Abstract
Down-regulation of cell surface growth factor receptors plays a key role in the tight control of cellular responses. Recent reports suggest that the ubiquitin system, in addition to participating in degradation by the proteasome of cytosolic and nuclear proteins, might also be involved in the down-regulation of various membrane receptors. We have previously characterized a signal in the cytosolic part of the interleukin 2 receptor β chain (IL2Rβ) responsible for its targeting to late endosomes/lysosomes. In this report, the role of the ubiquitin/proteasome system on the intracellular fate of IL2Rβ was investigated. Inactivation of the cellular ubiquitination machinery in ts20 cells, which express a thermolabile ubiquitin-activating enzyme E1, leads to a significant decrease in the degradation rate of IL2Rβ, with little effect on its internalization. In addition, we show that a fraction of IL2Rβ can be monoubiquitinated. Furthermore, mutation of the lysine residues of the cytosolic region of a chimeric receptor carrying the IL2Rβ targeting signal resulted in a decreased degradation rate. When cells expressing IL2Rβ were treated either by proteasome or lysosome inhibitors, a significant decrease in receptor degradation was observed. Our data show that ubiquitination is required for the sorting of IL2Rβ toward degradation. They also indicate that impairment of proteasome function might more generally affect intracellular routing.
INTRODUCTION
After endocytosis, many growth factor and hormone receptors are transported to late endocytic compartments and degraded. This leads to the down-regulation of receptors, which is important to control their cell surface expression, and thereby the transduction of signals delivered in response to growth factors and hormones. This process requires sorting of receptors in early endocytic compartments and further transport to late compartments, where they are degraded (Mukherjee et al., 1997).
We recently investigated the intracellular fate of a growth factor receptor, the interleukin 2 receptor (IL2R). The cytokine interleukin 2 (IL2) is produced by activated helper T lymphocytes and acts as a potent proliferation and differentiation factor on a variety of cells of the immune system. High-affinity IL2 receptors (Kd ≈ 10–100 pM) are composed of three distinct components, the α, β, and γ chains, that are associated in a noncovalent manner (Minami et al., 1993). Both the β and γ chains, but not the α chain, belong to the cytokine receptor superfamily (Bazan, 1990). This hematopoietic cytokine receptor family includes receptors for several cytokines such as erythropoietin, the granulocyte colony-stimulating factor, the granulocyte-macrophage colony-stimulating factor, the leukemia inhibitory factor, growth hormone, prolactin, and ciliary neurotrophic factor. Many receptor subfamily members share at least one component; thus, the receptors for IL2, 4, 7, 9, and 15 have a common γ chain, and the receptors for IL2 and IL15 share the β chain (reviewed by Sugamura et al., 1996).
One of the early events following IL2 binding to high-affinity receptors on the cell surface is the efficient internalization of IL2 receptor complexes (Duprez et al., 1988; Subtil et al., 1994). After endocytosis, the three subunits are sorted differently: the α chain recycles to the plasma membrane, whereas the β and γ chains are targeted to late endocytic compartments (Hémar et al., 1995).
Little is known about the molecular mechanisms controlling intracellular sorting of the subunits. We have previously shown that a motif of 10 amino acids in the cytosolic tail of IL2Rβ is sufficient to retarget a normally recycling receptor toward degradation compartments (Subtil et al., 1997). Accordingly, deletion of this signal strongly impaired IL2Rβ degradation. Further molecular characterization of this motif showed that it was different from the well-documented tyrosine and di-leucine families of trafficking signals (Subtil et al., 1998).
What are the cellular mechanisms responsible for the sorting of the β subunit to late compartments and its final degradation? It is known that cells degrade proteins generally by two major systems: the lysosomes and the ubiquitin/proteasome system. Lysosomes are in general responsible for the degradation of membrane and extracellular proteins that enter cells by endocytosis. The ubiquitin/proteasome pathway is involved in the degradation of cytosolic and nuclear proteins (Weissman, 1997). Ubiquitin is a 76 amino-acid polypeptide expressed in all eucaryotic cells and highly conserved from yeast to human. Classically, it is reported that degradation of proteins by the proteasome involves two distinct and successive steps (reviewed by Hershko and Ciechanover, 1998). First, multiple ubiquitin molecules are covalently linked to the target protein via a lysine residue, and then the ubiquitinated protein is degraded by the 26S proteasome. Ubiquitin molecules are added to target proteins through the sequential action of three enzymes; ubiquitin is first activated in an ATP-dependent manner by a specific activating enzyme (E1) and then transferred to an ubiquitin carrier protein (E2). Next, activated ubiquitin is linked to a lysine residue of the substrate protein, either directly or in cooperation with an ubiquitin ligase (E3). In most species, only one E1 enzyme but multiple E2 and E3 enzymes have been described.
Recently, it has been shown that besides its role in cytosolic and nuclear protein degradation, the ubiquitin-conjugating machinery and the proteasome may play a role in the sorting of various membrane anchored-proteins (reviewed by Bonifacino and Weissman, 1998; Hicke, 1999; Strous and Govers, 1999). A clear link between the ubiquitin/proteasome system and several membrane proteins' down-regulation has been established. Involvement of ubiquitin/proteasome pathway in the down-regulation of some mammalian cell surface receptors was demonstrated (reviewed by Bonifacino and Weissman, 1998; Hicke, 1999; Strous and Govers, 1999). Ubiquitination of some of these mammalian plasma membrane proteins might serve as a signal for their entry into the endocytic pathway. In yeast, mono- or diubiquitination in this process has been shown to be sufficient for several membrane proteins (Galan and Haguenauer-Tsapis, 1997; Terrell et al., 1998; Lucero et al., 2000). In some cases a direct role of the ubiquitin/proteasome system in the intracellular degradation of membrane proteins was suggested (Laing and Beyer, 1995; Mori et al., 1995; Jeffers et al., 1997; Staub et al., 1997).
In the present article, we have investigated the role of ubiquitination as well as of proteasomes in the down-modulation of IL2Rβ and of a chimera carrying its degradation targeting signal.
