Abstract
Colletotrichum acutatum is known as an important anthracnose pathogen of a wide range of host plants worldwide. Numerous studies have reported subgroups within the C. acutatum species complex. Multilocus molecular phylogenetic analysis (ITS, ACT, TUB2, CHS-1, GAPDH, HIS3) of 331 strains previously identified as C. acutatum and other related taxa, including strains from numerous hosts with wide geographic distributions, confirmed the molecular groups previously recognised and identified a series of novel taxa. Thirty-one species are accepted, of which 21 have not previously been recognised. Colletotrichum orchidophilum clusters basal to the C. acutatum species complex. There is a high phenotypic diversity within this complex, and some of the species appear to have preferences to specific hosts or geographical regions. Others appear to be plurivorous and are present in multiple regions. In this study, only C. salicis and C. rhombiforme formed sexual morphs in culture, although sexual morphs have been described from other taxa (especially as laboratory crosses), and there is evidence of hybridisation between different species. One species with similar morphology to C. acutatum but not belonging to this species complex was also described here as new, namely C. pseudoacutatum.
Taxonomic novelties:
New combinations - Colletotrichum limetticola (R.E. Clausen) Damm, P.F. Cannon & Crous, C. lupini (Bondar) Damm, P.F. Cannon & Crous, C. salicis (Fuckel) Damm, P.F. Cannon & Crous. New species - C. acerbum Damm, P.F. Cannon & Crous, C. australe Damm, P.F. Cannon & Crous, C. brisbanense Damm, P.F. Cannon & Crous, C. cosmi Damm, P.F. Cannon & Crous, C. costaricense Damm, P.F. Cannon & Crous, C. cuscutae Damm, P.F. Cannon & Crous, C. guajavae Damm, P.F. Cannon & Crous, C. indonesiense Damm, P.F. Cannon & Crous, C. johnstonii Damm, P.F. Cannon & Crous, C. kinghornii Damm, P.F. Cannon & Crous, C. laticiphilum Damm, P.F. Cannon & Crous, C. melonis Damm, P.F. Cannon & Crous, C. orchidophilum Damm, P.F. Cannon & Crous, C. paxtonii Damm, P.F. Cannon & Crous, C. pseudoacutatum Damm, P.F. Cannon & Crous C. pyricola Damm, P.F. Cannon & Crous, C. rhombiforme Damm, P.F. Cannon & Crous, C. scovillei Damm, P.F. Cannon & Crous, C. sloanei Damm, P.F. Cannon & Crous, C. tamarilloi Damm, P.F. Cannon & Crous, C. walleri Damm, P.F. Cannon & Crous. Typifications: Epitypifications - C. acutatum J.H. Simmonds, C. limetticola (R.E. Clausen) Damm, P.F. Cannon & Crous, C. nymphaeae (Pass.) Aa, C. phormii (Henn.) D.F. Farr & Rossman, C. salicis (Fuckel) Damm, P.F. Cannon & Crous. Lectotypifications - C. nymphaeae (Pass.) Aa, C. orchidearum Allesch.
Key words: anthracnose, Ascomycota, Colletotrichum acutatum, Gloeosporium, Glomerella, phylogeny, systematics
INTRODUCTION
Colletotrichum acutatum is one of the most frequently reported species of the genus and causes diseases commonly known as anthracnose on numerous host plants worldwide (Farr & Rossman 2012). Originally described from diseased tissues of Carica papaya, Capsicum frutescens and Delphinium ajacis in Australia by Simmonds (1965), the C. acutatum species complex is today known as especially destructive on fruits like strawberry (Garrido et al. 2009), citrus (Peres et al. 2008), apple (Lee et al. 2007), olive (Talhinhas et al. 2011), cranberry (Polashock et al. 2009) and blueberry (Wharton & Schilder 2008). It is also implicated in the “terminal crook” disease of pine (Dingley & Gilmour 1972) and in the anthracnose of leather leaf fern (Schiller et al. 2006). There are also reports of a disseminated infection of a sea turtle (Manire et al. 2002) and the infection of a scale insect (Marcelino et al. 2008). Reviews of the species in its broad sense and its pathology were published by Wharton & Diéguez-Uribeondo (2004) and Peres et al. (2005).
On strawberry, C. acutatum mainly causes black spot of fruit but can also attack crowns, roots and leaves (Freeman & Katan 1997), and is one of the most serious diseases in commercial fruit production. Largely due to its economic importance as a strawberry pathogen, C. acutatum was treated for many years as a regulated plant quarantine pest by the European and Mediterranean Plant Protection Organization (EPPO), though it is absent from the current list (EPPO 2011) – presumably due to its now widespread distribution in Europe. Inoculum sources are frequently transplant material, mostly with quiescent infections (Rahman & Louws 2008), infected plants, weeds and other hosts (McInnes et al. 1992, Parikka et al. 2006), while the survival rate of conidia in natural field soil is low (Freeman et al. 2002).
The most well-known morphological feature of C. acutatum (s. lat.) is the shape of its conidia, which have acute ends (Simmonds 1965). However, other conidial shapes, especially ± cylindrical with only one acute end, are frequently encountered, especially in strains that have been repeatedly subcultured, but these conidial shapes can also occur in species outside the C. acutatum species complex. Even the differentiation between C. acutatum (s. lat.) and C. gloeosporioides (s. lat.) is difficult, because many intermediate strains exist with a restricted number of typical fusiform conidia and many cylindrical ones (Van der Aa et al. 1990). On the host, conidia are formed in acervuli; in culture, conidia are often also produced in the aerial mycelium (Johnston & Jones 1997). Colletotrichum acutatum has also been observed to form secondary conidia on the surface of living strawberry leaves (Leandro et al. 2001) that were stimulated by strawberry plant extracts, especially flower extracts (Leandro et al. 2003). According to Buddie et al. (1999) secondary conidia may be produced directly from germinating primary conidia, and are smaller and more variable in shape, thus obscuring differences between taxa. Additionally, C. acutatum forms simple pigmented appressoria, but few or no setae (Simmonds 1965).
Guerber & Correll (1997, 2001) described Glomerella acutata, the sexual morph of C. acutatum, as the product of mating experiments, while some related species are homothallic, including Ga. acutata var. fioriniae (Marcelino et al. 2008), later regarded as a separate species (C. fioriniae, Shivas & Tan 2009) and an isolate of a Glomerella species related to C. acutata from Acer platanoides in the USA (LoBuglio & Pfister 2008). Talgø et al. (2007) observed the sexual morph Ga. acutata on naturally infected fruits of highbush blueberry in Norway. Numerous studies have shown that C. acutatum is morphologically and phylogenetically diverse (Sreenivasaprasad et al. 1994, Johnston & Jones 1997, Lardner et al. 1999, Freeman et al. 2001a, Nirenberg et al. 2002, Talhinhas et al. 2002, Guerber et al. 2003, Lubbe et al. 2004, Du et al. 2005, Peres et al. 2005, Sreenivasaprasad & Talhinhas 2005, Talhinhas et al. 2005, Johnston et al. 2008). Sreenivasaprasad et al. (1996) were the first to recognise that C. acutatum was unusually diverse, with strains showing divergence of 5.8 % in ITS-1 sequence compared with levels of 2–4 % frequently found within other fungal species, and they suggested splitting C. acutatum into two species. Johnston & Jones (1997) recognised four morphological groups, C. acutatum A–C and Glomerella miyabeana. Three of these groups were supported by 28S nuclear ribosomal large subunit rRNA (LSU) sequence data. Lardner et al. (1999), using a combination of RAPDs and morphological/cultural data, identified seven subordinate groups within C. acutatum. Sequences of a 200-bp intron of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and a 900-bp intron of the glutamine synthetase GS distinguished seven main clades and several subclades within strains that originated mainly from North America and New Zealand (Guerber et al. 2003). The recognition of infraspecific groups was more firmly established by Sreenivasaprasad & Talhinhas (2005), who distinguished clades A1 to A8 based on rDNA ITS and beta-tubulin DNA (TUB2) sequences. These clades were mostly correlated with the groups that had been distinguished previously. Whitelaw-Weckert et al. (2007) recognised an additional group A9.
At this point, it was widely presumed that C. acutatum was a species complex containing a number of constituent taxa, but there was substantial reluctance to recognise the clades involved as independent species. This was due to the lack of differential morphological and cultural characters. For example, C. lupini was not recognised as formally separate from C. acutatum by Talhinhas et al. (2002) or by Sreenivasaprasad & Talhinhas (2005). There were some attempts to address these concerns via adoption of formae speciales e.g. C. acutatum f. sp. pineum (Dingley & Gilmour 1972), C. acutatum f. sp. hakeae (Lubbe et al. 2004) and C. acutatum f. sp. fioriniae (Marcelino et al. 2008), but this mechanism for recognition of pathology-related taxa is now rarely used.
Gradually, separate species were recognised or accepted as part of the C. acutatum species complex, e.g. C. lupini (Nirenberg et al. 2002) and C. phormii (Farr et al. 2006). In a study using two genes, ITS and TUB2, combined with morphological data, Shivas & Tan (2009) recognised three distinct groups within C. acutatum strains from Australia and accepted two new species, C. simmondsii and C. fioriniae (formerly C. acutatum f. sp. fioriniae) for groups A2 and A3. Recently, a new species was described for group A4, C. clavatum (Faedda et al. 2011).
Our research aims to present a comprehensive revision of the C. acutatum species complex. We thoroughly survey the constituent taxa and delineate additional species where needed. We have examined a large number of C. acutatum s. lat. strains, isolated from various hosts and in various geographic areas. Multi-locus molecular analysis is the basis of species recognition, but morphological and cultural characters allowing alternative means of species recognition are given where possible.
MATERIALS AND METHODS
Isolates
A total of 331 strains have been studied, mostly previously identified as C. acutatum, as well as other related strains from the CBS, IMI and other culture collections. Type material (holotypes and epitypes) of the species studied are located in the Herbarium of the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands, in the IMI Fungarium, which is based in the Royal Botanic Gardens, Kew (IMI and K(M)), UK, US National Fungus Collections (BPI), Beltsville, Maryland, USA, the Botanic Garden and Botanical Museum Berlin-Dahlem, Freie Universität Berlin (B), Germany and in the dried collection of the Botanische Staatssammlung München (M), Germany. All descriptions are based on the ex-type, ex-epitype or ex-neotype culture as appropriate. Features of other strains are added if deviant. Subcultures of the types, epitypes and neotypes, respectively, as well as all other isolates used for morphological and sequence analyses are maintained in the culture collections of CBS and IMI (Table 1).
Table 1.
Strains of Colletotrichum spp. studied, with collection details and GenBank accessions.
CBS: Culture collection of the Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, Utrecht, The Netherlands; IMI: Culture collection of CABI Europe UK Centre, Egham, UK; MAFF: MAFF Genebank Project, Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Japan; BRIP: Plant Pathology Herbarium, Department of Employment, Economic, Development and Innovation, Queensland, Australia; ICMP: International Collection of Microorganisms from Plants, Auckland, New Zealand; STE-U: Culture collection of the Department of Plant Pathology, University of Stellenbosch, South Africa; HKUCC: The University of Hong Kong Culture Collection, Hong Kong, China; PD: Plantenziektenkundige Dienst Wageningen, Nederland; * ex-holotype or ex-epitype cultures.
Morphological analysis
To enhance sporulation, autoclaved filter paper and double-autoclaved stems of Anthriscus sylvestris were placed onto the surface of synthetic nutrient-poor agar medium (SNA; Nirenberg 1976). SNA and oatmeal agar (OA; Crous et al. 2009) cultures were incubated at 20 °C under near UV light with 12 h photoperiod for 10 d. Measurements and photographs of characteristic structures were made according to Damm et al. (2007). Appressoria on hyphae were observed on the reverse side of SNA plates. Microscopic preparations were made in clear lactic acid, with 30 measurements per structure and observed with a Nikon SMZ1000 dissecting microscope (DM) or with a Nikon Eclipse 80i microscope using differential interference contrast (DIC) illumination. In the C. acutatum species complex, conidia are usually formed in acervular conidiomata and additionally in the aerial mycelium. Unless mentioned otherwise, only conidia from conidiomata were used in this study for morphological examination.
Colony characters and pigment production on SNA and OA cultures incubated at 20 °C under near UV light with 12 h photoperiod were noted after 10 d. Colony colours were rated according to Rayner (1970). Growth rates were measured after 7 and 10 d.
Phylogenetic analysis
Genomic DNA of the isolates was extracted using the method of Damm et al. (2008). The 5.8S nuclear ribosomal gene with the two flanking internal transcribed spacers (ITS), a 200-bp intron of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and partial sequences of the chitin synthase 1 (CHS-1), histone3 (HIS3), actin (ACT) and beta-tubulin (TUB2) genes were amplified and sequenced using the primer pairs ITS-1F (Gardes & Bruns 1993) + ITS-4 (White et al. 1990) or V9G (de Hoog & Gerrits van den Ende 1998) + ITS-4, GDF1 + GDR1 (Guerber et al. 2003), CHS-354R + CHS-79F (Carbone & Kohn 1999), CYLH3F + CYLH3R (Crous et al. 2004b), ACT-512F + ACT-783R (Carbone & Kohn 1999) and BT2Fd + BT4R (Woudenberg et al. 2009) or T1 (O’Donnell & Cigelnik 1997) + Bt-2b (Glass & Donaldson 1995), respectively. The PCRs were performed in a 2720 Thermal Cycler (Applied Biosystems, Foster City, California) in a total volume of 12.5 μL. The GAPDH, CHS-1, HIS3, ACT and TUB2 PCR mixture contained 1 μL 20x diluted genomic DNA, 0.2 μM of each primer, 1x PCR buffer (Bioline, Luckenwalde, Germany), 2 mM MgCl2, 20 μM of each dNTP, 0.7 μL DMSO and 0.25 U Taq DNA polymerase (Bioline). Conditions for PCR of these genes constituted an initial denaturation step of 5 min at 94 °C, followed by 40 cycles of 30 s at 94 °C, 30 s at 52 °C and 30 s at 72 °C, and a final denaturation step of 7 min at 72 °C, while the ITS PCR was performed as described by Woudenberg et al. (2009). The DNA sequences generated with forward and reverse primers were used to obtain consensus sequences using Bionumerics v. 4.60 (Applied Maths, St-Marthens-Lathem, Belgium), and the alignment assembled and manually adjusted using Sequence Alignment Editor v. 2.0a11 (Rambaut 2002).
To determine whether the six sequence datasets were congruent and combinable, tree topologies of 70 % reciprocal Neighbour-Joining bootstrap with Maximum Likelihood distances (10 000 replicates) with substitution models determined separately for each partition using MrModeltest v. 2.3 (Nylander 2004) were compared visually (Mason-Gamer & Kellogg 1996). A maximum parsimony analysis was performed on the multilocus alignment (ITS, GAPDH, CHS-1, HIS3, ACT, TUB2) as well as for each gene separately with PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2000) using the heuristic search option with 100 random sequence additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Alignment gaps were treated as missing and all characters were unordered and of equal weight. No more than 10 trees of score (length) greater than or equal to 10 were saved in each replicate. The robustness of the trees obtained was evaluated by 10 000 bootstrap replications using the Fast-stepwise addition algorithm (Hillis & Bull 1993). Tree length, consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI) were calculated for the resulting tree. A Markov Chain Monte Carlo (MCMC) algorithm was used to generate phylogenetic trees with Bayesian probabilities using MrBayes v. 3.1.1 (Ronquist & Huelsenbeck 2003) for the combined sequence datasets. Models of nucleotide substitution for each gene determined by MrModeltest v. 2.3 were included for each gene partition. The analyses of two MCMC chains were run from random trees for 1000 000 generations and sampled every 100 generations. The likelihood score of the two runs were 2 500 and 2 200 and therefore, the first 2 350 (the average of both) trees were discarded as the burn-in phase of the analysis and posterior probabilities determined from the remaining trees. For additional comparison, a Neighbour-Joining analysis was performed on the multigene alignment using PAUP and 1000 bootstrap replications. Sequences derived in this study have been lodged at GenBank, the alignment in TreeBASE (www.treebase.org/treebase-web/home.html), and taxonomic novelties in MycoBank (Crous et al. 2004a).
RESULTS
Phylogeny
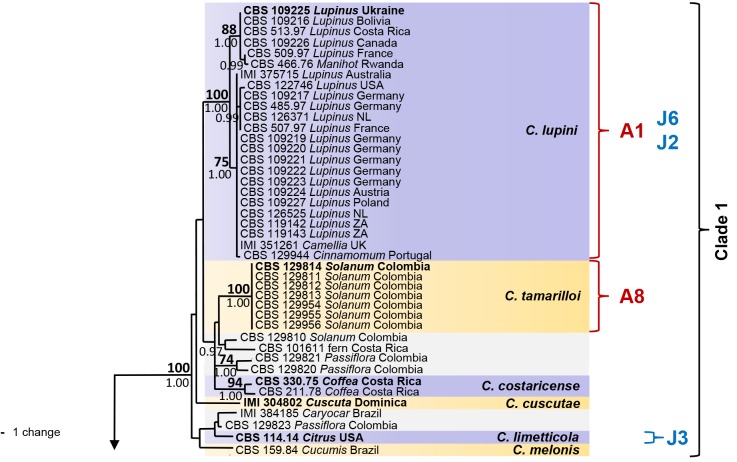
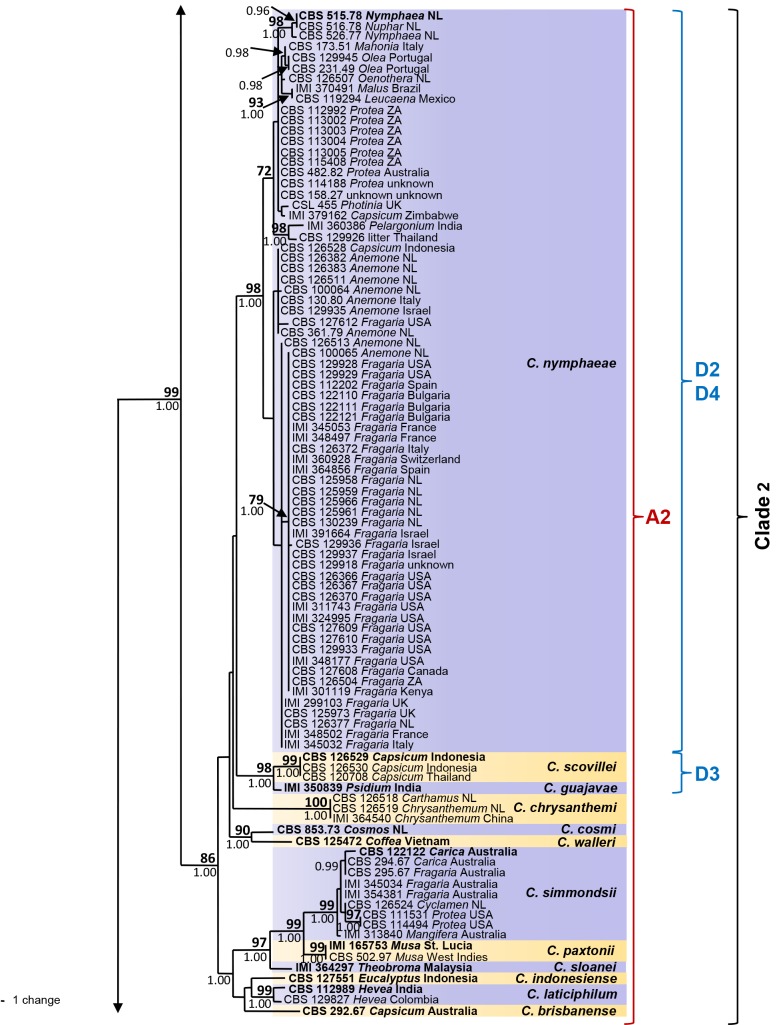
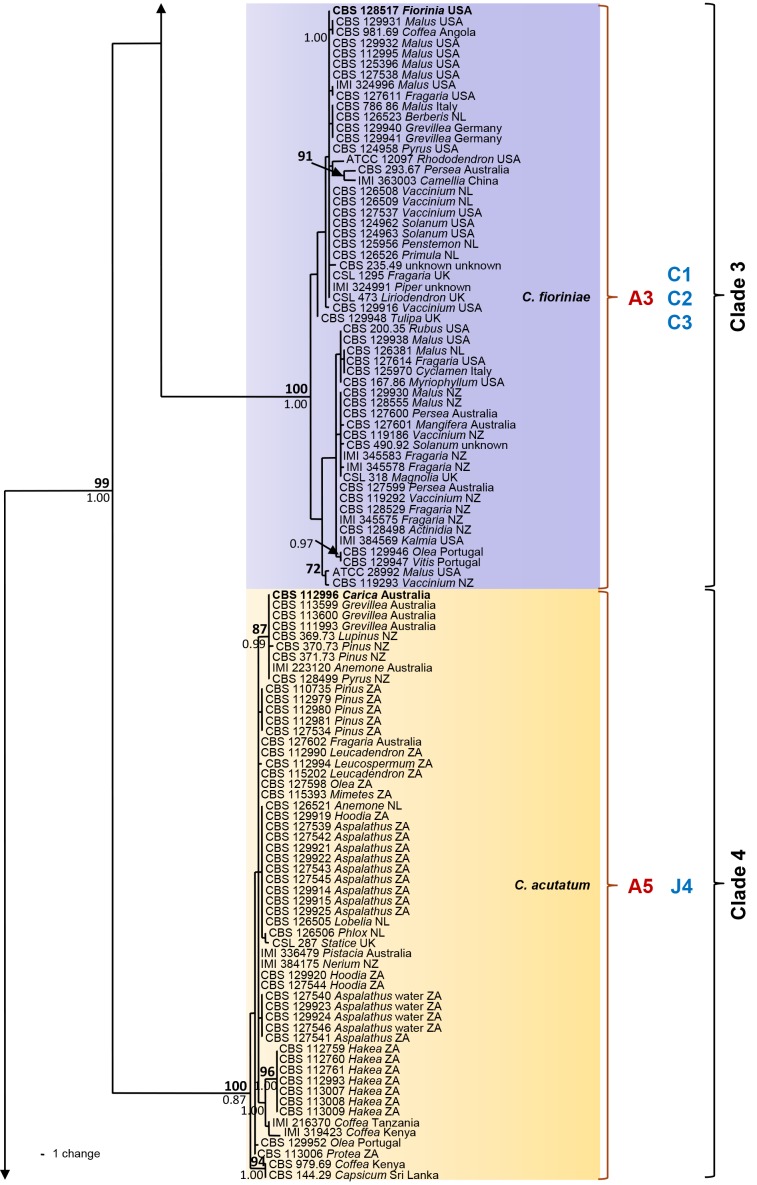
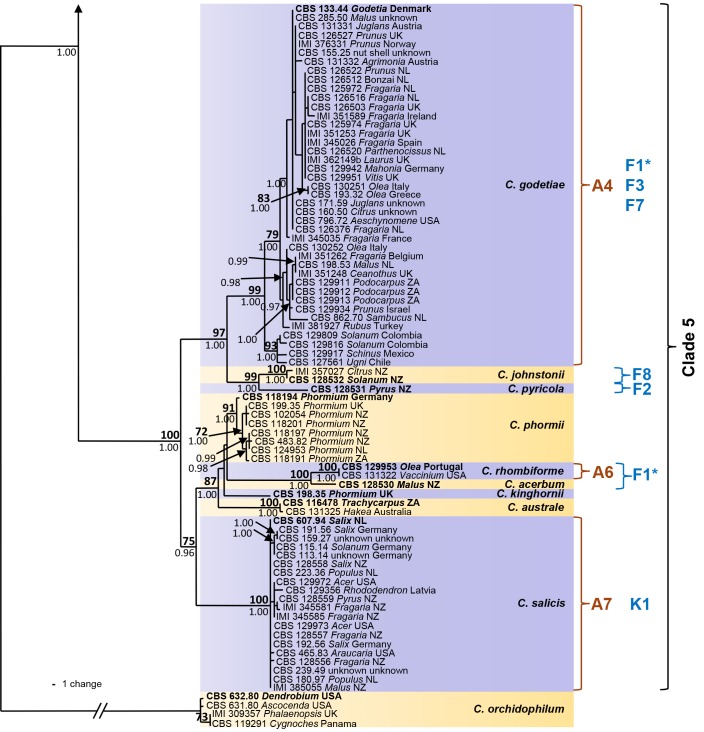
The six sequence data sets did not show any conflicts in tree topology for the 70 % reciprocal bootstrap trees, which allowed us to combine them. In the multigene analyses (gene boundaries of ITS: 1–546, GAPDH: 557–829, CHS-1: 840–1121, HIS3: 1131–1519, ACT: 1530–1786, TUB2: 1797–2290) of 330 isolates of C. acutatum and related Colletotrichum species including the outgroup (C. orchidophilum strains CBS 631.80, CBS 632.80, CBS 119291, IMI 309357), 2290 characters including the alignment gaps were processed, of which 468 characters were parsimony-informative, 65 parsimony-uninformative and 1757 constant. One strain that was revealed as not belonging to the C. acutatum species complex (CBS 436.77, C. pseudoacutatum) was not included in the analysis presented in Fig. 1. After a heuristic search using PAUP, 830 most parsimonious trees were retained (length = 1008 steps, CI = 0.643, RI = 0.981, RC = 0.681, HI = 0.357) of which one is shown in Fig. 1. The topology of the 830 trees was similar, which was verified for a large selection of trees. They differed only in the position of taxa within the subclades. For Bayesian analysis, a HKY+I model was selected for ITS, a HKY+G model for GAPDH and TUB2, a K80+I+G model for CHS-1, a HKY+I+G model for HIS3, a GTR+G model for ACT, and incorporated in the analysis. The consensus tree obtained from Bayesian analyses and the NJ tree (not shown) confirmed the tree topology obtained with parsimony. Bayesian posterior probability values agreed with bootstrap supports (Fig. 1).
Fig. 1.
One of 830 most parsimonious trees obtained from a heuristic search of the combined ITS, GAPDH, CHS-1, ACT, HIS3 and TUB2 sequences alignment of the Colletotrichum acutatum species complex. Bootstrap support values above 70 % (bold) and Bayesian posterior probability values above 0.95 are shown at the nodes. Colletotrichum orchidophilum CBS 632.80, CBS 631.80, IMI 309357 and CBS 119291 are used as outgroup. Numbers of ex-holotype, ex-neotype and ex-epitype strains are emphasised in bold. Strain numbers are followed by substrate (host genus) and country of origin, NL = Netherlands, NZ = New Zealand, ZA = South Africa. Branches that are crossed by diagonal lines are shortened by 50 %. Corresponding groups of Sreenivasaprasad & Talhinhas 2005 are emphasised in red, mtDNA RFLP haplotypes of Guerber et al. (2003) are emphasised in blue.
The analyses resulted in detection of five main clades and 29 subclades within C. acutatum s. lat., which we accept as representing different Colletotrichum species. The corresponding groups according to Sreenivasaprasad & Talhinhas (2005, numbers beginning with A) and Guerber et al. (2003, mtDNA RFLP haplotypes, numbers beginning with C...K), which are the most differential and comparable studies, are listed in brackets below and are indicated in the phylogenetic tree (Fig. 1). The first clade is well supported with a bootstrap support of 100 % and a Bayesian posterior probability value of 1.00. It consists of two frequently isolated, well-supported clades (bootstrap support/Bayesian posterior probability value of both 100/1.00) comprising several strains each, representing C. lupini (A1, J2/J6) and C. tamarilloi (A8). Other less frequently encountered subclades in the first clade include C. costaricense (94/1.00) with two strains, C. cuscutae and C. melonis both represented by single-strain clades on long branches, and several short-branched single-strain clades, including the known species C. limetticola (J3) and six further unnamed strains. The majority of strains in clade 2 (A2, 86/1.00) belong to C. nymphaeae (98/1.00, D2/D4), while most of the other 11 subclades of this clade are occupied by only one or few strains. The clade representing C. scovillei (99/1.00) consists of three strains and groups (98/1.00) with a single-strain clade formed by C. guajavae. These two adjacent clades probably correspond to clade D3 in Guerber et al. (2003). The other sister clades represent C. simmondsii (99/1.00), C. chrysanthemi (100/1.00), C. paxtonii (99/1.00), C. laticiphilum (99/1.00), C. cosmi, C. walleri, C. sloanei, C. indonesiense and C. brisbanense, the last five of which consist of single-strain clades. Clades 3 and 4 are well-supported (100/1.00 and 100/0.87) and on long branches; they represent C. fioriniae (A3, C1/C2/C3) and C. acutatum (A5, J4). Clade 5 consists of two sister clades. Colletotrichum godetiae (A4, F1*, 99/1.00), formed by a large number of strains, belongs to the first sister clade and groups (97/1.00) with C. johnstonii (F8, 100/1.00) and a single-strain clade representing C. pyricola (F2). The other sister clade (75/1.00) consists of six subclades: a large, long-branched and almost homogenous subclade representing C. salicis (A7, K1, 100/1.00); a short-branched subclade representing C. phormii (91/1.00); C. rhombiforme (A6, 100/1.00), which groups with a single-strain clade representing C. acerbum on a long branch (F1*, 100/1.00); plus C. australe (100/1.00) and C. kinghornii on long branches. Strains named F1 appear in the phylogeny of Guerber et al. (2003) in different subclades, corresponding to C. acerbum, C. godetiae and probably also C. rhombiforme. Colletotrichum pseudoacutatum is only distantly related to the C. acutatum complex and is therefore not included in the phylogeny, while C. orchidophilum was found to be more closely related and was therefore used as outgroup. The phylogenetic position of these and all other species included here is exhibited in fig. 1 and 2 of Cannon et al. (2012, this issue).
The individual alignments and maximum parsimony analyses of the six single genes were compared with respect to their performance in species recognition. With ITS and CHS-1, only 11 and 13 species, respectively, can be recognised. All subclades are recognised with TUB2 and GAPDH. TUB2 performs better than GAPDH due to higher numbers of base pair differences, but even with TUB2 there are clades with differerences of only 1 bp, which suggests that both genes should be used for identification. The performance of the other two genes is intermediate between ITS and TUB2/GAPDH.
Taxonomy
Based on DNA sequence data and morphology, the 331 strains studied (Table 1) are assigned to 31 species, of which 29 species are within the C. acutatum species complex and two outside this group, including 21 species that proved to be new to science. Two species formed sexual morphs in vitro. All species studied in culture are characterised below.
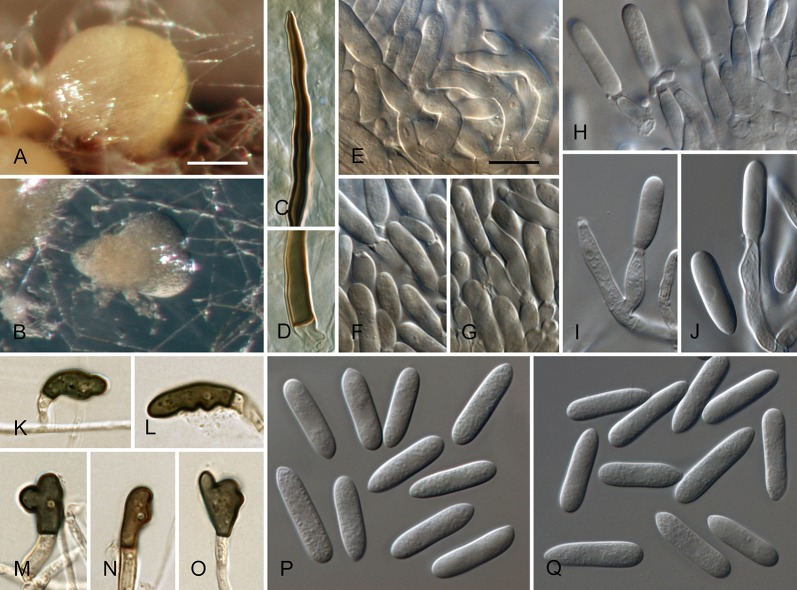
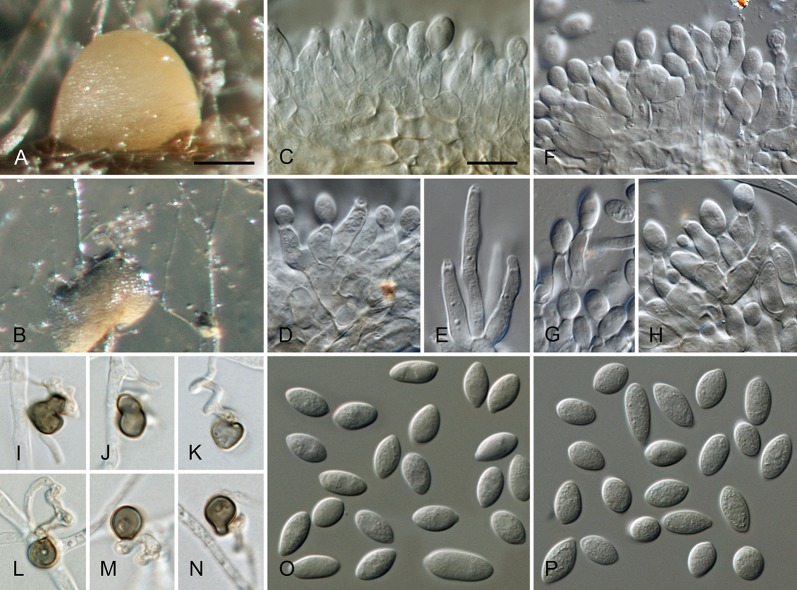
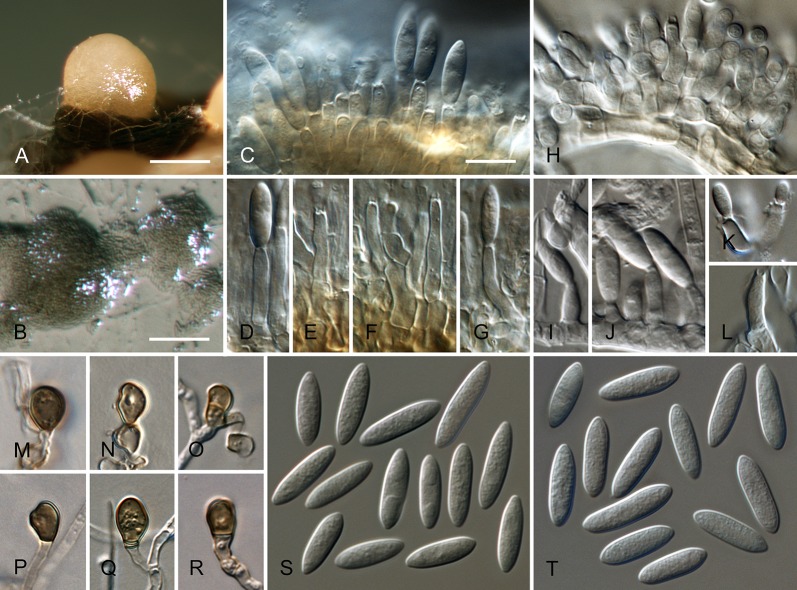
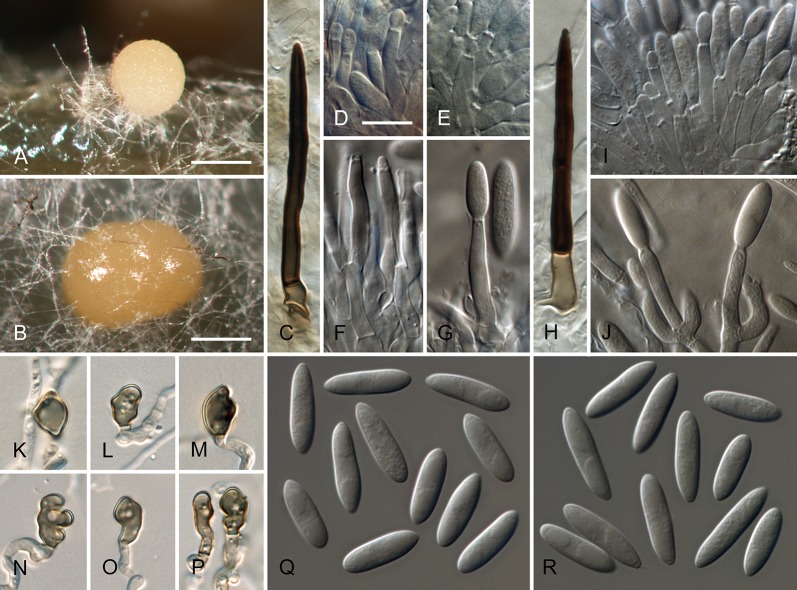
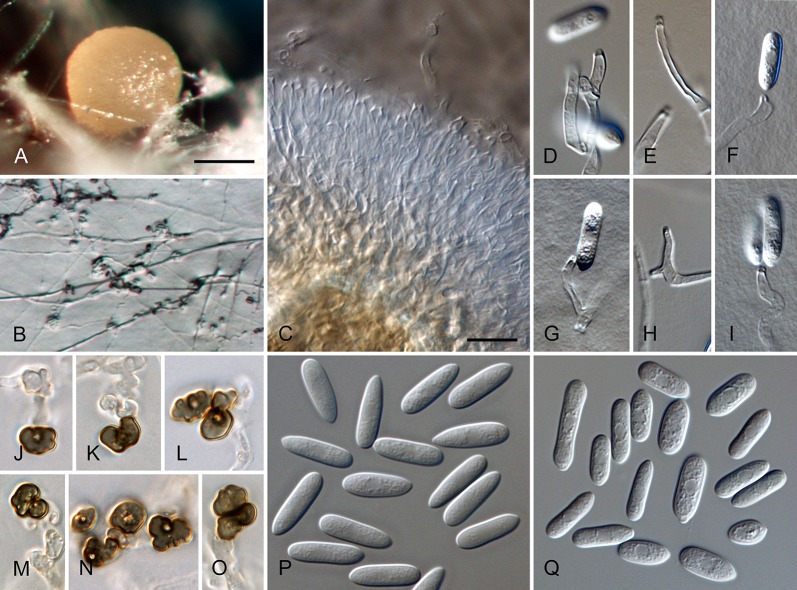
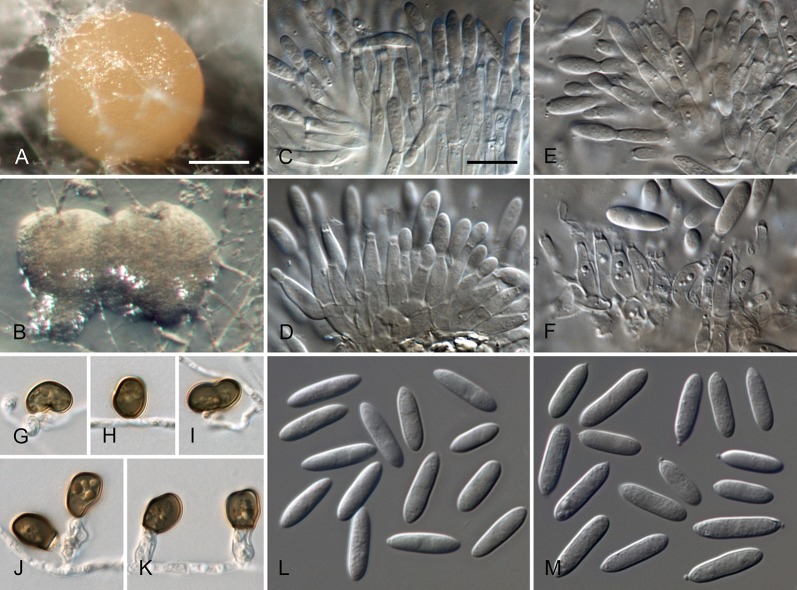
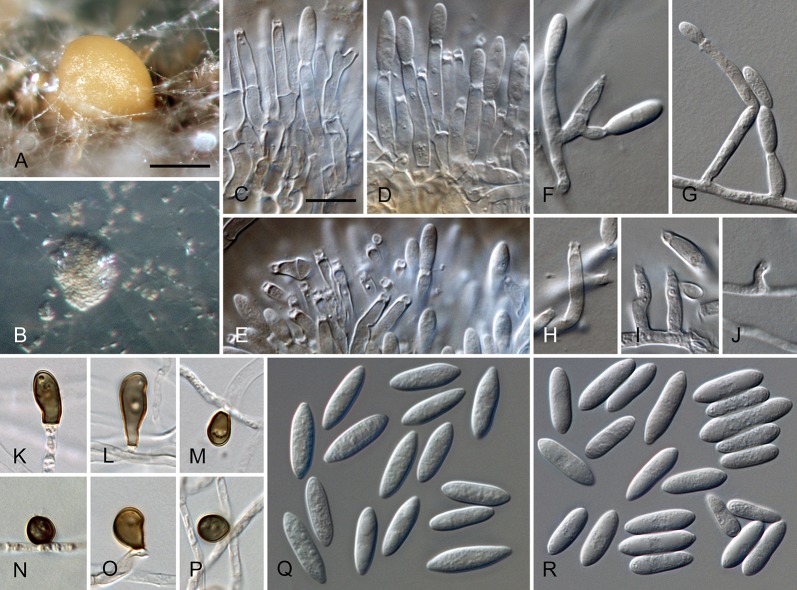
Colletotrichum acerbum Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800494. Fig. 2.
