto S, Humle T, Tanaka M. Chimpanzees’ flexible targeted helping based on an understanding of conspecifics’ goals. Proc Natl Acad Sci USA. 2012;109:3588–3592. doi: 10.1073/pnas.1108517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boesch C, Boesch-Achermann H. The Chimpanzees of Tai Forest: Behavioral Ecology and Evolution. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 16.de Waal FBM. The Age of Empathy: Nature’s Lessons for a Kinder Society. New York: Harmony; 2009. [Google Scholar]
- 17.Barrett L, Henzi SP, Weingrill T, Lycett JE, Hill RA. Market forces predict grooming reciprocity in female baboons. Proc Biol Sci. 1999;266:665–670. [Google Scholar]
- 18.Schino G. Grooming and agonistic support: A meta-analysis of primate reciprocal altruism. Behav Ecol. 2007;18:115–120. [Google Scholar]
- 19.de Waal FBM. The chimpanzee’s service economy: Food for grooming. Evol Hum Behav. 1997;18:375–386. [Google Scholar]
- 20.Koyama NF, Caws C, Aureli F. Interchange of grooming and agonistic support in chimpanzees. Int J Primatol. 2006;27:1293–1309. [Google Scholar]
- 21.Brosnan SF, Beran MJ. Trading behavior between conspecifics in chimpanzees, Pan troglodytes. J Comp Psychol. 2009;123:181–194. doi: 10.1037/a0015092. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto S, Humle T, Tanaka M, Plaistow S. Chimpanzees help each other upon request. PLoS ONE. 2009;4:e7416. doi: 10.1371/journal.pone.0007416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto S, Tanaka M. Do chimpanzees (Pan troglodytes) spontaneously take turns in a reciprocal cooperation task? J Comp Psychol. 2009;123:242–249. doi: 10.1037/a0015838. [DOI] [PubMed] [Google Scholar]
- 24.Brosnan SF, et al. Chimpanzees (Pan troglodytes) do not develop contingent reciprocity in an experimental task. Anim Cogn. 2009;12:587–597. doi: 10.1007/s10071-009-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cronin KA, Schroeder KKE, Snowdon CT. Prosocial behaviour emerges independent of reciprocity in cottontop tamarins. Proc Biol Sci. 2010;277:3845–3851. doi: 10.1098/rspb.2010.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Tanaka M. The Influence of kin relationship and reciprocal context on chimpanzees’ other regarding preferences. Anim Behav. 2010;79:595–602. [Google Scholar]
- 27.Brosnan SF, de Waal FBM. A proximate perspective on reciprocal altruism. Hum Nat. 2002;13:129–152. doi: 10.1007/s12110-002-1017-2. [DOI] [PubMed] [Google Scholar]
- 28.Schino G, Aureli F. Reciprocal altruism in primates: Partner choice, cognition and emotion. Adv Stud Behav. 2009;39:45–69. [Google Scholar]
- 29.Perry S. Intergroup encounters in wild white-faced capuchins. Int J Primatol. 1996;17:309–330. [Google Scholar]
- 30.Fragaszy DM, Visalberghi E, Fedigan LM. The Complete Capuchin: The Biology of the Genus Cebus. Cambridge, UK: Cambridge Univ Press; 2004. [Google Scholar]
- 31.de Waal FBM, Berger ML. Payment for labour in monkeys. Nature. 2000;404:563. doi: 10.1038/35007138. [DOI] [PubMed] [Google Scholar]
- 32.Hattori Y, Kuroshima H, Fujita K. Cooperative problem solving by tufted capuchin monkeys (Cebus apella): spontaneous division of labor, communication, and reciprocal altruism. J Comp Psychol. 2005;119:335–342. doi: 10.1037/0735-7036.119.3.335. [DOI] [PubMed] [Google Scholar]
- 33.Takimoto A, Fujita K. I acknowledge your help: Capuchin monkeys’ sensitivity to others’ labor. Anim Cogn. 2011;14:715–725. doi: 10.1007/s10071-011-0406-5. [DOI] [PubMed] [Google Scholar]