MATERIALS AND METHODS
Cells, Monoclonal Antibodies, and Reagents
IARC 301.5 is a subclone from a cell line derived from a human T lymphoma, which expresses high- and low-affinity IL2 receptors (Duprez et al., 1985, 1988). YT12881, a subclone from the natural killer cell line YT, which expresses the IL2R β and γ chains, was provided by Dr. Kendall Smith (Dartmouth Medical School, NH) (Teshigawara et al., 1987). IARC301.5, YT, and erythroleukemia K562 cells were grown in suspension in RPMI 1640. The Chinese lung cell line ts20 used in this study was kindly provided by Dr. G.J. Strous (Utrecht University, The Netherlands) and is characterized by an inactive ubiquitin conjugating system at the nonpermissive temperature of 42°C (Kulka et al., 1988). ts20 cells were maintained in culture at 33°C in DMEM. All culture media were supplemented with 10% decomplemented fetal calf serum and 2 mM l-glutamine. Stably transfected K562 or ts20 cells were grown in the same medium supplemented with 1.5 mg/ml G418 (Geneticin; Life Technologies, Gaithersburg, MD).
Mouse monoclonal antibodies (mAbs) 7G7B6 (IgG2a) and 2A3A1H (IgG1) directed against the α chain of the IL2 receptor (IL2Rα) were obtained from the American Type Culture Collection (Rockville, MD). Mouse mAb341 (IgG1) and 561 (IgG2a), directed against the β chain, were kind gifts from Dr. R. Robb (Dupont Merck Pharmaceutical, Wilmington, DE) (Voss et al., 1993). The rabbit polyclonal anti-ubiquitin antiserum was purchased from Sigma (St. Louis, MO), the mouse monoclonal anti-ubiquitin antibody (IgG1) was from Zymed Laboratories (San Francisco, CA), and the anti-human lysosome-associated membrane protein-1 (Lamp-1) H4A3 was from the Developmental Studies Hybridoma bank (University of Iowa, Iowa City, IA). Phycoerythrin-conjugated goat F(ab)′2 anti-murine IgG was obtained from Immunotech (Marseilles, France) and peroxidase-conjugated (sheep) anti-mouse immunoglobulins from Amersham Pharmacia Biotech (Uppsala, Sweden). Fluorescein-labeled anti-mouse IgG1 and Texas Red-labeled anti-mouse IgG2a were from Southern Biotechnology (Birmingham, AL.). Human transferrin was labeled with Cy5 dye (Amersham Pharmacia Biotech), by using the CyDye Fluorolink reactive dye kit according to the manufacturer's instructions. Enhanced chemiluminescence and enhanced chemifluorescence-Western Blotting detection reagents were from Amersham Pharmacia Biotech.
Chloroquine, leupeptin, and N-acetyl-l-l-leucyl-norleucinal (ALLN) were purchased from Sigma, MG132 from Calbiochem (La Jolla, CA), and lactacystin from Alexis (COGER, France). Lactacystin and ALLN were dissolved in ethanol and dimethyl sulfoxide, respectively. Throughout the experiments, control cells were preincubated with the same solvent concentration in the culture medium at a final concentration not exceeding 0.5%.
Plasmids
The cDNA coding for the IL2Rβ chain was isolated from plasmid pdKCRβ, kindly provided by Dr. T. Kono (Osaka University, Osaka, Japan) and subcloned in NT vector, a gift from Dr. C. Bonnerot (Institut Curie, Paris, France) as previously described (Subtil et al., 1997). The αYβ18-27 chimera was generated by polymerase chain reaction (PCR). Briefly, the transferrin receptor YTRF internalization signal and the β chain sorting signal were inserted in the cytosolic part of the IL2Rα chain, slightly modified by the introduction of a cloning cassette (Subtil et al., 1997). The primary sequence of the cytosolic part of the αYβ18-27 chimera is TWQRRQRKSEPLSYTRFQASSPDPSKFFSQL. Mutations of the two lysine residues at position 8 and 26 in the cytosolic portion of the chimera, introducing, respectively, a glutamic acid and an alanine at these positions, were also performed by PCR. The resulting mutated chimera αYβ18-27 (K8E/K26A) will be referred to as ΔLys.
Cell Transfection
To generate stably transfected K562 or ts20 cells, 7 × 106 cells were washed once in DMEM, 4.5 g/l glucose, and resuspended in 800 μl of the same medium, with 20 μg of the plasmid of interest. Electroporation was performed using the Easyject electroporator (Eurogentec, Seraing, Belgium) with a single pulse, 240 V, 1500 μF. Selection with 1.5 mg/ml G418 was initiated 2 d after transfection, and the cells were cloned in 96-well dishes. G418-resistant clones were assayed for expression by flow cytometry by using anti-α (2A3A1H) or anti-β (341) antibodies. The expression levels of recombinant proteins in all clones tested were the same as or less than their normal level in activated lymphocytes.
Internalization Assays
Internalization of 2A3A1H or 341 antibodies directed, respectively, against the chimeras and the β chain were quantitated by flow cytometry as previously described (Subtil et al., 1998). Briefly, cells were incubated at 4°C for 60 min with 2A3A1H or 341 antibody (1/2000 ascites fluid). The cells were washed in chilled phosphate-buffered saline (PBS) at 4°C, and then they were incubated at 37°C for the indicated times to allow internalization, rapidly cooled to 4°C, and washed twice in cold PBS, 2% fetal calf serum. The cells were then incubated at 4°C for 1 h with phycoerythrin-conjugated goat F(ab)′2 anti-murine IgG and washed once at 4°C. Expression of receptors remaining at the cell surface was assayed using a FACScan flowcytometer (Becton Dickinson, San Jose, CA). Internalization was monitored by the decrease in mean fluorescence as a function of time at 37°C. For the mutant ts20 cells, after 1-h preincubation at 30°C or at 42°C, the internalization assays were performed at the same temperatures. The background fluorescence intensity, determined with cells incubated only with the secondary antibody, was <10% and was substracted.