Fig. 2.
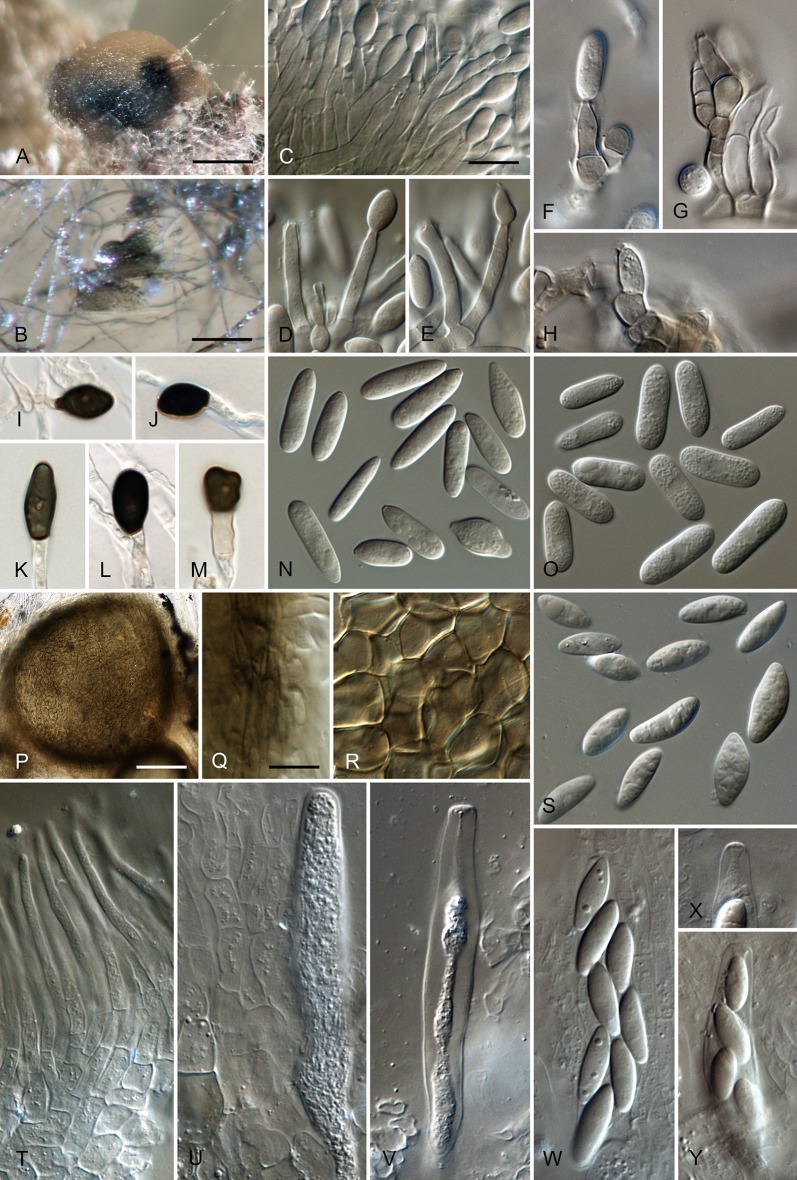
Colletotrichum acerbum (from ex-holotype strain CBS 128530). A–B. Conidiomata. C. Tip of a seta. D. Basis of a seta. E–J. Conidiophores. K–O. Appressoria. P–Q. Conidia. A, C–G, P. from Anthriscus stem. B, H–O, Q. from SNA. A–B. DM, C–Q. DIC, Scale bars: A = 100 μm, E = 10 μm. Scale bar of A applies to A–B. Scale bar of E applies to C–Q.
Etymology: acerbus = Latin for bitter; referring to bitter rot, the vernacular name for Colletotrichum disease of apple.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–6 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, sometimes lacking a basal septum and continuous with the conidiophore, sometimes extending to form new conidiogenous loci, polyphialides sometimes observed, discrete phialides measure 7–18 × 3–4.5 μm, opening 1.5–2 μm diam, collarette 0.5–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to clavate with one end round and one end slightly acute or both ends round, 15.5–20.5(–29) × (4–) 4.5–5 μm, mean ± SD = 17.9 ± 2.4 × 4.7 ± 0.2 μm, L/W ratio = 3.8. Appressoria single or in loose groups, medium to dark brown, smooth-walled, clavate, ovate or irregular outline, the edge entire or undulate, sometimes lobate, (8–)9–14(–16.5) × (4–)5–7.5(–9.5) μm, mean ± SD = 11.3 ± 2.4 × 6.2 ± 1.2 μm, L/W ratio = 1.8.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on a cushion of pale brown angular cells, 3–8.5 μm diam. Setae very few, medium brown, basal cell pale, smooth-walled, 1–2-septate, 45–85 μm long, base cylindrical, 3–4 μm diam, tip ± acute. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, 9–20 × 3.5–5 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end slightly acute, (12.5–)15–18.5(–20.5) × (4–)4.5–5 μm, mean ± SD = 16.8 ± 1.7 × 4.7 ± 0.3 μm, L/W ratio = 3.6.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to pale cinnamon, on filter paper, Anthriscus stem and medium partly covered with short floccose-felty white aerial mycelium and salmon, orange to olivaceous grey acervuli, reverse same colours, growth rate 20–21.5 mm in 7 d (32–33 mm in 10 d). Colonies on OA flat with entire margin; surface buff to honey, almost entirely covered with floccose-felty white to pale olivaceous grey aerial mycelium and olivaceous grey to salmon acervuli, reverse buff, pale olivaceous grey, grey olivaceous to iron-grey, growth rate 17.5–20 mm in 7 d (27.5–30 mm in 10 d). Conidia in mass salmon to orange.
Material examined: New Zealand, Nelson, from bitter rot on fruit of Malus domestica, 1 Aug. 1987, P.R. Johnston, (CBS H-20725 holotype, culture ex-type CBS 128530 = ICMP 12921 = PRJ 1199.3).
Notes: Bitter rot has been considered an economically signficant disease of apple for many years (Schrenk & Spaulding 1903b), and was initially ascribed to Gloeosporium fructigenum (Berkeley 1856). However, Berkeley’s type was examined by Vinnere (2004) and found to have falcate conidia, thus excluding it from the C. acutatum species complex. Currently, bitter rot is known to be caused primarily by fungi from the C. gloeosporioides species complex (González et al. 2006); that study focused on strains from the USA and Brazil, and we do not know whether their C. acutatum s. lat. strains are conspecific with C. acerbum. Those of Lee et al. (2007) from Korea are referable to C. acutatum clades 2 (C. nymphaeae and related species) and 3 (C. fioriniae), so the host certainly appears susceptible to a wide range of Colletotrichum pathogens.
The ex-type strain of C. acerbum is the only strain we included in our study that represents C. acutatum group B as delineated by Lardner et al. (1999). It may be common on Malus in New Zealand, but Lardner et al. (1999) found that more strains from fruit rot of apple, as well as from feijoa and fig, belonged to group C and had similar RAPD banding patterns (Lardner et al. 1999). It is possible that their group C includes more than one species. The GAPDH sequence of strain PJ9 (= PRJ 819 in Lardner et al. 1999), which was isolated from apple in New Zealand and was sequenced by Guerber et al. (2003), is identical to that of CBS 128530, the ex-type strain of C. acerbum.
Colletotrichum acerbum is distinguishable from C. rhombiforme and all other species in all gene sequences analysed except for CHS-1, and is most effectively distinguished with TUB2 and ITS. In morphological terms its conidia are longer and the appressoria are shorter and wider than those of C. rhombiforme. Based on our studies and blastn searches in GenBank, it seems that C. acerbum could be endemic to New Zealand. The closest match based on TUB2 sequence that we could find (with 99 % identity, 5 bp differences) was AJ748624 from isolate PT250 (= CBS 129953), derived from olive in Portugal (Talhinhas et al. 2005), which we assign to C. rhombiforme. The closest matches for the ITS sequence of C. acerbum (with 99 % identity, 3 bp differences), were with the ITS of C. phormii and C. salicis, which are all members of the same major clade.
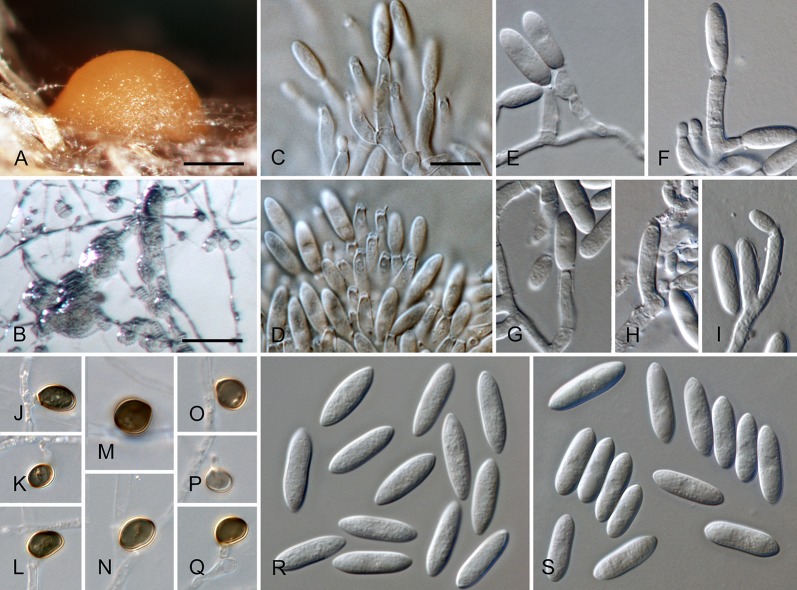
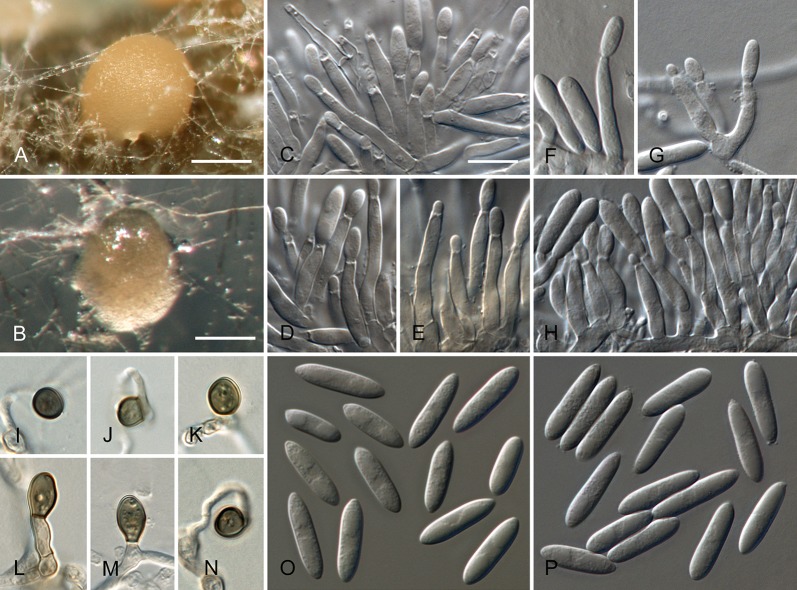
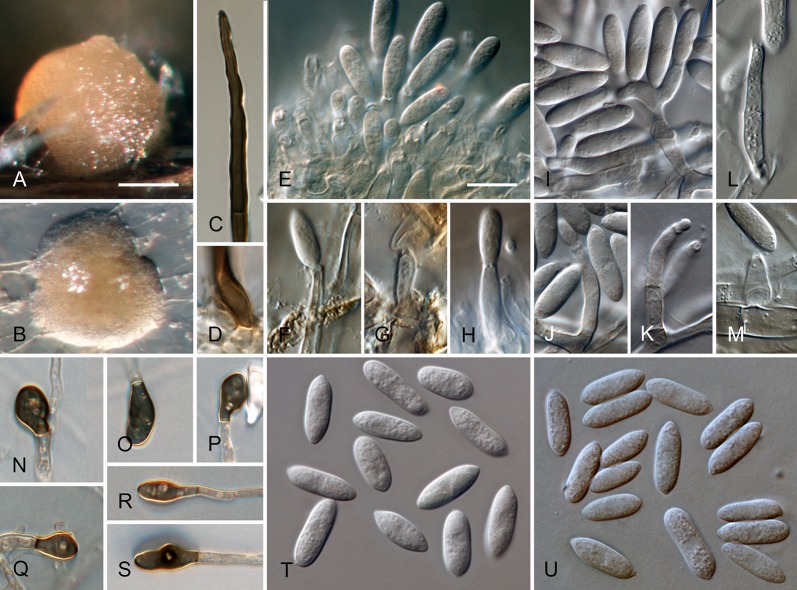
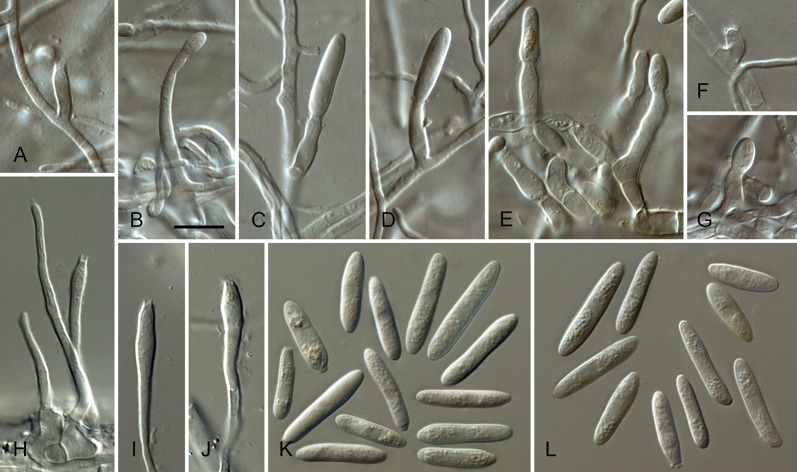
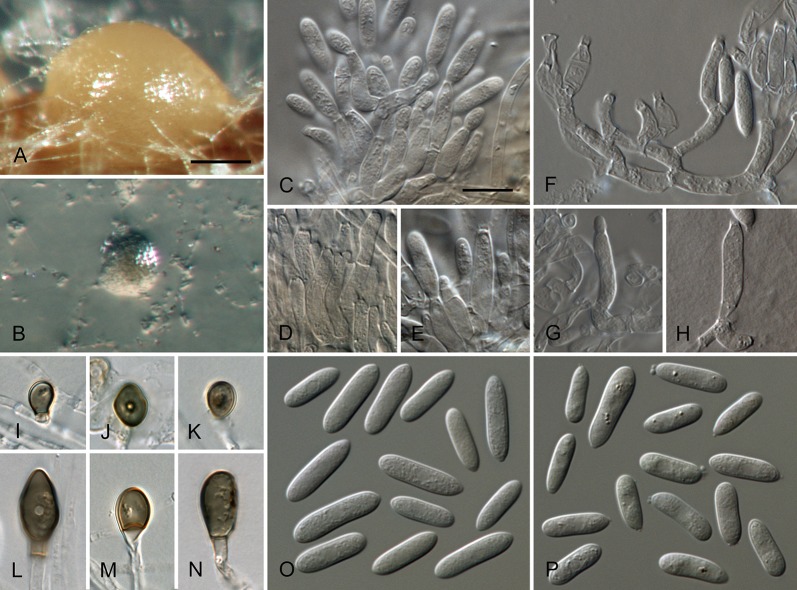
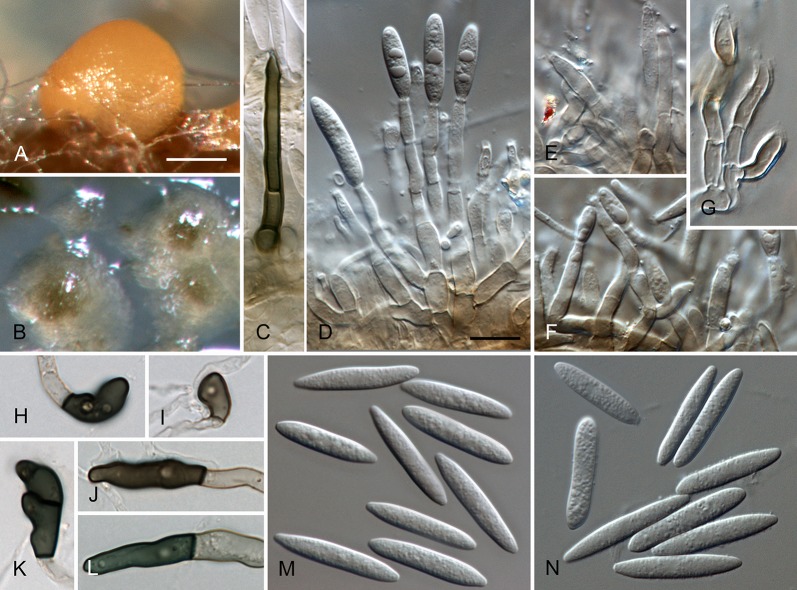
Colletotrichum acutatum J.H. Simmonds, Queensland J. agric. Anim. Sci. 25: 178A. 1968. Fig. 3.
Fig. 3.
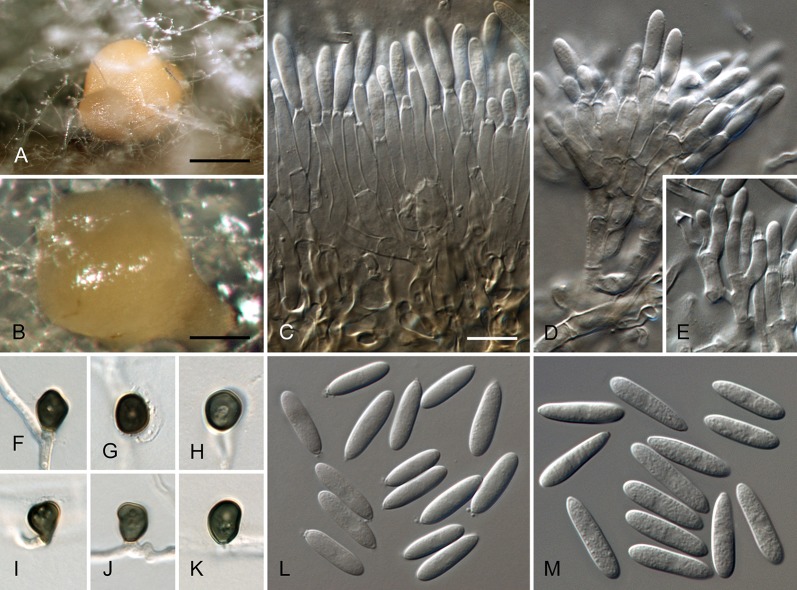
Colletotrichum acutatum (from ex-epitype strain CBS 112996). A–B. Conidiomata. C–I. Conidiophores. J–Q. Appressoria. R–S. Conidia. A, C–D, R. from Anthriscus stem. B, E–Q, S. from SNA. A–B. DM, C–S. DIC, Scale bars: A = 200 μm, B = 100 μm, C = 10 μm. Scale bar of C applies to C–S.
≡ Colletotrichum acutatum J.H. Simmonds, Queensland J. agric. Anim. Sci. 22: 458. 1965, nom. inval., Art. 37.1.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–5.5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on vegetative hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, mostly simple, sometimes septate and branched, to 25 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to slightly inflated, often not clearly separated from subtending hyphae by a septum, 3.5–20 × 2–3.5 μm, opening 1–1.5 μm diam, collarette distinct, 1–1.5 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends acute, (7.5–)11–14.5(–19) × 3.5–4(–4.5) μm, mean ± SD = 12.6 ± 1.8 × 3.9 ± 0.3 μm, L/W ratio = 3.2, conidia of strains CBS 112759, CBS 112979 and CBS 979.69 differ in being cylindrical to clavate and having one round and one acute end, e.g., conidia of strain CBS 112759 are smaller, measuring (6.5–)8.5–12(–13) × (2.5–)3–4 μm, mean ± SD = 10.3 ± 1.9 × 3.4 ± 0.5 μm, L/W ratio = 3.1. Appressoria solitary, medium brown, smooth-walled, ellipsoidal to obovate, entire edge, sometimes undulate, (4–)5.5–9(–13) × (3–)4–6.5(–9.5) μm, mean ± SD = 7.3 ± 2.0 × 5.4 ± 1.2 μm, L/W ratio = 1.3.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on a cushion of pale brown angular cells, 2–7 μm diam. Setae not observed. Conidiophores hyaline, septate, branched, smooth-walled, to 50 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, 9–18 × 3–3.5 μm, opening 1–1.5 μm diam, collarette distinct, 0.5 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, fusiform to cylindrical, apex and base uniformly acute, (8.5–)12–16.5(–17.5) × (3–)3.5–4.5(–5) μm, mean ± SD = 14.3 ± 2.1 × 4.1 ± 0.4 μm, L/W ratio = 3.5, conidia of strains CBS 112759, CBS 370.73 and CBS 112979 differ in being cylindrical to clavate and in having one round and one acute end, conidia of strain CBS 370.73 are smaller, measuring (5–)6.5–11(–12.5) × (2–)2.5–3.5(–4.5) μm, mean ± SD = 8.8 ± 2.1 × 3.2 ± 0.5 μm, L/W ratio = 2.7.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline with white aerial mycelium on Anthriscus stem and filter paper, reverse of filter paper partly pale ochreous; growth rate 21–24.5 mm in 7 d (30–36.5 mm in 10 d). Colonies on OA flat with entire margin; surface buff, rosy buff, salmon to peach due to sporulation, with olivaceous sectors in the centre, partly covered by white floccose aerial mycelium, reverse rosy buff to flesh, smoke grey to olivaceous grey in the centre; growth rate 20–25 mm in 7 d (31–33.5 mm in 10 d). Conidia in mass saffron to orange.
Material examined: Australia, Queensland, Ormiston, Redlands Research Station, from fruit rot of Carica papaya, 1 Oct. 1965, J.H. Simmonds (IMI 117617 = QDPI&F plant disease log book no. 16741B1 holotype, BRIP 4693, isotype of C. acutatum); Queensland, Brisbane, Ormiston, from fruit rot of Carica papaya, 5 Jul. 1965, J.H. Simmonds (deposited in CBS collection 2002 by P.W. Crous), (CBS-H 20723, epitype here designated, culture ex-epitype (and ex-paratype IMI 117620 of C. acutatum) CBS 112996 = ATCC 56816 = ICMP 1783 = STE-U 5292); New South Wales; Mount Annari, from Grevillea sp., 12 Oct. 1999, P.W. Crous, culture CBS 111993 = STE-U 3037; Western Australia, Wanneroo, from Fragaria × ananassa, 8 Aug. 1988, R.M. Floyd, culture CBS 127602 = BRIP 52691a = WAC 5416; from seedling of Pinus radiata, collection date and collector unknown (isolated Apr. 1971, deposited in CBS collection Sep. 1972 from Forest Research Institut Rotorua as C. acutatum f. sp. pineum), culture CBS 797.72. South Africa, from Leucadendron sp. cv. Safari Sunset, collection date unknown, J.E. Taylor, culture CBS 112990 = STE-U 4448; from Aspalathus linearis, collection date unknown, S. Lamprecht, culture CBS 129915 = CPC 15512; Southern Cape, Kruisfontein, from Pinus radiata, collection date unknown, Lundquist, culture CBS 110735 = STE-U 163; Kruisfontein, from Pinus radiata, collection date unknown, Lundquist, culture CBS 112979 = STE-U 160; Eastern Cape, Langkloof, from Hakea sericea, collector unknown (deposited in CBS collection 2002 by P.W. Crous), culture CBS 112759 = STE-U 4470; from Hakea sericea, collection date unknown (deposited in CBS collection 2002 by P.W. Crous), K. Lubbe, culture CBS 112761 = STE-U 4461. New Zealand, Tokoroa, from Pinus radiata, unknown collection date and collector (deposited in CBS collection Jan 1973 by J.M. Dingley), culture CBS 370.73 = NRCC 10088; Kenya, Yala, from berry of Coffea arabica, collection date unknown, D.M. Masaba, culture IMI 319423 = CPC 18877; from Coffea arabica, unknown collection date and collector (deposited in CBS collection Nov. 1969 by H. Vermeulen), culture CBS 979.69.
Notes: Colletotrichum acutatum was described by Simmonds (1965) from a range of different hosts from Australia. No type was designated, and the name was validated three years later (Simmonds 1968) with designation of a holotype, IMI 117617 from Carica papaya, and paratypes from C. papaya (IMI 117618 - IMI 117621), Capsicum frutescens (IMI 117622), and Delphinium sp. (IMI 117623).
Vinnere et al. (2002) sequenced the ITS region of the holotype specimen (AF411700) and one paratype specimen IMI 117619 (AF411701) and found morphological and cultural differences between Simmonds’s six holotype/paratype specimens of C. acutatum. There is no living ex-holotype culture available, but two ex-paratype strains, one from Carica papaya (IMI 117620 = QDPI&F plant disease log book no. 16633D = ATCC 56816 = CBS 112996 = STE-U 5292) and one from Capsicum frutescens (IMI 117622 = QDPI&F plant disease log book no. 11711A = CBS 292.67, see C. brisbanense) and an ex-topotype strain from Carica papaya (QDPI&F plant disease log book no. 13483-0 = CBS 294.67, see C. simmondsii) do exist in a living state.
Than et al. (2008b) epitypified C. acutatum with a strain from Carica papaya from the region in which the species was first collected (BRIP 28519 = CBS 122122). Not only was this action inadvisable bearing in mind that living cultures from two paratypes still exist, it was regrettable as it was subsequently discovered that their epitype was not conspecific with the type. Following an ITS and TUB2 analysis of the clade, Shivas & Tan (2009) described C. acutatum sensu Than et al. (2008b) as a separate species, C. simmondsii. They did not designate a further epitype for C. acutatum, but bearing in mind that only the ITS region of the holotype was sequenced, we feel that it is important to fix the application of that species name with an appropriate epitype that can be subject to multigene analysis. This has been done above, with one of Simmond’s original paratypes chosen for this purpose.
Colletotrichum acutatum s. str. causes diseases of a wide range of unrelated plants, some of which are economically significant, including papaya (Carica papaya), strawberry (Fragaria × ananassa), pine (Pinus spp.), Hakea spp. and rooibos (Aspalathus linearis). Two of these are associated with recognition of formae speciales. These are not accepted as a taxonomic rank in the International Code of Nomenclature for Algae, Fungi and Plants (ICN) as they are based on fungus/plant interactions rather than single species, and formal ICN-compliant taxa cannot use formae speciales as basionyms for new combinations.
Colletotrichum acutatum f. sp. pinea (Dingley & Gilmour 1972) was described for a malady of pines called terminal crook disease, with the fungus apparently causing malformation of growing tips. We have examined authentic cultures derived from Dingley & Gilmour’s work, which were also used by von Arx. Most of these cannot be distinguished in morphological or molecular terms from C. acutatum s. str., but strain CBS 436.77 (from Chile, not from New Zealand as are the authentic cultures of C. acutatum f. sp. pinea), belongs to a quite different species outside of the C. acutatum species complex (see C. pseudoacutatum). CBS 797.72 appears to show evidence of hybrid origin and is not included in the molecular analyses (see below).
Colletotrichum acutatum f. sp. hakeae (Lubbe et al. 2004) was introduced for an apparently strongly host-specific set of strains, one of which was being used as a potential biological control agent (Morris 1982, Gordon & Fourie 2011), but we have not found any morphological differences and there are few sequence-based differences (1 bp difference in ITS, 2 bp differences in HIS3) between these and other C. acutatum s. str. strains. We therefore do not feel confident to recognise this forma specialis on that basis as a segregate species. Colletotrichum acutatum f. sp. chromogenum was described by Baxter et al. (1983), based on strains from olive referred to as Gloeosporium fructigenum f. sp. chromogenum by Gorter (1962), for strains producing pink to purple pigments in culture. Such pigment production is common throughout the C. acutatum complex (e.g. Polashock et al. 2009) and is especially prominent in C. acutatum s. str. (as their clade A5) according to Sreenivasaprasad & Talhinhas (2005). In fact the only strain from olive in South Africa included in this study (CBS 127589) belongs to this species and could represent C. acutatum f. sp. chromogenum. But whatever the case, the rank used is inappropriate for this purpose.
A variety of C. acutatum, described on Fiorinia externa (a scale insect), C. acutatum var. fioriniae (Marcelino et al. 2008), was recognised as the separate species C. fioriniae by Shivas & Tan (2009) and is included below in this study.
A sexual morph was described for C. acutatum (Guerber & Correll 1997, 2001), based on mating compatible strains in the laboratory. The cross designated as type of Glomerella acutata was based on two cultures, ATCC 56816 and ATCC MYA-662. The first of these is derived from one of Simmonds’ original Queensland collections from papaya, IMI 117620 (here designated as epitype of C. acutatum). There is ongoing confusion regarding the provenance of this strain, however; Guerber & Correll (2001) and Than et al. (2008b) wrongly equated ATCC 56816 with IMI 117617, the holotype, and that congruence is recorded as such in the ATCC catalogue. The second strain ATCC MYA-662 was isolated from apple in Louisiana, USA (Guerber & Correll 2001), and is here assigned to C. fioriniae in clade 3. Fertile sexual morphs were also produced by Guerber & Correll (2001) and Guerber et al. (2003) by mating a series of different strains, including crosses between parents that are both assigned to C. fioriniae. None of the strains tested was self-fertile.
The holotype of Glomerella acutata is therefore an interspecific hybrid between C. acutatum and C. fioriniae. This might be construed as strong evidence that these two taxa constitute a single biological species, and therefore that the species concepts used in this paper are much too narrow. However, the parent strains of the holotype originate from highly distant populations in geographical terms, and there are instances in other fungal groups (e.g. Neurospora) where non-sympatric populations lose post-mating reproductive isolation barriers (Turner et al. 2010, 2011). Further research on population structures and mating-type barriers would be instructive.
Colletotrichum acutatum has subsequently been reported to produce a sexual morph in nature, on Vaccinium corymbosum (highbush blueberry) in Norway (Talgø et al. 2007). Sequence-based identification was apparently not carried out and so the identity of this population remains uncertain, however the blueberry pathogen is usually C. fioriniae, which has a known sexual morph (Marcelino et al. 2008). Its origin is also unknown; the crop is not native to Norway and the fungus may have been introduced from the USA along with planting material. The Glomerella sexual morph described from Acer platanoides in Massachusetts, USA is homothallic (LoBuglio & Pfister 2008), and belongs to C. salicis, not C. acutatum s. str. Two strains from this research are included in our study. Further discussion may be found in Cannon et al. (2012, this issue).
A further twist in the story may be provided by CBS 797.72; this is one of the strains on which C. acutatum f. sp. pinea was based (Dingley & Gilmour 1972). Sequences of three of the six genes sampled (ACT, HIS3 and CHS-1) indicate affinities with C. acutatum (clade 4) but the other three (ITS, GAPDH and TUB2) suggest that the strain belongs to C. fioriniae (clade 3). This was confirmed by repeating sequencing from a new subculture from the CBS collection and after re-singlesporing of one of the single spore isolates. This too may be of hybrid origin. The phylogeny by Guerber et al. (2003) includes strains from Pinus in New Zealand in both species (as groups J4 and C1). We do not know if they should be assigned to C. fioriniae, or are hybrids as well.
The widespread geographical range and economic importance of C. acutatum makes it likely that an earlier name exists for the species, probably listed as a synonym of C. gloeosporioides by von Arx (1957). Walker et al. (1991) noted that C. xanthii (Halsted 1893) is such a candidate based on the fusiform shape of conidia found on the type material, but no authentic cultures exist and no other strain from Xanthium with fusiform conidia was available to us. It is therefore impossible to determine whether this species provides an earlier name for C. acutatum s. str., for another species within the C. acutatum complex or belongs to the C. acutatum species complex at all (see C. pseudoacutatum).
Apart from C. acutatum and C. simmondsii, there have been other Colletotrichum and Gloeosporium species described on Carica papaya, all from Brazil. Conidia of C. papayae Henn. described from branches and petioles of papaya in Sao Paulo, are larger and differ in shape from C. acutatum s. str.; they are cylindrical, straight to curved, hyaline, and measure 12–20 × 5–7 μm (Saccardo et al. 1931), while those of C. acutatum measure on average 8.8–15.5 × 3.2–4.5 μm, depending on strain and medium. Gloeosporium papayae Henn., described from stems of papaya in Uberaba, Minas Gerais, forms cylindrical-oblong to subclavate, obtuse, straight, hyaline to pale yellowish conidia that measure 11–14 × 5–6 μm (Hennings 1895); conidia of C. acutatum are hyaline and broader. A presumed isotype in K(M) of G. papayae, “E. Ule n. 1947”, collected in June 1892, is actually a species of Phomopsis. Gloeosporium fructus-caricae Henn. forms conidia that overlap in size with those of C. acutatum, however their shape is described as oblong-cylindrical with both ends rounded, while conidia of C. acutatum usually have both ends acute. Even if the description matches completely with that of C. acutatum we could not be sure they are the same species; the morphology of most species in the C. acutatum species complex is highly variable and overlapping. There is no strain from papaya in Brazil included in this study, and there is no report of C. acutatum s. lat. from Brazil listed in Farr & Rossman (2012). We have tried to draw a reasonable balance between respect for the rules of priority and the need for nomenclatural stability, and in this case we feel that Simmonds’ name should be conserved if such a synonymy is established.
Colletotrichum acutatum is separated from other species by all genes. Closest matches in a blastn search with the TUB2 sequence of strain CBS 112996, with 100 % identity, were GU183307–GU183309 and GU183311–GU183314, from Boronia, Anemone, Fragaria, Pistacia, Anemone, Olea, Ranunculus and Mangifera in Australia (Shivas & Tan 2009), FJ788419 from Simmonds’ specimen 16633D from Carica papaya in Australia (Weir & Johnston, unpubl. data), AY376546–AY376549 and AY376558–AY376568 from Pinus, Leucadendron and Carica (STE-U 5292 = CBS 112996) and Hakea (Lubbe et al. 2004), AJ748627 and AJ748630 from Phlox and Statice (Talhinhas et al. 2005), HE573032 from Arbutus unedo (strawberry tree) (Polizzi et al. 2011) and with 99 % identity (1 or 2 bp difference) AY376550 and AY376545 from Protea and Leucospermum (Lubbe et al. 2004) and AJ748618 from Olea (Talhinhas et al. 2005). Colletotrichum acutatum s. str. can therefore be assumed to be a widespread species that causes disease symptoms on a wide range of plants.
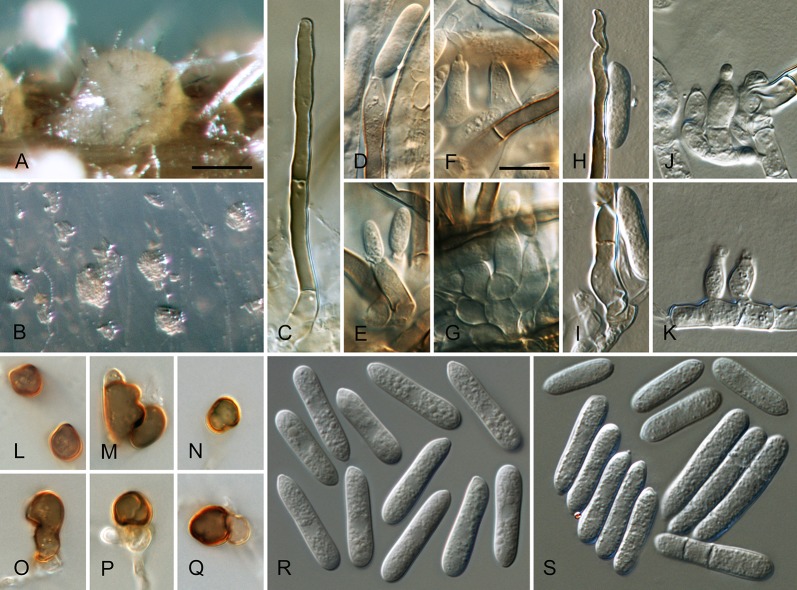
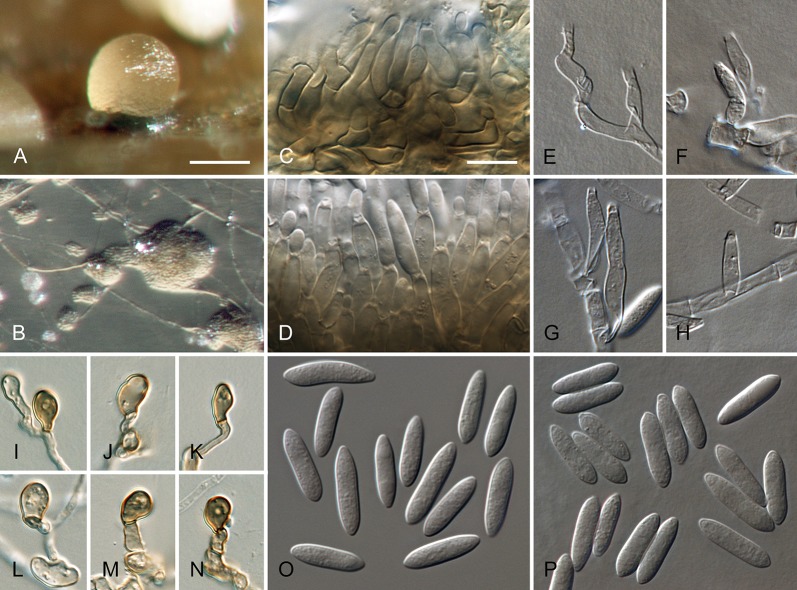
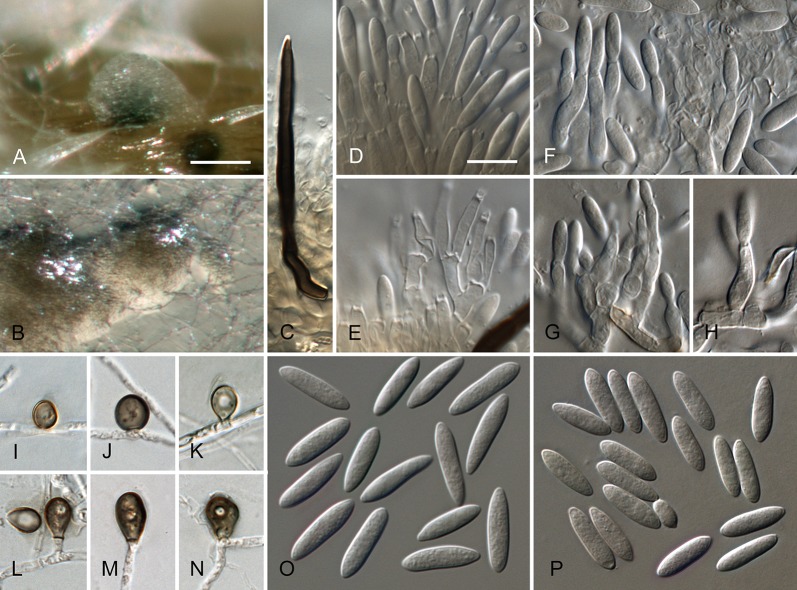
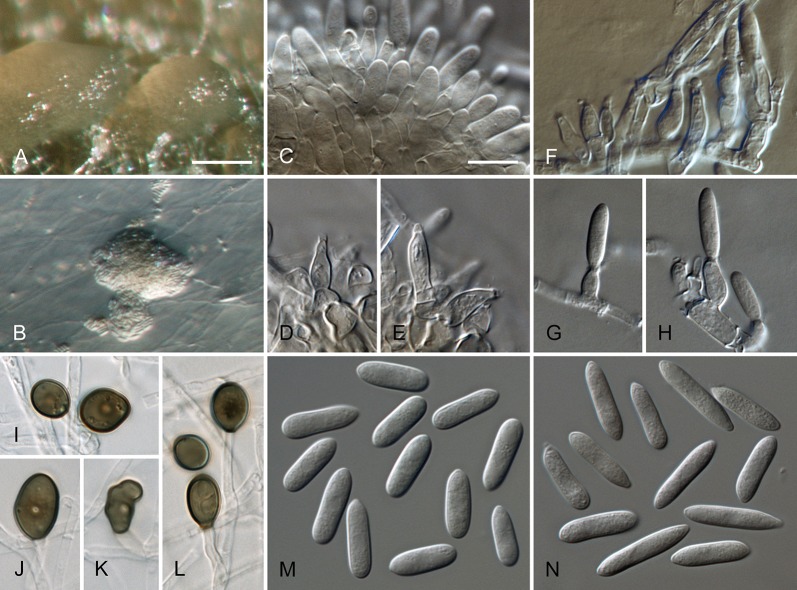
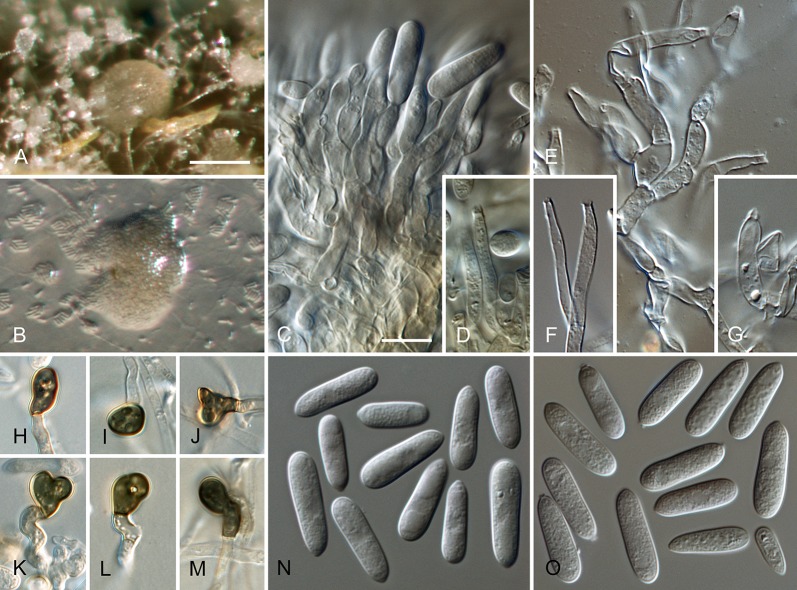
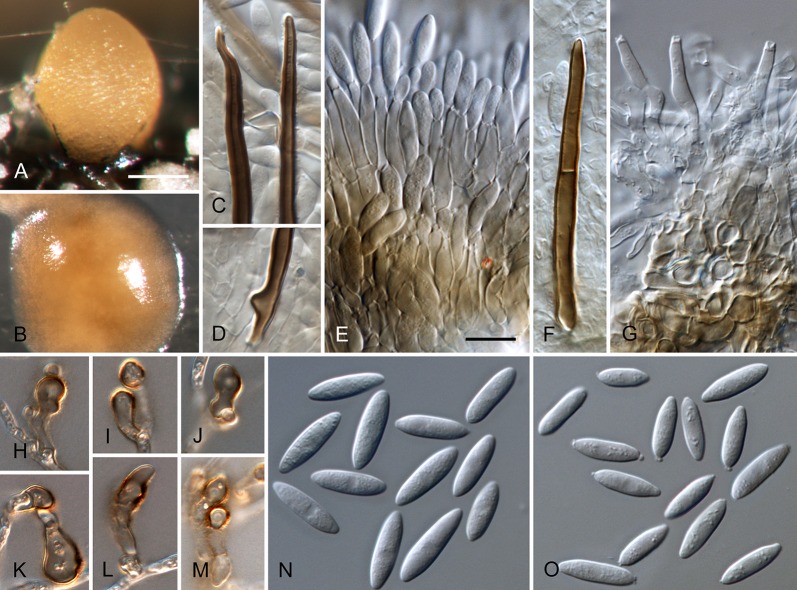
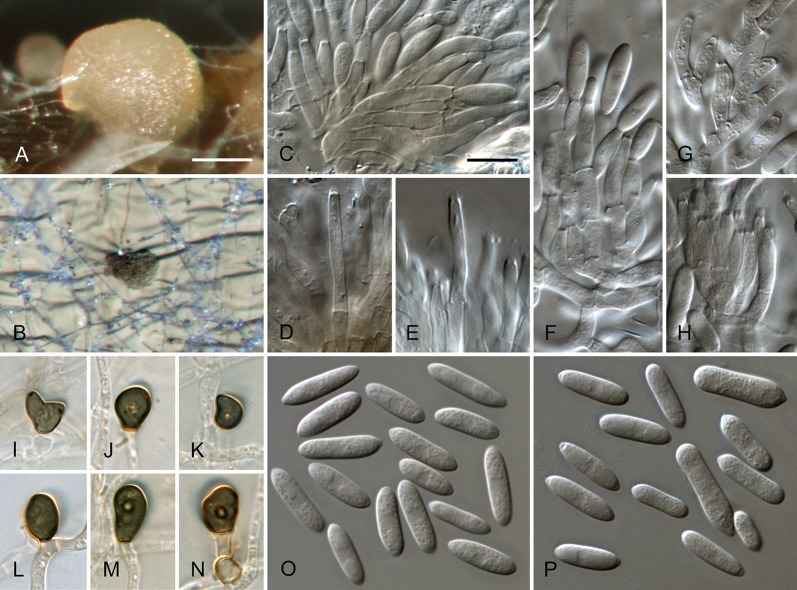
Colletotrichum australe Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800495. Fig. 4.