- 34.de Waal FBM, Luttrell LM. Mechanisms of social reciprocity in three primate species: Symmetrical relationship characteristics or cognition? Ethol Sociobiol. 1988;9:101–118. [Google Scholar]
- 35.Sabbatini G, De Bortoli Vizioli A, Visalberghi E, Schino G. Food transfers in capuchin monkeys: An experiment on partner choice. Biol Lett. 2012 doi: 10.1098/rsbl.2012.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper MA, Bernstein IS, Fragaszy DM, de Waal FBM. Integration of new males into four social groups of tufted capuchins (Cebus apella) Int J Primatol. 2001;22:663–683. [Google Scholar]
- 37.de Waal FBM, Dindo M, Freeman CA, Hall MJ. The monkey in the mirror: Hardly a stranger. Proc Natl Acad Sci USA. 2005;102:11140–11147. doi: 10.1073/pnas.0503935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fragaszy DM, Baer J, Adams-Curtis LE. Introduction and integration of strangers into captive groups of tufted capuchins (Cebus apella) Int J Primatol. 1994;15:399–420. [Google Scholar]
- 39.Pokorny JJ, de Waal FBM. Monkeys recognize the faces of group mates in photographs. Proc Natl Acad Sci USA. 2009;106:21539–21543. doi: 10.1073/pnas.0912174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnham TC, Johnson DDP. The biological and evolutionary logic of human cooperation. Anal Kritik. 2005;27:113–135. [Google Scholar]
- 41.Lehmann L, Rousset F, Roze D, Keller L. Strong reciprocity or strong ferocity? A population genetic view of the evolution of altruistic punishment. Am Nat. 2007;170:21–36. doi: 10.1086/518568. [DOI] [PubMed] [Google Scholar]
- 42.Levitt SD, List JA. What do laboratory experiments measuring social preferences reveal about the real world? J Econ Perspect. 2007;21:153–174. [Google Scholar]
- 43.Brosnan SF, De Waal FBM. Monkeys reject unequal pay. Nature. 2003;425:297–299. doi: 10.1038/nature01963. [DOI] [PubMed] [Google Scholar]
- 44.van Wolkenten M, Brosnan SF, de Waal FBM. Inequity responses of monkeys modified by effort. Proc Natl Acad Sci USA. 2007;104:18854–18859. doi: 10.1073/pnas.0707182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens JR, Cushman FA, Hauser MD. Evolving the psychological mechanisms for cooperation. Annu Rev Ecol Evol Syst. 2005;36:499–518. [Google Scholar]
- 46.Galloway AT, Addessi E, Fragaszy DM, Visalberghi E. Social facilitation of eating familiar food in tufted capuchins (Cebus apella): Does it involve behavioral coordination? Int J Primatol. 2005;26:181–189. [Google Scholar]
- 47.Paukner A, Suomi SJ, Visalberghi E, Ferrari PF. Capuchin monkeys display affiliation toward humans who imitate them. Science. 2009;325:880–883. doi: 10.1126/science.1176269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashton-James C, van Baaren RB, Chartrand TL, Decety J, Karremans J. Mimicry and me: The impact of mimicry on self-construal. Soc Cogn. 2007;25:518–535. [Google Scholar]
- 49.Lakin JL, Chartrand TL. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol Sci. 2003;14:334–339. doi: 10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- 50.van Baaren RB, Holland RW, Kawakami K, van Knippenberg A. Mimicry and prosocial behavior. Psychol Sci. 2004;15:71–74. doi: 10.1111/j.0963-7214.2004.01501012.x. [DOI] [PubMed] [Google Scholar]
- 51.de Waal FBM. de Waal FB Attitudinal reciprocity in food sharing among brown capuchin monkeys. Anim Behav. 2000;60:253–261. doi: 10.1006/anbe.2000.1471. [DOI] [PubMed] [Google Scholar]
- 52.de Waal FBM, Brosnan SF. Simple and complex reciprocity in primates. In: Kappeler PM, van Schaik CP, editors. Cooperation in Primates and Humans: Mechanisms and Evolution. Berlin: Springer; 2006. pp. 85–105. [Google Scholar]