Cell Surface Half-Life Measurements
To measure the half-life on the cell surface of IL2Rβ or of the chimeras, cells were incubated with 50 μM cycloheximide (Sigma) to prevent the synthesis of new receptors. After different times of incubation at 37°C in culture medium with cycloheximide, the cells were cooled to 4°C and cell surface expression of the remaining receptors was assayed by flow cytometry as described (Hémar and Dautry-Varsat, 1990). Time zero on the graph corresponds to a 30-min incubation in the presence of cycloheximide, a time length necessary for the newly synthesized IL2 receptor to reach the cell surface (Duprez and Dautry-Varsat, 1986). For the ts20 cell line, cells were preincubated 1 h at 30°C or at 42°C, and half-life measurement performed at these same temperatures. All experiments were done at least four times. The means ± SE are shown.
Immunofluorescence and Confocal Microscopy
K562 cells transfected with αYβ18–27 or with the ΔLys mutant were collected and incubated for 4 h at 37°C with or without various reagents, and then cycloheximide was added in the presence of Cy5-conjugated human transferrin for the last 30 min. The cells were washed twice in cold PBS and fixed in 3.7% paraformaldehyde and 0.03 M sucrose as previously described. Cells were then incubated with anti-α (7G7B6) and anti-Lamp-1 (H4A3) mAb diluted in the permeabilizing buffer. After two washes in permeabilizing buffer, the cells were further incubated for 1 h in the permeabilizing buffer containing labeled secondary antibodies. After washes and sample mounting in 100 mg/ml Mowiol (Calbiochem), 100 mg/ml 1.4-diazalbicyclo(2.2.2)octane (Sigma), 25% glycerol (vol/vol), 100 mM Tris-HCl, pH 8.5, the cells were examined under a confocal microscope (LSM 510; Zeiss, Jena, Germany). Fluorescein, Texas-Red and Cy5 dyes were excited by laser light at 488, 543, or 633 nm, respectively. To avoid bleed-through effect in multiple staining experiments, each dye was scanned independently using the multitracking function of the LSM 510 unit. Images were merged and colocalization evaluated by the LSM 510 software. Quantification of colocalization is expressed as the percentage of green pixels corresponding to the αYβ18–27 or the Δlys signal, which also contain the staining corresponding to the transferrin receptor or Lamp-1 signal relative to the total number of green pixels.
Immunoprecipitation and Western Blotting
IARC 301.5 cells or YT cells (2.5 × 107 cells/immunoprecipitate) were incubated with 12.5 μM ALLN or 10 μM MG132 or 200 μM chloroquine for 3 h. After two washes in cold PBS, the cells were lysed in 0.5% NP-40 (Sigma) complemented with 1% protease inhibitor cocktail (Sigma), 2 mM phenylmethylsulfonyl fluoride, and 5 μM ALLN for 10 min at 4°C. After a further 30-min incubation at 37°C in the presence of 1% Triton X-100 and 10 passages through a syringe needle, a postnuclear supernatant was collected by centrifugation (800 × g, 4°C). Lysate supernatants were immunoprecipitated with mAb 561 (anti-IL2Rβ), or with a rabbit anti-ubiquitin antiserum, or with a mouse monoclonal anti-ubiquitin antibody or with an irrelevant antibody. The immune complexes were recovered by the addition of protein A-Sepharose CL-4B (Amersham Pharmacia Biotech) and after five washes in lysis buffer, bound proteins eluted into electrophoresis sample buffer were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech). Membranes were probed with an anti-IL2Rβ mAb (341) and an alkaline phosphatase-conjugated or peroxidase-conjugated secondary antibody was used for immunodetection. After washing, the membranes were incubated with enhanced chemiluminescence or Vistra (enhanced chemifluorescence) reagent for 5–20 min. A quantitative analysis of Western blots was conducted for some experiments by scanning on a Storm Phosphorimager/Fluorimager (Molecular Dynamics, Sunnyvale, CA). Bands were quantitated and density values were in the linear range of the detection method.
RESULTS
An Active Ubiquitination System Is Required for Efficient Down-Regulation of IL2Rβ Chain
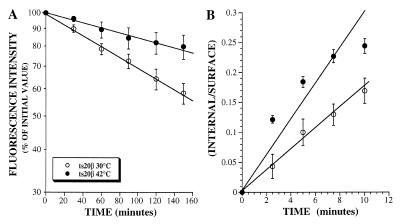
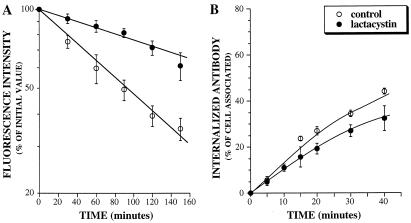
After internalization, the IL2Rβ chain is sorted to late endosomes (Hémar et al., 1995). To determine whether ubiquitination was involved in this process, we made use of the ts20 cell line carrying a temperature-sensitive ubiquitin-activating enzyme E1. This enzyme is fully functional when cells are incubated at 30°C, but becomes inactivated when cells are incubated at the nonpermissive temperature of 42°C, thereby impairing ubiquitination of proteins (Kulka et al., 1988). Stably transfected ts20 cells expressing IL2Rβ chain were thus preincubated at permissive or nonpermissive temperature before and during the assays. We have previously demonstrated that the follow up, by flow cytometry analysis, of the disappearance of receptors from the surface of cells incubated with cycloheximide accounts for the analysis of their intracellular degradation (Subtil et al., 1997). Results (Figure 1A) show that the half-life of IL2Rβ was significantly increased when the cells were incubated at 42°C in comparison to those incubated at 30°C. This difference was not due to a decrease in internalization rate, because IL2Rβ was internalized more rapidly at nonpermissive temperature (Figure 1B). It was checked that in the parental cell line E36, the IL2Rβ half-life was not significantly increased at nonpermissive temperature compared with permissive temperature (our unpublished results). These data strongly suggest that an active ubiquitination machinery is required for the efficient degradation of the β chain of the IL2R, but not for its internalization.
Figure 1.