Fig. 4.
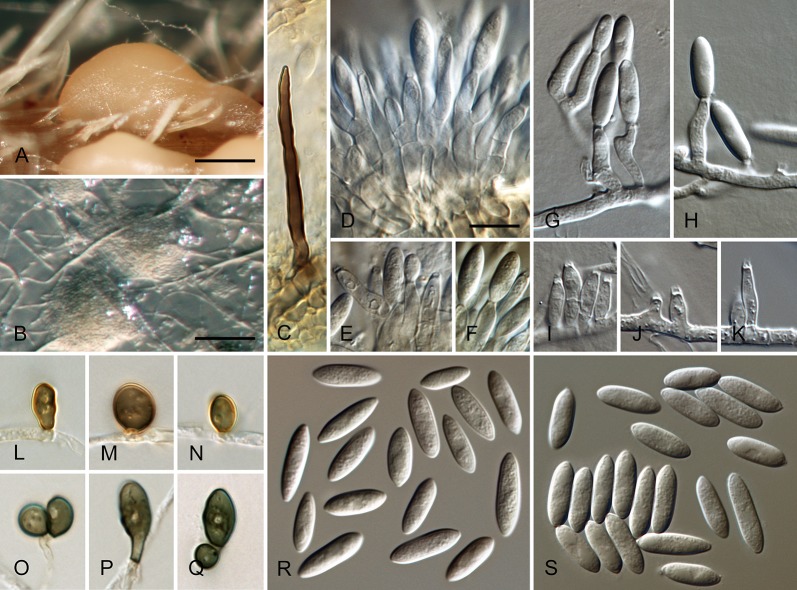
Colletotrichum australe (from ex-holotype strain CBS 116478). A–B. Conidiomata. C. Seta. D–G. Conidiophores. H. Tip of seta. I. Basis of seta. J–K. Conidiophores. L–Q. Appressoria. R–S. Conidia. A, C–G, R. from Anthriscus stem. B, H–Q, S. from SNA. A–B. DM, C–S. DIC, Scale bars: A = 100 μm, F = 10 μm. Scale bar of A applies to A–B. Scale bar of F applies to C–S.
Etymology: derived from the localities of collection in the Southern Hemisphere.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores and setae formed directly on hyphae. Setae rarely observed, pale to medium brown, smooth-walled to finely verruculose, 1–3-septate, 30–90 μm long, base cylindrical to conical, 3.5–5.5 μm diam, tip ± roundish and bent and function as a conidiogenous locus. Conidiophores hyaline to pale brown, smooth-walled, simple or septate and branched, to 30 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, conical to ampulliform, 4.5–15 × 2.5–5.5 μm, opening 0.5–1 μm diam, collarette 0.5–1.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, become septate with age, straight, cylindrical, with one end round and one end slightly acute to truncate, (10–)14.5–19.5(–25) × (3.5–)4–5(–6) μm, mean ± SD = 17.0 ± 2.4 × 4.4 ± 0.5 μm, L/W ratio = 3.9. Appressoria single or in small groups, medium brown, smooth-walled, outline mostly subglobose to elliptical, sometimes clavate, the edge entire or undulate, sometimes slightly lobate, (5–)6–11(–14) × (4–)4.5–7(–8.5) μm, mean ± SD = 8.5 ± 2.6 × 5.8 ± 1.1 μm, L/W ratio = 1.5.
Asexual morph on Anthriscus stem. Conidiomata acervular where present, conidiophores and setae formed directly on hyphae or on a cushion of pale brown angular cells. Setae pale to medium brown, smooth-walled to finely verruculose, 2–7-septate, 40–130 μm long, base cylindrical to conical, 3–5 μm diam, tip broadly rounded to somewhat acute, and may function as a conidiogenous locus. Conidiophores hyaline to pale brown, smooth-walled, septate, little branched, to 50 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical to ampulliform, 8–19 × 3–5 μm, opening 1–2.5 μm diam, collarette 0.5–1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical, sometimes slightly constricted in the middle, with one end round and one end slightly acute to truncate, (16–)17–20(–22) × (4–)4.5–5(–5.5) μm, mean ± SD = 18.6 ± 1.6 × 4.7 ± 0.4 μm, L/W ratio = 4.0, conidia of strain CBS 131325 smaller, measuring (13.5–)15–17.5(–18) × (3.5–)4–5(–5.5) μm, mean ± SD = 16.3 ± 1.1 × 4.4 ± 0.4 μm, L/W ratio = 3.7.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to honey, filter paper straw to pale olivaceous grey, aerial mycelium lacking, reverse same colours, growth rate 16–18 mm in 7 d (28.5–30 mm in 10 d). Colonies on OA flat with entire margin; surface pale luteous to amber, in the centre covered with floccose white aerial mycelium, reverse pale luteous to salmon, growth rate 16–20 mm in 7 d (25–29.5 mm in 10 d). Conidia in mass salmon.
Material examined: South Africa, Stellenbosch, university campus, from Trachycarpus fortunei, 2 Jan. 1998, J.E. Taylor, (CBS-H 20721 holotype, culture ex-type CBS 116478 = HKUCC2616). Australia, Western Australia, Alcoa, from Hakea sp., 12 Jul. 2011, W. Gams, culture CBS 131325.
Notes: Colletotrichum australe belongs to the clade that includes C. phormii, C. kinghornii, C. rhombiforme and C. acerbum. Setae are better developed (in cultures on Anthriscus stem) and conidia are larger than in most other species in the C. acutatum species complex. Only C. phormii forms larger conidia, which are fusiform, while those of C. australe are cylindrical. Additionally, appressoria of C. australe are shorter than those of C. phormii. Conidia of C. rhombiforme are shorter, while those of C. kinghornii are narrower.
It is possible that Fusarium hakeae (Hennings 1898), described from leaves of Hakea saligna from the Botanic Garden in Berlin, Germany, is the same species as C. australe. The description is short but largely corresponds with our species, but bearing in mind that most Colletotrichum species show a lack of host specificity, there is no strong reason to equate the two taxa in the absence of sequenceable material of F. hakeae. Wollenweber (1916) transferred F. hakeae to Gloeosporium, and von Arx (1957, 1970) included the name as a synonym of C. gloeosporioides. Bondarzeva-Monteverde et al. (1936) described a separate fungus as Gloeosporium hakeae from greenhouses in St Petersburg; this was reported to have straight to curved conidia and is unlikely to be a synonym of Hennings’ fungus. Lubbe et al. (2004) published C.acutatum f. sp. hakeae for isolates that caused a distinctive disease of Hakea in South Africa; these have shorter conidia than those of C. australe and group in C. acutatum s. str. Colletotrichum acutatum has been reported from Trachycarpus fortunei in Australia and Switzerland by Taylor & Hyde (2003); we do not know whether these collections represent further records of C. australe.
Colletotrichum australe is separated from other species by all gene sequences surveyed except for CHS-1, which is the same as that of C. phormii, and most effectively separated by HIS3. The closest match in a blastn search with the TUB2 sequence of strain CBS 116478 (with 98 % identity, 8 and 9 bp differences) were isolates PCF 459 (EU635504) from strawberry in Belgium (Debode et al. 2009) and PT250 (= CBS 129953, see C. rhombiforme), and AJ748624 from olive, Portugal (Talhinhas et al. 2005). We do not think that any of these sequences are derived from strains that are conspecific with C. australe. With the GAPDH sequence there was no closer match than 87 % identity. The closest matches with ITS sequence, with 99 % sequence identity, include Glomerella cingulata BBA 70991 from Salix (AJ301952, Nirenberg et al. 2002) and Glomerella sp. strain MP3 from Acer platanoides (EU622052, LoBuglio & Pfister 2008), which are both likely to be C. salicis. Other strains with 99 % ITS sequence homology include that deposited as Fusarium phormii strain CBS 198.35 (DQ286144, Farr et al. 2006) which we assign to C. kinghornii, and Ga. acutata PT715 from Olea europaea in Portugal (AM991135, Talhinhas et al. 2009).
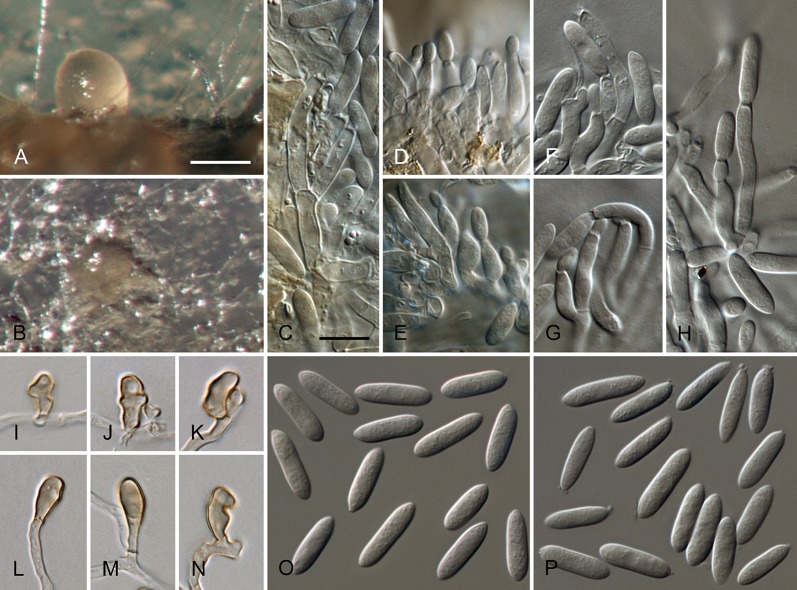
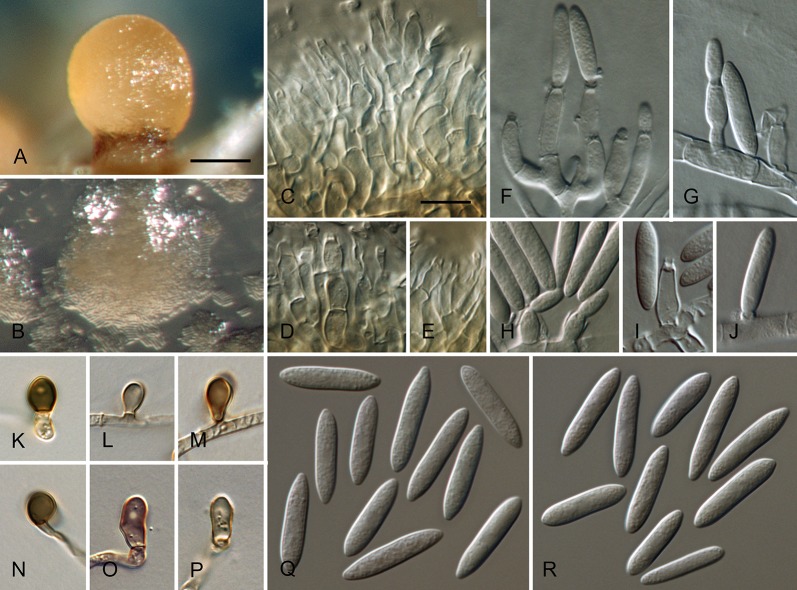
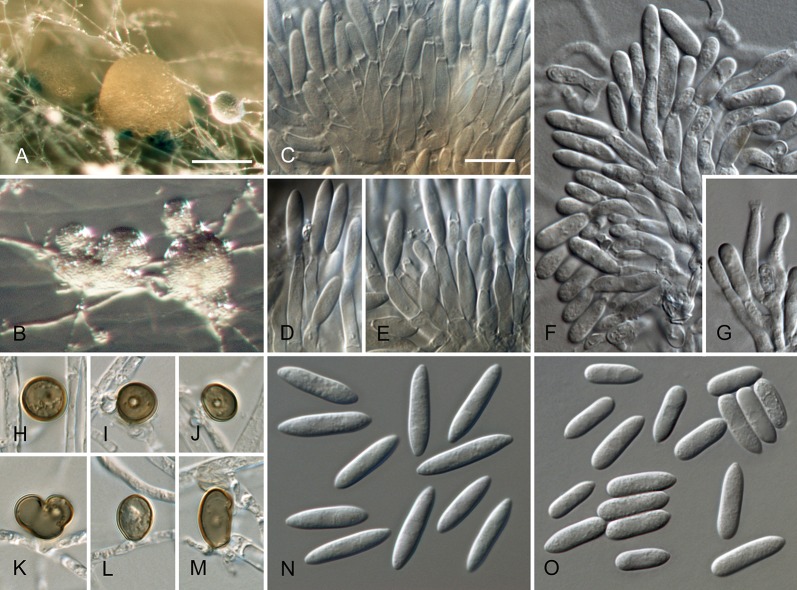
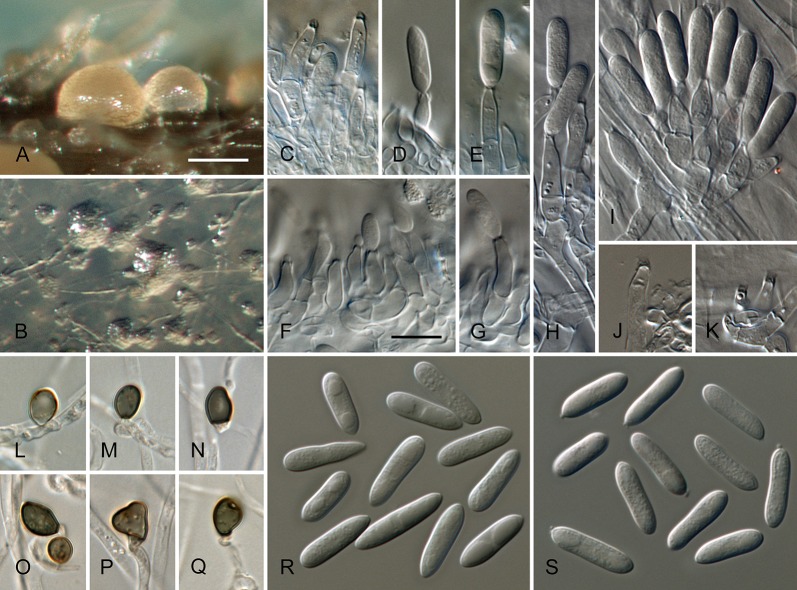
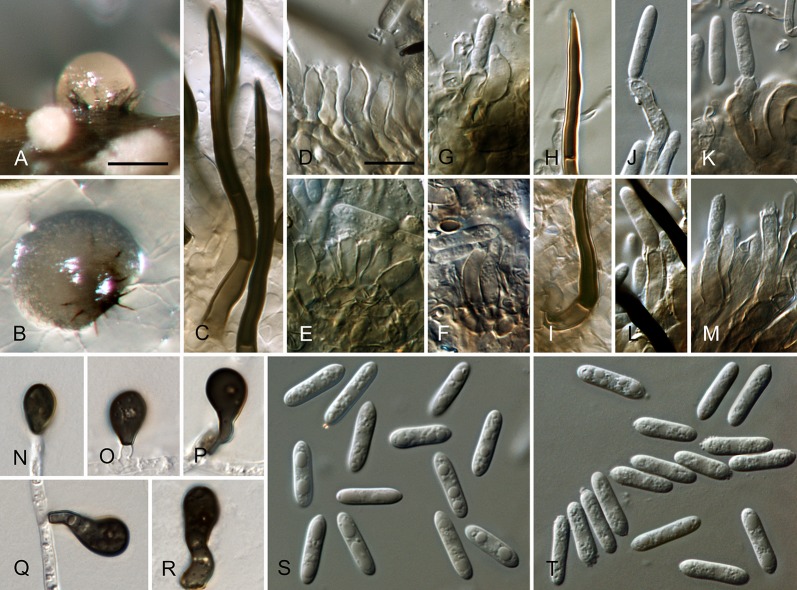
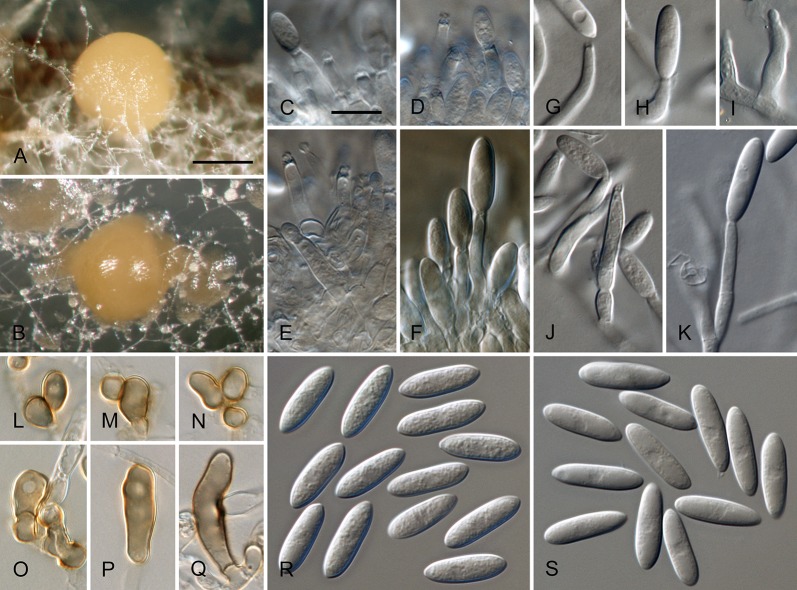
Colletotrichum brisbanense Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800497. Fig. 5.
Fig. 5.
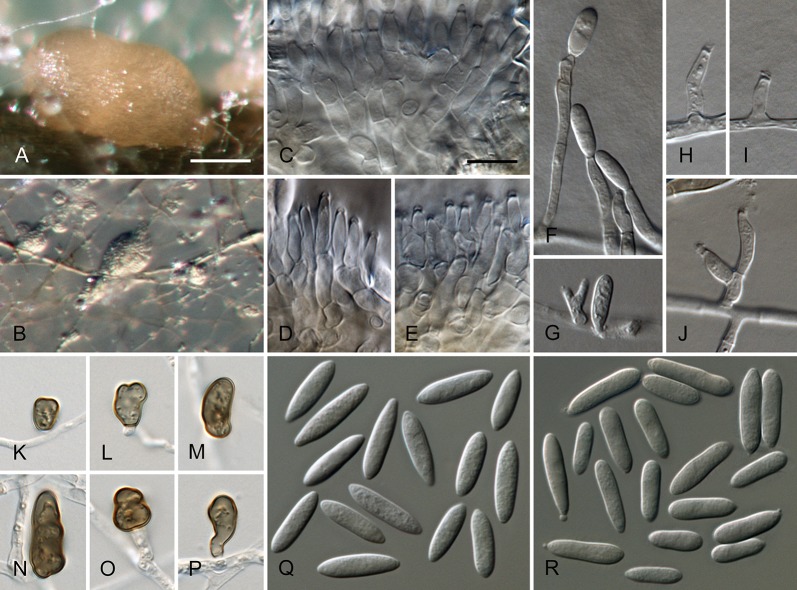
Colletotrichum brisbanense (from ex-holotype strain CBS 292.67). A–B. Conidiomata. C–H. Conidiophores. I–N. Appressoria. O–P. Conidia. A, C–E, O. from Anthriscus stem. B, F–N, P. from SNA. A–B. DM, C–P. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–P.
Etymology: Named after Brisbane, the city in Queensland, Australia where the species was collected.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–8 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to slightly inflated, sometimes lacking a basal septum and continuous with the conidiophore, sometimes proliferating and extending to form a new conidiogenous locus, discrete phialides measure 8.5–21 × 2.5–4 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with both ends slightly acute or one end round and one end slightly acute, (12–)12–17.5(–25) × (3–) 3.5–4(–5) μm, mean ± SD = 14.8 ± 2.8 × 3.8 ± 0.5 μm, L/W ratio = 3.9. Appressoria single or in loose groups, pale brown, smooth-walled, mostly clavate, the edge entire to undulate, (5–)7.5–14.5(–18) × (2.5–)3.5–5(–6) μm, mean ± SD = 11.1 ± 3.4 × 4.3 ± 0.9 μm, L/W ratio = 2.6.
Asexual morph on Anthriscus stem. Conidiomata possibly acervular, but no basal cells observed. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, sometimes proliferating and extending to form a new conidiogenous locus, sometimes polyphialidic, 8.5–23 × 2.5–4.5 μm, opening 1–2 μm diam, collarette 0.5–1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with both ends slightly acute, (9.5–)12–15(–17) × (3–)3.5–4 μm, mean ± SD = 13.5 ± 1.4 × 3.9 ± 0.3 μm, L/W ratio = 3.5.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to pale cinnamon, on filter paper partly pale saffron, agar medium partly covered with very short white aerial mycelium, reverse same colours; growth rate 18–20 mm in 7 d (26–29 mm in 10 d). Colonies on OA flat with entire margin; surface buff, rosy buff to pale saffron, covered with short white aerial mycelium, reverse same colours; growth rate 17.5–18.5 mm in 7 d (27.5–28.5 mm in 10 d). Conidia in mass salmon.
Material examined: Australia, Queensland, Brisbane, Eight Mile Plains, from fruit rot of Capsicum annuum, 14 Jul. 1955, J.H. Simmonds, (IMI 117622 holotype of C. brisbanense (also paratype of C. acutatum), CBS H-20801 isotype, culture ex-type CBS 292.67 = BRIP 4684).
Notes: The type and only confirmed strain of C. brisbanense was cited as one of the paratype strains of C. acutatum by Simmonds (1968), and assigned to C. simmondsii by Shivas & Tan (2009). Conidia and appressoria of C. brisbanense are larger overall than those of C. simmondsii as accepted in this treatment. The two species are easily separable using all sequence data except for ITS, and most effectively with TUB2 and GAPDH sequences. There is only one bp difference in CHS-1 sequence between C. brisbanense and C. indonesiense. There is a further species in clade 2 associated with Capsicum annuum, C. scovillei, possibly a species endemic to Southeast Asia. Colletotrichum brisbanense can be separated easily from C. scovillei based on appressorium measurements, as well as by most DNA data. See C. scovillei for further information.
A blastn search with the TUB2 sequence of strain CBS 292.67 resulted in a 100 % match with GU183275, the sequence of the same strain generated by Shivas & Tan (2009); next closest was DQ454064 from isolate S6 from Fragaria in Thailand with 99 % identity (four differences; Sang et al. 2011). With the GAPDH sequence there was no match with more than 95 % identity. The ITS sequence of strain CBS 292.67 matched 100 % with GU183315, a sequence of the same isolate generated by Shivas & Tan (2009).
Colletotrichum chrysanthemi (Hori) Sawada, Rep. Govt Res. Inst. Dep. Agric., Formosa 85: 81. 1943. Fig. 6.
Fig. 6.
Colletotrichum chrysanthemi (from strain CBS 126518). A–B. Conidiomata. C–H. Conidiophores. I–N. Appressoria. O–P. Conidia. A, C–E, O. from Anthriscus stem. B, F–N, P. from SNA. A–B. DM, C–P. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–P.
≡ Gloeosporium chrysanthemi Hori, in Takimoto, Jour. Hort. Japan 36(9): 27. 1924.
Sexual morph not observed. Asexual morph on SNA (CBS 126518). Vegetative hyphae 1.5–9 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate and branched, to 55 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, 7–15 × 3–4.5 μm, opening 1.5–2 μm diam, collarette distinct, 0.5–1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, broadly ellipsoidal to ovoid, with both ends acute, rarely clavate to cylindrical with one round end one acute end, (6–)7–9.5(–12) × (3–)4–5.5(–6) μm, mean ± SD = 8.3 ± 1.3 × 4.8 ± 0.6 μm, L/W ratio = 1.7, conidia from aerial mycelium shorter, measuring (3.5–)4.5–9(–15) × 3–5(–6.5) μm, mean ± SD = 6.7 ± 2.3 × 4.1 ± 0.8 μm, L/W ratio = 1.6. Appressoria single, medium brown, smooth-walled, subglobose, elliptical or irregular in outline, with entire, undulate or lobate margin, (5–)5.5–9.5(–11.3) × (3–)4.5–6.5(–7.5) μm, mean ± SD = 7.5 ± 1.8 × 5.4 ± 1.1 μm, L/W ratio = 1.4.
Asexual morph on Anthriscus stem (CBS 126518). Conidiomata acervular, conidiophores formed on a cushion of angular cells 3–8.5 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, 7–16.5 × 3.5–4.5 μm, opening 1–2 μm diam, collarette distinct, 0.5–1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, subglobose, broadly ellipsoidal to oval, with both ends ± acute, (3.5–)6.5–10.5(–13.5) × (3.5–)4–5(–5.5) μm, mean ± SD = 8.5 ± 1.8 × 4.5 ± 0.5 μm, L/W ratio = 1.9.
Culture characteristics (CBS 126518): Colonies on SNA flat with entire margin, hyaline to pale honey, on filter paper, Anthriscus stem and medium partly covered with floccose white aerial mycelium, reverse hyaline to pale honey; growth rate 14–17.5 mm in 7 d (23.5–27.5 mm in 10 d). Colonies on OA umbonate with entire margin; surface rosy buff to pale purplish grey, covered with woolly to floccose-felty white to pale grey aerial mycelium, reverse rosy buff, olivaceous grey to iron grey; growth rate 16–17.5 mm in 7 d (27.5–28 mm in 10 d). Conidia in mass pale salmon.
Material examined: Netherlands, Emmeloord, from twisted stem of Carthamus sp., unknown collection date and collector, culture CBS 126518 = PD 84/520; from vascular discoloration of Glebionis carinata, collection date and collector unknown, culture CBS 126519 = PD 85/694. China, Hong Kong, from leaf spot of Glebionis coronaria, (deposited in IMI 1994 by Wan-chi Ko as culture no. 1964), culture IMI 365540.
Notes: Gloeosporium chrysanthemi was described by Hori as causing severe anthracnose disease in Chrysanthemum coronarium (= Glebionis coronaria) in the Fukuoka prefecture in Japan (Takimoto 1924) and transferred to Colletotrichum by Sawada (1943). A pathogen of another Asteraceae plant, Carthamus tinctorius, was described in Japan by Fukui as Marssonia carthami (Fukui 1916, see also Tanaka 1917). The fungus was transferred to Gloeosporium by Hori & Hemmi.
Uematsu et al. (2012) re-examined authentic specimens of C. chrysanthemi collected by Takimoto in 1919 and of G. carthami collected by Hemmi in 1915 and sequenced the ITS1 and TUB2 regions of these specimens as well as of isolates from Carthamus, Chrysanthemum and Calendula species from Japan. The resulting sequences place the two species in the C. acutatum species complex. While all specimens and strains had almost identical ITS sequences, there were two groups in the TUB2 phylogeny, placing most of the Calendula isolates with the authentic specimen of Gm. carthami and the Chrysanthemum and Carthamus isolates as well as two Calendula isolates with the authentic specimen of Gm. chrysanthemi, suggesting the two species to be separate. In spite of this, the authors regard C. chrysanthemi as synonym of the older species G. carthami. Based on TUB2 sequences of the authentic specimens (AB696992, AB696993) and some of the strains from Calendula (AB688785, AB688787), Carthamus (AB688807, AB688811) and Chrysanthemum (AB688791) included in our alignment (not shown), isolates studied here group with the Japanese isolates from Carthamus and Chrysanthemum and the authentic specimen of Gm. chrysanthemi, and we therefore treat them here as C. chrysanthemi. The TUB2 sequences of the Calendula isolates and the authentic material of Gm. carthami appear to belong to a different clade that is not included in our study.
There are few additional reports of Colletotrichum on Carthamus, Chrysanthemum and Calendula. Sette et al. (1999) report C. acutatum on Carthamus tinctorius in Korea; the fungus formed strongly fusiform conidia (see fig. 2 in Sette et al. 1999), and formed setae at least occasionally on host plant and PDA medium. Vichova et al. (2011) found C. simmondsii on Carthamus tinctorius in the Czech Republic. There is another species that was also described on Chrysanthemum and Dahlia in Portugal, C. dahliae; this species however forms larger conidia with round ends, measuring 16–19 × 5.3–7 μm (Costa & Sousa da Câmara 1953).
Colletotrichum chrysanthemi is separated from other species by all diagnostic genes applied in this study except for ITS, best with TUB2, GAPDH and HIS3, and its very short acute-ended conidia differ from those of other species of the C. acutatum species complex. The ITS sequence of strain CBS 126518 matches with 100 % identity with AB042306 and AB042307 from isolates from Carthamus and Chrysanthemum in Japan (Moriwaki J, Tsukiboshi T, Sato T, Uematsu S, unpubl. data), and also with AJ749675 from isolates PD85/694 (= CBS 126519), sequenced by Talhinhas et al. (2005) and AY376508 Ga. acutata strain STE-U 5303 (= CBS 112989, C. laticiphilum) from Hevea (Lubbe et al. 2004). Closest match in a blastn search with the TUB2 sequence of strain CBS 126518 with 100 % identity was AJ748632 from isolate PD85/694 (= CBS 126519, included in this study), sequenced by Talhinhas et al. (2005). Closest matches with the GAPDH sequence with 95 % identity (12 and 13 differences) were HM038336 from isolate MFU09 0628 from Mangifera indica and HM038337 from isolate MFU09 0624 from Ziziphus mauritiana, both from Laos (Phoulivong et al. 2010).
Colletotrichum cosmi Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800498. Fig. 7.
Fig. 7.
Colletotrichum cosmi (from ex-holotype strain CBS 853.73). A–B. Conidiomata. C–H. Conidiophores. I–N. Appressoria. O–P. Conidia. A, C–E, O. from Anthriscus stem. B, F–N, P. from SNA. A–B. DM, C–P. DIC, Scale bars: A = 200 μm, B = 100 μm, C = 10 μm. Scale bar of C applies to C–P.
Etymology: Named after the host plant, Cosmos.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–7.5 μm diam, hyaline, sometimes pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, sometimes slightly inflated, 9–17 × 2.5–3.5 μm, opening 1–1.5 μm diam, collarette 1 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to clavate with both ends slightly acute or one end round, (7–)13–18.5(–19.5) × (3–)3.5–4.5 μm, mean ± SD = 15.8 ± 2.5 × 4.0 ± 0.4 μm, L/W ratio = 4.0. Appressoria very few, mostly single, pale to medium brown, smooth-walled, subglobose, elliptical or clavate, the edge entire, (5–)5.5–8(–11.5) × (4–)4.5–5.5 μm, mean ± SD = 6.8 ± 1.2 × 4.9 ± 0.4 μm, L/W ratio = 1.4.
Asexual morph on Anthriscus stem. Conidiomata either not developed, conidiophores formed directly on hyphae, or acervular, conidiophores formed on pale brown, angular, basal cells, 3–9 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, 9–24 × 3–3.5 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends ± acute, (12–)14–16.5(–18) × (3.5–)4–4.5 μm, mean ± SD = 15.3 ± 1.4 × 4.0 ± 0.3 μm, L/W ratio = 3.8.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, buff to pale honey, on filter paper partly pale olivaceous grey, the medium, filter paper and Anthriscus stem partly covered with floccose-felty whitish to pale olivaceous grey aerial mycelium and orange acervuli, reverse of filter paper partly pale cinnamon, pale olivaceous grey to olivaceous grey; growth rate 23–24 mm in 7 d (33.5–34 mm in 10 d). Colonies on OA flat with entire margin; surface entirely covered with thin floccose-felty white to pale olivaceous grey aerial mycelium and orange acervuli, reverse vinaceous buff, purplish grey to fuscous black; growth rate 23–25 mm in 7 d (33.5–37.5 mm in 10 d). Conidia in mass orange.
Material examined: Netherlands, Wageningen, from seed of Cosmos sp., collection date and collector unknown (deposited in CBS collection in Nov. 1973 by G.H. Boerema), (CBS H-20794 holotype, culture ex-type CBS 853.73 = PD 73/856).
Notes: Kwon et al. (1999) report C. acutatum (s. lat.) to cause sunken brownish spots on stems, as well as symptoms on leaves, flowers and floral axes of Cosmos bipinnatus in Korea. Morphological characters (conidia, appressoria) are similar to those of strain CBS 853.73, except for setae, which our strain did not develop in our standard culture conditions. It is therefore possible that the collection from Korea represents C. cosmi. Colletotrichum acutatum (s. lat.) is also known as an anthracnose pathogen of flowers and flower buds of Cosmos bipinnatus in Japan (Yaguchi et al. 1996). The shape of conidia and appressoria of the Japanese fungus are similar to our strain, but the conidia are smaller, measuring 11–14 × 2.8–3.5 μm. Two other species are reported from Cosmos bipinnatus in India, C. truncatum (as C. capsici) associated with seeds and causing seed and seedling rot (Srivastava et al. 1981) and C. gloeosporioides associated with leaves (Kumari et al. 1981). When strain CBS 853.73 was first accessed into CBS, von Arx identified it as C. gloeosporioides, but with the remark “deviating by longer, slender conidia”. Molecular data do not support this identification; the strain belongs to the C. acutatum complex, but it is possible that reports of C. gloeosporioides refer to this species.
Colletotrichum cosmi is part of clade 2. It can be separated from other species by all gene sequences, but mostly with only 1 bp divergence. There are more sequence divergences in GAPDH and HIS3; however, with these genes individually, the species sits within the very variable C. nymphaeae clade. The closest match in a blastn search with the TUB2 sequence of strain CBS 853.73 (with 99 % identity, 4 bp differences) was GU246633 from isolate R14 from Capsicum annuum from South Korea (Sang et al. 2011), while the closest match with the GAPDH sequence covering ± the full length sequence (with 98 % identity, 4 bp differences) was HQ846724 from isolate OBP6 from an unknown plant, probably from India (P. Chowdappa, C.S. Chethana, S. Madhura, unpubl. data). We do not consider that these data in isolation are sufficient evidence to identify these sequences as originating from C. cosmi. There are 22 sequences in GenBank that match the ITS sequence of strain CBS 853.73 with 99 % identity, all with 2 bp differences.
Colletotrichum costaricense Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800499. Fig. 8.
Fig. 8.
Colletotrichum costaricense (from ex-holotype strain CBS 330.75). A–B. Conidiomata. C–H. Conidiophores. I–N. Appressoria. O–P. Conidia. A, C–D, O. from Anthriscus stem. B, E–N, P. from SNA. A–B. DM, C–P. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–P.
Etymology: Named after the country where it was collected, Costa Rica.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–9.5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, polyphialides observed, 4.5–24 × 2–3.5 μm, opening 0.5–1.5 μm diam, collarette 0.5–1.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with both ends acute, (9–)11.5–18(–28) × (3–)3.5–4(–4.5) μm, mean ± SD = 14.6 ± 3.1 × 3.7 ± 0.3 μm, L/W ratio = 4.0. Appressoria sparse, single or in small groups, pale brown, smooth-walled, subglobose to elliptical, the edge entire to undulate, (4.5–)6–8.5(–10) × (3–)4–6(–6.5) μm, mean ± SD = 7.1 ± 1.2 × 4.9 ± 0.9 μm, L/W ratio = 1.4, appressoria of strain CBS 211.78 are medium brown.
Asexual morph on Anthriscus stem. Conidiomata not developed, conidiophores and setae formed directly on hyphae. Setae medium to dark brown, smooth-walled to finely verruculose, 0–2-septate, 50–60 μm long, base cylindrical, 3.5–4.5 μm diam, the tip ± acute. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical to ampulliform, 8–22 × 3–5 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with both ends acute, (12.5–)13.5–16(–18) × 3.5–4 μm, mean ± SD = 14.8 ± 1.4 × 3.8 ± 0.3 μm, L/W ratio = 3.9.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to pale cinnamon, on Anthriscus stem partly olivaceous grey to iron-grey, on filter paper pale olivaceous grey to olivaceous grey, with short or woolly white aerial mycelium and few salmon acervuli on filter paper and on Anthriscus stem, reverse of filter paper same colours; growth rate 19–22.5 mm in 7 d (31–34 mm in 10 d). Colonies on OA flat with entire margin; surface olivaceous with pale olivaceous grey to olivaceous grey sectors, the sectors covered with short white aerial mycelium and salmon acervuli or culture completely covered with short felty whitish aerial mycelium, reverse honey, olivaceous grey to iron-grey, growth rate 22–23 mm in 7 d (28.5–34.5 mm in 10 d). Conidia in mass salmon to saffron.
Material examined: Costa Rica, Meseta Central, from berry of Coffea arabica cv. Typica, collection date and collector unknown (deposited in CBS collection Jun. 1975 by D. Mulder, Wageningen), (CBS H-20811 holotype, culture ex-type CBS 330.75); Turrialba, from twig of Coffea sp., collection date and collector unknown (deposited in CBS collection Apr. 1978 by C. Bianchini), culture CBS 211.78 = IMI 309622.
Notes: Von Arx (in litt.) identified the strain CBS 330.75 as C. acutatum but with the remark “deviating by lack of pigment and less fusiform conidia”. While the main causal agent of coffee berry disease (CBD) is C. kahawae (Waller et al. 1993) that belongs to the C. gloeosporioides species complex (Weir et al. 2012, this issue), strains from the C. acutatum aggregate are not frequently encountered associated with coffee. Hindorf (1973) studied Colletotrichum populations from Coffea arabica in Kenya and illustrated conidia or ascospores of some strains diverging from each other in morphology and culture appearance, including a strain identified as C. acutatum and another as C. gloeosporioides with conidia some of which are ellipsoidal and acute-ended. One of the two strains from western Kenya that are assigned to C. acutatum s. str. is derived from a suspected disease symptom on a coffee berry from Kenya that did not cause CBD (Gielink & Vermeulen, 1983). One of the endophytic strains from Coffea robusta in Brazil studied by Sette et al. (2006) showing antimicrobial activity against Staphylococcus aureus belongs to the C. acutatum species complex; since only a short ITS sequence of this strain was generated (DQ123614), the species cannot be identified. Colletotrichum walleri (clade 2) is known from a single strain from coffee, from Vietnam. Colletotrichum costaricense is quite distinct from either of these taxa based on molecular sequence data.
Two Colletotrichum species have previously been described from leaves of Coffea sp. in Costa Rica, C. brachysporum and C. coffeophilum. Conidia of the first are smaller than those of C. costaricense and have a different shape; they are subglobose-ovoid and measure 7–8 × 4–6 μm (Saccardo et al. 1931), while those of C. costaricense measure on average 14.6 × 3.7 μm or 14.8 × 3.8 μm depending on the medium. Conidia of C. coffeophilum are wider than those of C. costaricense, being ellipsoidal and straight or slightly curved (navicular), and measuring 13–15 × 6–8 μm (Saccardo et al. 1931).
Colletotrichum costaricense may be differentiated from the other species accepted here by TUB2, GAPDH and ACT sequences, and most effectively with TUB2. The ACT sequences of the two strains differ by 2 bp, but have only 1 bp in common to separate them from C. lupini and some of the unnamed single strains. The closest match in a blastn search with the TUB2 sequence of strain CBS 330.75 with 99 % identity (3 bp differences) was FN611028 from a Citrus sinensis isolate (Ramos et al. 2006), while the closest matches with the GAPDH sequence with 99 % identity (2 differences) were EU647322 and EU647324 from leatherleaf fern isolates (MacKenzie et al. 2009). All isolates were from Florida, USA. The closest matches with the ITS sequence with 100 % identity were FN566877 from isolate DPI from Citrus aurantifolia in Florida, USA (Ramos et al. 2006) and isolate c2 from Citrus sp. in Brazil (Giaretta et al. 2010).
Colletotrichum cuscutae Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800500. Fig. 9.
Fig. 9.
Colletotrichum cuscutae (from ex-holotype strain IMI 304802). A–B. Conidiomata. C–J. Conidiophores. K–P. Appressoria. Q–R. Conidia. A, C–E, Q. from Anthriscus stem. B, F–P, R. from SNA. A–B. DM, C–R. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–R.
Etymology: Named after the host plant, Cuscuta.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–5.5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched, to 35 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, often integrated, polyphialides occasionally observed, discrete phialides measuring 4–14.5 × 2.5–4.5 μm, opening 1.5–2 μm diam, collarette 0.5–1.5 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with both ends acute, (15.5–)17.5–21(–27) × (3–)3.5–4.5 μm, mean ± SD = 19.2 ± 1.7 × 4.0 ± 0.3 μm, L/W ratio = 4.8. Appressoria single or in loose clusters, pale brown, smooth-walled, elliptical to clavate, entire edge (3.5–)5.5–11.5(–15.5) × (2–)3.5–5.5(–6.5) μm, mean ± SD = 8.5 ± 3.2 × 4.6 ± 0.9 μm, L/W ratio = 1.8.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on pale brown angular basal cells, 3–8 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to fusiform with both ends acute, 8–21 × 2–3.5 μm, opening 1–2 μm diam, collarette 0.5–1 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with both ends acute, (15–)17–20(–21) × (3.5–)4–4.5 μm, mean ± SD = 18.6 ± 1.5 × 4.2 ± 0.2 μm, L/W ratio = 4.5.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to buff, on filter paper and Anthriscus stem partly covered with woolly to felty white to pale grey aerial mycelium and orange acervuli, reverse hyaline to buff, under filter paper pale olivaceous grey; growth 20 mm in 7 d (30 mm in 10 d). Colonies on OA flat to raised with entire margin; surface partly covered with woolly white to pale olivaceous grey aerial mycelium and olivaceous grey to orange acervuli appearing in rings, reverse buff, pale olivaceous grey to olivaceous grey with orange sectors; growth 19–21 mm in 7 d (27.5–31 mm in 10 d). Conidia in mass orange.
Material examined: Dominica, Castle Comfort, from Cuscuta sp., 1986, C. Prior (IMI 304802 holotype, CBS H-20784 isotype, culture ex-type IMI 304802).
Notes: Colletotrichum cuscutae is known from a single strain, reported from Dominica. The multigene analysis indicates that it occupies a single subclade within clade 1, quite distinct from the principal subclade of C. lupini. Its conidia are substantially longer than is typical for C. lupini (mean length 18.6 μm as opposed to 12 μm for C. lupini), though the length range for the latter species is considerable. The appressoria of C. cuscutae are narrower than those of C. lupini and also greater in length/width ratio.
Colletotrichum species have been reported previously as parasitising Cuscuta species, which are themselves non-photosynthetic parasites of other plants. Colletotrichum destructivum was found to affect Cuscuta campestris parasitising alfalfa crops in NW USA (Leach 1958). A strain identified as C. gloeosporioides f. sp. cuscutae was apparently used widely as a biological control agent “Lu Bao no. 1” of Cuscuta in China after its adoption in the 1960s (Zhang 1985, Gao & Gan 1992), but its current commercial status is unknown and it may no longer be in production (Watson et al. 2000). According to Watson et al. (2000) and Dinoor et al. (2009) the Lu Bao strain belongs to C. acutatum rather than C. gloeosporioides. However, no detailed morphological data are available and the identification as C. acutatum was made by means of primers that at that time were considered to be species-specific for the two species that are both now recognised as species complexes.
Guerber et al. (2003) studied isolates from Cuscuta in the USA and China that belong to two different species, neither of which is conspecific with C. cuscutae. Based on GAPDH sequences (Guerber et al. 2003), isolates FRC2 and FRC7 from dodder in the USA are C. fioriniae, while strain 783 from China (apparently identical with strain Lu Bao no. 1) belongs to a subclade of clade 2 that is not included in this study. Strain 783 was found to have a haplotype of MspI mtDNA identical to those of two Australian strains causing terminal crook disease of pine, and distinct from those of other Cuscuta strains.
In an attempt to compare endophytes of a Cuscuta parasite and its hosts in India, Suryanarayanan et al. (2000) isolated 44 fungal endophytes from Cuscuta reflexa, including C. gloeosporioides, C. truncatum and a “Colletotrichum sp.” that was not further characterised. None of the strains is included in this study, and there are no corresponding sequences available on GenBank.
Colletotrichum cuscutae is separated from other species by all genes studied except for ITS, most effectively by TUB2 and ACT. In blastn searches with the ITS, TUB2 and GAPDH sequences of the ex-type strain IMI 304802, no sequence matched with 100 % homology. Closest matches with the TUB2 sequence (with 98 % identity, 8 bp differences) were FN611029 and FN611028 from Citrus aurantifolia and Citrus sinensis from Florida, USA (Ramos et al. 2006) and the closest matches with the GAPDH sequence (with 98 % identity and 4 bp differences) were EU168905, EU647318 and EU647319 from sweet orange (Peres et al. 2008, MacKenzie et al. 2009). In a blastn search with the ITS sequence a large number of strains were 99 % identical with that of strain IMI 304802 including several ITS sequences from Key lime isolates, e.g. EU647307 and EU647308 (MacKenzie et al. 2009).
Colletotrichum fioriniae (Marcelino & Gouli) R.G. Shivas & Y.P. Tan, Fungal Diversity 39: 117. 2009. Fig. 10.
Fig. 10.
Colletotrichum fioriniae (from ex-holotype strain CBS 128517). A–B. Conidiomata. C–L. Conidiophores. M–R. Appressoria. S–T. Conidia. A, C–G, S. from Anthriscus stem. B, H–R, T. from SNA. A–B. DM, C–T. DIC, Scale bars: A = 200 μm, B = 100 μm, C = 10 μm. Scale bar of C applies to C–T.
Basionym: Colletotrichum acutatum var. fioriniae Marcelino & Gouli, Mycologia 100: 362. 2008.
≡ Glomerella fioriniae (Marcelino & Gouli) R.G. Shivas & Y.P. Tan, Fungal Diversity 39: 117. 2009.
≡ Glomerella acutata var. fioriniae Marcelino & Gouli, Mycologia 100: 362. 2008.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1.5–7.5 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, up to 35 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical to ampulliform, sometimes lacking a basal septum and continuous with the conidiophore, sometimes covert with a mucous coating, discrete phialides measure 4–12 × 2.5–3.5 μm, opening 1–2 μm diam, collarette 1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, fusiform to cylindrical with both ends acute, (10–)13.5–16.5(–19.5) × 4–5(–5.5) μm, mean ± SD = 15.0 ± 1.6 × 4.5 ± 0.3 μm, L/W ratio = 3.3 μm, conidia of strain CBS 129947 are smaller, measuring (10.5–)12–15(–17) × 3.5–5(–6) μm, mean ± SD = 13.5 ± 1.7 × 4.1 ± 0.8 μm, L/W ratio = 3.3 μm. Appressoria solitary or in loose groups, pale to medium brown, smooth-walled, ellipsoidal, clavate to irregular outline, entire edge or undulate, (4.5–)7–11.5(–15.5) × (4–)4.5–7(–10.5) μm, mean ± SD = 9.2 ± 2.2 × 5.6 ± 1.2 μm, L/W ratio = 1.6.
Asexual morph on Anthriscus stem. Conidiomata forming a cushion of pale brown, thick-walled, angular cells, 3–6.5 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, up to 35 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, 10–22 × 3–4 μm, opening 1.5–2 μm diam, collarette 0.5–1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, fusiform to cylindrical with both ends acute, (12.5–)14–18.5(–24.5) × 4–5 μm, mean ± SD = 16.1 ± 2.2 × 4.4 ± 0.4 μm, L/W ratio = 3.6, conidia of CBS 200.35 differ in sometimes having one round and one slightly acute end, conidia of strain CBS 129947 are smaller, measuring (13–)14–16(–17) × (3.5–)4–4.5(–5) μm, mean ± SD = 15.0 ± 1.0 × 4.3 ± 0.4 μm, L/W ratio = 3.5 μm.
Culture characteristics: Colonies on SNA filter paper, Anthriscus stem covered with orange acervuli, on filter paper covered with white to pale olivaceous grey aerial mycelium and partly with salmon to orange acervuli, reverse filter paper with pale olivaceous grey to olivaceous grey patches and spots, growth rate 22.5–23 mm in 7 d (32.5–34 mm in 10 d). Colonies on OA flat with entire margin; surface saffron with olivaceous spots (mottled), covered with salmon acervuli, aerial mycelium lacking, reverse salmon, pale vinaceous, olivaceous to purplish grey, growth rate 22–22.5 mm in 7 d (34–35 mm in 10 d). Conidia in mass salmon to orange
Material examined: USA, New York, Ward Pound Ridge Reserve, on mummified adult Fiorinia externa (elongate hemlock scale, insect), 2005, J.A.P. Marcelino and S. Gouli, culture ex-holotype of C. fioriniae CBS 128517 = EHS58 = ARSE 10222; Michigan, from Vaccinium sp. (blueberry), collection date and collector unknown (isolated by A. Schilder), culture CBS 129916 = CPC 16823; Unknown country (probably USA), from Rubus sp., collection date and collector unknown (deposited in CBS collection Apr. 1935 by K.J. Kadow as Glomerella rubicola), culture CBS 200.35. Australia, Queensland, Mount Tamborine, from fruit rot of Persea americana, 4 Sep. 2002, K.G. Pegg, culture CBS 127599 = BRIP 29284a; Queensland, Brisbane, from Persea americana, collection date and collector unknown (isolated J.H. Simmonds, No. 13120, 25 Jun 1958) culture CBS 293.67 = DPI 13120; Queensland, Yarwun, endophytic from stem of Mangifera indica, 16 Feb. 1994, G.I. Johnson, culture CBS 127601 = BRIP 28761a. Portugal, Lisbon, from Vitis vinifera, 2000, collector unknown, culture CBS 129947.
Notes: Colletotrichum fioriniae is the only representative of clade 3, which is supported by all six genes individually (including ITS). The clade has been recognised as distinct within the C. acutatum species complex for some years now (Sreenivasaprasad & Talhinhas 2005), and was accepted as a separate species by Shivas & Tan (2009).
In the current study, a large number of strains (over 50) has been found to belong to this species. They were isolated from a wide variety of host plants, primarily in the temperate zones. There is some evidence of heterogeneity within the species, as two subclades are apparent in the phylogenetic analysis, but neither bootstrap support nor Bayesian probability values are sufficiently high to justify their recognition at species level. Also, strains from the major hosts and countries appear throughout the clade.
The name C. fioriniae is based on C. acutatum var. fioriniae (Marcelino et al. 2008), named for a series of strains isolated from an epizootic infection of the exotic scale insect Fiorinia externa in the New England region. Implication of Colletotrichum species as entomopathogens might be considered surprising. However, the insects in question are sap-suckers and C. fioriniae was found to occur widely as an endophyte (Marcelino et al. 2009), both in the host plant of the scale insect (Tsuga canadensis) and in a phylogenetically diverse set of associated plants. This appears to represent a further case of mutualism between Colletotrichum and its host plants, with endophytic strains acting as natural protectants against insect herbivory. A similar case was reported for a strain labelled C. gloeosporioides f. sp. ortheziidae (probably belonging to C. nymphaeae, see notes there) parasitising the economically important citrus scale insect Orthezia praelonga in Brazil (Cesnik et al. 1996). Endophytic Colletotrichum strains have been demonstrated to protect Theobroma cacao plants against Phytophthora pathogens (Arnold et al. 2003, Mejía et al. 2008, Rojas et al. 2010).
Strains referred to as C. acutatum and identified here as C. fioriniae have been implicated in fruit rot of cranberry and blueberry throughout the northern USA and in British Columbia (MacKenzie et al. 2009, Polashock et al. 2009).
In fruit-rot assays by Freeman & Shabi (1996), isolates from apple and peach (based on ITS sequence, probably identifiable as C. fioriniae) produced lesions on many different fruits, “suggesting that isolates of this group have the ability to cross-infect fruit from multiple hosts”. All of the fruits tested in the study (almond, apple, avocado, mango, nectarine) are host plants of C. fioriniae (Guerber et al. 2003, Table 1). In pathogenicity tests MacKenzie et al. (2009) showed that isolates from blueberry (= C. fioriniae) did not cause lesions on strawberry leaves but caused anthracnose on strawberry fruits, though lesions were smaller than those caused by isolates from strawberry (= C. nymphaeae). MacKenzie et al. (2009) concluded that therefore the probability of an epidemic on strawberry in Florida caused by blueberry isolates is rather low, but added that the climate could also play a role; in Florida, ripe rot of blueberry fruits is predominantly caused by C. gloeosporioides (s. lat.), while further north in temperate regions, it is most frequently caused by C. acutatum (s. lat.) (Smith et al. 1996). According to our study, both species occur on strawberry, but based on the number of strains we have seen, C. fioriniae seems to be of rather minor importance compared to C. nymphaeae.
Marcelino et al. (2008) found that strains of C. fioriniae could be crossed to form a sexual morph and that some appeared to be self-fertile, though it is not clear whether the self-fertile strains were derived from single spores. We did not see sexual production in the strains examined during the present study.
An earlier name may exist for C. fioriniae, in Gnomoniopsis rubicola (Stoneman 1898), one of a group of five species (including Ga. cingulata) on which the genus Glomerella was based (Schrenk & Spaulding 1903a, b). That species was described from diseased leaves of Rubus strigosus in West Virginia. No cultures are available and the description of the asexual morph is not detailed, but Marcelino et al. (2009) showed that C. fioriniae is widespread in the region and both taxa produce a sexual morph. Kadow (1935) ascribed a disease of raspberry from the same region to Ga. rubicola, and a culture derived from his work (CBS 200.35) has been examined in the current study and confirmed as belonging to C. fioriniae. Without sequence-based evidence from type material, however, we are reluctant to adopt this earlier name. As far as we can tell, a combination into Colletotrichum has never formally been made. The name C. rubicola was cited on herbarium labels by Ellis & Everhart but only as an asexual name for Glomerella rubicola; apparently it was never accompanied by a description.
Colletotrichum fioriniae is separated from other species by all gene sequences studied. The closest matches in a blastn search with the TUB2 sequence of strain CBS 128517 with 100 % identity were AY376557 (from apple in the USA, strain STE-U 5287; Lubbe et al. 2004) and AJ748628 from Liriodendron tulipifera in the UK (Talhinhas et al. 2005). With 99 % identity (and 1–3 bp differences) a series of matches could be made, including AJ748610 and AJ748623 from olive in Portugal, AJ748626 from Nandina domestica and AJ748634 from Magnolia in the UK (Talhinhas et al. 2005). Further sequences with the same level of homology include AJ311668 from Vitis vinifera (Talhinhas et al. 2002), EF593320–EF593326 from Fiorinia externa, EF593329 from blueberry and EF593330 tomato (all from the USA; Marcelino et al. 2008), GU183274 from Acacia acuminata, GU183273, GU183270, and GU183268 from Persea americana, GU183267 Actinidia chinensis and GU183269 from Mangifera indica (all from Australia; Shivas & Tan 2009), AB618092 from Apium graveolens var. dulce (celery) in Japan (Fujinaga et al. 2011) and AB273716 from grape in Japan (Nakaune & Nakano 2007). All of these are likely to represent strains of C. fioriniae, further emphasising its widespread distribution and presumably also its wide host range as a pathogen.
Colletotrichum godetiae Neerg., Friesia 4: 72. 1950. Fig. 11.
Fig. 11.
Colletotrichum godetiae (F–G, L–M, T–U from ex-holotype strain CBS 133.44. A–E, H–K, N–S from strain CBS 125972). A–B. Conidiomata. C. Tip of a seta. D. Basis of a seta. E–M. Conidiophores. N–S. Appressoria. T–U. Conidia. A, C–H, T. from Anthriscus stem. B, I–S, U. from SNA. A–B. DM, C–U. DIC, Scale bars: A = 100 μm, E = 10 μm. Scale bar of A applies to A–B. Scale bar of E applies to C–U.
≡ Colletotrichum godetiae Neerg., Aarsberetn. J. E. Ohlens Enkes plantepatol. Lab. 1 April 1942–31 Marts 1943: 8. 1943, nom. inval., Art. 36.1.
= Colletotrichum clavatum Agosteo, Faedda & Cacciola, Fungal Diversity 50: 292. 2011.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–7 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, simple, to 14 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, often with only short necks, 4–14 × (1.5–)3–6 μm, opening 1.5–2 μm diam, collarette 0.5 μm long, periclinal thickening observed. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends acute or one end round and one end slightly acute, (7–)10.5–14.5(–15.5) × (3.5–)4–5(–5.5) μm, mean ± SD = 12.4 ± 2.0 × 4.3 ± 0.5 μm, L/W ratio = 2.9, strains CBS 127561, CBS 129917, CBS 193.32 and CBS 129951 differ in forming cylindrical to clavate conidia with one round and one acute end, conidia of strain CBS 862.70 are larger, measuring (8–)14–19(–24) × (4–)4.5–5(–5.5) μm, mean ± SD = 16.4 ± 2.4 × 4.9 ± 0.4 μm, L/W ratio = 3.4. Appressoria solitary, medium brown, smooth-walled, clavate to elliptical, the edge entire or undulate (8–)9–12.5(–14.5) × (3–)4–5.5(–6) μm, mean ± SD = 10.7 ± 1.9 × 4.7 ± 0.7 μm, L/W ratio = 2.3.
Asexual morph on Anthriscus stem. Conidiomata absent, conidiophores formed directly on hyphae in aerial mycelium (in strain CBS 125972 present as a cushion of angular to roundish cells 4–10 μm diam). Setae not observed (in strain CBS 125972 very few setae present, medium brown, smooth-walled, 2–3-septate, 70–110 μm long, base cylindrical, 4–5 μm diam, tip ± acute). Conidiophores hyaline, septate, branched, smooth-walled. Conidiogenous cells hyaline, smooth-walled, cylindrical, 9–20 × 3 μm, opening 1.5 μm diam, collarette < 0.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, cylindrical to fusiform with both ends acute, (9.5–)10.5–15(–20.5) × 4–5 μm, mean ± SD = 12.8 ± 2.3 × 4.5 ± 0.4 μm, L/W ratio = 2.8, strain CBS 127561 differs in forming clavate conidia with one round and one acute end and strains CBS 129917, CBS 193.32 and CBS 129911 in forming cylindrical to clavate conidia with one round and one acute end, conidia of strain CBS 862.70 are larger, measuring (12.5–)15.5–18(–19.5) × 4.5–5(5.5) μm, mean ± SD = 16.8 ± 1.4 × 4.9 ± 0.2 μm, L/W ratio = 3.4, conidia of strain CBS 129911 are smaller, measuring (7–)9–13(–15.5) × (2.5–)3–4 μm, mean ± SD = 11.0 ± 2.0 × 3.5 ± 0.3 μm, L/W ratio = 3.1.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, with little low white aerial mycelium, on Anthriscus stem growth rate 21–21.5 mm in 7 d (30.5–31.5 mm in 10 d). Colonies on OA flat with entire margin; surface salmon to hazel, no aerial mycelium, reverse salmon to vinaceous buff; growth rate 21–24 mm in 7 d (30–33.5 mm in 10 d). Conidia in mass not observed in strain CBS 133.44, but in strain CBS 125972 orange.
Material examined: Denmark, from seed of Clarkia (syn. Godetia) hybrida cv. Kelvedon Glory 463 C in seed disinfection experiment, 17 Jun. 1943, P. Neergaard, culture ex-holotype of C. godetiae CBS 133.44. Italy, Calabria, Rizziconi, from rotten fruit of Olea europaea, Oct. 1992, G.E. Agosteo and G. Magnano di San Lio, culture ex-holotype of C. clavatum CBS 130251 = OL10 = IMI 398854. Greece, from Olea europaea, collection date and collector unknown (deposited in CBS collection Jan. 1932 by L. Petri), culture CBS 193.32. Netherlands, Tilburg, from Fragaria × ananassa, collection date and collector unknown, culture CBS 125972 = PD 85/456; near Meerssen, from fruit of Sambucus nigra, collection date and collector unknown (deposited in CBS collection Oct. 1970), culture CBS 862.70. South Africa, from Podocarpus sp., collection date unknown, A. Wood, culture CBS 129911. Colombia, Cundinamarca, from fruit anthracnose of Solanum betaceum, 13 Aug. 2010, J. Molina, culture CBS 129809 = T.A.1; Cundinamarca, from fruit anthracnose of Solanum betaceum, 13 Aug. 2010, J. Molina, culture CBS 129816 = T.A.8. Chile, Puerto Saavedra, from tip necrosis on twig of Ugni molinae, 1 Oct. 2008, A. Schilder, culture CBS 127561. Mexico, Montecillo, from Schinus molle, unknown collection date, M. de Jesus Yarez-Morales, culture CBS 129917. USA, Arkansas, Fayetteville, from Aeschynomene virginica (but see notes), collection date and collector unknown (deposited in CBS collection Aug. 1972 by G.E. Templeton as C. gloeosporioides f. sp. aeschynomenes), G.E. Templeton, culture CBS 796.72.
Notes: Colletotrichum godetiae was described from seed of Clarkia (syn. Godetia) hybrida cv. Kelvedon Glory by Neergard (1943), and validated with a Latin description seven years later (Neergard 1950). Colletotrichum godetiae corresponds to C. acutatum group A4 as recognised by Sreenivasaprasad & Talhinhas (2005) and to part of clade F as defined by Guerber et al. (2003). According to Sreenivasaprasad & Talhinhas (2005), group A4 corresponds to group B from New Zealand (Lardner et al. 1999). However, the only C. acutatum group B strain from New Zealand that we have studied belongs to C. acerbum (A6-2). Faedda et al. (2011) described strains from group A4 that cause olive anthracnose in Italy as C. clavatum, not knowing that an older name for this species exists, of which the ex-holotype culture is available in the CBS culture collection. The ex-holo- and ex-paratype strains are included in this study (Fig. 1). Von Arx (1957) regarded C. godetiae as a synonym of Ga. cingulata.
Colletotrichum godetiae also occurs on hosts such as Fragaria, Malus, and Prunus, mainly in Europe and the Near East, causing fruit, leaf or stem (cane, twig) diseases. Most of the isolates of C. acutatum s. lat. from Rhododendron in Sweden and Latvia (Vinnere et al. 2002) belong to this species, based on ITS data. One of their strains (S1) is included in Guerber et al. (2003); its GAPDH sequence groups with C. godetiae, thus confirming this placement. Additionally, several strains from Latin America have been studied. These occupy a subclade that has comparitively high bootstrap support, but as the subclades cannot be separated using any single gene of the set we have used, we amalgamate them into the one species.
Faedda et al. (2011) named C. clavatum to highlight the shape of the conidia in the constituent strains. However, conidia of the ex-type strain of C. godetiae, CBS 133.44, are rarely clavate and mostly fusiform or short cylindrical. Additionally, conidia of CBS 125972 from strawberry on SNA are uniformly fusiform, while those of CBS 193.32 from olive are mainly clavate, and those of CBS 129911 from Podocarpus are fusiform on SNA and mainly clavate on Anthriscus stem. According to Vinnere et al. (2002) isolates from Rhododendron in Sweden and Latvia also form mainly clavate conidia. The conidial shape is therefore an unreliable character for species recognition and seems to depend on the host/origin of the isolate or the growth medium.
One of the isolates we studied, CBS 796.72, was deposited in the CBS collection in August 1972 by G.E. Templeton as C. gloeosporioides f. sp. aeschynomenes and would appear to be an authentic strain of this forma specialis (Daniel et al. 1973). Colletotrichum gloeosporioides f. sp. aeschynomenes caused an epidemic anthracnose disease of northern jointvetch (Aeschynomene virginica) in 1969 in Arkansas, USA and was in the following years successfully applied as a biological control agent against this weed. According to our multigene phylogeny, this isolate belongs to the C. godetiae clade. According to Daniel et al. (1973) C. gloeosporioides f. sp. aeschynomenes is specific for Aeschynomene species, was considered to be more virulent to A. virginica than to A. indica and did not affect rice, soybeans, cotton or 12 other common crops tested. The fungus developed as the weed biocontrol agent Collego (TeBeest 1988, Ditmore et al. 2008) against A. virginica was also named as C. gloeosporioides f. sp. aeschynomenes, and is genetically distinct from C. godetiae. It belongs to the C. gloeosporioides species complex and is newly described in this volume as C. aeschynomenes (Weir at al. 2012, this issue). This is probably the reason for differences noted in the host range by TeBeest (1988). There is also some confusion about the host plant. Aeschynomene virginica as a weed of soybean and rice fields is actually misidentified A. indica, while the true A. virginica is rare and threatened and became a federally listed threatened species in the United States in 1992 (www.wikipedia.org).
Colletotrichum godetiae is separated from other species in the C. acutatum species complex by all genes except CHS-1, which has the same sequence as in C. johnstonii; TUB2, ACT and HIS3 separate the species best. With all genes, the interspecific variation is high. Blastn searches with the TUB2 seqence of CBS 133.44 resulted in 100 % identity with several GenBank accessions from olive isolates studied by Talhinhas et al. (2005) and one (AJ409294) from a Fragaria isolate (Talhinhas et al. 2002), followed with 99 % identity by AJ409302 from a Ceanothus isolate in France (Talhinhas et al. 2002). These are all probably referable to C. godetiae.
Colletotrichum guajavae Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800501. Fig. 12.
Fig. 12.
Colletotrichum guajavae (from ex-holotype strain IMI 350839). A–B. Conidiomata. C. Seta. D–H. Conidiophores. I–N. Appressoria. O–P. Conidia. A, C–E, O. from Anthriscus stem. B, F–N, P. from SNA. A–B. DM, C–P. DIC, Scale bars: A = 100 μm, D = 10 μm. Scale bar of A applies to A–B. Scale bar of D applies to C–P.
Etymology: Named after the host plant, Psidium guajava.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–6 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, some polyphialides observed, 7–19 × 3–4 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends slightly acute, (6–)10.5–16.5(–23.5) × (2.5–)3–4(–5) μm, mean ± SD = 13.4 ± 3.0 × 3.5 ± 0.5 μm, L/W ratio = 3.8. Appressoria formed singly, medium brown, smooth-walled, subglobose or elliptical to clavate, the outline entire, (4.5–)5–8(–10.5) × (3.5–)4.5–6(–6.5) μm, mean ± SD = 6.6 ± 1.4 × 5.2 ± 0.7 μm, L/W ratio = 1.3.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on pale brown, angular, basal cells 2.5–8 μm diam. Setae medium brown, smooth-walled, 0–2-septate, 40–75 μm long, base cylindrical, sometimes inflated, 3–6 μm diam at the widest part, tip ± acute. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, sometimes extending to form a new conidiogenous locus, 7–18 × 2–3.5 μm, opening 1–1.5 μm diam, collarette 0.5–1.5 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends ± acute, (11–)13–16(–17) × (3–)3.5–4 μm, mean ± SD = 14.6 ± 1.7 × 3.8 ± 0.3 μm, L/W ratio = 3.9.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, buff to pale honey, on filter paper partly pale olivaceous grey, on medium, filter paper and Anthriscus stem partly covered with whitish to pale olivaceous grey floccose-felty aerial mycelium, reverse of filter paper smoke grey to grey olivaceous; growth rate 22–24 mm in 7 d (31.5–34 mm in 10 d). Colonies on OA flat with entire margin; surface white, pale olivaceous grey to rosy buff, covered with thin floccose-felty whitish to pale olivaceous grey aerial mycelium, reverse rosy buff, grey olivaceous to olivaceous black; growth rate 24–26.5 mm in 7 d (35.5–37 mm in 10 d). Conidia in mass salmon.
Material examined: India, Assam, Silchar, from fruit of Psidium guajava, collection date and collector unknown (deposited in IMI 1991 by M. Das as isolate India No. 1), (IMI 350839 holotype, CBS H-20793 isotype, culture ex-type IMI 350839).
Notes: Anthracnose and fruit canker of guava are serious diseases in the Indian subcontinent, and according to Misra (2004) are caused in part by C. psidii. However, the identity of the guava pathogen in the sense of Misra is unclear as the conidia are described as sickle-shaped. Curzi (1927) described the conidia of C. psidii as cylindrical with both ends rounded, straight, sometimes slightly curved and measuring 12–15 × 3.5–4.5 μm. Based on study of an authentic strain from Psidium sp. from Italy, C. psidii belongs to the C. gloeosporioides species complex (Weir et al. 2012, this issue). A separate taxon, Glomerella psidii (apparently based on Gloeosporium psidii), causing the “mummy disease” of guava, has uncertain relationships. The sexual morph was formed on apple agar and resembled Glomerella. The Gloeosporium stage it links to may well fall within the C. acutatum complex: Gm. psidii, described from Psidium pomiferi (= Psidium guajava) in Mexico, forms ellipsoidal-ovoid conidia measuring 10–16 × 4–6 μm (Saccardo 1906); its conidia are thus broader than those of C. guajavae. Gloeosporium fructus-psidii was found on fruits of Psidium in Sao Paulo, Brazil, and was described as forming oblong, subfusoid to clavate, hyaline conidia, measuring 14–20 × 5–6 μm (Saccardo et al. 1931). The shape of the conidia of that species points also at the C. acutatum complex, however, there is no species in this complex with conidia on average generally wider than 5 μm. Conidia of C. guajavae are substantially smaller, measuring on average 13.4 × 3.5 μm on SNA and 14.6 × 3.8 μm on Anthriscus stem.
Peres et al. (2002) isolated C. acutatum (s. lat.) from a guava fruit in Brazil. It caused lesions on guava fruits that were slightly larger than those caused by a C. acutatum (s. lat.) isolate from strawberry. Based on the ITS sequences they generated, the isolates from guava and strawberry from Brazil belong to the same major clade as C. guajavae; the ITS sequence is in fact identical to that of C. guajavae, but also the same as a number of other species in this complex, making an identification to species level impossible without additional information. Based on a phylogeny from combined GAPDH and GS sequences in the study by Guerber et al. (2003), both strains belong to clade D (= clade 2 in this study), but not to the same subclade. The GAPDH sequence generated in Guerber et al. (2003) differs in 5 bp from that of C. guajavae ex-holotype strain IMI 350839. A strain from guava from New Zealand, included in the same study, belongs to clade J3 sensu Guerber et al. (2003) (= C. acutatum s. str.). Apart from C. acutatum s. lat. and C. psidii, Farr & Rossman (2012) list reports from Psidium for C. coccodes in Myanmar, C. gloeosporioides in Brazil, China, Cuba, India, Mexico, Puerto Rico, South Africa, USA, Virgin Islands and Mexico, and Colletotrichum sp. in Brazil, Jamaica and Mexico; it is possible that some of these reports should be referred to C. guajavae.
Colletotrichum guajavae can be distinguished from other species of clade 2 of the C. acutatum complex using TUB2, GAPDH and ACT sequences, most effectively with GAPDH. With data from GAPDH alone the species sits within the very variable C. nymphaeae cluster. With TUB2 and ACT there is only 1 bp difference between C. guajavae and C. scovillei, while CHS-1 and HIS3 sequences are the same as those of C. scovillei. Colletotrichum guajavae is not reliably distinguishable from these species using morphological characteristics. Blastn searches with the GAPDH sequence of strain CBS 853.73 shows 100 % identity with HM038337 from Colletotrichum sp. isolate MFU 09 0624 from Ziziphus mauritiana (jujube) from Laos (Phoulivong et al. 2010), and it is therefore probable that this strain also belongs to C. guajavae. The closest match with the TUB2 sequence of strain CBS 853.73, with 100 % identity, was GU246633 from isolate R14 from Capsicum annuum from South Korea (Sang et al. 2011). We identify that isolate as C. scovillei; the available sequence does not include the region containing the single nucleotide polymorphism that distinguishes TUB2 sequences of C. guajavae and C. scovillei.
Colletotrichum indonesiense Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800502. Fig. 13.
Fig. 13.
Colletotrichum indonesiense (from ex-holotype strain CBS 127551). A–B. Conidiomata. C–G. Conidiophores. H–M. Appressoria. N–O. Conidia. A, C–E, N. from Anthriscus stem. B, F–M, O. from SNA. A–B. DM, C–O. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–O.
Etymology: Named after the country of origin, Indonesia.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–7 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched, to 40 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ± inflated, 8–21 × 2–3.5 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end acute, (8–)10–14.5(–18) × (2.5–)3.5–4(–4.5) μm, mean ± SD = 12.3 ± 2.4 × 3.8 ± 0.3 μm, L/W ratio = 3.2. Appressoria single, pale to medium brown, smooth-walled, elliptical, to subglobose in outline, the edge entire, sometimes undulate, 5.5–9(–14.5) × (5–)5.5–7.5(–9) μm, mean ± SD = 7.5 ± 1.8 × 6.3 ± 1.0 μm, L/W ratio = 1.2.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on pale brown, angular, basal cells 2.5–6 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 60 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, sometimes ± inflated, 9–25 × 2–4 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends acute, (10.5–)13–17.5(–19) × (3–)3.5–4 μm, mean ± SD = 15.4 ± 2.2 × 3.7 ± 0.2 μm, L/W ratio = 4.1.
Culture characteristics: Colonies on SNA flat, partly raised with entire margin, hyaline, buff to pale honey, on medium, filter paper and Anthriscus stem partly covered with irregular white aerial mycelium, Anthriscus stem partly covered with orange acervuli, reverse hyaline, white, buff to cinnamon, filter paper partly olivaceous grey; growth rate 18.5–20 mm in 7 d (30–31 mm in 10 d). Colonies on OA flat, partly raised with entire margin; surface covered with irregular floccose to woolly white to olivaceous grey aerial mycelium and few orange acervuli, reverse buff, cinnamon to dark purplish grey; growth rate 22.5–24 mm in 7 d (32.5–34 mm in 10 d). Conidia in mass orange.
Material examined: Indonesia: Sumatra, Tele, from leaf spots developing after herbicide treatment of Eucalyptus sp., 1 Jan. 2008, M.J. Wingfield, (CBS H-20798 holotype, culture ex-type CBS 127551 = CPC 14986).
Notes: Eucalyptus is not a well-known disease reservoir for Colletotrichum species. Colletotrichum eucalypti was described from Brazil by Bitancourt (1927) and noted again by Viégas (1946) from the Campinas region, causing anthracnose of Eucalyptus leaves. Viégas described the species as having conidia that are elongate-fusiform to oblong and 10–20 × 3–5 μm in size; the description is reminiscent of the C. acutatum species complex, but cultures are not available, we have not seen type material, and the species was described from a different continent.
There is a number of Gloeosporium species that were described on Eucalyptus spp. in different countries, but none was described in Asia, and most differ considerably from C. indonesiense. Gloeosporium eucalypti was described on E. corynocalyx in Australia, and forms shorter conidia than C. indonesiense, measuring 8–10 × 3–4 μm (Saccardo 1906) compared to those of C. indonesiense that average 12.3 × 3.8 μm and 15.4 × 3.7 μm on SNA and Anthriscus stem, respectively. Gloeosporium eucalyptorum, described on leaves and twigs of Eucalyptus spp. in Italy, has larger conidia, measuring 18–26 × 5–6 μm. They have a different shape, cylindrical to cylindric-clavate, straight to slightly curved, with both ends obtuse (see Tavola VIII, fig. 5 in Turconi 1924), while conidia of C. indonesiense are straight and cylindrical, with one acute end when formed on SNA and both ends acute when formed on Anthriscus stem. Gloeosporium capsularum was described on Eucalyptus sp. in California, USA; it has longer and narrower conidia, measuring 18–20 × 2.5 μm. They are straight and cylindrical with both sides obtuse (Saccardo 1884). Conidia of Gm. nigricans described from leaves of E. pauciflora in Australia are ovoid and wider than those of C. indonesiense, measuring 12 × 7 μm (Cooke 1891). Gloeosporium ochrostictum from E. rostrata in Australia has oblong-clavate, inaequilateral conidia measuring 9–12 × 4–5 μm (Saccardo 1899); conidia of C. indonesiense are narrower and aequilateral. We have not examined authentic material of any of these taxa, but bearing in mind that none have associated cultures and that type material would be too old to yield multigene sequences, we prefer to leave them in obscurity.
There are Colletotrichum species in the C. boninense species complex known on Eucalyptus: C. boninense and C. karstii have both been found on Eucalyptus in South Africa, and C. karstii also occurs on the related host genus Eugenia in Brazil (Damm et al. 2012, this issue).
Colletotrichum indonesiense is separated from other species by TUB2, ACT, GAPDH and CHS-1 sequences, and most effectively with TUB2. With CHS-1 there is only one bp difference from C. laticiphilum, and the HIS3 sequence is the same as that of that species. The closest match in a blastn search with the TUB2 sequence of strain CBS 127551 with 99 % identity (6 differences) was GU246633 from isolate R14 from Capsicum annuum from South Korea (Sang et al. 2011; identified by us as C. scovillei). The closest match with the GAPDH sequence (with 97 % identity, 7 bp differences) is isolate OCC95 from an unspecified crop in India (HQ846719; P. Chowdappa, C.S. Chethana, S. Madhura, unpubl. data). There are more than 40 ITS sequences in GenBank with 99 % identity (1 bp difference) to the ITS sequence of C. indonesiense.
Colletotrichum johnstonii Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800503. Fig. 14.
Fig. 14.
Colletotrichum johnstonii (A–B, D–G, I–R. from ex-holotype strain CBS 128532. C, H. from IMI 357027). A–B. Conidiomata. C, H. Setae. D–G, I–J. Conidiophores. K–P. Appressoria. Q–R. Conidia. A, C–G, Q. from Anthriscus stem. B, H–P, R. from SNA. A–B. DM, C–R. DIC, Scale bars: A = 100 μm, B = 200 μm, D = 10 μm. Scale bar of D applies to C–R.