Cell surface half-life (A) and endocytosis (B) of IL2Rβ on stably transfected ts20 mutant cells. Adherent ts20β cells were harvested using a 10-min incubation with PBS containing 5 mM EDTA at 33°C and, after one wash, preincubated for 1 h at permissive (30°C) or nonpermissive (42°C) temperature before and during the assays. (A) Cell surface expression of IL2Rβ on cells treated for different times with of 50 μM of cycloheximide was assayed by flow cytometry by using mAb 341 as described in MATERIALS AND METHODS. (B) For internalization assays, after 1-h preincubation at 30°C or 42°C, cells were labeled with the antibody at 4°C for 1 h, washed once at 4°C, and incubated for the indicated times at permissive or nonpermissive temperature. Disappearance of anti-IL2Rβ mAb from the cell surface upon internalization was assessed by cytofluorimetry and the percentage of internalized antibody was calculated. The y-axis is the ratio of the antibody internalized to the amount of antibody bound to the cell surface at each time point. The slope of this plot is the internalization rate constant (Wiley and Cunningham, 1982). The results presented in A and B are the means ± SE of at least three independent experiments.
Biochemical Study of IL2Rβ Chain Ubiquitination
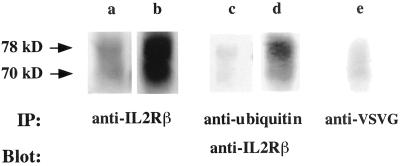
It was previously reported that, after internalization, the IL2Rβ chain is sorted to late endosomes/lysosomes and that its degradation is inhibited in the presence of weak bases (Weissman et al., 1986; Hémar et al., 1995). We therefore treated lymphocytic cells with chloroquine, a weak base that inhibits lysosomal function by increasing the pH of acidic intracellular compartments, and immunoprecipitated cell lysates with an anti-IL2Rβ. After electrophoresis, the presence of IL2Rβ chain was revealed and quantitated by immunoblotting with an anti-IL2Rβ antibody and fluorimager analysis. The intensity of the band corresponding to IL2Rβ (70 kDa) increased fourfold following chloroquine treatment, compared with control cells (Figure 2, lanes a and b), confirming the involvement of the acidic compartments in the degradation of this receptor. The presence of a band of higher molecular weight (78 kDa) was also observed and this upper band was also more intense when chloroquine was used (lane b). Because ubiquitin is a small protein of ∼7.5 kDa, the 78-kDa form of the β chain could be attributed to a ubiquitin moiety appended to the IL2Rβ chain. To test this hypothesis, the lysates of cells incubated with or without chloroquine were immunoprecipitated with a rabbit polyclonal anti-ubiquitin antibody and immunoblotting was performed with an IL2Rβ-specific antibody. The anti-ubiquitin antibody was able to precipitate the 78-kDa form of the β chain that accumulates when cells are incubated with chloroquine. It indicates that this band corresponds to the ubiquitinated β chain (Figure 2, lanes c and d). No higher molecular weight bands were detectable, suggesting that IL2Rβ is only monoubiquitinated.
Figure 2.
Western blot analysis of lysates of IARC301.5 cells preincubated for 3 h with control medium (lanes a, c, and e), or 200 μM chloroquine (lanes b and d). Immunoprecipitation (IP) was performed using anti-IL2Rβ (lanes a and b), a rabbit polyclonal anti-ubiquitin antibody (lanes c and d) or an irrelevant antibody (lane e) and analyzed by immunoblotting (Blot) by using anti-IL2Rβ (341 mAb). The upper arrow indicates a ubiquitinated form of the IL2Rβ chain migrating at 78 kDa.
Effect of Mutating Lysine Residues
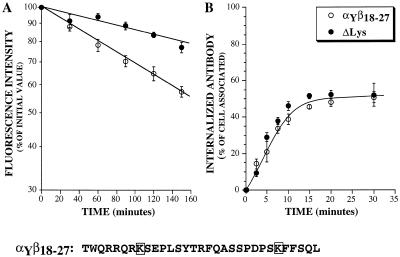
It is well established that ubiquitin is coupled to proteins on their lysine residues (Hershko and Ciechanover, 1998). To assess the role of the presence of potential ubiquitination sites in the function of the IL2Rβ chain degradation signal, we made use of a chimeric receptor, αYβ18-27, carrying this sorting signal and containing only two cytosolic lysine residues. We had previously shown that a 10 amino-acid motif in this chain, including residues 18 to 27 (β18-27 sequence), was sufficient to mediate the efficient sorting of a recycling receptor, αY, toward degradation. The αY chimera was constructed by inserting the strong tyrosine-based internalization signal of the transferrin receptor YTRF at the carboxyl end of the IL2Rα chain cytosolic domain. This chimera is efficiently internalized and recycled back to the cell surface. In contrast, when the degradation motif β18-27 was added to the cytosolic domain of the αY chimera, the resulting receptor, αYβ18-27, was rapidly degraded after endocytosis (Subtil et al., 1997). We mutated the two lysine residues contained in the cytosolic part of the αYβ18-27 chimera, which are potential ubiquitination sites. The sorting of this lysine-mutated receptor was analyzed by measuring its internalization rate and its half-life at the cell surface. As shown in Figure 3, the mutated chimeric receptor was internalized with the same kinetics as αYβ18-27, but was less efficiently degraded because its half-life was doubled. The chimera carrying only one of the two mutations had the same degradation rate as the nonmutated αYβ18-27 chimera (our unpublished results), showing that the absence of both lysine residues was necessary to increase the half-life. These results imply that the sorting signal of the IL2Rβ chain toward degradation needs the presence of lysine residues to function.
Figure 3.
Effect of mutating the cytosolic lysine residues of the chimeric receptor αYβ18-27 on its cell surface half-life (A) and endocytosis (B). Experiments were performed as described in Figure 1, except that the incubations were performed at 37°C. The half-life values (A) or the percentage of internalized antibody (B) are shown. The results are the means ± SE of at least three independent experiments. The sequence of the cytosolic tail of the αYβ18-27 chimera is shown with its two lysine residues boxed.