Etymology: Named after Peter R. Johnston (Landcare Research), a major contributor to recent improvements in Colletotrichum systematics.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–7 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed in type, but present in strain IMI 357027, medium brown, basal cell sometimes pale brown, smooth-walled, 0–1-septate, 35–55 μm long, base cylindric-conical, often constricted at septum, 3.5–4 μm diam, the tip ± acute. Conidiophores hyaline, smooth-walled, septate, branched. Conidiogenous cells hyaline smooth-walled, cylindrical, sometimes slightly inflated, sometimes lacking a basal septum and continuous with the conidiophore, some polyphialides observed, discrete phialides measure 6–27 × 2.5–4 μm, opening 1–2 μm diam, collarette 1–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with one end slightly acute and one end round or slightly acute, (13.5–)14.5–19(–21.5) × (3.5–)4.5–5(–6) μm, mean ± SD = 16.7 ± 2.1 × 4.7 ± 0.4 μm, L/W ratio = 3.6. Appressoria sparse, single or in loose groups, pale to medium brown, smooth-walled, elliptical to clavate or irregular, the outline undulate or entire, (6–) 8–11.5(–14) × (2–)4–7.5(–10.5) μm, mean ± SD = 9.6 ± 1.7 × 5.8 ± 1.9 μm, L/W ratio = 1.7.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on pale brown, angular, basal cells, 3.5–7.5 μm diam. Setae not observed in type, but present in strain IMI 357027, medium brown, basal cell pale brown, smooth-walled, 0–1-septate, 40–60 μm long, base cylindric-conical to slightly inflated, 2.5–5 μm diam, the tip ± acute to ± roundish, sometimes with a constriction. Conidiophores hyaline, smooth-walled, septate, branched, to 60 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, 11–26 × 2.5–4 μm, opening 1–2 μm diam, collarette 1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with one end slightly acute and one end round or slightly acute, (14.5–)15.5–17(–18) × 4.5–5(–5.5) μm, mean ± SD = 16.3 ± 1.0 × 4.9 ± 0.3 μm, L/W ratio = 3.3.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline; medium, filter paper and Anthriscus stem partly covered with thin floccose white to pale grey aerial mycelium and orange acervuli, reverse hyaline with orange to grey acervuli shining through; growth rate 23–24.5 mm in 7 d (36–37 mm in 10 d). Colonies on OA flat to raised, with entire margin; surface covered with floccose whitish to pale olivaceous grey aerial mycelium and orange acervuli, reverse rosy buff, olivaceous grey to iron-grey in the centre; growth rate 22.5–24.5 mm in 7 d (37.5–39 mm in 10 d). Conidia in mass orange.
Material examined: New Zealand, AK, Auckland, from fruit rot of Solanum lycopersicum, 29 Feb. 1990, J.M. Dingley, (CBS H-20809 holotype, culture ex-type CBS 128532 = ICMP 12926 = PRJ 1139.3); Takaka, from fruit rot of Citrus sp., 1989, collector unknown (deposited in IMI 1993 by P.R. Johnston, No. 1125.5), culture IMI 357027 = PRJ 1125.5.
Notes: Colletotrichum johnstonii is part of clade 4 but has slightly longer conidia than those of C. godetiae, and can be separated from other species on the basis of ACT, HIS3, TUB2, and GAPDH sequences. The gene that performs best as a differential test is ACT. The GAPDH sequence is only 1 bp different from that of C. godetiae, while the CHS-1 sequences of both species are the same. The two C. johnstonii strains from citrus and tomato and a strain from pear that is newly described here as C. pyricola were included in C. acutatum group C by Lardner et al. (1999). Two of their tamarillo strains, also in Lardner’s group C, had near-identical RAPD banding patterns to that of the ex-type strain of C. johnstonii. There are two strains from citrus (PJ50 = PRJ 1125.5 and PJ49 = PRJ 1124.5) and one from tamarillo (Pj18 = PRJ 979.9) from New Zealand included in the study of Guerber et al. (2003) that have the same GAPDH sequence, and these strains also were assigned to to group C in Lardner et al. (1999). From the evidence we have to date, C. johnstonii appears to be endemic to New Zealand, but is not host-specific.
The closest matches from GenBank with the TUB2 sequence of strain CBS 128532 with (98 % identity, 10 bp differences) were AJ409294 from Fragaria in the UK (Talhinhas et al. 2002) as well as AJ748609, AJ748612-AJ748614, AJ748619–AJ748622 and AJ748625, isolates from olive (Talhinhas et al. 2005). We do not believe that any of these sequences represent further records of C. johnstonii. With the GAPDH sequence of strain CBS 128532, there was no closer match than 88 % identity. The ITS sequence of strain CBS 128532 is identical to those of C. salicis, C. pyricola and C. phormii.
Colletotrichum kinghornii Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800504. Fig. 15.
Fig. 15.
Colletotrichum kinghornii (from ex-holotype strain CBS 198.35). A–J. Conidiophores. K–L. Conidia. H–K. from Anthriscus stem. A–G, L. from SNA. A–L. DIC, Scale bars: B = 10 μm. Scale bar of B applies to A–L.
Etymology: Named after W.O. Kinghorn, who previously studied this fungus.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae hyaline, smooth-walled, septate, branched, 1–6 μm diam. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched, up to 45 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, conical or ± inflated, 5.5–18 × 2–3.5 μm, opening 1–1.5 μm diam, collarette 0.5–1.5, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with one round and one truncate end, (11–)15.5–21(–22.5) × (3–)3.5–4(–4.5) μm, mean ± SD = 18.3 ± 2.9 × 3.8 ± 0.4 μm, L/W ratio = 4.9. Appressoria not observed.
Asexual morph on Anthriscus stem. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores, hyaline, smooth-walled, septate, sometimes branched, up to 50 μm long. Conidiogenous cells, hyaline, smooth-walled, cylindrical to clavate, 20–27 × 2.5–4 μm, opening 1–1.5 μm diam, collarette 1 μm, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with one round and one truncate end, (n = 18) measure (15–)16–20.5(–23) × 3.5–4.5 μm, mean ± SD = 18.1 ± 2.3 × 4.0 ± 0.4 μm, L/W ratio = 4.6.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, medium partly covert with very short white aerial mycelium, reverse same colours; 14.5–15.5 mm in 7 d (21–24 mm in 10 d). Colonies on OA flat with entire margin, white, pale olivaceous grey to greyish sepia, surface covert with thin, short floccose white aerial mycelium, reverse white to pale olivaceous grey; 11–16.5 mm in 7 d (16–24 mm in 10 d). Conidia in mass not observed.
Material examined: UK, Scotland, from Phormium tenax, unknown collection date, N.L. Alcock (deposited in CBS collection Feb. 1935 by W.O. Kinghorn as Glomerella phacidiomorpha), (CBS H-20909 holotype, culture ex-type CBS 198.35).
Notes: Kinghorn (1936) worked on two strains isolated from Phormium from material collected in Scotland by N.L. Alcock. Both of these were identified as C. phormii by Farr et al. (2006). One of these is confirmed as C. phormii in this study, but we have found the other (CBS 198.35) to be distinct in molecular terms. Kinghorn named his material Glomerella phacidiomorpha, but Farr et al. (2006) examined the type of that name and found it to be a species of Phaeosphaeriopsis.
Colletotrichum kinghornii is one of the two species in the C. acutatum complex with the largest conidia; only those of C. phormii are bigger. However, strain CBS 198.35 hardly sporulates, and the conidia measured were mostly formed in the aerial mycelium. According to the molecular analyses, strain CBS 198.35 must be considered separate at species rank from C. phormii, with several sequence differences in almost every gene, and a single bp difference in the ITS sequence (this was not detected in the Farr et al. study). Colletotrichum kinghornii is most effectively separated from other species using HIS3.
Closest match in blastn searches with the TUB2 sequence of strain CBS 198.35 (with 98 % identity, 7 bp differences) was Glomerella acutata isolate PCF 459 (EU635504) from strawberry in Belgium (Debode et al. 2009) and with 98 % identity (8 bp differences) isolate PT250 (= CBS 129953) AJ748624 from olive in Portugal (see A6-1) (Talhinhas et al. 2005). This last strain is assigned to C. rhombiforme in this study. With the GAPDH sequence of strain CBS 198.35 there was no closer match from GenBank than with 86 % identity.
Colletotrichum laticiphilum Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800505. Fig. 16.
Fig. 16.
Colletotrichum laticiphilum (from ex-holotype strain CBS 112989). A–B. Conidiomata. C–H. Conidiophores. I–L. Appressoria. M–N. Conidia. A, C–E, M. from Anthriscus stem. B, F–L, N. from SNA. A–B. DM, C–N. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–N.
Etymology: latex = Greek for milk, latex and -philus = Greek for loving; referring to the economically significant feature of the host plant.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–7.5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched. Conidiogenous cells hyaline, smooth-walled, ampulliform to conical, sometimes lacking a basal septum and continuous with the conidiophore, discrete phialides measuring 6.5–15 × 3–4.5 μm, opening 1–1.5 μm diam, collarette 0.5–1.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with both ends ± acute or one end round and one end slightly acute, (9.5–)13.5–19.5(–25.5) × (3–) 3.5–4(–4.5) μm, mean ± SD = 16.6 ± 3.1 × 3.8 ± 0.4 μm, L/W ratio = 4.4, conidia of CBS 129827 smaller, measuring (5–)8–15(–18.5) × (1.5–)2.5–4.5(–5.3) μm, mean ± SD = 11.5 ± 3.4 × 3.6 ± 0.9 μm, L/W ratio = 3.2. Appressoria single, medium brown, smooth-walled, subglobose, elliptical to clavate, the edge entire or rarely slightly undulate, (5–)6.5–12(–16) × (4–)6–8(–8.5) μm, mean ± SD = 9.2 ± 2.8 × 7.2 ± 1.0 μm, L/W ratio = 1.3, appressoria of CBS 129827 smaller, measuring (4–)5–7(–8) × (2.5–)3.5–5.5(–6) μm, mean ± SD = 6.0 ± 1.1 × 4.5 ± 0.8 μm, L/W ratio = 1.3.
Asexual morph on Anthriscus stem. Conidiomata possibly acervular, but no basal cells observed. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, simple or septate and branched, to 25 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, ampulliform to cylindrical, 9–15 × 3.5–5.5 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end slightly acute, (10–)12–15(–19.5) × 4–5(–5.5) μm, mean ± SD = 13.6 ± 1.7 × 4.5 ± 0.3 μm, L/W ratio = 3.0.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to pale honey, filter paper pale olivaceous grey; growth rate 22.5 mm in 7 d (33.5 mm in 10 d). Colonies on OA flat with entire margin; surface white, buff to pale isabelline, covered with short felty white aerial mycelium, reverse buff to honey; growth rate 22.5–23 mm in 7 d (32.5–35 mm in 10 d). Conidia in mass whitish.
Material examined: India, Kerala, Kottayam, Rubber Research Institute campus, from raised spots on leaf of Hevea brasiliensis, 1999, unknown collector, (CBS H-20799 holotype, culture ex-type CBS 112989 = IMI 383015 = STE-U 5303 = CG6). Colombia, Meta, Villavicencio, from leaf, anthracnose of Hevea brasiliensis, 14 Aug. 2010, O. Castro, culture CBS 129827 = CH2.
Notes: Colletotrichum leaf disease (CLD) has been considered to be a major cause of declining yields of Hevea brasiliensis in Southeast Asia (Brown & Soepena 1994, Jayasinghe et al. 1997, Saha et al. 2002). The pathogen was at first routinely identified as C. heveae (Petch 1906) and then assumed to be C. gloeosporioides (s. lat.) (Carpenter & Stevenson 1954, von Arx 1957).
Jayasinghe and colleagues found that the majority of strains examined from Sri Lanka belonged to C. acutatum (s. lat.), and Saha et al. (2002) reported this species from India as well; it is likely that similar strains are widespread in the region. Saha et al. (2002) revealed that C. acutatum (s. lat.) causes the raised spot symptom, while C. gloeosporioides (s. lat.) causes both anthracnose and papery lesions on Hevea leaves in India. In a study from Sri Lanka, Thambugala & Deshappriya (2009) found that C. acutatum causes larger lesions and can act synergistically in combination with C. gloeosporioides to cause CLD. Strain IMI 383015 is one of the strains causing the raised spots on Hevea leaves in India. It was included in the study of Saha et al. (2002) and also in the study of Lubbe et al. (2004), who generated its ITS and TUB2 sequences. The TUB2 sequence of strain IMI 383015 (AY376556) was also included in the TUB2 phylogeny by Shivas & Tan (2009); the strain was identified there as C. simmonsii.
It is necessary to consider the possible conspecificity of C. laticiphilum with three previously described taxa, all published by Petch in the same paper (Petch 1906) from collections made from Hevea in Sri Lanka. These were named as C. heveae, Gloeosporium heveae [nomenclaturally unrelated to C. heveae] and Gm. alborubrum. All three species were regarded as synonyms of C. gloeosporioides by von Arx (1957).
Colletotrichum heveae was described with very wide conidia, measuring 18–24 × 7.5–8 μm (Petch 1906), larger than any of the species in the C. acutatum species complex, and possibly belonging to the C. crassipes group as accepted by Sutton (1980). No type details were given in the original description. There is a probable type specimen of C. heveae in K(M), collected from leaves of Hevea (Petch 2228) on 7 Oct. 1905, presumably from Sri Lanka. It is fragmentary and also contains a fungus identified on the label as Gloeosporium brunneum. According to Petch (1927), C. heveae causes an indeterminate leaf spot, and is perhaps an invader following mechanical damage; it was not considered to be a significant disease of rubber at that time. No fungus corresponding to the description of C. heveae was found on the type specimen, and a slide previously made from this material (IMI 80135) also does not contain this species. Glomerella phyllanthi (from the related plant Phyllanthus acidus) was initially regarded as the sexual morph of C. heveae (Pai 1970), but was later revealed to belong to the C. boninense species complex, as was another species on Hevea, C. annellatum (Damm et al. 2012, this issue).
The conidia of Gm. heveae are about the same size as those of C. laticiphilum (12–17 × 3.5–5 μm); however the spores extrude in a pale brown mass, which would be unusual for a Colletotrichum. Also the size range of the “basidia” (= conidiogenous cells) is given as 20–34 × 2 μm; corresponding structures of C. laticiphilum are shorter and much wider. There is no material in K(M) identified as Gm. heveae. It is possible that the fungus identified as Gm. brunneum in the type collection of C. heveae is actually Gm. heveae, as Gm. brunneum is a completely unrelated fungus originating from Populus leaves in the USA (Ellis & Everhart 1889). Petch could have realised after writing the packet label, but before publication, that naming his fungus Gm. brunneum would create a later homonym. Petch (1927) indicated that Gm. heveae was found only in one isolated instance in 1905, when it caused leaf fall in young nursery-grown plants and resulted in general discoloration and death of the whole leaf blade. The disease was successfully controlled by reducing exposure to shade. Synonymy of Gm. heveae with our fungus would not affect the naming of C. laticiphilum as a combination into Colletotrichum based on Gm. heveae would be a later homonym of C. heveae.
It seems possible that Gm. alborubrum might be referable to the species described here. According to Saha et al. (2002) a symptom consisting of raised spots had been attributed to this species. The fungus was originally described from green stems of Hevea brasiliensis, but Petch (1927) stated that it caused abnormal leaf fall and appeared to spread to green ends of the branches to cause dieback. He thought that it might be a secondary invader following Phytophthora infection. These symptoms do not seem to correspond well with those described by Saha and colleagues. The conidia of Gm. alborubrum were measured as 15–20 × 3–4 μm, and described as oblong with rounded ends, straight or slightly curved, issuing in thick pink or white tendrils (Petch 1906). The size is similar to C. laticiphilum; we also observed slightly curved conidia, especially in the isolate from Colombia (CBS 129827). The conidial shape of both C. laticiphilum isolates on Anthriscus stem is not fusiform, but cylindrical with one end round and one end only slightly acute. Therer are three specimens in K(M) identified by Petch as belonging to Gm. alborubrum, but none can be type material as they were all collected after publication of the name.
Bearing in mind that definite type material of all three names is either missing or fragmentary and that none of the authentic material would be likely to yield good sequences, we think that it is more practical to publish a new taxon rather than to epitypify or neotypify one of the earlier names with a specimen that we are not confident is conspecific with the type.
Colletotrichum laticiphilum is separated from other species by its TUB2, GAPDH and CHS-1 sequences, and most differentially with TUB2. With CHS-1 there is only one bp difference from C. indonesiense, while the HIS3 sequence is the same as that of that species. The closest match with the GAPDH sequence (with 99 % identity, 1 bp difference) was HQ846719 from an unnamed plant, probably from India (P. Chowdappa, C.S. Chethana, S. Madhura, unpubl. data). The ITS sequence of strain CBS 112989 matches 100 % with AB042306 and AB042307 from isolates from Carthamus and Glebionis from Japan (J. Moriwaki, T. Tsukiboshi, T. Sato, S. Uematsu, unpubl. data), with AJ749675 from isolate PD85/694 (= CBS 126519, C. chrysanthemi), and with AB219024 from strawberry in Japan (Chung et al. 2006).
Colletotrichum limetticola (R.E. Clausen) Damm, P.F. Cannon & Crous, comb. nov. MycoBank MB455483. Fig. 17. Basionym: Gloeosporium limetticola [as Gm. limetticolum] R.E. Clausen, Phytopathology 2: 231. 1912.
Fig. 17.
Colletotrichum limetticola (from ex-epitype strain CBS 114.14). A–B. Conidiomata. C–K. Conidiophores. L–Q. Appressoria. R–S. Conidia. A, C–G, R. from Anthriscus stem. B, H–Q, S. from SNA. A–B. DM, C–S. DIC, Scale bars: A = 100 μm, F = 10 μm. Scale bar of A applies to A–B. Scale bar of F applies to C–S.
Sexual morph not observed. Asexual morph on leaf of Citrus aurantifolia (BPI 394978). Conidiomata conidiophores formed on a cushion of pale brown angular cells 3–6 μm diam. Setae not observed. Conidiophores hyaline, smooth-walled, septate and branched, up to 75 μm. Conidiogenous cells hyaline, smooth-walled, cylindrical, sometimes slightly inflated, 10–18 × 2.5–4 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible, sometimes distinct. Conidia hyaline, smooth-walled, aseptate, straight, sometimes slightly flexuous, cylindrical with one end round and one end slightly acute to truncate, or both ends slightly acute, (10–)12.5–17.5(–20) × (3.5–)4–4.5(–4.5) μm, mean ± SD = 15.1 ± 2.4 × 4.1 ± 0.3 μm, L/W ratio = 3.7. Appressoria few observed on specimen, pale to medium brown, smooth-walled, subglobose, ovoid to ellipsoidal outline, entire edge.
Asexual morph on SNA (CBS 114.14). Vegetative hyphae 1–8.5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched, up to 45 μm. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, sometimes integrated (not separated from fertile hyphae by a septum, polyphialides rarely observed, 8.5–20 × 3–5.5 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible, sometimes distinct. Conidia hyaline, smooth-walled, aseptate, straight, sometimes slightly curved, cylindrical to clavate with one end round and one end slightly acute to truncate, or both ends slightly acute, sometimes slightly constricted in the middle, (9–)12–20.5(–29) × (3–)4–5(–6) μm, mean ± SD = 16.3 ± 4.2 × 4.5 ± 0.6 μm, L/W ratio = 3.6. Appressoria single or in loose groups, pale to medium brown, smooth-walled, subglobose, ovoid to ellipsoidal outline, entire or undulate edge (5–)6–8.5(–11) × (4–)4.5–6(–7) μm, mean ± SD = 7.4 ± 1.3 × 5.3 ± 0.7 μm, L/W ratio = 1.4.
Asexual morph on Anthriscus stem (CBS 114.14). Conidiomata conidiophores formed directly on hyphae or on a cushion of pale brown angular cells 3.5–6.5 μm diam. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched, to 80 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical slightly inflated, 6–13 × 2.5–4.5 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical, clavate, cylindrical to fusiform with one end round and one end (often only slightly) acute or both ends acute, (12–)13–18(–24) × (3.5–)4–4.5(–5.5) μm, mean ± SD = 15.5 ± 2.3 × 4.3 ± 0.4 μm, L/W ratio = 3.6.
Culture characteristics: Colonies on SNA flat to low convex with entire margin, hyaline, filter paper partly pale salmon to straw, partly covered with felty white aerial mycelium, reverse hyaline to pale ochreous, filter paper partly straw; 18.5–20 mm in 7 d (26–30.5 mm in 10 d). Colonies on OA flat with entire margin; surface moist, white to pale luteous, saffron towards the centre due to sporulation, aerial mycelium lacking, reverse whitish, buff to rosy buff, 18–21.5 mm in 7 d (26–29 mm in 10 d). Conidia in mass salmon.
Material examined: Cuba, Herradura, inoculation experiment XV in Berkeley, Alameda Co., California, from twig of Citrus medica var. acida (= Citrus aurantifolia), unknown collection date, (inoculated 30 Jan. 1912, photographed 20 Mar. 1912 by R.E. Clausen), Earle (UC 302386 lectotype [not seen], BPI 394978 isolectotype). USA, Florida, from young twig of Citrus aurantifolia, collection date and collector unknown (deposited in CBS collection Feb. 1914 by R.E. Clausen as Gloeosporium limetticola), (CBS H-20910 epitype, here designated, culture ex-epitype CBS 114.14).
Notes: Gloeosporium limetticola was described by Clausen (1912) following pathogenicity trials in California on young sour lime (Citrus medica var. acida = Citrus aurantifolia, Key lime) trees inoculated with strains from sour lime from Cuba and with strains from orange, lemon, pomelo and tangerine from Cuba, California, and Florida. The Cuban sour lime strain from Herradura consistently caused wither tip disease symptoms on tester plants from that species, and another Cuban strain (from Santiago de las Vegas) caused broadly similar symptoms on both sour lime and lemon (Citrus limon) trees. Clausen stated that a virulent form of wither tip occurred in Florida also, but this author was unable to access diseased material to compare with the Cuban pathogen.
Type material of Gm. limetticola was deposited by Clausen in the dried fungus collections at the University of California (UC) and Washington DC (BPI). However, its identity (in particular its local geographical origin, i.e. from Herradura or Santiago de las Vegas) was not specified in the original paper. The species was described [translated from the Latin] as occurring “in young leaves and stems of Citrus medica var. acida, acting as a pathogen naturally in Cuba, and also artificially inoculated in greenhouses in California on leaves and stems of C. medica var. acida, C. limetta and C. limon)”.
The relevant accession at UC consists of a single packet (UC 302386) containing three further packets. One is from Clausen’s Experiment XV and is marked “lime type”; another is from lemon (Experiment XXVII) and Cuban lime material (presumably the original diseased sample) and is marked “type material”. The lemon sample is definitely from a genetic source different from that of the lime collections, and, it was not marked as type material. The two lime samples may well be genetically identical and could be regarded collectively as the holotype, but on balance we feel that treating them as two syntypes is more reasonable. That conclusion was also reached by Tavares et al. (1997), who designated the collection from Experiment XV in UC as lectotype of Gm. limetticola.
Cultures from the type material have not been preserved. However, strain CBS 114.14 from Florida was deposited in the CBS collection in Feb. 1914 by R.E. Clausen, as Gm. limetticola. The strain was not specified as being an ex-type strain, and we suppose that it was one of the samples requested by Clausen from wither tip of lime in Florida. It is reasonable to consider the culture as authentic material, and we therefore designate a dried subculture as epitype for Gm. limetticola.
The wither tip disease of Citrus aurantifolia is apparently identical with Key lime anthracnose (KLA), a specific disease of leaves, twigs, flowers and fruits of Key lime (Citrus aurantifolia) and has been well studied in recent years (Brown et al. 1996, Agostini et al. 1992, Timmer & Brown 2000, Peres et al. 2008, MacKenzie et al. 2009). While the causal organism of KLA was identified as C. gloeosporioides by Agostini et al. (1992), following von Arx (1957) who listed the fungus as a synonym of that taxon, Brown et al. (1996) assigned the fungus to C. acutatum based on ITS sequence data. According to Farr & Rossman (2012), Gm. limetticola has been reported from Citrus aurantifolia in Barbados, California, Cuba, Fiji, Florida, Hawaii, India, Jamaica, Philippines and Tanzania.
Colletotrichum strains from anthracnose on leaves of Key lime in Florida, USA (KLA-Anderson, HM-1, Ss) and MTR-KLA-A1 (Belize) included in the study of Peres et al. (2008) and MacKenzie et al. (2009) have the same ITS and GAPDH sequences as strain CBS 114.14. Additionally, the ITS sequences of isolates DPI from Citrus aurantifolia in Florida, USA (FN566877, Ramos et al. 2006) and c2 from Citrus sp. in Brazil (EU008878, Giaretta et al. 2010) match that of CBS 144.14 with 100 % identity. Probable C. limetticola strains are also included in Guerber et al. (2003) as mtDNA RFLP haplotype J3; the GAPDH sequences of two Key lime strains (MD33, MD15) are almost identical to that of CBS 114.14. The closest match with the TUB2 sequence of strain CBS 114.14 with 100 % identity is GenBank accession FN611029 from isolate DPI as well (Ramos et al. 2006). In their study on Citrus in Portugal, Ramos et al. (2006) did not find any C. acutatum s. lat.; C. gloeosporioides (s. lat.) seems to be the major anthracnose pathogen.
According to MacKenzie et al. (2009), Key lime isolates differ significantly from isolates from flowers of postbloom fruit drop (PFD) affecting sweet orange (Citrus sinensis) in Florida, USA (STF-FTP-10, OCO-ARC-4, ALB-IND-25). The differences are found in their ITS, GAPDH and GS sequences. Based on ITS and GAPDH sequences of 69 PFD and KLA strains from different countries (Belize, Brazil, Costa Rica, Dominican Republic, USA (Florida), Mexico), Peres et al. (2008) recognised the causal agents of the two citrus diseases as two distinct phylogenetic lineages of C. acutatum with few or no sequence differences in both the ITS and GAPDH genes. We did not include PFD isolates in our study, but according to ITS and GAPDH sequences, PFD and KLA strains are related to each other, but seem to belong to different species. Agostini et al. (1992) noticed morphological and cultural differences between PFD and KLA isolates: appressoria of PFD isolates were clavate and deeply pigmented and those of KLA isolates round, smaller and less pigmented. Also, KLA strains grew slightly more slowly than PFD isolates.
Pathogenicity tests by MacKenzie et al. (2009) had basically the same results as those by Clausen (1912); only Colletotrichum isolates from key lime caused leaf necrosis on key lime, while isolates from PFD, strawberry (= C. nymphaeae, according to this study), blueberry (= C. fioriniae, according to this study) and leatherleaf fern did not. Key lime isolates caused necrosis of flowers on Orlando tangelo flower clusters as well, but the percentage of affected flowers was lower than those inoculated with PFD isolates. Chen et al. (2005) identified a gene (KLAP1 gene) that was required for causing KLA, particularly for the infection of Key lime leaves, but not for the infection of flower petals.
Colletotrichum limetticola is distinguished from other species by TUB2, GAPDH and HIS3, most effectively with TUB2, which is only 2 bp different from the sequence seen in CBS 129823 (Colletotrichum sp. from Passiflora in Colombia, occupying an unnamed subclade within Clade 1).
Colletotrichum lupini (Bondar) Damm, P.F. Cannon & Crous, comb. nov. MycoBank MB800519. Fig. 18.
Fig. 18.
Colletotrichum lupini (from ex-neotype strain CBS 109225). A–B. Conidiomata. C–I. Conidiophores. J–O. Appressoria. P–Q. Conidia. A, C, P. from Anthriscus stem. B, D–O, Q. from SNA. A–B. Dissecting microscope (DM), C–Q. Differential interference contrast illumination (DIC), Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–Q.
Basionym: Gloeosporium lupini [as Gm. lupinus] Bondar, Boln Agric., São Paulo 13: 427. 1912.
≡ Colletotrichum lupini (Bondar) Nirenberg, Feiler & Hagedorn, Mycologia 94(2): 309. 2002, nom. inval. (Art. 33.3).
≡ Colletotrichum lupini var. setosum Nirenberg, Feiler & Hagedorn, Mycologia 94(2): 309. 2002, nom. inval. (Art. 43.1).
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–6.5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae on the surface of the medium and in the aerial mycelium. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched, rapidly degenerating. Conidiogenous cells hyaline, smooth-walled, cylindrical, 2.5–20 × 1.5–2.5 μm, often integrated (not separated from fertile hyphae by a septum), opening 0.5 μm diam, collarette 0.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, rather variable in shape, usually cylindrical to clavate with one end round and one end acute, 9–15(–26.5) × (3–)3.5–4.5(–6) μm, mean ± SD = 12.0 ± 3.2 × 4.1 ± 0.6 μm, L/W ratio = 2.9, conidia of strain CBS 109221 are slightly larger, measuring 11.5–15.5(–19) × (3.5–)4–4.5(–5) μm, mean ± SD = 13.5 ± 1.9 × 4.3 ± 0.4 μm, L/W ratio = 3.2. Appressoria single or in small dense clusters, medium brown, round to elliptical in outline with an undulate to lobate margin, (4–)6–12(–20.5) × (4.5–) 6–9(–11.5) μm, mean ± SD = 9.0 ± 2.8 × 7.4 ± 1.7 μm, L/W ratio = 1.2. Appressoria of strain CBS 109221 differ in being arranged singly or in rows along hyphae and mostly having an entire margin (rarely undulate to lobate).
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on a cushion of pale brown angular cells 3–6.5 μm diam. Setae not observed in the ex-neotype strain, but in strain CBS 109221 where a few setae were observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, sometimes ± inflated, 7–15 × 2.5–3.5 μm, opening 1–1.5 μm diam, collarette 0.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to clavate with one end round and one end acute, (10–)12.5–16(–18.5) × (3–)3.5–4.5 μm, mean ± SD = 14.2 ± 1.7 × 4.0 ± 0.3 μm, L/W ratio = 3.6.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, on filter paper and on Anthriscus stem partly covered with short white to pale grey aerial mycelium, reverse of filter paper white to pale luteous; growth 15–21 mm in 7 d (25–31 mm in 10 d). Colonies on OA flat with entire margin; surface covered with felty to woolly white to pale olivaceous grey aerial mycelium, reverse buff to smoke grey; growth 15–19 mm in 7 d (24–27 mm in 10 d), strain CBS 466.76 grows faster 23.5–27.5 mm in 7 d (36–37.5 mm in 10 d). Conidia in mass salmon.
Material examined: Ukraine, from seed of Lupinus albus, unknown date, H.I. Nirenberg, culture ex-neotype of C. lupini, CBS 109225 = BBA 70884. Germany, from Lupinus albus, unknown date, U. Feiler, culture ex-holotype of C. lupini var. setosum, CBS 109221 = BBA 70352.
Notes: Two studies on the causal agent of lupin anthracnose published in 2002 arrived at different results: while Talhinhas et al. (2002) regarded the causal agent of lupin anthracnose as C. acutatum, Nirenberg et al. (2002) concluded that the causal isolates belonged to a separate species, C. lupini. Nirenberg and her colleagues based this new name on Gloeosporium lupini (Bondar 1912), but their combination is invalid because the basionym was not cited correctly according to the ICBN. We therefore validate the combination here. Nirenberg et al. (2002) designated a dried culture derived from BBA 70884 (= CBS 109225) as a neotype of Gm. lupini, since no type material was designated by Bondar (1912); this action is nomenclaturally correct.
Nirenberg et al. (2002) additionally described a variety of the lupin pathogen, C. lupini var. setosum. They noted few morphological and physiological differences between the two varieties: strains of var. lupini were observed to produce more conidia than var. setosum in the aerial mycelium, as well as to grow slightly slower on PDA and to have a lower optimum growth temperature. In addition, var. lupini isolates usually formed concentric growth rings in culture, while var. setosum did not. The authors rarely observed setae in var. lupini, but these were regularly seen in var. setosum. In our study, the ex-holotype strain of C. lupini var. setosum formed a few setae. Nirenberg and colleagues indicated that the ITS sequences of the two varieties differ in only one base. Our study, based on analysis of six genes, showed few other bp differences, blurring the distinction between the two varieties. The name C. lupini var. setosum was also invalidly published (Art. 43.1). As the species name C. lupini was invalid itself at the time, and as we do not accept the variety as a distinct taxon, we do not validate the name here.
According to Nirenberg et al. (2002), a typical feature of C. lupini is the conidial morphology, with spores having one end pointed and one rounded. We also observed this feature clearly when the fungus was growing on Anthriscus stem. However, the conidia of the ex-neotype strain observed in this study on SNA are from simple or branched conidiophores at the agar surface and from the aerial mycelium rather than from conidiomata, because the strain no longer produces defined acervuli on this medium. Conidia from aerial mycelium are ± cylindrical, sometimes with both ends rounded. They are very variable in size (Nirenberg et al. 2002).
Colletotrichum lupini was originally described from Lupinus albus in the São Paulo region of Brazil, presumably introduced to South America along with its host plant, which is native to the Mediterranean region (Kurlovich 2002). The only isolates from South and Central America (Bolivia and Costa Rica) included in our study have sequences identical to that of the ex-neotype strain of C. lupini. The same is true for the strains studied from Europe and elsewhere. The species now appears to have no restriction to particular continents or climatic zones.
Colletotrichum lupini is an economically significant pathogen of lupin crops worldwide, and there is substantial interest in breeding resistant host cultivars (e.g. Adhikari et al. 2011). While C. lupini shows a clear host preference based on the strains we have examined, a few cultures were derived from hosts other than lupins, namely from Manihot, Camellia and Cinnamomum. Sreenivasaprasad & Talhinhas (2005) also listed Urtica dioica as a host. A study by Nirenberg & Gerlach (2000) showed that a strain of C. lupini var. setosum was able to infect Bergenia in greenhouse tests. Pathogenicity tests by Sreenivasaprasad & Talhinhas (2005) also failed to show host specificity of C. acutatum strains from lupins (= C. lupini), though Lardner et al. (1999) found that the strains they placed in C. acutatum Group D (now known to belong to C. lupini) did not infect pine seedlings in the manner of C. acutatum f. sp. pineum (now regarded as C. acutatum s. str.). The strain from Camellia in the UK (IMI 351261) was deposited 1992 in IMI by R. Cook and is most likely one of the avirulent C. acutatum strains reported from ornamental Camellia species by Dickens and Cook (1989).
Our phylogeny clearly supports C. lupini as a distinct species within the C. acutatum species complex. Colletotrichum lupini is separated from other species by all genes included, except for ACT, with TUB2 providing the best differential test.
Colletotrichum melonis Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800506. Fig. 19.
Fig. 19.
Colletotrichum melonis (from ex-holotype strain CBS 159.84). A–B. Conidiomata. C–H. Conidiophores. I–N. Appressoria. O–P. Conidia. A, C–E, O. from Anthriscus stem. B, F–N, P. from SNA. A–B. DM, C–P. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–P.
Etymology: Named after host plant, Cucumis melo.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–6.5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly from vegetative hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched, degenerating rapidly. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, 7–19 × 2.5–4 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, some slightly curved, cylindrical with one end round and one end slightly acute to round, rarely both ends acute, (7–)9–16.5(–23.5) × (3–)3.5–4.5(–5) μm, mean ± SD = 12.8 ± 3.6 × 3.9 ± 0.4 μm, L/W ratio = 3.3 μm, L/W ratio = 3.7. Appressoria formed singly, medium brown, smooth-walled, subglobose, elliptical or clavate, the edge entire, rarely slightly undulate, (4.5–)6–11(–13.5) × (3.5–)4.5–6.5(–7.5) μm, mean ± SD = 8.3 ± 2.4 × 5.5 ± 1.0 μm, L/W ratio = 1.5.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on pale brown angular basal cells, 3–7 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 50 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, sometimes polyphialidic, 10–20 × 3–4.5 μm, opening 1–2 μm diam, collarette 0.5–1(–1.5) μm long, periclinal thickening visible, sometimes distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end slightly acute to round, (9–)12–17(–20) × (3.5–)4–4.5(–5) μm, mean ± SD = 14.5 ± 2.3 × 4.2 ± 0.3 μm, L/W ratio = 3.5.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to pale honey, on filter paper and Anthriscus stem partly covered with floccose-felty white aerial mycelium, reverse same colours; growth 20.5–21.5 mm in 7 d (27.5–32 mm in 10 d). Colonies on OA flat with entire margin; surface buff, honey to saffron, partly covered with floccose-felty white aerial mycelium and saffron to isabelline acervuli, reverse buff, honey to rosy buff; growth 22–24 mm in 7 d (34–34.5 mm in 10 d). Conidia in mass saffron.
Material examined: Brazil, from peel of fruit of Cucumis melo, unknown collector and collection date (isolated by H.A. van der Aa, No. 9014 and deposited in CBS collection 1 Mar. 1984), (CBS H-20785 holotype, culture ex-type CBS 159.84).
Notes: Colletotrichum melonis belongs to clade 1 of the C. acutatum species complex but occupies a distinct subclade that is supported by multiple genes. The sole strain that we are aware of has appressoria with a significantly larger length/width ratio than those of C. lupini (mean L/W = 1.5 versus 1.2), the most frequently encountered species of clade 1. These appressoria form singly rather than in clusters.
The pathogenicity of C. melonis is not known. This appears to be the first report of a Colletotrichum species from the C. acutatum species complex as an associate of cucurbits. There are various reports of disease caused by members of the C. boninense and C. gloeosporioides clades, but the principal cucurbit pathogens appear to be Glomerella magna and C. orbiculare (von Arx & van der Velden 1961, Jenkins & Winstead 1964, Du et al. 2005, Hyde et al. 2009, Cannon et al. 2012, this issue).
Colletotrichum melonis is separated from other species by GAPDH, ACT and HIS3 sequences, with GAPDH performing best as a differential gene, while the TUB2 sequence is the same as that of strain IMI 384185 (unnamed strain in clade 1). Closest matches in blastn search with the GAPDH sequence of strain CBS 159.84 (with 97 % identity, 6 bp differences) were EU168905, EU647318 and EU647319 from sweet orange (Peres et al. 2008, MacKenzie et al. 2009), while the closest published matches with the TUB2 sequence (with 99 % identity, 4 bp differences) were FN611029 and FN611028 from Citrus aurantifolia and Citrus sinensis from USA, Florida (Ramos et al. 2006). The ITS sequence matched 100 % with EU008864–EU008866 from Malus domestica in Brazil (Giaretta et al. 2010).
Colletotrichum nymphaeae (Pass.) Aa, Netherlands J. Pl. Pathol., Supplement 1 84: 110. 1978. Fig. 20.
Fig. 20.
Colletotrichum nymphaea (from ex-epitype strain CBS 515.78). A–B. Conidiomata. C–G. Conidiophores. H–M. Appressoria. N–O. Conidia. A, C–D, N. from Anthriscus stem. B, E–M, O. from SNA. A–B. DM, C–O. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–O.
Basionym: Ascochyta nymphaeae Pass., in Rabenh., Fungi Europaei edn 2: 2251 (1876, in sched.); Hedwigia 16: 120. 1877.
= Colletotrichum mahoniae Fabric., Atti Imp. Regia Accad. Rovereto, ser. 3, 6: 139. 1950.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1.5–5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate and branched, to 60 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, 10.5–20 × 2–4 μm, opening 1 μm diam, collarette distinct, 1–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to cylindric-clavate with one end round and one end rounded to ± acute, (10–)14–18.5(–19.5) × (3–)4–5.5(–6) μm, mean ± SD = 16.1 ± 2.3 × 4.9 ± 0.7 μm, L/W ratio = 3.3. Strain CBS 526.77 has smaller conidia, measuring (8.5–)9–13(–16) × (3–)3–4.5(–5) μm, mean ± SD = 11.0 ± 2.0 × 3.8 ± 0.6 μm, L/W ratio = 2.9, while conidia of strain CBS 112202 differ in being cylindrical to fusiform with both ends acute. Appressoria single, medium brown, smooth-walled, elliptical, clavate or irregular in outline, entire, undulate to lobate margin, (4.5–)6–11(–15) × (3–)4.5–6.5(–8) μm, mean ± SD = 8.7 ± 2.5 × 5.5 ± 1.0 μm, L/W ratio = 1.6.
Asexual morph on Anthriscus stem. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 60 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, ± cylindrical, sometimes polyphialidic, 12–30 × 2.5–3.5 μm, opening 0.5 μm diam, collarette distinct, 0.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to cylindric-clavate with one end round and one end rounded to ± acute, (12.5–)14–18.5(–22.5) × (4–)4.5–5.5(–6) μm, mean ± SD = 16.3 ± 2.1 × 4.8 ± 0.5 μm, L/W ratio = 3.4. Strain CBS 526.77 has wider conidia, measuring (9.5–)13.5–19(–21.5) × (3.5–)5–6(–6.5) μm, mean ± SD = 16.1 ± 2.7 × 5.6 ± 0.7 μm, L/W ratio = 2.9, while conidia of strain 173.51 are smaller, measuring (7.5–)10–14.5(–16) × (3–)3.5–4.5 μm, mean ± SD = 12.3 ± 2.0 × 3.9 ± 0.4 μm, L/W ratio = 3.2, conidia of most of the isolates studied differ in shape from the ex-epitype strain, being cylindrical to fusiform with both ends acute, e.g. CBS 173.51 and CBS 112202.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline with low white aerial mycelium on filter paper and Anthriscus stem, on filter paper partly pale olivaceous grey on both sides; growth rate 16.5 mm in 7 d (20 mm in 10 d); some strains grow faster, e.g. CBS 126382 25–26 mm in 7 d (35–37 mm in 10 d). Colonies on OA flat with entire margin; surface isabelline, cinnamon to honey, white at the margin, aerial mycelium lacking, reverse greyish sepia to buff; growth rate 14.5 mm in 7 d (20 mm in 10 d); some other strains grow faster, e.g. CBS 126382 23.5–29 mm in 7 d (37.5–40 mm in 10 d). Colony surface of strains CBS 516.78 and CBS 526.77 is dark olivaceous to iron-grey. Conidia in mass pale salmon.
Material examined: Italy, Parma, in horto botanico, from leaf of Nymphaea alba, summer 1875, G. Passerini, in Rabenhorst, Fungi Europaei exsiccati edn 2: 2251 (holotype not selected by the original author and location uncertain; K(M) 176820 isotype, here designated as lectotype; K(M) 99741 isolectotype; CBS H-00769 isolectotype). Netherlands, Oude Waal near Nijmegen, Gem. Ubbergen, from leaf spots of Nymphaea alba, 7 Aug. 1978, G. van der Velde, (CBS H-20787 epitype, here designated, culture ex-epitype CBS 515.78 = van der Aa No. 6573); Kortenhoefse Plassen from leaf of Nymphaea alba, collection date and collector unknown (isolated Aug. 1977 by H.A. van der Aa), culture CBS 526.77; from curl disease of Anemone coronaria De Caen, collection date and collector unknown, CBS 126382 = PD 79/648. Italy, Rome, from leaf of Mahonia aquifolium, collection date and collector unknown (deposited in CBS collection Jun. 1951 by R. Ciferri), culture CBS 173.51; Rome, from Fragaria x ananassa, cv. Idea, collection date and collector unknown (send to Plantenziektenkundige Dienst Wageningen by L Corazza), culture CBS 126372 = PD 93/1666A. South Africa Western Cape, Stellenbosch, Elsenberg Farm, from Protea magnifica, 1 Apr. 2001, K. Lubbe, culture CBS 112992 = STE-U 4452. Spain, from fruit lesions of Fragaria sp., Mar. 2002, H.A. van der Aa, culture CBS 112202.
Notes: Colletotrichum nymphaeae was described in detail in morphological and pathological terms by van der Aa (1978). Its basionym Ascochyta nymphaeae was first validly published in 1876 in Rabenhorst’s Fungi Europaei edn nova, exsiccatum no. 2251 (Stevenson 1971), and the label data was published in the journal Hedwigia in the following year. The name A. nymphaeae was ascribed to Passerini on the exsiccatum label as an unpublished herbarium name. Individuals of this exsiccatum can therefore be regarded as type material, but it is not clear where the holotype resides. We interpret individuals of Fungi Europaei no. 2251 as isotypes, and select one of the three examples in Kew, K(M) 176820 (labelled as purchased 1/1886) as lectotype of A. nymphaeae. We also designate an epitype with a living culture from the material studied by van der Aa.
Van der Aa (1978) investigated possible synonyms of C. nymphaeae, finding that Ramularia nymphaeae (syn. Ovularia nymphaeae) was conspecific with that species. Gloeosporium nymphaearum (Allescher 1895) is the type of the genus Ovulariella (considered as a nom. nud. by von Arx (1970) but with an indirect reference to a description in the original publication). Von Arx considered it to be a later synonym of Ramularia nymphaeae, and van der Aa (1978) confirmed the synonymy. To our knowledge, there are no living cultures derived from authentic material of either of these taxa. We have no reason to doubt van der Aa’s synonymy, but we have not examined type material and there is no strong reason to designate epitypes.
We have examined a culture from Mahonia aquifolia from Italy, which was sent to CBS by R. Ciferri as C. mahoniae the year after the species had been described, and this could have been derived from the type of C. mahoniae, but we do not have enough information to be sure. Another species from Mahonia, Gloeosporium japonicum (Hemmi 1920), was described as having wider conidia (10–18 × 5–7 μm) with a different shape (ellipsoidal, short-cylindrical or ovoidal, both ends rounded). However, Hemmi mentioned that conidia in culture have very variable size and shape, measuring 9–20 × 3.6–6 μm in size. We have not located authentic material for this taxon, but even if it were conspecific with C. nymphaeae its name would not have priority. Von Arx (1957) considered Gm. japonicum to be a synonym of C. gloeosporioides.
Another possible synonym of C. nymphaeae is C. nymphaeicola (Kelkar 1972, as C. “nymphicola”). Judging from the description and illustration this is certainly a species of Colletotrichum, but the conidia were claimed to be oblong and to measure 5–15 × 1.5–3 μm. This wide variation in size makes it impossible to attempt a placement in any species as currently circumscribed. The type was reputedly deposited in HCIO but apparently no cultures were obtained. Gloeosporium nymphaeae (Hemmi & Kawase 1954) causes symptoms similar to those of C. nymphaeae, but setae were found to be present. These were not seen in C. nymphaeae either by ourselves or by van der Aa (1978). The conidia of Gm. nymphaeae were described as rounded at both ends, 9–17 × 3–6 μm. We have not been able to locate type material or living cultures of this fungus.
A further species of Colletotrichum associated with waterlilies, C. nupharicola, was described by Johnson et al. (1997). This species appears to have substantially longer and wider conidia than C. nymphaeae with mean widths of individual strains ranging between 6.5 and 7.5 μm (the figures are difficult to interpret and the overall range of conidial size in the discription surprising). It has been found to belong within the C. gloeosporioides complex (Weir et al. 2012, this issue).
In pathogenicity tests MacKenzie et al. (2009) showed that Colletotrichum isolates from petiole, fruit and crown of strawberry with anthracnose from Florida, USA (based on ITS and GAPDH: C. nymphaeae) caused anthracnose on strawberry fruits. Lesions were larger than those caused by isolates from blueberry (based on ITS and GAPDH: C. fioriniae). These differences in virulence should be attributed to the different species the pathogens belong to rather than to the different host plants; both species occur on strawberries, but based on the number of strains in this study, C. nymphaeae seems to be the more important strawberry anthracnose pathogen within the C. acutatum species complex.
Colletotrichum nymphaeae is well separated from other species with TUB2, but not in its ITS. With all other genes the intraspecific variability is very high. The closest matches (100 % identity) in a blastn search using the TUB2 sequence of the ex-epitype strain were AB618090 from Apium in Japan (Fujinaga et al. 2011); AY376551–AY376555 from Protea (Lubbe et al. 2004); AJ409296, AJ314716, AJ314718 from Fragaria in USA, Portugal and Australia; AJ314722, AJ409300, AJ748636 from Lupinus and Anemone (Talhinhas et al. 2002, 2005); and DQ454063, DQ454064 from Fragaria in Thailand (Than et al. 2008a). With 99 % identity reflecting 1 bp difference, the search yielded AJ748605, AJ748607, AJ748608, AJ748611, AJ748615 from olive; AM992148, AM992147 probably also from olive; AJ748633 from Photinia (Talhinhas et al. 2005, 2009); and GQ369612 from a strain identified as C. caudatum (Chen H, Feng Y and Hyde KD, unpubl. data). With 99 % identity reflecting 2 bp differences, we got EF593327 and EF593328 from strains ARSEF4360 and EMA26, respectively, from Orthezia praelonga in Brazil (Marcelino et al. 2008). These two strains were identified as C. gloeosporioides f. sp. ortheziidae and have entomopathogenic activity to the scale insect Orthezia praelonga. They are apparently being used effectively as a biological control agent against this insect in Brazil (Cesnik et al. 1996, Cesnik & Ferraz 2000). Several of these strains listed above are included in this study.
Colletotrichum orchidophilum Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800507. Fig. 21.
Fig. 21.
Colletotrichum orchidophilum (A–M, S–T from ex-holotype strain CBS 632.80. N–R from IMI 305357). A–B. Conidiomata. C. Setae. D–F. Conidiophores. H. Tip of seta. I. Basis of seta. J–M. Conidiophores. N–R. Appressoria. S–T. Conidia. A, C–F, S. from Anthriscus stem. B, H–R, T. from SNA. A–B. DM, C–T. DIC, Scale bars: A = 100 μm, D = 10 μm. Scale bar of A applies to A–B. Scale bar of D applies to C–T.
Etymology: Named for the host plants from which the species is known, all of which belong to the Orchidaceae.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1.5–7 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores and setae formed directly on hyphae. Setae abundant, medium brown, basal cell often paler, smooth-walled, 1–2-septate, 40–80 μm long, base cylindrical, 2–4.5 μm diam, tip somewhat acute. Conidiophores pale to medium brown, septate, branched, smooth-walled, to 60 μm long. Conidiogenous cells hyaline to medium brown, usually smooth-walled, but some warted conidiogenous cells observed, cylindrical with a slime sheath, 7–18 × 2.5–5 μm, opening 1–1.5 μm diam, collarette 0.5 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end somewhat acute, (10.5–)11.5–14(–16.5) × (2–)3–3.5(–4) μm, mean ± SD = 12.7 ± 1.1 × 3.1 ± 0.3 μm, L/W ratio = 4.1, conidia of strain CBS 631.80 are larger, measuring (13–)13.5–17.5(–19) × 2.5–3.5 μm, mean ± SD = 15.4 ± 2.1 × 3.0 ± 0.3 μm, L/W ratio = 5.1. Appressoria not observed in type, but present in strain IMI 305357, single or in periodic intervals along hyphae, dark brown, smooth-walled, elliptical, pyriform or spathulate, (5.5–)7.5–15.5(–20.5) × (4.5–)5.5–8.5(–12) μm, mean ± SD = 11.6 ± 3.9 × 7.0 ± 1.6 μm, L/W ratio = 1.6, appressoria of strain CBS 631.80 smaller, measuring (4.5–)5.5–11(–18) × (4–)4.5–6(–7) μm, mean ± SD = 8.2 ± 2.8 × 5.2 ± 0.8 μm, L/W ratio = 1.6. In SNA cultures of strains IMI 305357 and CBS 119291 no setae were observed.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores and setae formed directly on hyphae or on pale brown basal cells 3–6 μm diam. Setae abundant, dark brown, basal cell often paler, smooth-walled, 0–2-septate, 40–70 μm long, base cylindrical to conical, sometimes inflated, 3–6.5 μm wide, tip somewhat acute, setae of strain CBS 631.80 only up to 40 μm long, with a round tip or functioning as conidiogenous cells. Conidiophores pale brown, septate, branched, to 40 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, 7–16 × 3–5 μm, opening 1.5–2 μm diam, collarette 0.5–1 μm long, distinct, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end somewhat acute, (11.5–)12.5–14(–15.5) × (2.5–)3–3.5(–4) μm, mean ± SD = 13.2 ± 0.9 × 3.3 ± 0.3 μm, L/W ratio = 4.0.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, aerial mycelium lacking, medium, filter paper and on Anthriscus stem partly covered with acervuli appearing as tiny black spots, which are also visible from the reverse side; growth rate 17.5–22.5 mm in 7 d (32.5–36 mm in 10 d). Colonies on OA flat with entire margin; surface olivaceous to grey olivaceous, covered with black or salmon acervuli, aerial mycelium lacking, reverse olivaceous to olivaceous grey; growth rate 18–26 mm in 7 d (30–37.5 mm in 10 d). Conidia in mass salmon. Colonies of isolate IMI 309357 differ in forming felty white to olivaceous buff or grey aerial mycelium on OA and Anthriscus stem.
Material examined: USA, Hawaii, Oahu, Manoa, from Dendrobium sp., unknown collection date and collector (deposited in CBS collection Oct. 1980 by M. Aragaki, isolated 1978 as No. 826), (CBS H-20718 holotype, culture ex-type CBS 632.80); Hawaii, Kona, from × Ascocenda sp., unknown collection date and collector (deposited in CBS collection Oct. 1980 by M. Aragaki, isolated 1978 as No. 828), culture CBS 631.80. UK, from Phalaenopsis sp., unknown collection date and collector, culture IMI 305357. Panama, APHIS interception Miami 223820, from Cycnoches aureum, collection date unknown (isolated 11 Apr. 2003 by M.E. Palm), D. Begley, culture CBS 119291 = MEP1545. Germany, Munich, glasshouses of Botanical Garden, on dead and dying leaves of Eria javanica (syn. E. stellata), April 1895, J.E. Weiss (M-0140831 syntype of C. orchidearum (named as forma eriae) and lectotype of C. orchidearum, here designated); Munich, glasshouses of Botanical Garden, on both sides of dying leaves of Cymbidium aloifolium (syn. C. pendulum), Apr. 1895, J.E. Weiss (M-0140830 syntype of C. orchidearum (named as C. orchidearum forma cymbidii)); Munich, glasshouses of Botanical Garden, on dead and dying leaves of Stelis emarginata (syn. Physosiphon loddigesii), April 1895, J.E. Weiss (M-0140832 syntype of C. orchidearum (named as forma physosiphonis)).
Notes: Diagnostic features for C. orchidophilum include its very narrow (usually 3–3.5 μm wide) cylindrical conidia, abundantly formed setae and dark brown, uniformly shaped, pyriform to spathulate appressoria. Colletotrichum orchidophilum is basal to the C. acutatum species complex (fig. 1 in Cannon et al. 2012, this issue) and therefore used as outgroup in the phylogeny of the C. acutatum complex (Fig. 1). The species is associated with a range of genera in the Orchidaceae. According to blastn searches with ITS sequences, C. orchidophilum has possibly also been found on other orchids and in other countries: on Cycnoches aureum in Panama (DQ286148, Farr et al. 2006), on Pleione sp. (AJ301980, Nirenberg et al. 2002) and as an endophyte of Dendrobium nobile in China (FJ042519, Yuan et al. 2009). As far as we can tell, C. orchidophilum is restricted to the Orchidaceae.
The description of C. cinctum provided by Stoneman (1898), with its narrow conidia and abundant setae, seems similar to that of the strains we have identified as C. orchidophilum, and her strain originated from the same habitat and geographical region as Berkeley & Curtis’s fungus Gloeosporium cinctum (Berkeley 1874). Stoneman referred to the binomial C. cinctum but only in synonymy with the sexual morph Gnomoniopsis cincta (= Glomerella cincta), and it is therefore invalidly published. The connection between Gloeosporium cinctum and Gnomoniopsis cincta does seem to be doubtful; Stoneman referred to the Colletotrichum morph “found associated with a pycnidial stage and also a minute pyrenomycetous form”. The former was not described (and is presumably a co-coloniser rather than genetically linked), and the latter was described as having spores measuring only 6–7 × 2–3 μm – much smaller than typical Glomerella ascospores. This contrasts with the Gnomoniopsis morph described by Stoneman from old cultures of the asexual morph, which had ascospores measuring 15–20 × 3 μm; we interpret this as the true sexual morph. We have not seen a sexual morph associated with C. orchidophilum, and we think it is more likely that Stoneman’s fungus (and that of Berkeley & Curtis) belong to the C. gloeosporioides species complex.
Colletotrichum orchidophilum differs from C. orchidearum in forming much narrower conidia; those of the type of C. orchidearum forma eriae measure (13.5–)15.5–19.5 × 5–6 μm, mean ± SD = 17.2 ± 1.6 × 5.6 ± 0.3 μm, L/W ratio = 3.1, n = 20, and those of forma physosiphonis measure (14–)16–18.5 × 5–6 μm, mean ± SD = 17.2 ± 1.1 × 5.5 ± 0.3 μm, L/W ratio = 3.1, n = 20. Colletotrichum orchidearum was described by Allescher (1902) from three diseased orchid plants in the glasshouses of the Munich botanic garden. Each of the collections was given a separate name at forma rank, as listed above, and while no forma orchidearum was listed, that name automatically comes into existence on description of the other forms (Art. 26). The species account included a description of the overall taxon, and compared it with C. macrosporum, another species from orchids described by Saccardo (1896) from a Brazilian collection. That species was found by Saccardo to have substantially larger conidia than those of C. orchidearum (measurements of 28–32 × 8–10 μm were given), and its affinities are currently unknown. One of the forms introduced by Allescher, forma cymbidii, is invalidly published as no description was given.
There are three dried specimens in the Allescher collections in M, all gathered by Dr J.E. Weiss in April 1895, which clearly constitute type material; M-0140830 named as C. orchidearum forma cymbidii from Cymbidium aloifolium (syn. C. pendulum), M-0140831 named as forma eriae from Eria javanica (syn. E. stellata), and M-0140832 named as forma physosiphonis from Stelis emarginata (syn. Physosiphon loddigesii). The collection on Cymbidium appears to be effete, and while a few of the conidiomata contained setae, no conidia or conidiogenous cells were seen. This may be why C. orchidearum forma cymbidii was not described in the original publication. It is necessary to designate one of these authentic collections as lectotype of C. orchidearum in order to fix the application of that name, and we therefore choose M-0140831 for this purpose as M-0140830 is effete and M-0140832 is rather depauperate. That has the effect that C. orchidearum forma eriae becomes an obligate synonym of C. orchidearum forma orchidearum. There are no significant morphological differences between the material from Eria and that from Physosiphon. The conidiomata of the invalid forma cymbidii are substantially larger than those of the two validly published forms, and the host plant material is strongly blackened in their immediate vicinity. We are unable to establish the significance of this distinction; it may be host-rather than fungus-related.
Yang et al. (2011) reviewed species of Colletotrichum from orchids in south-western China. They identified one clade as C. orchidearum, and their Chinese strain does seem to have close similarities with the type of that species. None of the species treated in Yang et al. (2011) belong to the C. acutatum complex.
Colletotrichum paxtonii Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800508. Fig. 22.
Fig. 22.
Colletotrichum paxtonii (from ex-holotype strain IMI 165753). A–B. Conidiomata. C–F. Conidiophores. G–K. Appressoria. L–M. Conidia. A, C–D, L. from Anthriscus stem. B, E–K, M. from SNA. A–B. DM, C–M. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–M.
Etymology: Named after Sir Joseph Paxton, gardener to William Spencer Cavendish, 6th Duke of Devonshire, who first brought the Cavendish banana into cultivation.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–8 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ± inflated, 5–10 × 2–4 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end slightly acute both ends slightly acute, (5–)10.5–15.5(–19.5) × (2.5–)3.5–4(–4.5) μm, mean ± SD = 13.0 ± 2.6 × 3.7 ± 0.3 μm, L/W ratio = 3.5. Appressoria single or in loose groups, medium brown, smooth-walled, subglobose, elliptical or clavate, the edge undulate or entire, (5–)6–11.5(–16.5) × (3.5–)5.5–7.5(–8.5) μm, mean ± SD = 8.8 ± 2.7 × 6.5 ± 1.1 μm, L/W ratio = 1.4, strain CBS 502.97 forms smaller appressoria, measuring (3.5–)4.5–7.5(–10.5) × (3–)3.5–5(–5.5) μm, mean ± SD = 6.0 ± 1.7 × 4.2 ± 0.7 μm, L/W ratio = 1.4.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on pale brown, angular, basal cells 3.5–7.5 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, 7–19 × 2–3 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends ± acute, sometimes one end round, (6.5–)12–15.5(–17) × (3–)3.5–4 μm, mean ± SD = 13.7 ± 1.8 × 3.8 ± 0.3 μm, L/W ratio = 3.6.
Culture characteristics: Colonies on SNA flat to raised with entire margin, hyaline, on filter paper partly pale olivaceous grey, on medium, filter paper and Anthriscus stem partly covered with thin, floccose white to pale olivaceous grey aerial mycelium and orange acervuli, reverse hyaline to pale cinnamon, filter paper partly pale olivaceous grey; 23–24.5 mm in 7 d (34–36.5 mm in 10 d). Colonies on OA flat with entire margin; surface covered with floccose rosy buff to pale olivaceous grey aerial mycelium and orange acervuli, reverse pale vinaceous, hazel, olivaceous grey to iron grey; growth rate 22.5–23 mm in 7 d (33.5–35.5 mm in 10 d). Conidia in mass orange.
Material examined: St. Lucia, from Musa sp., 1972, P. Griffee (IMI 165753 holotype, CBS H-20797 isotype, culture ex-type IMI 165753). Unknown country (West Indies), from Musa nana, unknown collection date (deposited in CBS collection Feb. 1997 by J.A. Bailey), P. Spencer-Phillips, CBS 502.97 = LARS 58 [sterile on receipt at CBS, judging from information in Sherriff et al. (1994) these two strains originate from the same isolate].
Notes: The most prominent species of Colletotrichum associated with Musa species is C. musae, a central species in one of the major clades of the C. gloeosporioides species complex (Weir et al. 2012, this issue). It was recently epitypified with a strain from Florida (Su et al. 2011). One of the strains (CBS 502.97 = LARS 58) that we have examined of C. paxtonii was first studied by Sherriff et al. (1994) using the misapplied name C. musae; however, Johnston & Jones (1997) confirmed that it was a member of the C. acutatum complex. Colletotrichum paxtonii does not appear to produce setae at all, while C. musae rarely does so, and this may have led to confusion between the two species in the past.
There are no records of C. acutatum (s. lat.) on Musa in Farr & Rossman (2012); however, some other species have been described on Musa spp. Colletotrichum cavendishii was described by Petrak (1925) with “elongated oblong, ellipsoid, oblong or ovate, almost cylindrical” conidia that measure 10–19 × 4.5–7 μm. This certainly suggests that Petrak’s species belongs to the C. acutatum species complex and it could provide an earlier name for C. paxtonii, but its conidia are described as substantially wider than those of that the latter species - conidia in the C. acutatum species complex are rarely wider than 5 μm (Table 2). No cultures are available to allow evaluation of the synonymy.
Table 2.
Conidia measurements of Colletotrichum strains studied.
| Species | Accession No. |
Conidia on SNA |
Conidia on Anthriscus stem |
||||
|---|---|---|---|---|---|---|---|
| length × width (μm)2 |
length × width (μm) mean ± stdev |
L/W ratio | length × width (μm)2 |
length × width (μm) mean ± stdev |
L/W ratio | ||
| C. acerbum | CBS 128530* | 15.5–20.5(–29) × (4–)4.5–5 | 17.9 ± 2.4 × 4.7 ± 0.2 | 3.8 | (12.5–)15–18.5(–20.5) × (4–)4.5–5 | 16.8 ± 1.7 × 4.7 ± 0.3 | 3.6 |
| C. acutatum | CBS 112996* | (7.5–)11–14.5(–19) × 3.5–4(–4.5) | 12.6 ± 1.8 × 3.9 ± 0.3 | 3.2 | (8.5–)12–16.5(–17.5) × (3–)3.5–4.5(–5) | 14.3 ± 2.1 × 4.1 ± 0.4 | 3.5 |
| CBS 111993 | (10.5–)12–16(–20) × (3–)3.5–4.5(–5) | 14.0 ± 1.8 × 4.0 ± 0.5 | 3.5 | (12.5–)14–17(–18) × (3.5–)4–4.5(–5) | 15.5 ± 1.3 × 4.2 ± 0.3 | 3.7 | |
| CBS 127602 | (8–)11.5–14.5(–15) × (2.5–)3.5–4 | 13.0 ± 1.5 × 3.8 ± 0.4 | 3.5 | (8–)12.5–18.5(–22) × 4–4.5 | 15.5 ± 2.9 × 4.2 ± 0.3 | 3.7 | |
| CBS 112990 | (8.5–)12.5–15.5(–17.5) × (2.5–)4–5 | 14.1 ± 1.6 × 4.3 ± 0.5 | 3.3 | (8.5–)11.5–15.5(–17) × (3.5–)4–4.5(–5) | 13.5 ± 1.8 × 4.2 ± 0.3 | 3.2 | |
| CBS 129915 | (7–)12–15.5(–16.5) × (3.5–)4–4.5(–5) | 13.8 ± 1.9 × 4.1 ± 0.3 | 3.3 | (6.5–)11–14.5(–15.5) × (3.5–)4–4.5(–5) | 12.8 ± 2.0 × 4.3 ± 0.3 | 2.9 | |
| CBS 112759 | (6.5–)8.5–12(–13) × (2.5–)3–4 | 10.3 ± 1.9 × 3.4 ± 0.5 | 3.1 | (8–)11–15(–19) × (3–)4–5 | 12.9 ± 1.9 × 4.5 ± 0.4 | 2.9 | |
| CBS 112761 | (7.5–)10–15.5(–20.5) × (3–)3.5–4.5(–6) | 12.7 ± 2.7 × 4.1 ± 0.5 | 3.1 | ||||
| CBS 370.73 | (5–)6.5–11(–12.5) × (2–)2.5–3.5(–4.5) | 8.8 ± 2.1 × 3.2 ± 0.5 | 2.7 | ||||
| CBS 797.72 | (6–)8.5–13(–18) × 3.5–4.5(–5) | 10.8 ± 2.3 × 4.0 ± 0.3 | 2.7 | (5.5–)10–14(–15.5) × (3–)4–5 | 12.0 ± 2.2 × 4.4 ± 0.5 | 2.7 | |
| CBS 110735 | (7.5–)11–16.5(–21) × (3–)3.5–4(–4.5) | 13.7 ± 2.5 × 3.8 ± 0.4 | 3.6 | (12.5–)14–17(–17.5) × (3.5–)4–4.5(–5) | 15.5 ± 1.5 × 4.4 ± 0.3 | 3.5 | |
| CBS 112979 | (7.5–)9–13(–15) × (2–)3–3.5(–4) | 11 ± 2.0 × 3.3 ± 0.4 | 3.3 | (9–)10.5–15.5(–16.5) × (3–)3.5–4.5(–5) | 13.1 ± 2.4 × 4.2 ± 0.5 | 3.1 | |
| CBS 979.69 | (5–)8.5–14.5(–16.5) × (2.5–)3–4(–4.5) | 11.5 ± 3.1 × 3.5 ± 0.4 | 3.3 | (12–)13–15.5(–17) × (3.5–)4–4.5(–5) | 14.1 ± 1.3 × 4.1 ± 0.3 | 3.4 | |
| IMI 319423 | (8–)11.5–14.5(–16) × (3–)3.5–4.5 | 13.2 ± 1.5 × 4.0 ± 0.3 | 3.3 | (7.5–)11.5–15.5(–19.5) × (3–)3.5–4.5 | 13.6 ± 2.0 × 4.0 ± 0.4 | 3.4 | |
| C. australe | CBS 116478* | (10–)14.5–19.5(–25) × (3.5–)4–5(–6) | 17.0 ± 2.4 × 4.4 ± 0.5 | 3.9 | (16–)17–20(–22) × (4–)4.5–5(–5.5) | 18.6 ± 1.6 × 4.7 ± 0.4 | 4.0 |
| CBS 131320 | (14–)15–19(–26) × (3.5–)4–5(–5.5) | 17.0 ± 2.0 × 4.5 ± 0.3 | 3.8 | (13.5–)15–17.5(–18) × (3.5–)4–5(–5.5) | 16.3 ± 1.1 × 4.4 ± 0.4 | 3.7 | |
| C. brisbanense | CBS 292.67* | (12–)12–17.5(–25) × (3–)3.5–4(–5) | 14.8 ± 2.8 × 3.8 ± 0.5 | 3.9 | (9.5–)12–15(–17) × (3–)3.5–4 | 13.5 ± 1.4 × 3.9 ± 0.3 | 3.5 |
| C. chrysanthemi | CBS 126518 | (6–)7–9.5(–12) × (3–)4–5.5(–6) | 8.3 ± 1.3 × 4.8 ± 0.6 | 1.7 | (3.5–)6.5–10.5(–13.5) × (3.5–)4–5(–5.5) | 8.5 ± 1.8 × 4.5 ± 0.5 | 1.9 |
| CBS 1265181 | (3.5–)4.5–9(–15) × 3–5(–6.5) | 6.7 ± 2.3 × 4.1 ± 0.8 | 1.6 | ||||
| CBS 1265191 | (4–)5–10(–14) × (3–)3.5–5(–6) | 7.4 ± 2.6 × 4.1 ± 0.6 | 1.8 | (6.5–)8–10.5(–14.5) × (3–)4–5(–5.5) | 9.2 ± 1.4 × 4.5 ± 0.4 | 2.0 | |
| IMI 364540 | (4.5–)6.5–13(–26) × (3–)3.5–5(–11) | 9.8 ± 3.2 × 4.3 ± 0.9 | 2.3 | (5–)6.5–12.5(–21) × (2–)3.5–5(–6) | 9.4 ± 2.9 × 4.3 ± 0.7 | 2.2 | |
| C. cosmi | CBS 853.73* | (7–)13–18.5(–19.5) × (3–)3.5–4.5 | 15.8 ± 2.5 × 4.0 ± 0.4 | 4.0 | (12–)14–16.5(–18) × (3.5–)4–4.5 | 15.3 ± 1.4 × 4.0 ± 0.3 | 3.8 |
| C. costaricense | CBS 330.75* | (9–)11.5–18(–28) × (3–)3.5–4(–4.5) | 14.6 ± 3.1 × 3.7 ± 0.3 | 4.0 | 12.5–)13.5–16(–18) × 3.5–4 | 14.8 ± 1.4 × 3.8 ± 0.3 | 3.9 |
| CBS 211.78 | (11.5–)12–16(–20) × (3.5–)4–4.5 | 13.9 ± 1.9 × 4.3 ± 0.2 | 3.2 | (12–)14–16.5(18.5) × (3.5–)4–4.5 | 15.2 ± 1.4 × 4.2 ± 0.2 | 3.6 | |
| C. cuscutae | IMI 304802* | (15.5–)17.5–21(–22.5) × (3–)3.5–4.5 | 19.2 ± 1.7 × 4.0 ± 0.3 | 4.8 | (15–)17–20(–21) × (3.5–)4–4.5 | 18.6 ± 1.5 × 4.2 ± 0.2 | 4.5 |
| C. fioriniae | CBS 128517* | (10–)13.5–16.5(–19.5) × 4–5(–5.5) | 15.0 ± 1.6 × 4.5 ± 0.3 | 3.3 | (12.5–)14–18.5(–24.5) × 4–5 | 16.1 ± 2.2 × 4.4 ± 0.4 | 3.6 |
| CBS 200.35 | (8–)10.5–17.5(–30.5) × 3.5–5(–6.5) | 14.1 ± 3.4 × 4.3 ± 0.6 | 3.3 | (9–)12.5–17.5(–23) × (3.5–)4–5 | 15.0 ± 2.3 × 4.4 ± 0.4 | 3.4 | |
| CBS 127599 | (7.5–)12–16.5(–17) × (2.5–)4–5(–5.5) | 14.4 ± 2.4 × 4.5 ± 0.6 | 3.2 | (10.5–)13.5–17(–17.5) × (3.5–)4–5 | 15.1 ± 1.7 × 4.4 ± 0.4 | 3.4 | |
| CBS 129916 | (6.5–)11.5–16 × (3–)3.5–4.5(–5) | 13.8 ± 2.2 × 3.9 ± 0.4 | 3.5 | (10–)13–16.5(–18) × (4–)4.5–5(–5.5) | 14.8 ± 1.7 × 4.7 ± 0.3 | 3.2 | |
| CBS 127601 | (4.5–)10.5–18(–20) × (3.5–)4–5(–5.5) | 14.2 ± 4.0 × 4.5 ± 0.4 | 3.2 | (7–)13.5–18.5(–19) × (3.5–)4–4.5(–5) | 15.9 ± 2.3 × 4.4 ± 0.4 | 3.6 | |
| CBS 129947 | (10.5–)12–15(–17) × 3.5–5(–6) | 13.5 ± 1.7 × 4.1 ± 0.8 | 3.3 | (13–)14–16(–17) × (3.5–)4–4.5(–5) | 15.0 ± 1.0 × 4.3 ± 0.4 | 3.5 | |
| CBS 293.67 | (8.5–)12.5–16(–17.5) × (3–)3.5–4.5(–5) | 14.4 ± 1.7 × 4.0 ± 0.5 | 3.6 | (13–)14–16.5(–18) × (4–)4.5–5(–5.5) | 15.2 ± 1.3 × 4.7 ± 0.3 | 3.2 | |
| C. godetiae | CBS 133.44* | (7–)10.5–14.5(–15.5) × (3.5–)4–5(–5.5) | 12.4 ± 2.0 × 4.3 ± 0.5 | 2.9 | (9.5–)10.5–15(–20.5) × 4–5 | 12.8 ± 2.3 × 4.5 ± 0.4 | 2.8 |
| CBS 125972 | (5.5–)12–16.5(–17) × (3.5–)4–4.5(–5) | 14.2 ± 2.2 × 4.3 ± 0.4 | 3.3 | (13.5–)14–16(–17) × (4–)4.5–5 | 15.1 ± 0.9 × 4.7 ± 0.3 | 3.2 | |
| C. godetiae | CBS 193.32 | (8–)11.5–15(–19.5) × (2.5–)3.5–4.5(–5) | 13.2 ± 1.9 × 4.2 ± 0.5 | 3.1 | (6.5–)9.5–14(–15.5) × (3–)3.5–4.5(–5.5) | 11.8 ± 2.2 × 4.1 ± 0.6 | 2.9 |
| CBS 129911 | (10.5–)12.5–15.5(–17.5) × (3–)4–5(–6.5) | 13.9 ± 1.4 × 4.5 ± 0.6 | 3.1 | (7–)9–13(–15.5) × (2.5–)3–4 | 11.0 ± 2.0 × 3.5 ± 0.3 | 3.1 | |
| CBS 862.70 | (8–)14–19(–24) × (4–)4.5–5(–5.5) | 16.4 ± 2.4 × 4.9 ± 0.4 | 3.4 | (12.5–)15.5–18(–19.5) × 4.5–5(5.5) | 16.8 ± 1.4 × 4.9 ± 0.2 | 3.4 | |
| CBS 129809 | (10.5–)13.5–17(–22.5) × (3.5–)4–5(–5.5) | 15.3 ± 1.9 × 4.4 ± 0.3 | 3.4 | (14–)14–17.5(–23) × 4–4.5(–5) | 15.8 ± 1.6 × 4.4 ± 0.3 | 3.6 | |
| CBS 129816 | (13–)13.5–16.5(–20.5) × 4–4.5(–5) | 15.1 ± 1.4 × 4.4 ± 0.3 | 3.4 | (13–)14–16(–17.5) × 4.5–5 | 15.1 ± 1.1 × 4.7 ± 0.2 | 3.2 | |
| CBS 127561 | (12.5–)14–15.5(–16.5) × 4.5–5(–5.5) | 14.7 ± 0.9 × 4.9 ± 0.3 | 3.0 | (11.5–)13.5–17.5(–20) × (4–)4.5–5.5 | 15.5 ± 1.8 × 5.0 ± 0.4 | 3.1 | |
| CBS 129917 | (8–)10–15(–18.5) × 3–4.5(–5.5) | 12.5 ± 2.3 × 3.8 ± 0.6 | 3.3 | (9.5–)13–16(–17) × (4–)4.5–5.5(–6) | 14.6 ± 1.5 × 4.9 ± 0.5 | 3.0 | |
| C. guajavae | IMI 350839* | (6–)10.5–16.5(–23.5) × (2.5–)3–4(–5) | 13.4 ± 3.0 × 3.5 ± 0.5 | 3.8 | (11–)13–16(–17) × (3–)3.5–4 | 14.6 ± 1.7 × 3.8 ± 0.3 | 3.9 |