Effect of Proteasome Inhibitors on IL2Rβ Chain Down-Modulation
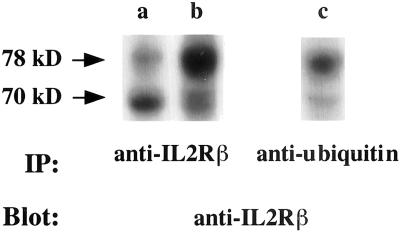
We then wanted to evaluate the contribution of proteasomes. To this end, we performed immunoblot experiments on lymphocytic cell lysates incubated with or without MG132, a strong inhibitor of proteasome (Lee and Goldberg, 1998). Lysates were immunoprecipitated with mouse monoclonal anti-IL2Rβ or anti-ubiquitin antibodies and immunoblotting was performed with an IL2Rβ-specific antibody. Treatment with MG132 increased the total amount of IL2Rβ in the cell lysate, but more particularly the upper band (Figure 4, lanes a and b). This upper band is the ubiquitinated form of the receptor as shown by immunoprecipitation with an anti-ubiquitin mAb (Figure 4, lane c). The same results were obtained when another proteasome inhibitor, ALLN (Lee and Goldberg, 1998), was used (our unpublished results). These results show that proteasome inhibitors lead to the accumulation of the monoubiquitinated form of the β chain.
Figure 4.
Lysates of YT cells preincubated for 3 h with control medium (lanes a and c) or with 10 μM MG132 (lane b) were immunoprecipitated with an anti-IL2Rβ antibody (lanes a and b) or with a mouse monoclonal anti-ubiquitin antibody (lane c) and analyzed by immunoblotting with anti-IL2Rβ antibody. The upper arrow indicates a ubiquitinated form of the IL2Rβ chain migrating at 78 kDa.
Effect of Lysosome or Proteasome Inhibitors on Down-Regulation of IL2Rβ or of a Chimera Carrying Its Degradation Sorting Signal
We next measured the effect of proteasome inhibitor on IL2Rβ chain half-life and endocytosis. We observed that treating human T cells with the specific proteasome inhibitor lactacystin (Lee and Goldberg, 1998) led to a significant decrease in the degradation rate of the IL2Rβ chain, as shown by the increase of its half-life (300 min instead of 90 min) (Figure 5A). This confirms the observation that the use of proteasome inhibitors leads to an accumulation of IL2Rβ. In contrast, the internalization rate (Figure 5B) was only slightly affected. Therefore, proteasome inhibitors affect the intracellular routing of IL2Rβ toward degradation without significantly altering its internalization.
Figure 5.
Cell surface half-life (A) and internalization rate (B) of IL2Rβ on IARC301.5 cells treated with or without the proteasome inhibitor lactacystin. Cells were preincubated for 3 h at 37°C before and during the assays with 12.5 μM lactacystin or with control medium and the experiments were performed as described in Figure 1. The results presented in A and B are the means ± SE of at least three independent experiments.
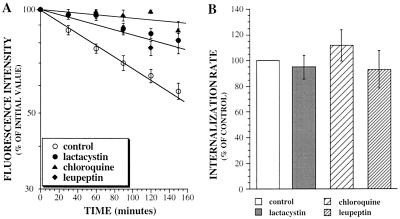
The effects of lactacystin on the degradation and internalization rates of the αYβ18-27 chimera were also assayed. The degradation rate of the chimera was reduced in the presence of lactacystin but not its uptake (Figure 6). We also studied the effect of the lysosomal protease inhibitor leupeptin and of chloroquine. These compounds strongly impaired the degradation of αYβ18-27 (Figure 6A) without significantly affecting the internalization rate of the chimera (Figure 6B). These results show that the two different intracellular proteolytic systems, lysosomes and proteasomes, are involved in the turnover of IL2Rβ chain or of the chimeric receptor containing its degradation signal.
Figure 6.
Cell surface half-life (A) and endocytosis (B) of the chimeric receptor αYβ18-27 in cells preincubated with or without (control) proteasome inhibitor (lactacystin), chloroquine, or the lysosomal proteases inhibitor leupeptin. Cells were incubated with 25 μM lactacystin, 200 μM chloroquine, or 100 μM leupeptin for 3 h at 37°C and experiments performed as described in Figure 1. Cell surface expression of the chimera on cells treated for different times with 50 μM cycloheximide was assayed by flow cytometry (A) with mAb 2A3A1H. The kinetics of endocytosis was measured and results expressed as internalization rate constants (B). The results presented in A and B are the means ± SE of at least three independent experiments.
Analysis of Intracellular Localization of Receptors by Confocal Microscopy
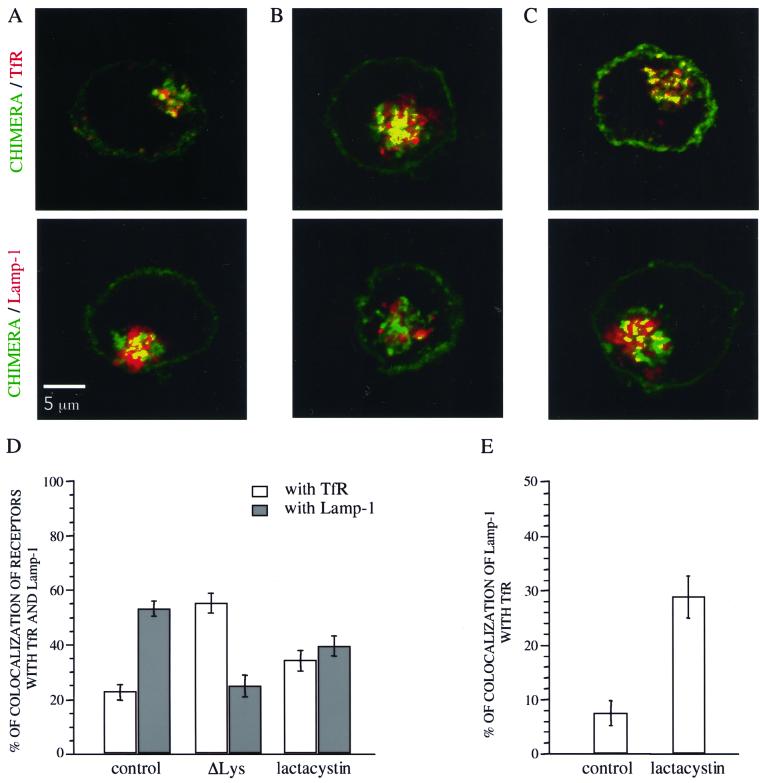
The intracellular localization of the chimeric αYβ18-27 receptor in cells treated with lactacystin and of the Δlys mutant was analyzed by confocal microscopy. After treatment with lactacystin when indicated, the cells were fixed and permeabilized and the chimeric receptor or Δlys mutant was stained. In parallel, the transferrin receptor (TfR), a marker of early and recycling endosomes or the late endosome/lysosome marker Lamp-1 were labeled. The cells were observed by confocal microscopy (Figure 7, A–C) and the extent of colocalization of the chimeric αYβ18-27 or Δlys mutant receptor with the TfR or Lamp-1 was measured as described in MATERIALS AND METHODS (Figure 7D). In control cells expressing αYβ18-27, the extent of colocalization with the Tf receptor was low, ∼20%, whereas with Lamp-1, it was higher, ∼60% (Figure 7, A and D). This result was expected because this chimera is first internalized in early endosomes and then sorted to late endosomes/lysosomes (Subtil et al., 1997). In contrast, the Δlys mutant chimera displayed a different pattern: the mutated chains accumulated in a compartment strongly stained with the transferrin receptor, but poorly with the lysosomal marker (Figure 7, B and D), suggesting some retention in the early/recycling compartment and/or less efficient sorting to late endosomes.