| C. indonesiense | CBS 127551* | (8–)10–14.5(–18) × (2.5–)3.5–4(–4.5) | 12.3 ± 2.4 × 3.8 ± 0.3 | 3.2 | (10.5–)13–17.5(–19) × (3–)3.5–4 | 15.4 ± 2.2 × 3.7 ± 0.2 | 4.1 |
| C. johnstonii | CBS 128532* | (13.5–)14.5–19(–21.5) × (3.5–)4.5–5(–6) | 16.7 ± 2.1 × 4.7 ± 0.4 | 3.6 | (14.5–)15.5–17(–18) × 4.5–5(–5.5) | 16.3 ± 1.0 × 4.9 ± 0.3 | 3.3 |
| IMI 357027 | (13–)14.5–17(–19) × (4–)4.5–5(–5.5) | 15.6 ± 1.3 × 4.7 ± 0.3 | 3.3 | (8.5–)14.5–17.5(–19) × (3–)4–5 | 15.9 ± 1.6 × 4.6 ± 0.4 | 3.8 | |
| C. kinghornii | CBS 198.35* | (11–)15.5–21(–22.5) × (3–)3.5–4(–4.5) | 18.3 ± 2.9 × 3.8 ± 0.4 | 4.9 | (15–)16–20.5(–23) × 3.5–4.5 | 18.1 ± 2.3 × 4.0 ± 0.4 | 4.6 |
| C. laticiphilum | CBS 112989* | (9.5–)13.5–19.5(–25.5) × (3–)3.5–4(–4.5) | 16.6 ± 3.1 × 3.8 ± 0.4 | 4.4 | (10–)12–15(–19.5) × 4–5(–5.5) | 13.6 ± 1.7 × 4.5 ± 0.3 | 3.0 |
| CBS 129827 | (5–)8–15(–18.5) × (1.5–)2.5–4.5(–5.3) | 11.5 ± 3.4 × 3.6 ± 0.9 | 3.2 | (10–)12.5–17.5(–20) × 4–4.5(–5) | 15.1 ± 2.5 × 4.4 ± 0.3 | 3.4 | |
| C. limetticola | CBS 114.14* | (9–)12–20.5(–29) × (3–)4–5(–6) | 16.3 ± 4.2 × 4.5 ± 0.6 | 3.6 | (12–)13–18(–24) × (3.5–)4–4.5(–5.5) | 15.5 ± 2.3 × 4.3 ± 0.4 | 3.6 |
| C. lupini | CBS 109225* | 9–15(–26.5) × (3–)3.5–4.5(–6) | 12.0 ± 3.2 × 4.1 ± 0.6 | 2.9 | (10–)12.5–16(–18.5) × (3–)3.5–4.5 | 14.2 ± 1.7 × 4.0 ± 0.3 | 3.6 |
| IMI 375715 | (7.5–)9.5–16(–25) × 3.5–5(–6.5) | 12.8 ± 3.3 × 4.3 ± 0.6 | 3.0 | (10–)11.5–15(–17) × (3.5–)4–4.5(–5) | 13.3 ± 1.8 × 4.2 ± 0.3 | 3.1 | |
| CBS 109221 | 11.5–15.5(–19) × (3.5–)4–4.5(–5) | 13.5 ± 1.9 × 4.3 ±0 .4 | 3.2 | (11.5–)13.5–16.5(–18.5) × (3.5–)4–4.5 | 15.0 ± 1.5 × 4.3 ± 0.3 | 3.5 | |
| C. melonis | CBS 159.84* | (7–)9–16.5(–23.5) × (3–)3.5–4.5(–5) | 12.8 ± 3.6 × 3.9 ± 0.4 | 3.3 | (9–)12–17(–20) × (3.5–)4–4.5(–5) | 14.5 ± 2.3 × 4.2 ± 0.3 | 3.5 |
| C. nymphaeae | CBS 173.51 | (6–)9.5–13.5(–15) × (2–)3–4.5 | 11.5 ± 1.8 × 3.7 ± 0.5 | 3.1 | (7.5–)10–14.5(–16) × (3–)3.5–4.5 | 12.3 ± 2.0 × 3.9 ± 0.4 | 3.2 |
| CBS 112992 | (5.5–)8–14.5(–20.5) × (2.5–)3–4(–4.5) | 11.2 ± 3.4 × 3.5 ± 0.5 | 3.2 | (7.5–)11–16(–20) × (2.5–)3.5–4(–4.5) | 13.6 ± 2.7 × 3.8 ± 0.4 | 3.6 | |
| CBS 126372 | (9.5–)13–17.5(–21.5) × (2.5–)3.5–4.5(–5.5) | 15.3 ± 2.4 × 3.9 ± 0.7 | 3.9 | (12.5–)13.5–17(–18.5) × (3.5–)4–4.5 | 15.3 ± 1.7 × 4.2 ± 0.3 | 3.7 | |
| CBS 112202 | (10–)14–17(–18.5) × (3–)4–4.5 | 15.7 ± 1.6 × 4.1 ± 0.3 | 3.8 | (12–)13.5–17.5(–19.5) × (3.5–)4(–4.5) | 15.5 ± 1.8 × 4.0 ± 0.2 | 3.9 | |
| CBS 126382 | (3–)7.5–14(–17.5) × 3–4(–5) | 10.8 ± 3.4 × 3.6 ± 0.5 | 3.0 | (11–)12.5–16(–18) × (3–)3.5–4.5 | 14.4 ± 1.8 × 4.0 ± 0.3 | 3.6 | |
| CBS 515.78* | (10–)14–18.5(–19.5) × (3–)4–5.5(–6) | 16.1 ± 2.3 × 4.9 ± 0.7 | 3.3 | (12.5–)14–18.5(–22.5) × (4–)4.5–5.5(–6) | 16.3 ± 2.1 × 4.8 ± 0.5 | 3.4 | |
| CBS 526.77 | (8.5–)9–13(–16) × (3–)3–4.5(–5) | 11.0 ± 2.0 × 3.8 ± 0.6 | 2.9 | (9.5–)13.5–19(–21.5) × (3.5–)5–6(–6.5) | 16.1 ± 2.7 × 5.6 ± 0.7 | 2.9 | |
| C. orchidophilum | CBS 632.80* | (10.5–)11.5–14(–16.5) × (2–)3–3.5(–4) | 12.7 ± 1.1 × 3.1 ± 0.3 | 4.1 | (11.5–)12.5–14(–15.5) × (2.5–)3–3.5(–4) | 13.2 ± 0.9 × 3.3 ± 0.3 | 4.0 |
| IMI 305357 | (8.5–)11.5–17(–25) × (1.5–)2.5–4(–4.5) | 14.2 ± 2.7 × 3.3 ± 0.6 | 4.4 | (10–)12–14.5(–15) × 3–3.5(–4) | 13.3 ± 1.0 × 3.5 ± 0.3 | 3.8 | |
| CBS 119291 | (13.5–)14–15.5(–16) × 3–3.5(–4) | 14.8 ± 0.7 × 3.3 ± 0.3 | 4.5 | (10.5–)11.5–13.5(–14.5) × 3–4 | 12.7 ± 1.0 × 3.5 ± 0.3 | 3.6 | |
| CBS 631.80 | (13–)13.5–17.5(–19) × 2.5–3.5 | 15.4 ± 2.1 × 3.0 ± 0.3 | 5.1 | (10.5–)11.5–13.5(–14.5) × (3–)3.5–4 | 12.4 ± 0.9 × 3.6 ± 0.3 | 3.4 | |
| C. paxtonii | IMI 165753* | (5–)10.5–15.5(–19.5) × (2.5–)3.5–4(–4.5) | 13.0 ± 2.6 × 3.7 ± 0.3 | 3.5 | (6.5–)12–15.5(–17) × (3–)3.5–4 | 13.7 ± 1.8 × 3.8 ± 0.3 | 3.6 |
| C. phormii | CBS 118194* | (17–)20–26(–35.5) × 4–5(–6.5) | 23.0 ± 3.2 × 4.6 ± 0.6 | 5.1 | (14–)20–24.5(–25.5) × 4–4.5(–5) | 22.3 ± 2.3 × 4.3 ± 0.2 | 5.2 |
| CBS 102054 | (180.5–)20–24(–29) × (4–)4.5–5(–5.5) | 22.1 ± 2.1 × 4.8 ± 0.4 | 4.6 | (19–)20.5–24(–25) × (4–)4.5–5.5 | 22.2 ± 1.6 × 4.9 ± 0.4 | 4.5 | |
| CBS 118197 | 19.5–25(–33.4) × (3.5–)4–5(–6) | 22.3 ± 2.6 × 4.5 ± 0.4 | 5.0 | 21.5–26(–30) × (4–)4.5–5(–6) | 23.7 ± 2.1 × 4.9 ± 0.4 | 4.9 | |
| C. phormii | CBS 118201 | (21–)21.5–24(–24.5) × 4–4.5 | 22.9 ± 1.2 × 4.4 ± 0.3 | 5.2 | (20–)20.5–23.5(–25) × 4.5–5(–5.5) | 21.9 ± 1.4 × 4.8 ± 0.2 | 4.5 |
| CBS 118191 | (18–)18.5–30(–39.5) × (3–)3.5–4.5(–5) | 24.1 ± 5.5 × 4.2 ± 0.3 | 5.7 | (14–)18.5–22(–24) × (4–)4.5–5(–5.5) | 20.3 ± 1.9 × 4.9 ± 0.4 | 4.1 | |
| CBS 124953 | (13.5–)18–26.5(–28) × 4–4.5(–5) | 22.3 ± 4.2 × 4.4 ± 0.3 | 5.0 | (20.5–)21–23(–23.5) × 4.5–5 | 22.0 ± 1.2 × 4.9 ± 0.2 | 4.5 | |
| CBS 483.82 | (18–)19–28(–33.5) × 4–5(–6.5) | 23.3 ± 4.5 × 4.5 ± 0.6 | 5.2 | (19–)20–22(–23) × 4.5–5(–5.5) | 20.9 ± 1.0 × 4.7 ± 0.3 | 4.4 | |
| C. pseudoacutatum | CBS 436.77* | (9.5–)11.5–13.5(–14.5) × 3.5–4 | 12.7 ± 1.1 × 3.8 ± 0.2 | 3.4 | (9.5–)11.5–13.5(–14.5) × 3.5–4 | 15.0 ± 1.2 × 4.2 ± 0.3 | 3.5 |
| C. pyricola | CBS 128531* | (10–)14.5–18.5(–24) × (3.5–)4.5–5(–5.5) | 16.7 ± 2.1 × 4.7 ± 0.4 | 3.5 | (9.5–)14–17(–18.5) × (4–)4.5–5(–5.5) | 15.4 ± 1.6 × 4.8 ± 0.4 | 3.2 |
| C. rhombiforme | CBS 129953* | (12–)12.5–17(–24) × (4–)4.5–5.5(–6) | 14.7 ± 2.1 × 5.0 ± 0.7 | 2.9 | (7.5–)10.5–17.5(–21) × (3.5–)4–5.5(–6) | 14.1 ± 3.5 × 4.8 ± 0.6 | 2.9 |
| C. salicis | CBS 607.94* | (8.5–)10.5–15.5(–19.5) × (3.5–)3–4.5(–5) | 13.0 ± 2.4 × 4.0 ± 0.5 | 3.2 | (14.5–)16–18.5(–20) × (4–)4.5–5(–5.5) | 17.1 ± 1.3 × 4.9 ± 0.3 | 3.5 |
| CBS 115.14 | (9–)10.5–15(–17) × 2.5–3.5(–4) | 12.7 ± 2.3 × 3.1 ± 0.5 | 4.1 | (9.5–)11.5–16(–18.5) × (2.5–)3–4(–4.5) | 14.0 ± 2.3 × 3.3 ± 0.4 | 4.2 | |
| CBS 465.83 | (7.5–)9.5–15.5(–22) × 3–3.5(–4.5) | 12.4 ± 3.1 × 3.3 ± 0.4 | 3.8 | not observed | |||
| C. scovillei | CBS 126529* | (10.5–)12.5–15(–16.5) × (3–)3.5–4(–4.5) | 13.7 ± 1.3 × 3.8 ± 0.3 | 3.6 | (9–)14.5–18(–19.5) × 3.5–4.5 | 16.0 ± 1.8 × 4.0 ± 0.3 | 4.0 |
| CBS 120708 | (11.5–)12.5–14.5(–15) × 3–3.5 | 13.5 ± 0.8 × 3.3 ± 0.2 | 4.1 | (12.5–)13–16(–18) × (3–)3.5–4 | 14.6 ± 1.4 × 3.6 ± 0.3 | 4.1 | |
| C. simmondsii | CBS 122122*,1 | (4.5–)6.5–10(–11.5) × (2–)2.5–3.5(–4) | 8.1 ± 1.7 × 2.9 ± 0.4 | 2.7 | (6–)7–10(–12.5) × (2–)2.5–3.5(–4.5) | 8.4 ± 1.5 × 3.0 ± 0.5 | 2.8 |
| CBS 294.67 | (6–)10.5–14(–16.5) × 3.5–4.5(–5.5) | 12.3 ± 1.8 × 4.0 ± 0.4 | 3.0 | (11–)12–14.5(–15.5) × (3–)4–4.5(–5) | 13.3 ± 1.2 × 4.1 ± 0.4 | 3.2 | |
| CBS 114494 | (6–)9.5–14.5(–15.5) × (2.5–)3–4(–4.5) | 12.1 ± 2.7 × 3.6 ± 0.5 | 3.3 | (10–)13–17(–18) × (3–)3.5–4.5(–5) | 14.9 ± 1.9 × 3.8 ± 0.4 | 3.9 | |
| IMI 354381 | (8.5–)11–15(–16) × (3.5–)4–4.5 | 13.0 ± 1.8 × 4.2 ± 0.2 | 3.1 | (12–)13.5–17(–19) × (3.5–)4–4.5(–5) | 15.4 ± 1.7 × 4.2 ± 0.3 | 3.7 | |
| C. sloanei | IMI364297* | (8.5–)12–17(–22) × (3–)3.5–4(–4.5) | 14.4 ± 2.5 × 3.7 ± 0.3 | 3.9 | (9–)11.5–15.5(–19.5) × (3–)3.5–4(–4.5) | 13.4 ± 1.8 × 3.9 ± 0.3 | 3.5 |
| C. tamarilloi | CBS 129814* | (8.5–)11.5–14.5(–15) × (2.5–)3–4(–4.5) | 13.0 ± 1.4 × 3.5 ± 0.4 | 3.7 | (10.5–)12–16(–22) × (3–)3.5–4.5(–5) | 14.0 ± 1.9 × 4.0 ± 0.4 | 3.5 |
| CBS 129811 | (9.5–)12–15.5(–19.5) × (3–)3.5–4(–4.5) | 13.7 ± 1.6 × 3.7 ± 0.3 | 3.7 | (12.5–)13.5–16.5(–17.5) × (3–)3.5–4 | 15.1 ± 1.4 × 3.8 ± 0.3 | 4.0 | |
| CBS 129955 | (10.5–)11.5–14.5(–17.5) × 3–4(–5) | 13.2 ± 1.5 × 3.6 ± 0.4 | 3.6 | (11.5–)13.5–17(–18.5) × 3.5–4(–4.5) | 15.3 ± 1.7 × 3.8 ± 0.3 | 4.0 | |
| C. walleri | CBS 125472* | (6–10.5)15.5–(–19.5) × (3–)3.5–4.5(–5.5) | 13.0 ± 2.7 × 4.0 ± 0.5 | 3.3 | (10.5–)12–16(–18.5) × 3.5–4(–4.5) | 13.9 ± 1.8 × 4.0 ± 0.3 | 3.5 |
| Colletotrichum sp. | CBS 129821 | (9–)12–14.5(–15.5) × (3–)3.5–4.5(–5.5) | 13.2 ± 1.4 × 4.0 ± 0.5 | 3.3 | (10–)13–17(–20) × 3.5–4(–4.5) | 14.9 ± 2.0 × 4.0 ± 0.2 | 3.8 |
| CBS 129820 | (9.5–)11–15(–19.5) × (2.5–)3.5–4(–4.5) | 13.1 ± 1.9 × 3.7 ± 0.4 | 3.5 | (9.5–)12–14.5(–16) × (3–)4–4.5 | 13.3 ± 1.3 × 4.0 ± 0.2 | 3.3 | |
| CBS 129823 | (7–)10.5–15.5(–18) × (2.5–)3–4(–4.5) | 13.1 ± 2.3 × 3.5 ± 0.6 | 3.7 | (9–)12–15.5(–17) × (2.5–)3.5–4(–4.5) | 14.0 ± 1.8 × 3.8 ± 0.4 | 3.7 | |
| IMI 384185 | (9–)12–14(–14.5) × (2.5–)3–4(–4.5) | 12.3 ± 1.5 × 3.6 ± 0.4 | 3.4 | (6–)10–16.5(–19.5) × (3–)3.5–4.5(–6) | 13.5 ± 3.2 × 4.0 ± 0.6 | 3.4 | |
| CBS 101611 | (13–)15–19(–22) × (3.5–)4–5(–5.5) | 16.9 ± 2.0 × 4.5 ± 0.4 | 3.7 | (14–)16.5–20(–23.5) × (4–)4.5–5(–5.5) | 18.3 ± 1.8 × 4.6 ± 0.3 | 4.0 | |
| CBS 129810 | (12.5–)13–17(–23.5) × (2.5–)3.5–4(–4.5) | 15.1 ± 2.1 × 3.9 ± 0.3 | 3.9 | (7.5–)9.5–12.5(–15) × 2.5–3.5(–4) | 10.8 ± 1.5 × 2.9 ± 0.5 | 3.7 | |
ex-type strain
aerial mycelium
(min–)min-stdev–max-stdev(–max)
Another species on banana was described by Sawada (1959), C. liukiuensis, on leaves of Musa liukiuensis in Taiwan. The conidia of this species are described as ellipsoid or oblong-ellipsoid with rounded ends, measuring 12–14 × 4.8–5.5 μm. The fungus forms dark brown 1–2-septate setae, which seem to be prominent, because they were included in the sketchy drawing (Pl. II: 30-31 of the publication) Sawada provided. This drawing showed a seta present as well as conidia with broadly rounded ends. Together with the width of the conidia, these characters exclude the name of this fungus from contention as an earlier synonym of C. paxtonii.
Additional species on Musa have been described in Gloeosporium. Gloeosporium musarum Cooke & Massee has elongate-ellipsoidal conidia with both ends rounded, measuring 12 × 4 μm. It was collected from ripe bananas in Brisbane, Australia (Cooke 1887). Apart from the rounded ends of the conidia, the fungus has features that tend to place it in the C. acutatum complex. Glomerella musarum was described from Musa paradisiaca in Sri Lanka and was observed to be associated with Gm. musarum and other fungi (Petch 1917). We could not locate the type material of either of these species to confirm their taxonomic positions. Gloeosporium musarum var. importatum, described in 1910 on fruits of Musa sapinea in Germany, has conidia larger than those of C. paxtonii, measuring 9–24 × 5–7 μm (Saccardo 1913). Gloeosporium lagenaria var. musarum was published without any morphological information; the paper merely stated that this fungus did not differ from the forms found on Cucurbitaceae (Ellis & Everhart 1889). The lack of description means that the name is invalidly published. Gloeosporium lagenaria var. lagenaria again has conidia larger than those of C. paxtonii, measuring 16–18 × 5–6 μm. It is widely believed to be a synonym of C. orbiculare (Cannon et al. 2012, this issue).
Colletotrichum paxtonii is separated from other species by TUB2 and GAPDH, with TUB2 performing best as a diagnostic sequence. With the GAPDH sequence there is only one bp difference from C. sloanei, while ACT, HIS3 and CHS-1 sequences are the same as C. simmondsii. The closest matches in a blastn search with the TUB2 sequence of strain IMI 165753 (with 99 % identity, 2 bp differences) were AJ748635 from isolate PD 89/582 (= CBS 126524, C. simmondsii) from Cyclamen in the Netherlands (Talhinhas et al. 2005), EU635505 from isolate DAR 32068 (as A9 from Whitelaw-Weckert et al. 2007) from Fragaria in Australia (Debode et al. 2009), EF143968 from isolate BRIP 4704a from Fragaria in Australia (Than et al. 2008a) and FJ907443 from isolate BRIP 28519 (ex-holotype culture of C. simmondsii) from Carica papaya in Australia (Prihastuti et al. 2009). With the GAPDH sequence of strain IMI 165753 there are no closer matches than 97 % identity covering ± the full of the length sequence. Since the ITS sequence of C. paxtonii strain IMI 165753 is the same as that of several other Colletotrichum spp., there is a long list of 100 % matching sequences in GenBank. These sequences, however, are all from isolates with hosts other than Musa.
Colletotrichum phormii (Henn.) D.F. Farr & Rossman, Mycol. Res. 110(12): 1403. 2006. Fig. 23.
Fig. 23.
Colletotrichum phormii (from ex-epitype strain CBS 118194). A–B. Conidiomata. C. Seta. D–G. Conidiophores. H–L. Appressoria. M–N. Conidia. A, C–D, M. from Anthriscus stem. B, E–L, N. from SNA. A–B. DM, C–N. DIC, Scale bars: A = 100 μm, D = 10 μm. Scale bar of A applies to A–B. Scale bar of D applies to C–N.
Basionym: Fusarium phormii Henn., Verh. bot. Ver. Prov. Brandenb. 40: 175. 1898. [1899].
≡ Gloeosporium phormii (Henn.) Wollenw., Fus. Auto Delin. no. 498. 1916, non Sacc. 1915.
= Gloeosporium phormii Sacc., Nuovo Giorn. Bot. Ital. n.s. 22: 67. 1915.
-
= Cryptosporium rhodocyclum Mont. ex Almeida & Souza da Camara, Bol. Soc. Brot. 25: 190. 1909.
≡ Gloeosporidium rhodocyclum (Mont. ex Almeida & Souza da Camara) Höhn., Annls mycol. 18(1/3): 92. 1920.
≡ Colletotrichum rhodocyclum (Mont. ex Almeida & Souza da Camara) Petr., Annls mycol. 25(3/4): 251. 1927.
-
= Physalospora phormii J. Schröt., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.2(3): 347. 1894.
≡ Hypostegium phormii (J. Schröt.) Theiss., Verh. zool.-bot. Ges. Wien 66: 384. 1916.
≡ Glomerella phormii (J. Schröt.) D.F. Farr & Rossman, Mycol. Res. 110(12): 1403. 2006.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to very pale brown, smooth-walled to finely verruculose, simple or septate and branched, to 40 μm in length. Conidiogenous cells hyaline to pale brown, smooth-walled to finely verruculose, cylindrical, elongate ampulliform to ampulliform, 7.5–16.5 × 2.2–4.5 μm, opening 1–1.5 μm diam, collarette 1–2 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled or verruculose, aseptate, straight, cylindrical to fusiform with both ends acute or one end round and one end acute, (17–)20–26(–35.5) × 4–5(–6.5) μm, mean ± SD = 23.0 ± 3.2 × 4.6 ± 0.6 μm, L/W ratio = 5.1. Appressoria single or in loose groups, medium to dark brown, outline mostly oblong to irregular, the edge entire or undulate, rarely lobate, (4–)8.5–20.5(–32) × (2.5–)4–6(–8) μm, mean ± SD = 14.5 ± 6.2 × 5.1 ± 1.0 μm, L/W ratio = 2.9, appressoria of strain CBS 102054 are shorter, measuring (5.5–)8–13(–14.5) × 5–6.5(–8) μm, mean ± SD = 10.4 ± 2.4 × 5.8 ± 0.8 μm, L/W ratio = 1.8.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores and setae formed on a cushion of pale brown roundish to angular cells, 2.5–10 μm diam. Setae few, hyaline to medium brown, smooth-walled, 0–1-septate, 25–70 μm long, base cylindrical, to 5 μm diam, tip ± roundish to ± acute. Conidiophores pale brown, smooth-walled, septate, branched, to 50 μm long. Conidiogenous cells pale brown, smooth-walled, usually cylindrical, sometimes elongate ampulliform to ampulliform, 8–25 × 2.5–3.5(–5.5) μm, opening 1–1.5 μm diam, collarette 1 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends acute, (14–)20–24.5(–25.5) × 4–4.5(–5) μm (one conidium measured 47 × 5 μm), mean ± SD = 22.3 ± 2.3 × 4.3 ± 0.2 μm, L/W ratio = 5.2, conidia of most other strains are slightly broader and those of strain CBS 118191 are additionally shorter than conidia of the ex-epitype strain, measuring (14–)18.5–22(–24) × (4–)4.5–5(–5.5) μm, mean ± SD = 20.3 ± 1.9 × 4.9 ± 0.4 μm, L/W ratio = 4.1.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline with felty white aerial mycelium on Anthriscus stem and filter paper, filter paper on both sides partially olivaceous to pale olivaceous grey; growth rate 15–19 mm in 7 d (27.5–32.5 mm in 10 d). Colonies on OA flat with entire margin; surface buff, with olivaceous to grey olivaceous sectors, and roundish olivaceous grey structures embedded in the medium, surface partly covered with floccose-felty white to pale olivaceous grey aerial mycelium, reverse buff with pale to dark olivaceous grey sectors; growth rate 15–21 mm in 7 d (27.5–33.5 mm in 10 d). Conidia in mass salmon.
Material examined: Germany, Berlin, Botanical garden, Kalthaus, from Phormium tenax, Apr. 1889, P. Hennings, (B 70 0005220 holotype of Fusarium phormii [not seen]); APHIS interception Port Orlando 007160, from Phormium sp., 6 Nov. 2000, W. Sheta, (CBS-H 20720 epitype, here designated, culture ex-epitype CBS 118194 = AR 3546). New Zealand, Auckland, Blockhouse Bay, from leaf spot of Phormium sp., Jun. 1999, C.F. Hill, culture CBS 102054; from leaf of Phormium sp., APHIS interception Los Angeles, California 134866, 1 May 1997, M.A. Abdelshife, culture CBS 118197 = AR 3389; APHIS interception Los Angeles, California 105828), from leaf of Phormium sp., 4 May 1993, N. Suzuki, culture CBS 118201 = MEP 1334; from Phormium tenax, unknown collection date and collector (deposited in CBS 1 Aug. 198), culture CBS 483.82. South Africa, from leaf of Phormium sp., APHIS interception Miami, Florida, 223143, 26 Feb. 2002, H. Ruiz, culture CBS 118191 = AR 3787. Netherlands, from leaf of Phormium sp., unknown collection date and collector, culture CBS 124953.
Notes: The synonymy given for this species follows Farr et al. (2006), and this work should be consulted for details. Fusarium phormii was described by Hennings (1898) on leaves of Phormium tenax in the Botanical Garden in Berlin, Germany, as forming sporodochia with oblong-cylindrical to fusoid, straight to slightly curved, multiguttulate, hyaline conidia, measuring 18–25 × 4–6 μm. Hennings (1898) found this fungus together with Physalospora phormii, and assumed the two belonged together. Fusarium phormii is formed on the leaf surface, while the perithecia of P. phormii appear on the undersurface. Kinghorn (1936) observed structures considered to be the sexual morph of C. phormii on leaves of Phormium plants but not in culture, as did von Arx (in litt.). We have found, however, that Kinghorn was looking at two species; part of this material belongs to a species that is named in the present publication as C. kinghornii. The sexual morph, originally named as Physalospora phormii, was originally found by Schröter (1894) on dead leaves of Phormium tenax in Breslau, Germany (today: Wrocław, Poland).
The sexual morph was not observed in our study. Farr et al. (2006) gave the following description: “Ascomata on upper and lower surface of leaves in large, elliptical, discoloured areas similar to those bearing acervuli, with or without a narrow, black margin, subepidermal, sometimes partially erumpent, solitary, scattered to crowded or aggregated, black, shiny when exposed, globose to ellipsoid, flattened. Ascomatal walls of thin-walled, brown cells, 9–15 μm diam. Paraphyses sparse, inflated, hyaline. Asci unitunicate, narrowly clavate with a rounded apex and short stipe, 56–70 × 15–20 μm, with an indistinct apical ring in immature asci, 8-spored, obliquely seriate. Ascospores hyaline, non-septate, ellipsoidal, 15–22 × 4.5–6 μm.”
Von Arx (1957) regarded Gloeosporium phormii as a synonym of C. gloeosporioides. However in the phylogeny of Farr et al. (2006), strains of this species cluster with C. acutatum and C. lupini. Morphological and cultural differences revealed C. phormii as a distinct lineage. We have confirmed this in our study. Colletotrichum phormii can be distinguished from the closely related C. salicis (and indeed from all other species in the C. acutatum complex) by its elongate, large conidia and large appressoria (Tables 2, 3). The species appears to be host-specific to Phormium spp. Recently, Takeuchi & Hori (2006) reported C. gloeosporioides from Phormium in Japan. However, based on dimensions of conidia (10–16.5 × 4–6 μm) and appressoria (7–17 × 4–11.5 μm) and the shape of the conidia – cylindrical with broadly rounded ends – (fig. 3 of that paper), the fungus seems to be a species in the C. gloeosporioides complex rather than one of the two C. acutatum complex members from Phormium treated in this study.
Table 3.
Appressoria measurements of Colletotrichum strains studied.
| Species | Accession No. |
Appressoria on SNA |
||
|---|---|---|---|---|
| length × width (μm)1 |
length × width (μm) mean ± stdev |
L/W ratio | ||
| C. acerbum | CBS 128530* | (8–)9–14(–16.5) × (4–)5–7.5(–9.5) | 11.3 ± 2.4 × 6.2 ± 1.2 | 1.8 |
| C. acutatum | CBS 112996* | (4–)5.5–9(–13) × (3–)4–6.5(–9.5) | 7.3 ± 2.0 × 5.4 ± 1.2 | 1.3 |
| CBS 111993 | (3–)5–8.5(–10.5) × (2–)4–6(–7) | 6.7 ± 1.8 × 4.9 ± 1.0 | 1.4 | |
| CBS 112759 | (4–)5–7.5(–10) × (3.5–)4–6(–8) | 6.3 ± 1.1 × 5.0 ± 1.0 | 1.3 | |
| IMI 319423 | (5.5–)6–13(–19.5) × (4–)5–6(–7.5) | 9.5 ± 3.6 × 5.5 ± 0.7 | 1.7 | |
| C. australe | CBS 116478* | (5–)6–11(–14) × (4–)4.5–7(–8.5) | 8.5 ± 2.6 × 5.8 ± 1.1 | 1.5 |
| CBS 131320 | (5–)7–10(–11) × (4.5–)5–7(–9) | 8.6 ± 1.6 × 6.1 ± 1.1 | 1.4 | |
| C. brisbanense | CBS 292.67* | (5–)7.5–14.5(–18) × (2.5–)3.5–5(–6) | 11.1 ± 3.4 × 4.3 ± 0.9 | 2.6 |
| C. chrysanthemi | CBS 126518 | (5–)5.5–9.5(–11.5) × (3–)4.5–6.5(–7.5) | 7.5 ± 1.8 × 5.4 ± 1.1 | 1.4 |
| IMI 364540 | (5.5–)6–10(–14) × (4.5–)5–6.5(–7.5) | 7.8 ± 2.0 × 5.5 ± 0.8 | 1.4 | |
| C. cosmi | CBS 853.73* | (5–)5.5–8(–11.5) × (4–)4.5–5.5 | 6.8 ± 1.2 × 4.9 ± 0.4 | 1.4 |
| C. costaricense | CBS 330.75* | (4.5–)6–8.5(–10) × (3–)4–6(–6.5) | 7.1 ± 1.2 × 4.9 ± 0.9 | 1.4 |
| CBS 211.78 | (4–)5.5–9(–11) × (3–)4–6(–6.5) | 7.3 ± 1.8 × 4.9 ± 1.2 | 1.5 | |
| C. cuscutae | IMI 304802* | (3.5–)5.5–11.5(–15.5) × (2–)3.5–5.5(–6.5) | 8.5 ± 3.2 × 4.6 ± 0.9 | 1.8 |
| C. fioriniae | CBS 128517* | (4.5–)7–11.5(–15.5) × (4–)4.5–7(–10.5) | 9.2 ± 2.2 × 5.6 ± 1.2 | 1.6 |
| CBS 200.35 | (6–)7.5–10.5(–12) × (4–)5–7(–9) | 8.8 ± 1.5 × 6.0 ± 1.0 | 1.5 | |
| CBS 129916 | (5–)5.5–11.5(–18) × (4–)4.5–6.5(–8) | 8.5 ± 3.1 × 5.4 ± 0.8 | 1.6 | |
| C. godetiae | CBS 133.44* | (8–)9–12.5(–14.5) × (3–)4–5.5(–6) | 10.7 ± 1.9 × 4.7 ± 0.7 | 2.3 |
| CBS 125972 | (6–)8–13(–17) × (3.5–)5–6.5(–7) | 10.3 ± 2.4 × 5.8 ± 0.7 | 1.8 | |
| CBS 129911 | (6–)7–10.5(–14.5) × (4.5–)5–7(–9) | 9.0 ± 1.7 × 6.1 ± 1.0 | 1.5 | |
| CBS 862.70 | (4–)5.5–12.5(–17.5) × (3.5–)4–6(–8) | 9.0 ± 3.4 × 5.1 ± 1.2 | 1.8 | |
| CBS 129809 | (6–)7.5–12(–15) × (4–)5–8(–11.5) | 9.6 ± 2.2 × 6.5 ± 1.5 | 1.5 | |
| CBS 127561 | (6–)7–12(–16.5) × 4–6(–6.5) | 9.4 ± 2.5 × 5.0 ± 0.9 | 1.9 | |
| C. guajavae | IMI 350839* | (4.5–)5–8(–10.5) × (3.5–)4.5–6(–6.5) | 6.6 ± 1.4 × 5.2 ± 0.7 | 1.3 |
| C. indonesiense | CBS 127551* | 5.5–9(–14.5) × (5–)5.5–7.5(–9) | 7.5 ± 1.8 × 6.3 ± 1.0 | 1.2 |
| C. johnstonii | CBS 128532* | (6–)8–11.5(–14) × (2–)4–7.5(–10.5) | 9.6 ± 1.7 × 5.8 ± 1.9 | 1.7 |
| IMI 357027 | (4.5–)6.5–10.5(–14) × (3–)4–7(–9.5) | 8.4 ± 1.9 × 5.4 ± 1.6 | 1.6 | |
| C. laticiphilum | CBS 112989* | (5–)6.5–12(–16) × (4–)6–8(–8.5) | 9.2 ± 2.8 × 7.2 ± 1.0 | 1.3 |
| CBS 129827 | (4–)5–7(–8) × (2.5–)3.5–5.5(–6) | 6.0 ± 1.1 × 4.5 ± 0.8 | 1.3 | |
| C. limetticola | CBS 114.14* | (5–)6–8.5(–11) × (4–)4.5–6(–7) | 7.4 ± 1.3 × 5.3 ± 0.7 | 1.4 |
| C. lupini | CBS 109225* | (4–)6–12(–20.5) × (4.5–)6–9(–11.5) | 9.0 ± 2.8 × 7.4 ± 1.7 | 1.2 |
| CBS 109221 | (4.5–)5.5–11.5(–19.5) × (3.5–)5–7.5(–8.5) | 8.6 ± 3.0 × 6.2 ± 1.1 | 1.4 | |
| C. melonis | CBS 159.84* | (4.5–)6–11(–13.5) × (3.5–)4.5–6.5(–7.5) | 8.3 ± 2.4 × 5.5 ± 1.0 | 1.5 |
| C. nymphaeae | CBS 173.51 | (4–)5.5–11(–17) × (3–)4–6.5(–9) | 8.2 ± 2.7 × 5.2 ± 1.3 | 1.6 |
| CBS 112992 | (4.5–)6–11(–16.5) × (4–)4.5–6(–7.7) | 8.5 ± 2.3 × 5.2 ± 0.9 | 1.6 | |
| CBS 112202 | (5–)6.5–10(–13.5) × (4–)5–6.5(–8) | 8.2 ± 1.9 × 5.8 ± 0.8 | 1.4 | |
| CBS 126382 | (5.5–)5.5–10(–17.5) × (3.5–)4.5–6.5(–9) | 7.8 ± 2.4 × 5.5 ± 1.1 | 1.4 | |
| CBS 515.78* | (4.5–)6–11(–15) × (3–)4.5–6.5(–8) | 8.7 ± 2.5 × 5.5 ± 1.0 | 1.6 | |
| CBS 526.77 | (4.5–)6–9(–12) × (3.5–)4.5–6.5(–7.5) | 7.4 ± 1.6 × 5.6 ± 1.1 | 1.3 | |
| C. orchidophilum | IMI 305357 | (5.5–)7.5–15.5(–20.5) × (4.5–)5.5–8.5(–12) | 11.6 ± 3.9 × 7.0 ± 1.6 | 1.6 |
| CBS 631.80 | (4.5–)5.5–11(–18) × (4–)4.5–6(–7) | 8.2 ± 2.8 × 5.2 ± 0.8 | 1.6 | |
| C. paxtonii | IMI 165753* | (5–)6–11.5(–16.5) × (3.5–)5.5–7.5(–8.5) | 8.8 ± 2.7 × 6.5 ± 1.1 | 1.4 |
| CBS 502.97 | (3.5–)4.5–7.5(–10.5) × (3–)3.5–5(–5.5) | 6.0 ± 1.7 × 4.2 ± 0.7 | 1.4 | |
| C. phormii | CBS 118194* | (4–)8.5–20.5(–32) × (2.5–)4–6(–8) | 14.5 ± 6.2 × 5.1 ± 1.0 | 2.9 |
| CBS 102054 | (5.5–)8–13(–14.5) × 5–6.5(–8) | 10.4 ± 2.4 × 5.8 ± 0.8 | 1.8 | |
| C. pseudoacutatum | CBS 436.77* | (3–)5.5–18.5(–25) × (2.5–)3.5–7(–9.5) | 12.0 ± 6.3 × 5.1 ± 1.7 | 2.3 |
| C. pyricola | CBS 128531* | (4.5–)6–16(–22) × (3.5–)4.5–7(–8.5) | 11.1 ± 5.1 × 5.7 ± 1.2 | 2.0 |
| C. rhombiforme | CBS 129953* | (5.5–)8–13(–17.5) × (4.5–)6–8(–9.5) | 10.6 ± 2.4 × 7.0 ± 1.1 | 1.5 |
| C. salicis | CBS 607.94* | (6–)8–15(–19.5) × (5–)6.5–8.5(–9.5) | 11.5 ± 3.5 × 7.6 ± 1.0 | 1.5 |
| CBS 115.14 | (3.5–)6.5–12(–16.5) × (3–)4–5.5(–7.5) | 9.3 ± 2.7 × 4.9 ± 0.9 | 1.9 | |
| C. salicis | CBS 465.83 | (7–)8–14(–18) × (5–)5.5–8(–11) | 11.1 ± 2.9 × 6.9 ± 1.3 | 1.6 |
| C. scovillei | CBS 126529* | (3.5–)5–7.5(–10.5) × (3.5–)5–6.5(–7) | 6.3 ± 1.2 × 5.6 ± 0.8 | 1.1 |
| CBS 120708 | (4.5–)6.5–9(–10.5) × (4.5–)6–7.5(–7.5) | 7.7 ± 1.2 × 6.7 ± 0.7 | 1.2 | |
| C. simmondsii | CBS 122122* | (4.5–)6–9.5(–11.5) × (3.5–)4–6.5(–9.5) | 7.8 ± 1.9 × 5.3 ± 1.1 | 1.5 |
| CBS 294.67 | (6–)6.5–10(–14) × (4.5–)5–7(–8.5) | 8.3 ± 1.8 × 6.0 ± 0.8 | 1.4 | |
| CBS 114494 | (4–)5.5–9.5(–12.5) × (3–)4–6(–8) | 7.5 ± 1.8 × 5.0 ± 1.1 | 1.5 | |
| C. sloanei | IMI 364297* | (4–)5–11(–17.5) × (4–)4.5–6.5(–8) | 8.0 ± 3.0 × 5.4 ± 0.9 | 1.5 |
| C. tamarilloi | CBS 129814* | (4–)5–10.5(–16) × (3.5–)4.5–6.5(–8) | 7.8 ± 2.6 × 5.5 ± 0.9 | 1.4 |
| CBS 129811 | (4–)5–10(–15) × (3.5–)4.5–6(–7) | 7.5 ± 2.4 × 5.2 ± 0.9 | 1.5 | |
| C. walleri | CBS 125472* | (4.5–)5.5–12.5(–18.5) × (3.5–)4.5–7.5(–10.5) | 9.0 ± 3.3 × 5.9 ± 1.4 | 1.5 |
| Colletotrichum sp. | CBS 129821 | (5.5–)6.5–9(–11) × (4.5–)5.5–7.5(–8.5) | 7.9 ± 1.3 × 6.5 ± 0.9 | 1.2 |
| CBS 129820 | (6.5–)8.5–11.5(–13.5) × (5–)6–8.5(–10.5) | 10.0 ± 1.6 × 7.2 ± 1.2 | 1.4 | |
| CBS 129823 | (5–)5.5–10.5(–15.5) × (3.5–)4.5–6.5(–8) | 7.9 ± 2.4 × 5.4 ± 1.1 | 1.5 | |
| IMI 384185 | (4.5–)5.5–9.5(–12.5) × (4.5–)5–7(–8) | 7.6 ± 2.1 × 6.0 ± 0.8 | 1.3 | |
| CBS 101611 | (5.5–)6.5–10(–14) × (5–)6–8(–8.5) | 8.2 ± 1.8 × 6.8 ± 1.0 | 1.2 | |
| CBS 129810 | (6–)6.5–9(–10.5) × (5.5–)6–7(–7.5) | 7.8 ± 1.1 × 6.3 ± 0.6 | 1.2 | |
ex-type strain
(min–)min-stdev–max-stdev(–max)
Colletotrichum phormii is separated from other species by TUB2, GAPDH, HIS3 and ACT sequences, and most effectively with HIS3. The CHS-1 sequence is the same as that of C. australe. The closest matches in a blastn search with the TUB2 sequence of strain CBS 118194 (with 99 % identity, 4 bp differences) was Ga. acutata isolate PCF 459 (EU635504) from strawberry in Belgium (Debode et al. 2009) and with 99 % identity (5 bp differences), isolate PT250 (= CBS 129953) AJ748624 from olive, Portugal (Talhinhas et al. 2005), which is here referred to C. rhombiforme. With the GAPDH sequence of strain CBS 118194 there was no match closer than 89 % identity. The closest matches in a blastn search with the ITS sequence with 100 % identity were the same GenBank accessions as those obtained in blastn searches of C. salicis, C. pyricola and C. johnstonii.
Colletotrichum pseudoacutatum Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800509. Fig. 24.
Fig. 24.
Colletotrichum pseudoacutatum (from ex-holotype strain CBS 436.77). A–B. Conidiomata. C. Tips of setae. D. basis of seta. E. Conidiophores. F. Seta. G. Conidiophores. H–M. Appressoria. N–O. Conidia. A, C–E, N. from Anthriscus stem. B, F–M, O. from SNA. A–B. DM, C–O. DIC, Scale bars: A = 100 μm, B = 200 μm, E = 10 μm. Scale bar of E applies to C–O.
Etymology: Named refers to the morphology that is similar to C. acutatum, which is not closely related.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–5 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata acervular, conidiophores and setae formed on a cushion of pale brown angular cells 3–8 μm diam. Setae rare (only one found), medium brown, smooth-walled, 2-septate, 57 μm long, base cylindrical, constricted at basal septum, 4 μm diam, tip somewhat round. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 50 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, often ± bent or partly inflated, 9–22 × 2–3.5 μm, opening 1 μm diam, collarette distinct, 0.5–1 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, fusiform to cylindrical with both ends acute, (9.5–)11.5–13.5(–14.5) × 3.5–4 μm, mean ± SD = 12.7 ± 1.1 × 3.8 ± 0.2 μm, L/W ratio = 3.4. Appressoria in loose groups to dense clusters, pale brown, verruculose, irregular shape, (3–)5.5–18.5(–25) × (2.5–)3.5–7(–9.5) μm, mean ± SD = 12.0 ± 6.3 × 5.1 ± 1.7 μm, L/W ratio = 2.3.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores and setae formed on a cushion of pale brown angular cells 4–10 μm diam. Setae abundant, medium brown, basal cell often paler, smooth-walled, 65–130 μm long, mostly with one septum close to the base, (0–)1(–2)-septate, base cylindrical to conical, often ± bent, often looking like an outgrowth or like beginning to branch, 3–5 μm wide, tip somewhat acute to slightly roundish. Conidiophores hyaline, septate, branched, smooth-walled, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to elongate ampulliform, 5.5–17 × 2.5–4(–5) μm, opening 1 μm diam, collarette distinct, 0.5–1 μm long, periclinal thickening visible, sometimes conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, fusiform to cylindrical with both ends acute, (9.5–)11.5–13.5(–14.5) × 3.5–4 μm, mean ± SD = 15.0 ± 1.2 × 4.2 ± 0.3 μm, L/W ratio = 3.5.
Culture characteristics: Colonies on SNA flat with undulate to lobate margin, hyaline, pale honey in the centre, aerial mycelium lacking, filter paper grey and mottled, on Anthriscus stem partly covered with salmon to apricot acervuli; growth rate 13.5–17.5 mm in 7 d (23–26 mm in 10 d). Colonies on OA flat with undulate to lobate margin; surface buff, sectors isabelline mottled and covered with salmon to apricot acervuli, aerial mycelium lacking, reverse salmon and mottled olivaceous grey, centre iron-grey; growth rate 15–21 mm in 13.5–21 mm in 7 d (21–27.5 mm in 10 d). Conidia in mass salmon to apricot.