Figure 7.
Intracellular localization of internalized receptors in K562 cells transfected with αYβ18-27 and incubated for 4 h at 37°C with or without 25 μM lactacystin or in cells expressing the ΔLys mutant. Cells were incubated for 30 min with 50 μM cycloheximide in the presence of 100 nM Tf-Cy5. After fixation and permeabilization, cells were first incubated with anti-chimera antibody (7G7B6) and with anti-Lamp-1 antibody (H4A3). The cells were then incubated with secondary labeled-antibody (anti-IgG2a-Texas Red and anti-IgG1-FITC) and analyzed by confocal microscopy. Merged images are shown where chimeric receptors are stained in green and the immunodetected intracellular markers (TfR or Lamp-1) in red. The yellow color indicates colocalization. (A) Control cells. (B) Cells expressing the ΔLys mutant. (C) Lactacystin-treated cells. Bar, 5 μM. (D) colocalization of the chimeric receptors with TfR or Lamp-1 was quantified as described in MATERIALS AND METHODS. (E) Colocalization of Lamp-1 with TfR in K562 αYβ18-27 cells incubated with or without lactacystin.-32767.
In cells incubated with lactacystin (Figure 7, C and D), the chimera appeared to be colocalized equally with Lamp-1 and the transferrin receptor, ∼50% suggesting that in the presence of lactacystin it is less efficiently targeted to late endosomes/lysosomes.
In the course of this study, we noticed that in cells treated with lactacystin αYβ18-27 receptors, in some areas, colocalized with both Lamp-1 and TfR. This can be due to the fact that the proteasome inhibitor might also alter trafficking of Lamp-1. The extent of colocalization between Lamp-1 and TfR was quantified and we found that only 7% of Lamp-1 colocalized with TfR in control cells versus 28% in cells treated with lactacystin (Figure 7E). The increase in the colocalization of these intracellular markers suggests that proteasome inhibitors might cause a global disturbance of protein trafficking in endocytic pathway.
DISCUSSION
Various growth factor receptors are found to be ubiquitinated at the plasma membrane and a direct involvement of the ubiquitin/proteasome system in the down-regulation of some of them has been demonstrated (Bonifacino and Weissman, 1998; Hicke, 1999; Strous and Govers, 1999). In this article, we have investigated its role in the down-modulation of the IL2Rβ. We have shown that a fraction of IL2Rβ can be monoubiquitinated and that indeed, ubiquitination of IL2Rβ or of the chimera containing its degradation signal, αYβ18-27, is required for the sorting toward late degradative compartments. Our results also indicate that impairment of proteasome function, by the use of inhibitors, affects the IL2Rβ degradation. Thus, the IL2Rβ chain joins the increasing number of membrane receptors shown to be down-regulated, at least in part, by their ubiquination and/or by proteasomes. However, the mechanism by which the ubiquitin/proteasome machinery acts on the intracellular fate of receptors is still unclear. From our current knowledge of how degradation of cell surface receptors occurs, there are three steps at which ubiquitin/proteasome system can be involved: internalization, intracellular routing, and processing of the receptor by itself.
Ubiquitination-dependent endocytosis has been reported for several yeast membrane proteins: the ABC peptide transporter, the α-factor receptor, the a-factor receptor, uracyl permease Fur4p, and the maltose permease transporter (reviewed by Bonifacino and Weissman, 1998; Hicke, 1999; Strous and Govers, 1999). A role for mono- or diubiquitination of some of these receptors at the internalization step of endocytosis has been shown (Galan and Haguenauer-Tsapis, 1997; Terrell et al., 1998; Lucero et al., 2000). Recently, it has been demonstrated that the monoubiquitin chain appended to the α-factor receptor (Shih et al., 2000) or to a-factor receptor (Roth and Davis, 2000) itself carries the information sufficient for internalization. Similarly, in mammalian cells, a recent report showed that a signal within a single ubiquitin moiety appended to a chimeric receptor is involved in its internalization (Nakatsu et al., 2000). For two other mammalian membrane proteins, ubiquitination was clearly involved in their internalization; the epithelial sodium channel (Staub et al., 1997) and the growth hormone receptor (GHR) (Strous et al., 1996). However, the mechanism by which ubiquitination initiates their uptake is still obscure. The interaction between the ubiquitin/proteasome system and cell surface receptors was further analyzed for the GHR, which belongs to the same cytokine receptor family as the IL2Rβ chain. For GHR, ubiquitination of the receptor itself does not appear to be necessary (Govers et al., 1999). One possibility is that the GHR needs to bind to a factor, regulated by the ubiquitin/proteasome pathway, before endocytosis can occur (van Kerkhof et al., 2000). From the experiments reported here, different conclusions can be drawn for the IL2Rβ chain. Impairment of ubiquitination machinery in ts20 cells shows that ubiquination of receptors is not required for their cellular uptake. Use of proteasome inhibitors also revealed that the proteasomes are not involved in the internalization step of IL2Rβ or of the chimera αYβ18-27, carrying its sorting signal. Similarly, the overall turnover, but not the internalization, of the PDGF receptor was shown to be ubiquitin/proteasome-dependent (Mori et al., 1995).
The second step at which ubiquitination or proteasome action may be implicated is after internalization, in routing of the receptor from early endocytic to late degradation compartments. Our studies on the chimera αYβ18-27, which is degraded after endocytosis, as is the IL2Rβ receptor, show that mutation of the lysine residues (Δlys) impairs receptor degradation and causes its accumulation in early/recycling endosomes. This is a strong argument in favor of a direct role of receptor ubiquitination as a signal in sorting from early/recycling to late endocytic compartments. Interestingly, ubiquitination of proteins can be regulated by specific ubiquitin proteases (Wilkinson, 1997). In this respect, it is worth noting that one of these deubiquitinating enzymes (named DUB-2) is induced by IL2 and down-regulated after the initiation of T-cell activation (Zhu et al., 1997).
The role of ubiquitination in IL2Rβ sorting could be independent of proteasome function. In fact, in agreement with this we detected only a monoubiquitinated form of IL2Rβ, whereas at least four residues of ubiquitin appear to be required for recognition by the proteasome (Deveraux et al., 1994; Thrower et al., 2000). However, treatment of cells with proteasome inhibitors also affected IL2Rβ and αYβ18-27 degradation. Moreover, we showed that the sorting of this receptor seemed to be altered because, in the presence of lactacystin, the αYβ18-27, which is normally directed toward degradation (Subtil et al., 1997), colocalizes equally with TfR and the lysosomal marker Lamp-1. These data suggest that the proteasome function is necessary for a proper sorting of receptors carrying IL2Rβ signal. However, we observed that, in the presence of the proteasome inhibitor, the extent of colocalization of two markers of normally distinct compartments, TfR and Lamp-1, was increased. This indicates that the proteasome inhibitor might have a general effect on intracellular trafficking, compromising several cellular compartments, which may explain the effect on the degradation of IL2Rβ. Effect of proteasome malfunction in the overall defect of vacuolar integrity was also one of the models proposed for the proteasome-dependent degradation of the a-factor transporter in yeast (Loayza and Michaelis, 1998). Interestingly, a link between ubiquitin system and maintenance of intracellular integrity has also previously been described (Lenk et al., 1992). In this report, it was shown, using ts20 mutant cell line, that a functional ubiquitination machinery is necessary for the maturation of autophagic vacuoles. The role of proteasome in the routing of membrane proteins from the endocytic to degradation compartments is unclear. One possible mechanism is that proteasome function might be involved in the regulation of a protein playing a crucial role in trafficking through the endocytic pathway. One potential candidate might be the recently described sorting nexin SNX15 (Barr et al., 2000).
The third possible action of ubiquitin/proteasome system is on the degradation of the receptor itself. Proteasomal degradation of some mammalian receptors has previously been described. For the platelet-derived growth factor (Mori et al., 1995), the Met Tyrosine kinase receptor (Jeffers et al., 1997), and the GHR (van Kerkhof et al., 2000), it has been proposed that both proteasomes and lysosomes might function to degrade different parts of given receptors. Herein, we have confirmed that lysosomes were involved in IL2Rβ degradation because degradation was inhibited by chloroquine or leupeptin. Because, as discussed above, proteasome inhibitors affected the trafficking of intracellular markers, we could not question whether proteasomes were also directly involved in the degradation of IL2Rβ. Again, the fact that only monoubiquitinated form of the β chain was detected argues against this possibility (Deveraux et al., 1994; Thrower et al., 2000).
In conclusion, our data, together with other recent reports, indicate that the ubiquitin/proteasome system, in addition to its involvement in cell cycle and signal transduction, in the elimination of endoplasmic reticulum-retained proteins, is also a key player in the modulation of the expression of cell surface growth factor receptors. Its role in many aspects of cell life (Bonifacino and Weissman, 1998; Ciechanover, 1998; Hershko and Ciechanover, 1998) supports the emerging idea that the proteasome/ubiquitin system might play an essential role in general intracellular protein routing and turnover.
ACKNOWLEDGMENTS
We are grateful to Raymond Hellio and Pascal Roux for help with confocal microscopy, Annick Dujeancourt for skillfull technical assistance. The confocal microscope was purchased with a donation from Marcel and Liliane Pollack. This work was supported by the Association pour la Recherche sur le Cancer (no. 5260)
Abbreviations used:
- ALLN
N-acetyl-l-l-leucyl-norleucinal
- GHR
growth hormone receptor
- IL2
interleukin 2
- IL2Rα
interleukin 2 receptor α chain
- IL2Rβ
interleukin 2 receptor β chain
- Lamp-1
lysosome-associated membrane protein-1
- mAb
monoclonal antibody
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- TfR
transferrin receptor
REFERENCES
- Barr VA, Phillips SA, Taylor SI, Renfrew Haft C. Overexpression of a novel sorting nexin, SNX15, affects endosome morphology and protein trafficking. Traffic. 2000;1:904–916. doi: 10.1034/j.1600-0854.2000.011109.x. [DOI] [PubMed] [Google Scholar]
- Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux Q, Ustrel l V, Pickart C, Rechsteiner M. A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem. 1994;269:7059–7061. [PubMed] [Google Scholar]
- Duprez V, Cornet V, Dautry-Varsat A. Down regulation of high affinity interleukin 2 receptors in a human tumor T cell line: IL2 increases the rate of surface receptor decay. J Biol Chem. 1988;263:12860–12865. [PubMed] [Google Scholar]
- Duprez V, Dautry-Varsat A. Receptor mediated endocytosis of interleukin 2 in a human tumor T cell line: degradation of interleukin 2 and evidence for the absence of recycling of interleukin 2 receptors. J Biol Chem. 1986;261:15450–15454. [PubMed] [Google Scholar]
- Duprez V, Lenoir G, Dautry-Varsat A. Autocrine growth stimulation of a human T-cell lymphoma line by IL2. Proc Natl Acad Sci USA. 1985;82:6932–6936. doi: 10.1073/pnas.82.20.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J-M, Haguenauer-Tsapis R. Ubiquitin Lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 1997;16:5847–5854. doi: 10.1093/emboj/16.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers R, ten Broeke T, van Kerkhof P, Schwartz AL, Strous GJ. Identification of a novel ubiquitin conjugation motif, required for ligand-induced internalization of the growth hormone receptor. EMBO J. 1999;18:28–36. doi: 10.1093/emboj/18.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hémar A, Dautry-Varsat A. Cyclosporin A inhibits the interleukin 2 receptor α chain gene transcription but not its cell surface expression: the α chain stability can explain this discrepancy. Eur J Immunol. 1990;20:2629–2635. doi: 10.1002/eji.1830201216. [DOI] [PubMed] [Google Scholar]
- Hémar A, Subtil A, Lieb M, Morelon E, Hellio R, Dautry-Varsat A. Endocytosis of interleukin 2 receptors in human T lymphocytes: distinct intracellular localization and fate of the receptor α, β and γ chains. J Cell Biol. 1995;129:55–64. doi: 10.1083/jcb.129.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hicke L. Gettin'down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- Jeffers M, Taylor GA, Weidner KM, Omura S, Vande Woude GF. Degradation of the met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol Cell Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka RG, Raboy B, Schuster R, Parag HA, Diamond G, Ciechanover A, Marcus M. A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J Biol Chem. 1988;263:15726–15731. [PubMed] [Google Scholar]
- Laing JG, Beyer EC. The Gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem. 1995;270:26399–26403. doi: 10.1074/jbc.270.44.26399. [DOI] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Lenk SE, Dunn WA, Jr, Trausch JS, Ciechanover A, Schwartz AL. Ubiquitin-activating enzyme, E1, is associated with maturation of autophagic vacuoles. J Cell Biol. 1992;118:301–308. doi: 10.1083/jcb.118.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loayza D, Michaelis S. Role for the ubiquitin-proteasome system in the vacuolar degradation of Ste6p, the a-factor transporter in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:779–789. doi: 10.1128/mcb.18.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero P, Penalver E, Vela L, Lagunas R. Monoubiquitination is sufficient to signal internalization of the maltose transporter in Saccharomyces cerevisiae. J Bacteriol. 2000;182:241–243. doi: 10.1128/jb.182.1.241-243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–267. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Mori S, Tanaka K, Omura S, Saito Y. Degradation process of ligand-stimulated platelet-derived growth factor β-receptor involves ubiquitin-proteasome proteolytic pathway. J Biol Chem. 1995;270:29447–29452. doi: 10.1074/jbc.270.49.29447. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Nakatsu FN, Sakuma M, Matsuo Y, Arase H, Yamasaki S, Nakamura N, Saito T, Ohno H. A di-leucine signal in the ubiquitin moiety. J Biol Chem. 2000;275:26213–26219. doi: 10.1074/jbc.M907720199. [DOI] [PubMed] [Google Scholar]
- Roth AF, Davis N. Ubiquitination of the PEST-like endocytosis signal of the yeast a-factor. J Biol Chem. 2000;275:8143–8153. doi: 10.1074/jbc.275.11.8143. [DOI] [PubMed] [Google Scholar]
- Shih SC, Sloper-Mold KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 2000;19:187–198. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub O, Gautschi I, Ishikawa T, Breitschpf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na-channel (ENaC) by ubiquitination. EMBO J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous GJ, Govers R. The ubiquitin-proteasome system and endocytosis. J Cell Sci. 1999;112:1417–1423. doi: 10.1242/jcs.112.10.1417. [DOI] [PubMed] [Google Scholar]
- Strous GJ, van Kerkhof P, Govers R, Ciechanover A, Schwartz AL. The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO J. 1996;15:3806–3812. [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Delepierre M, Dautry-Varsat A. An α-helical signal in the cytosolic domain of the interleukin 2 receptor β chain mediates sorting towards degradation after endocytosis. J Cell Biol. 1997;136:583–595. doi: 10.1083/jcb.136.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Hémar A, Dautry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J Cell Sci. 1994;107:3461–3468. doi: 10.1242/jcs.107.12.3461. [DOI] [PubMed] [Google Scholar]
- Subtil A, Rocca A, Dautry-Varsat A. Molecular characterization of the signal responsible for the targeting of the IL2 receptor β chain toward intracellular degradation. J Biol Chem. 1998;273:29424–29429. doi: 10.1074/jbc.273.45.29424. [DOI] [PubMed] [Google Scholar]
- Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin 2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell. 1998;1:193–202. doi: 10.1016/s1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- Teshigawara K, Wang H, Kato K, Smith KA. Interleukin 2 high-affinity receptors expression requires two distinct binding proteins. J Exp Med. 1987;165:223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof P, Govers R, Alves dos Santos C, Strous GJ. Endocytosis and degradation of the growth hormone receptor are proteasome-dependent. J Biol Chem. 2000;275:1575–1580. doi: 10.1074/jbc.275.3.1575. [DOI] [PubMed] [Google Scholar]
- Voss SD, Leary TP, Sondel PM, Robb RJ. Identification of a direct interaction between interleukin 2 and the p64 interleukin 2 receptor γ chain. Proc Natl Acad Sci USA. 1993;90:2428–2432. doi: 10.1073/pnas.90.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AM. Regulating protein degradation by ubiquitination. Immunol Today. 1997;18:189–198. doi: 10.1016/s0167-5699(97)84666-x. [DOI] [PubMed] [Google Scholar]
- Weissman AM, Harford JB, Svetlik PB, Leonard WL, Depper JM, Waldmann TA, Greene WC, Klausner RD. Only high affinity receptors for interleukin 2 mediate internalization of ligand. Proc Natl Acad Sci USA. 1986;83:1463–1466. doi: 10.1073/pnas.83.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS, Cunningham DD. The endocytic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982;257:4222–4229. [PubMed] [Google Scholar]
- Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997;11:1245–1256. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Lambert K, Corless C, Copeland NG, Gilbert DJ, Jenkins NA, D'Andrea AD. DUB-2 is a member of a novel family of cytokine-inducible deubiquitinating enzymes. J Biol Chem. 1997;272:51–57. doi: 10.1074/jbc.272.1.51. [DOI] [PubMed] [Google Scholar]