Material examined: Chile, Valdivia, San Patricio forest nursery of the Corporación Nacional Forestal near San José de la Mariquina, from seedlings of Pinus radiata, between Dec 1976 and Feb 1977, unknown collector (isolated and deposited in CBS collection Aug. 1977 by H. Peredo López), (CBS H-20729 holotype, culture ex-holotype CBS 436.77).
Notes: Peredo et al. (1979) reported a disease of Pinus radiata seedlings in a nursery in Chile. The seedlings bent leaders in a similar manner to the “terminal crook” disease in New Zealand (Dingley & Gilmor 1972) and the affected part of the stem became pinkish. The disease resulted in small seedlings with a thick stem, restricted terminal growth and increased growth of lateral shoots. Two strains with differing colony characteristics were isolated and were used in pathogenicity tests on 4-month-old seedlings of Pinus radiata; 11.5 % of the seedlings inoculated with the salmon orange culture and 92 % of the seedlings with the grey culture showed the symptoms. The two strains were sent to CBS, and both were identified as C. acutatum f. pineum by von Arx, presumably because of the identity of the host plant and some of their morphological features, especially the conidia with acute ends typical for C. acutatum. One of the strains was kept in the CBS collection as CBS 436.77; unfortunately we can only suppose it was the salmon orange culture.
Strain CBS 436.77 turns out not to be closely related to C. acutatum f. pineum, which belongs to C. acutatum s. str. (Fig. 1). Colletotrichum pseudoacutatum is at best basal to the C. acutatum species complex and forms a sister group to a clade containing the C. acutatum complex and C. orchidophilum (fig. 2 in Cannon et al. 2012, this issue). The closest matches in a blastn search on the ITS sequence of strain CBS 436.77 (with only 94 % identity) are unidentified Colletotrichum isolates, e.g. from Podocarpaceae in New Zealand (Joshee et al. 2009), plus several C. coccodes strains including the ex-epitype strain CBS 164.49 (HM171678, Liu et al. 2011), C. trichellum strains MEP1535 (= CBS 118198, DQ286152, Farr et al. 2006) and DAOM 188792 (= CBS 125343, EU400142, wrongly identified as C. dematium, Chen YY, Conner R, Babcock C, Penner W, unpubl. data) and “C. gloeosporioides” strains DAOM 183087 (EU400145, probably C. coccodes, Chen YY, Conner R, Babcock C, Penner W, unpubl. data) and BBA 71369 from Pleione (AJ301980, probably C. orchidophilum, Nirenberg et al. 2002). The closest matches with the TUB2 sequence showed only 82 % identity, including C. trichellum strains HKUCC 10378, CBS 217.64 and CBS 118198 (GQ849447, Yang et al. 2009, GU228106, GU228107, Damm et al. 2009). There is no match over the whole span of the GAPDH sequence of this species.
In morphological terms, C. pseudoacutatum mainly differs from species in the C. acutatum complex by the formation of pale brown, verruculose, irregular shaped appressoria, and also by the more abundant formation of setae.
Colletotrichum pyricola Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800510. Fig. 25.
Fig. 25.
Colletotrichum pyricola (from ex-holotype strain CBS 128531). A–B. Conidiomata. C–K. Conidiophores. L–Q. Appressoria. R–S. Conidia. A, C–F, R. from Anthriscus stem. B, G–Q, S. from SNA. A–B. DM, C–S. DIC, Scale bars: A = 200 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–S.
Etymology: Named after the host plant Pyrus communis.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–8 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched. Conidiogenous cells hyaline smooth-walled, cylindrical, 9–25 × 2.5–3.5 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, fusiform to cylindrical with one end slightly acute and one end round or slightly acute, (10–)14.5–18.5(–24) × (3.5–) 4.5–5(–5.5) μm, mean ± SD = 16.7 ± 2.1 × 4.7 ± 0.4 μm, L/W ratio = 3.5. Appressoria single or in small dense clusters, pale brown, smooth-walled, ellipsoidal, clavate to cylindrical, the edge entire or undulate, (4.5–)6–16(–22) × (3.5–)4.5–7(–8.5) μm, mean ± SD = 11.1 ± 5.1 × 5.7 ± 1.2 μm, L/W ratio = 2.0.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on pale brown, angular, basal cells 3–7 μm diam. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, 11–20 × 3–4 μm, opening 1.5–2 μm diam, collarette 0.5–2 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, fusiform to cylindrical with both ends acute, (9.5–)14–17(–18.5) × (4–)4.5–5(–5.5) μm, mean ± SD = 15.4 ± 1.6 × 4.8 ± 0.4 μm, L/W ratio = 3.2.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, on medium, filter paper and Anthriscus stem partly covered with thin floccose white to pale grey aerial mycelium and orange acervuli, reverse hyaline with orange to grey acervuli shining through; growth rate 24–25 mm in 7 d (35–37 mm in 10 d). Colonies on OA flat to raised with entire margin; surface partly covered with floccose to woolly whitish to pale olivaceous grey aerial mycelium and orange acervuli mainly appearing in growth rings, reverse buff, olivaceous buff to grey olivaceous with olivaceous grey to iron grey rings; growth rate 21.5–22.5 mm in 7 d (35–37.5 mm in 10 d). Conidia in mass orange.
Material examined: New Zealand, WO, Waikato, from fruit rot of Pyrus communis, 1 Jun. 1988, unknown collector (deposited in ICMP collection by P.R. Johnston), (CBS H-20810 holotype, culture ex-type CBS 128531 = ICMP 12924 = PRJ 977.1).
Notes: This is a third species within clade 4, not clearly distinct from C. johnstonii using morphological or cultural characteristics but with unique ACT, TUB2, CHS-1, GAPDH and HIS3 sequences. The ITS sequence of C. pyricola is identical with those of C. salicis, C. johnstonii and C. phormii.
As with C. johnstonii, C. pyricola appears to be endemic to New Zealand, but more data are needed to confirm its distribution. Strain CBS 128531 (= PRJ 977.1) is the only strain of this species available to us and was included in C. acutatum group C by Johnston & Jones (1997) and Lardner et al. (1999) and in group F2 by Guerber et al. (2003). In the combined GS and GAPDH phylogeny in Guerber et al. (2003), there is a second strain grouping with C. pyricola that they assigned as the only representative of their F5 group. This strain (PRJ 823) however belongs to group B in Lardner et al. (1999), with a completely different RAPD banding pattern.
In contrast to apple, for which Colletotrichum species are listed as major pathogens causing bitter rot (González et al. 2006), pear trees seem to be rarely affected by anthracnose. Colletotrichum piri Noack was actually described from apple (listed as Pyrus malus, a synonym of Malus pumila) in Brazil, rather than from pear as its name suggests.
The closest match in a blastn search with the TUB2 sequence of strain CBS 128531 (with 98 % identity, 10 bp differences) were AJ409294 isolate 90 from Fragaria in the UK (Talhinhas et al. 2002) as well as AJ748609, AJ748612–AJ748614, AJ748619–AJ748622, AJ748625 from olive isolates (Talhinhas et al. 2005). With the GAPDH sequence there was no closer match than 89 % identity.
Colletotrichum rhombiforme Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800511. Fig. 26.
Fig. 26.
Colletotrichum rhombiforme (from ex-holotype strain CBS 129953). A–B. Conidiomata. C–H. Conidiophores. I–M. Appressoria. N–O. Conidia. P. Ascomata. Q. Peridium in cross section. R. Outer surface of peridium. S. Ascospores. T. Paraphyses. U–W, Y. Asci. X. Apical region of ascus. A, C–E, N, P–Y. from Anthriscus stem. B, F–M, O. from SNA. A–B. DM, C–Y. DIC, Scale bars: A = 200 μm, B, P = 100 μm, C, Q = 10 μm. Scale bar of C applies to C–O. Scale bar of Q applies to Q–Y.
Etymology: Named after the shape of the ascospores, which can be rhomboidal.
Sexual morph developed on Anthriscus stem. Ascomata globose to subglobose, pale brown, 300–400 × 400–500 μm, glabrous, ostiolate. Peridium 8–14 μm thick, composed of pale to medium brown flattened angular cells, 6–16 μm diam. Ascogenous hyphae hyaline, smooth-walled, delicate. Interascal tissue composed of paraphyses, hyaline, septate, branched at the base, 35–80 × 3–5 μm, widest part at the base, tips round. Asci cylindrical, 55–73 × 9–11 μm, 8-spored. Ascospores arranged uni-to bi-seriately, aseptate, hyaline, smooth-walled, oval, fusiform, or rhomboidal, one end ± acute and one end round or both ends round, sometimes slightly curved, (11–)12.5–16(–17) × 4–)4.5–6(–7.5) μm, mean ± SD = 14.1 ± 1.6 × 5.2 ± 0.8 μm, L/W ratio = 2.7.
Asexual morph on SNA. Vegetative hyphae 1–8 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate and branched, to 50 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical to ampulliform, often lacking a basal septum and continuous with the conidiophore, discrete phialides measure 4–13 × 3–5 μm, opening 1–2 μm diam, collarette distinct, 1–2 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight cylindrical with one end round and one end slightly acute or both ends round, (12–)12.5–17(–24) × (4–)4.5–5.5(–6) μm, mean ± SD = 14.7 ± 2.1 × 5.0 ± 0.7μm, L/W ratio = 2.9. Appressoria single or in loose groups, medium to dark brown, smooth-walled, the outline mostly clavate, elliptical or ovate, the edge entire or undulate, rarely lobate, (5.5–)8–13(–17.5) × (4.5–) 6–8(–9.5) μm, mean ± SD = 10.6 ± 2.4 × 7.0 ± 1.1 μm, L/W ratio = 1.5.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on a cushion of pale brown angular cells 4–9 μm diam. Setae very few, pale brown, smooth-walled, 3–4-septate, 50–80 μm long, base cylindrical, 3–3.5 μm diam, tip ± rounded or ending with a conidiogenous locus. Conidiophores pale brown, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells pale brown, smooth-walled, cylindrical, sometimes polyphialides, 12–28 × 2–3.5 μm, opening 1–2 μm diam, collarette 0.5–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, very variable in shape, cylindrical, clavate, ellipsoidal or limoniform with one end round and one end slightly acute to truncate, (7.5–)10.5–17.5(–21) × (3.5–)4–5.5(–6) μm, mean ± SD = 14.1 ± 3.5 × 4.8 ± 0.6 μm, L/W ratio = 2.9.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to pale cinnamon, on filter paper, Anthriscus stem and medium covered with short floccose-felty pale olivaceous grey aerial mycelium, on Anthriscus stem covered with pale grey to black structures, reverse medium hyaline to pale cinnamon, filter paper pale cinnamon to olivaceous grey, growth rate 20–22.5 mm in 7 d (32.5–37.5 mm in 10 d). Colonies on OA flat with entire margin; surface honey, pale olivaceous grey, grey olivaceous to olivaceous, almost entirely covered with floccose-felty pale olivaceous grey aerial mycelium, reverse pale olivaceous grey, grey olivaceous to iron grey, growth rate 19–21 mm in 7 d (29–32.5 mm in 10 d). Conidia in mass whitish to pale salmon.
Material examined: Portugal, Mirandela, Torre de D. Chama, from anthracnose on fruit of Olea europaea, Dec. 2003, P. Talhinhas (CBS H-20724 holotype, culture ex-type CBS 129953 = PT250). USA, Washington, Long Beach, from Vaccinium macrocarpon, 1993, Carris, culture CBS 131322 = DAOM 233523.
Notes: Talhinhas et al. (2005, 2009, 2011) found a diverse range of C. acutatum isolates from olive fruit with anthracnose symptoms in Portugal. One of these strains, PT250 (=CBS 129953) was found to be significantly divergent from other groups within C. acutatum based on ITS and beta-tubulin sequences, and was placed in the new clade A6. Talhinhas’s olive strain here forms the type of C. rhombiforme. A second strain that we identified as C. rhombiforme and included here was isolated from Vaccinium macrocarpon (American cranberry) in the USA, and was studied by Robideau et al. (2008). Further representatives of this clade are likely to be some of those isolated from Rhododendron in Sweden and Latvia (strains S2, L3, L4, L5, L6) by Vinnere et al. (2002) that were reported to belong to clade A6 by Sreenivasaprasad & Talhinhas (2005) based on ITS sequencing. Since ITS does not distinguish between all species, sequences of additional genes would be necessary to confirm this placement.
A variety of Glomerella rufomaculans, Ga. rufomaculans var. vaccinii Shear was described from leaves of Vaccinium macrocarpon in New Jersey, USA with conidia and ascospores that agree in size with C. rhombiforme. Its conidia were described as oblong-cylindric, subclavate, sometimes slightly curved (Shear 1907). The variety was wrongly listed as Ga. fructigena var. vaccinii in Sylloge Fungorum (Saccardo & Trotter 1913); MycoBank and Index Fungorum list this taxon as separate species, Ga. rufomaculans-vaccinii Shear, MycoBank also as Ga. rufomaculansvaccinii (orthographic variant) and additionally as Ga. fructigena var. vaccinii. However a strain (CBS 124.22) deposited 1922 in the CBS collection by L.C. Shear as Ga. rufomaculans var. vaccinii is lacking host information and belongs to the C. gloeosporioides complex (Weir et al. 2012, this issue).
Colletotrichum rhombiforme is closely related to C. acerbum, C. australe, C. kinghornii and C. phormii, which together form a sister clade to C. salicis. In this study, only strains of C. rhombiforme and C. salicis formed sexual morphs in culture. The ascospores of the two species have the same size, but differ in shape. Additionally, conidia of C. salicis formed on SNA are smaller than those of C. rhombiforme, and conidia of C. rhombiforme formed on Anthriscus stem are sometimes ellipsoidal or limoniform while those of C. salicis are uniformly cylindrical.
Colletotrichum rhombiforme is separated from other species by all sequences studied except the CHS-1 sequence, which is the same as that of C. acerbum. It can best be identified with TUB2 and ITS. The closest match in a blastn search with the TUB2 sequence of CBS 129953 with 100 % identity was AJ748624, the sequence generated from the same isolate by Talhinhas et al. (2005), all other isolates showed ≤ 97 % sequence identity. With the GAPDH sequence there was no closer match than 88 % identity. Closest matches with the ITS sequence (with 100 % identity) were AJ749700 from isolate PT250 (= CBS 129953) (Talhinhas et al. 2005), AF411704, AF411706, AF411707 and AF411719 from Rhododendron isolates L3, L5, L6, S2 from Latvia and Sweden (Vinnere et al. 2002) and with 99 % identity (1 bp difference) AF411705 from Rhododendron isolate L4 (Vinnere et al. 2002) and EF672241 from Vaccinium isolate DAOM 233253 (= CBS 131322, the other isolate of C. rhombiforme included in this study) (Robideau et al. 2008).
Colletotrichum salicis (Fuckel) Damm, P.F. Cannon & Crous, comb. nov. MycoBank MB800518. Fig. 27.
Fig. 27.
Colletotrichum salicis (from ex-epitype strain CBS 607.94). A–B. Conidiomata. C–H. Conidiophores. I–N. Appressoria. O–P. Conidia. Q–R. Ascomata. S. Peridium in cross section. T. Outer surface of peridium. U. Ascospores. V. Paraphyses. W–Y. Asci. A, C–D, O, Q–Y. from Anthriscus stem. B, E–N, P. from SNA. A–B, Q. DM, C–P, R–Y. DIC, Scale bars: A, Q, R = 100 μm, C, S = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–P. Scale bar of S applies to S–Y.
Basionym: Sphaeria salicis Fuckel, Jahrb. nass. Ver. Naturk. 23–24: 115. 1870.
≡ Sphaeria salicis Auersw., in Fuckel, Fungi Rhen. no. 913, in sched. 1864, nom. nud.
≡ Physalospora salicis (Fuckel) Sacc., Syll. fung. (Abellini) 1: 439. 1882.
≡ Physosporella salicis (Fuckel) Höhn., Annls mycol. 16: 58. 1918.
≡ Anisostomula salicis (Fuckel) Petr., Hedwigia 65: 198. 1925.
≡ Plectosphaera salicis (Fuckel) Arx & E. Müll., Beitr. Kryptfl. Schweiz 11 (no. 1): 204. 1954.
≡ Glomerella salicis (Fuckel) L. Holm, in Holm & Ryman, Thunbergia 30: 6. 2000.
-
= Phyllachora amenti Rostr., Skr. Christiana Vidensk.-Selsk. Forhandl. 9: 5. 1891.
≡ Haplothecium amenti (Rostr.) Theiss. & Syd., Annls Mycol. 13: 615. 1915.
≡ Glomerella amenti (Rostr.) Arx & E. Müll., Beitr. Kryptfl. Schweiz 11 (no. 1): 197. 1954.
-
= Glomerella lycopersici F. Krüger, Arbeiten Kaiserl. Biol. Anst. Land-Forstw. 9: 308. 1913.
≡ Gloeosporium lycopersici F. Krüger, Arbeiten Kaiserl. Biol. Anst. Land-Forstw. 9: 308. 1913.
≡ Colletotrichum kruegerianum Vassiljevsky, Fungi Imperfecti Parasitici 2: 321. 1950 [non C. lycopersici Chester 1891].
-
= Physalospora miyabeana Fukushi, Annls phytopath. Soc. Japan 1 (no. 4): 7. 1921.
≡ Glomerella miyabeana (Fukushi) Arx, Phytopath. Z. 29: 448. 1957.
Sexual morph developed on Anthriscus stem. Ascomata globose to pyriform, ostiolate, medium brown, darker towards the ostiole, 150–200 × 185–250 μm. Peridium 10–15 μm thick, composed of pale to medium brown flattened angular cells 5–15 μm diam. Ascogenous hyphae hyaline, smooth-walled, delicate. Interascal tissue composed of paraphyses, hyaline, septate, 30–80 × 2–3.5 μm, widest part at the base, tips round. Asci cylindrical, 55–88 × 8–12 μm, 8-spored. Ascospores arranged uni- to biseriately, aseptate, hyaline, smooth-walled, ovoid, fusiform, cigar-shaped or cylindrical, one end acute and one end obtuse or both ends obtuse, sometimes very slightly curved, (12.5–)13–15(–17) × (4.5–)5–6(–6.5) μm, mean ± SD = 14.1 ± 1.1 × 5.4 ± 0.5 μm, L/W ratio = 2.6.
Asexual morph on SNA. Vegetative hyphae 1–8 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched. Conidiogenous cells hyaline, smooth-walled, cylindrical to elongate ampulliform, sometimes intercalary (necks not separated from hyphae by a septum), 5–20 × 2–3.5 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to clavate with one end round and one end ± acute to truncate, (8.5–)10.5–15.5(–19.5) × (3.5–)3–4.5(–5) μm, mean ± SD = 13.0 ± 2.4 × 4.0 ± 0.5 μm, L/W ratio = 3.2, conidia of strains CBS 115.14 and CBS 465.83 narrower, measuring (9–) 10.5–15(–17) × 2.5–3.5(–4) μm, mean ± SD = 12.7 ± 2.3 × 3.1 ± 0.5 μm, L/W ratio = 4.1 and (7.5–)9.5–15.5(–22) × 3–3.5(–4.5) μm, mean ± SD = 12.4 ± 3.1 × 3.3 ± 0.4 μm, L/W ratio = 3.8. Appressoria single or in small groups, medium brown, outline mostly clavate, elliptical or ovate, the edge entire or undulate, rarely lobate, (6–)8–15(–19.5) × (5–)6.5–8.5(–9.5) μm, mean ± SD = 11.5 ± 3.5 × 7.6 ± 1.0 μm, L/W ratio = 1.5.
Asexual morph on Anthriscus stem. Conidiomata acervular, only formed after ca. 14 d, the conidiophores, formed on a cushion of pale brown angular cells, 3.5–8.5 μm diam. Setae not observed. Conidiophores hyaline, smooth-walled, simple or septate and branched, to 30 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, 11–18 × 2.5–4 μm, opening 1–2 μm diam, collarette 0.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end slightly acute to truncate, (14.5–)16–18.5(–20) × (4–)4.5–5(–5.5) μm, mean ± SD = 17.1 ± 1.3 × 4.9 ± 0.3 μm, L/W ratio = 3.5, conidia of strain CBS 115.14 smaller, measuring (9.5–)11.5–16(–18.5) × (2.5–)3–4(–4.5) μm, mean ± SD = 14.0 ± 2.3 × 3.3 ± 0.4 μm, L/W ratio = 4.2.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, filter paper rose to iron-grey, with felty to woolly, white to olivaceous grey aerial mycelium on Anthriscus stem and filter paper, reverse same colours; growth rate 18–24 mm in 7 d (32.5–36 mm in 10 d). Colonies on OA flat with entire margin; surface pale amber, ochraceous to apricot, almost entirely covered by felty to floccose-felty, white, pale luteous to very pale olivaceous aerial mycelium, reverse rosy buff, ochraceous, cinnamon to buff; growth rate 21–27 mm in 7 d (34–37.5 mm in 10 d). Conidia in mass pale salmon.
Material examined: Germany, Hessen, near Oestrich (Hostrichia), on dry branches of Salix fragilis, collection date and collector unknown (Fuckel, Fungi Rhenani no. 913 (G holotype [not seen], K(M) isotype of Sphaeria salicis). Netherlands, Z.-Flevoland, Salix forest near Blocq van Kuffeler, from leaf spot of Salix sp., 11 Sep. 1994, H.A. van der Aa, (CBS H-20730 epitype of Sphaeria salicis, here designated, culture ex-epitype CBS 607.94). Sweden, Uppland, Uppsala, Bondkyrka parish, Nåsten forest, between Lurbo bridge and Predikstolen cliff, 22 Jun. 1946, S. Lundell (Fungi exsiccati Suecici, praesertim Upsalienses no. 3613a; K(M) 85441), stated by L. Holm to “agree perfectly” with the type of Sphaeria salicis. Germany, Berlin, Dahlem, from fruit of Solanum lycopersicum, collection date and collector unknown (deposited in CBS collection Feb. 1914 by F. Krüger), culture ex-syntype of Glomerella lycopersici CBS 115.14. Japan, Sapporo, on stems of Salix purpurea, 20 Oct. 1920, Fukushi (K(M) 110218), authentic and possible type material of Physalospora miyabeana, sent to Kew via R.M. Nattrass. USA, Ithaca, New York State College of Agriculture, in office, Plant Sci. Bldg, Cornell Univ., from anthracnose and dieback of Araucaria excelsa, 22 Apr. 1983, J. E. Carol, culture CBS 465.83.
Notes: Colletotrichum salicis is unusual among Colletotrichum species in the prominence of sexual structures relative to asexual structures; it is one of the few species to produce fertile ascomata in culture. The ascomata are not infrequently encountered on dead and dying, weakly lignified tissues including young stems, bracts and flower/fruit stalks of Salix species. A lack of distinctive features has caused this species to be described as new several times.
Colletotrichum salicis was first described as Sphaeria salicis by Fuckel (1870), based on an exsiccatum in his series Fungi Rhenani issued in 1864. Its label ascribed the species name to Auerswald, but no description was provided and Auerswald was not credited with the name when it was subsequently validly published.
Sphaeria salicis has been transferred to a range of different sexual morph genera. In 1954, it was moved to the rather confused genus Plectosphaera (von Arx & Müller 1954, Cannon 1991) and later, in 2000, to Glomerella (Holm & Ryman 2000).
Phyllachora amenti was described from Salix reticulata in Dovre, Norway by Rostrup (1891). Von Arx & Müller (1954) transferred the species to Glomerella (apparently not noticing the similarities with Plectosphaera salicis). We have not seen Rostrup’s type, but his description and that of von Arx & Müller are highly reminiscent of C. salicis and we are confident of the synonymy. Rostrup also described a putative asexual morph of Phyllachora amenti with filiform septate conidia, 35–45 × 1 μm in size, formed in pycnidia. This is most likely to be an accompanying species rather than a genetically linked morph. It may be Septoria didyma.
Physalospora miyabeana was described from Salix purpurea var. angustifolia in Japan by Fukushi (1921), and combined into Glomerella by von Arx (1957) as Glomerella miyabeana. The pathology of this fungus was described in detail by Nattrass (1928) based on British collections from Salix viminalis. He noted that the species showed similarities to Physalospora salicis. He identified his collections as P. miyabeana due to the presence of a Gloeosporium (i.e. Colletotrichum) asexual morph as noted by Fukushi (1921), and considered that the species was more closely related to Glomerella than to Physalospora. Further information on pathology has been contributed by Butin (1960).
Glomerella lycopersici was described from a mummified fruit of Solanum lycopersicum (= Lycopersicon esculentum) in Germany. The ex-syntype strain CBS 115.14 hardly sporulates and did not form a sexual morph in culture, but molecular data confirm the synonymy. The ascospore measurements (15–17.3 × 5.8–6.9 μm) in the original description by Krüger (1913) differ somewhat from our measurements, and those of conidia differ even more (20–22 × 4.7–6.9 μm) from our own; the discrepancy could be due to the use of different growth media. However, the shapes of the ascospores (one side often nearly straight and one side convex or irregularly biconvex) and of the conidia (often clavate) correspond to those of strain CBS 607.94. A further synonym may be Guignardia salicina (syn. Physalospora salicina, Glomerella salicina), but we have not been able to source the original description or examine type material.
Colletotrichum lucidae was described on living leaves Salix lucida in Wisconsin, USA by Greene (1956). It forms obtuse cylindrical conidia (13–19 × 4–6.5 μm) and 1–2-septate setae (50–65 × 4–5 μm). It might also be a synonym of C. salicis. Greene (1964) also found the species a few years later on S. pyrifolia. The strains we studied did not form setae, but if C. lucidae is conspecific, it will just be a later synonym of C. salicis.
Johnston & Jones (1997) found that C. salicis (as Ga. miyabeana) had a close genetic affinity to C. acutatum. Vinnere (2004) regarded Ga. miyabeana as the sexual morph of one of the biological groups within C. acutatum s. lat., and suggested that it should be recognised as a separate species. This is confirmed by our study. Colletotrichum salicis forms a sister clade to a clade formed by C. phormii, C. rhombiforme, C. acerbum, C. australe and C. kinghornii.
Fruit-inhabiting strains of C. salicis (as Ga. miyabeana) are known to be homothallic (Johnston & Jones 1997), and those from Acer platanoides in USA (which also belong here), were also determined as homothallic by LoBuglio & Pfister (2008). In this study, only strains of C. salicis and C. rhombiforme formed sexual morphs in culture. The ascospores of the two species are the same size, but differ in shape. Conidia of C. salicis formed on SNA are smaller than those of C. rhombiforme, and those formed on Anthriscus stem are uniformly cylindrical, with no ellipsoidal or limoniform conidia as found in C. rhombiforme. Other closely related species i.e. C. acerbum, C. australe, C. kinghornii and C. phormii form conidia on SNA, measuring on average 17.9 × 4.7 μm, 17 × 4.4 μm and 18.3 × 3.8 μm and 23 × 4.6 μm respectively, that are larger than those of C. salicis, measuring 13.0 × 4.0 μm.
According to our study, C. salicis is not restricted to a single host genus but seems to have a preference for woody hosts (Acer, Araucaria, Malus, Populus, Pyrus and especially Salix). According to Farr & Rossman (2012), Glomerella amenti has been recorded on Salix polaris and S. reticulata in Norway (Holm & Holm 1994) and Ga. miyabeana was recorded on Salix amygdaloides, S. babylonica, S. daphnoides, S. fragilis, S. gooddingii, S. lasiolepis, S. × alba-matsudana, Fragaria × ananassa, Malus domestica and Pyrus pyrifolia in New Zealand (Pennycook 1989, Guerber et al. 2003, Gadgil 2005) on Salix sp. in Poland (Mulenko et al. 2008) and UK (Dennis 1986) and on Acer truncatum in China (Sun et al. 2011). The species is also reported from leaf lesions of Salix fragilis, S. alba var. vitellina, S. cinerea in Australia (Cunnington et al. 2007). Johnston & Jones (1997) suggested that Ga. miyabeana which causes the distinctive disease “twig canker” on Salix spp., only occurs on fruits (strawberry, apple, nashi, tomato) as an opportunistic secondary invader, becoming infected from willow trees that, in New Zealand, are commonly used as orchard shelter belts. Cunnington et al. (2007) therefore tested the pathogenicity of strains from Salix spp. in Australia on apple and nashi fruits. They were shown to be positive for pathogenicity but less aggressive than a different C. acutatum s. lat. strain that originated from an apple fruit. All Colletotrichum strains from Salix spp. in the CBS collection belong to the former species.
Colletotrichum salicis is separated from other species by all genes, except for ITS; it forms a well-supported clade (bootstrap support 98–99 %) with little sequence variation in HIS3, TUB2, GAPDH and ACT. The closest match in a blastn search with the TUB2 sequence of CBS 607.94 (with 97 % identity, 13 bp differences) was Ga. acutata isolate PCF 459 (EU635504) from strawberry in Belgium (Debode et al. 2009). With the GAPDH sequence of CBS 607.94 no match closer than 87 % identity was found. In blastn searches with the ITS sequence, numerous matches with 100 % identity were found, some of which we know to belong to distinct species.
Colletotrichum scovillei Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800512. Fig. 28.
Fig. 28.
Colletotrichum scovillei (from ex-holotype strain CBS 126529). A–B. Conidiomata. C–E. Conidiophores. F–K. Appressoria. L–M. Conidia. A, C, L. from Anthriscus stem. B, D–K, M. from SNA. A–B. DM, C–M. DIC, Scale bars: A = 200 μm, B = 100 μm, C = 10 μm. Scale bar of C applies to C–M.
Etymology: Named after Wilbur Lincoln Scoville (1865–1942) who devised the Scoville scale for measuring the “hotness” of chilli peppers, the host plant of this species.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–5.5 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, acervuli not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled to verruculose, septate, branched, to 50 μm long. Conidiogenous cells hyaline smooth-walled, cylindrical to slightly inflated, 8–18 × 3–4 μm, opening 1–2 μm diam, collarette 1–1.5(–2) μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to clavate with one end round and one end ± acute, (10.5–)12.5–15(–16.5) × (3–)3.5–4(–4.5) μm, mean ± SD = 13.7 ± 1.3 × 3.8 ± 0.3 μm, L/W ratio = 3.6, conidia of strain CBS 120708 narrower, measuring (11.5–) 12.5–14.5(–15) × 3–3.5 μm, mean ± SD = 13.5 ± 0.8 × 3.3 ± 0.2 μm, L/W ratio = 4.1. Appressoria single or in loose groups, medium to dark brown, smooth-walled, subglobose, ovoid to ellipsoidal, the outline entire, sometimes undulate, (3.5–)5–7.5(–10.5) × (3.5–)5–6.5(–7) μm, mean ± SD = 6.3 ± 1.2 × 5.6 ± 0.8 μm, L/W ratio = 1.1.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on pale brown, angular, basal cells 3–8.5 μm diam. Setae not observed in strain CBS 126529, however in strain CBS 120708 medium brown, smooth-walled, 1–2-septate, 40–60 μm long, base cylindrical to inflated, 3.5–8 μm diam, rounded. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, 9–23 × 2.5–3.5 μm, opening 1.5–2 μm diam, collarette 1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends slightly acute or one end round, (9–)14.5–18(–19.5) × 3.5–4.5 μm, mean ± SD = 16.0 ± 1.8 × 4.0 ± 0.3 μm, L/W ratio = 4.0, conidia of strain CBS 120708 smaller, measuring (12.5–)13–16(–18) × (3–)3.5–4 μm, mean ± SD = 14.6 ± 1.4 × 3.6 ± 0.3 μm, L/W ratio = 4.1.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, on filter paper pale olivaceous grey, on medium, filter paper and Anthriscus stem partly covered with floccose whitish to pale olivaceous grey aerial mycelium and on Anthriscus stem with few orange acervuli, reverse hyaline, rosy buff to greyish sepia, on filter paper and Anthriscus stem partly fuscous black; growth rate 22–22.5 mm in 7 d (33.5–35 mm in 10 d). Colonies on OA flat with entire margin; surface covered with short floccose whitish to pale olivaceous grey aerial mycelium, margin rosy buff, reverse rosy buff, olivaceous grey to iron grey in the centre; growth rate19–20 mm in 7 d (33–35 mm in 10 d). Conidia in mass salmon.
Material examined: Indonesia, from Capsicum sp., collection date and collector unknown, (CBS H-20792 holotype, culture ex-type CBS 126529 = BBA 70349 = PD 94/921-3). Thailand, Chiang Mai, Sansai, from anthracnose on fruit of Capsicum annuum (chilli), 2005, P.P. Than, culture CBS 120708 = HKUCC 10893.
Notes: Colletotrichum scovillei belongs to clade 2 of the C. acutatum species complex, and can be separated from other species by TUB2, GAPDH and ACT sequences, (with GAPDH being most clearly differential), while CHS-1 and HIS3 sequences are the same as those of C. guajavae. The conidia are slightly longer than is typical for C. simmondsii and C. nymphaeae, with a larger length/width ratio. However, those characters are variable within the clade, and sequence data are required to distinguish between the constituent taxa on a reliable basis.
The ex-type strain was included in the study of Nirenberg et al. (2002) as C. acutatum, and one of the strains studied (CBS 120708) was included in a paper on Colletotrichum diseases of chilli in Thailand (Than et al. 2008a), in which ITS and TUB2 sequences were generated. The strain was identified there as C. acutatum, a representative of one of two clades of that species complex associated with chilli. In drop inoculation tests, strains from that clade were found to cause typical anthracnose symptoms on chilli fruits. Two other species (or species complexes) were reported to cause disease of chilli by Than et al. (2008a), with isolates identified also as C. gloeosporioides and C. capsici. The latter taxon was found to be a synonym of C. truncatum by Damm et al. (2009). Other Colletotrichum species were also reported from the C. boninese species complex, namely C. novae-zelandiae and C. karstii in New Zealand, both occuring also on other host plants (Damm et al. 2012, this issue). There are several reports of C. coccodes, inclusive of its synonym C. atramentarium and of C. nigrum, on Capsicum in different countries (Farr & Rossman 2012). These species do not belong to the C. acutatum complex. Colletotrichum coccodes is more closely related to some of the curved-spored species (fig. 1 in Cannon et al. 2012, this issue). The identity of C. nigrum has not been studied recently, and it is most probably either a further synonym of C. coccodes or a member of the C. gloeosporioides complex. Another species on Capsicum annuum from Australia belonging to the C. acutatum species complex, C. brisbanense, is described above. Apart from earlier reports of the strains included in this study, C. acutatum (s. lat.) has also been reported on Capsicum in Bulgaria (Jelev et al. 2008), India (Kaur & Singh 1990), Korea (Cho & Shin 2004) and Taiwan (Liao et al. 2012).
The closest match in a blastn search with the GAPDH sequence of strain CBS 126529 (with 100 % identity) was HM038335 from Colletotrichum sp. isolate MFU 090619 from Capsicum annuum (chilli) from Laos (Phoulivong et al. 2010). Among the closest matches with the TUB2 sequence were 100 % identity matches with DQ454059–DQ454060 from Capsicum annuum isolates obtained in Thailand (Than et al. 2008a). One of these isolates is included in this study. Another 100 % match was with GU246633 from isolate R14 from Capsicum annuum in South Korea (Sang et al. 2011). All of these strains are likely to belong to C. scovillei. Based on the GAPDH sequence of strain LLB17, C. scovillei also occurs on Capsicum annuum in Taiwan (as part of group D3 in Guerber et al. 2003).
Colletotrichum simmondsii R.G. Shivas & Y.P. Tan, Fungal Diversity 39: 119. 2009. Fig. 29.
Fig. 29.
Colletotrichum simmondsii (A–K, R-S from strain CBS 294.67. L–Q from ex-holotype strain CBS 122122). A–B. Conidiomata. C. Seta. D–K. Conidiophores. L–Q. Appressoria. R–S. Conidia. A, C–F, R. from Anthriscus stem. B, G–Q, S. from SNA. A–B. DM, C–S. DIC, Scale bars: A = 200 μm, B = 100 μm, D = 10 μm. Scale bar of D applies to C–S.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–5 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores formed directly on hyphae on the surface of the medium and in the aerial mycelium. Setae not observed. Conidiophores hyaline, smooth-walled, rather irregular in form, sometimes septate. Conidiogenous cells formed singly or in clusters of 2–3 apically or as lateral branches of conidiophores, hyaline, smooth-walled, cylindrical, thread-like, 7–23 × 1–2.2 μm, opening 0.5 μm diam, collarette sometimes visible, < 0.5 μm long, periclinal thickening not observed, conidiogenous cells of other strains differ, e.g. conidiophores of CBS 294.67 are cylindrical, sometimes slightly inflated and usually wider than the ex-type strain, measuring 4.5–18 × 1.5–4 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical with one end round and one end acute or both ends acute, (4.5–)6.5–10(–11.5) × (2–)2.5–3.5(–4) μm, mean ± SD = 8.1 ± 1.7 × 2.9 ± 0.4 μm, L/W ratio = 2.7, conidia of other strains differ in shape and size from the ex-type strain, e.g. conidia of CBS 294.67 are cylindrical to fusiform with both ends acute and measure (6–)10.5–14(–16.5) × 3.5–4.5(–5.5) μm, mean ± SD = 12.3 ± 1.8 × 4.0 ± 0.4 μm, L/W ratio = 3. Appressoria in loose groups or dense clusters of 2–6, medium brown, round, elliptical to clavate in outline, the margine entire to undulate, (4.5–) 6–9.5(–11.5) × (3.5–)4–6.5(–9.5) μm, mean ± SD = 7.8 ± 1.9 × 5.3 ± 1.1 μm, L/W ratio = 1.5.
Asexual morph on Anthriscus stem. Conidiomata not observed, conidiophores formed on aerial hyphae only. Setae not observed in the ex-type strain, but few setae observed in strain CBS 294.67, medium brown, smooth-walled, 0–1-septate, 20–40 μm long, base 2–3 μm diam, cylindrical, tip ± acute. Conidiophores hyaline, smooth-walled, septate, branched, to 65 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, thred-like, 19–30 × 1 μm, opening 0.5 μm diam, collarette < 0.5 μm long, periclinal thickening not observed, conidiogenous cells of other strains differ, e.g. conidiophores of CBS 294.67 are cylindrical to slightly inflated and usually wider than the ex-type strain, measuring 5–18 × 2.5–4.5 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends acute or one end round and one end acute, (6–)7–10(–12.5) × (2–)2.5–3.5(–4.5) μm, mean ± SD = 8.4 ± 1.5 × 3.0 ± 0.5 μm, L/W ratio = 2.8, conidia of other strains differ in shape and size from the ex-type strain, e.g. conidia of CBS 294.67 are cylindrical to fusiform with both ends acute and (11–)12–14.5(–15.5) × (3–)4–4.5(–5) μm, mean ± SD = 13.3 ± 1.2 × 4.1 ± 0.4 μm, L/W ratio = 3.2.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to to pale isabelline, on filter paper and on Anthriscus stem partly covered with short white to pale grey felty aerial mycelium, reverse of filter paper white to olivaceous grey, growth 10–16 mm in 7 d (16–26 mm in 10 d), other strains differ from the type strain by growing faster, e.g. CBS 294.67 grows 20.5–31 mm in 7 d (34–40 mm in 10 d). Colonies on OA flat with entire margin; surface covered with felty white aerial mycelium, becoming pale olivaceous grey towards the centre, margin white or rosy buff, reverse dark olivaceous grey or salmon and purplish to iron-grey towards the margin, growth 10–16 mm in 7 d (14–24 mm in 10 d), other strains differ from the type strain by growing faster, e.g. CBS 294.67 grows 21–27 mm in 7 d (32.5–40 mm in 10 d). Conidia in mass not observed in the ex-type strain, those of other strains are salmon-orange.
Material examined: Australia, Queensland, Yandina, from fruit anthracnose of of Carica papaya, May 1987, L.M. Coates, culture ex-holotype CBS 122122 = BRIP 28519 = BCC 28680 = HKUCC 10928 = ICMP 17298 = KACC 43258; Queensland, Brisbane, Ormiston, from fruit rot of Carica papaya, 1959, J.H. Simmonds, culture ex-topotype of C. acutatum CBS 294.67 = BRIP 11084; Queensland, Brisbane, Nambour, from fruit rot of Fragaria × ananassa, 30 Mar. 1965, J.H. Simmonds, (according to BRIP database: K.G. Pegg), culture CBS 295.67 = BRIP 11086; Western Australia, Wanneroo, from rotting fruit of Fragaria × ananassa, collection date and collector unknown (deposited in IMI in 1992 by R.M. Floyd, Western Australia Department of Agriculture, Australia, No. WA 2768), culture IMI 354381 = CPC 18923. USA, Hawaii, from Protea cynaroides, 8 Dec. 1998, P.W. Crous & M.E. Palm, culture CBS 114494 = STE-U 2964 = STE-U 2088.
Notes: Colletotrichum simmondsii was described by Shivas & Tan (2009) to accommodate strains of the C. acutatum aggregate assigned to group A2 by Sreenivasaprasad & Talhinhas (2005). The type of C. simmondsii was erroneously designated as an epitype of C. acutatum (i.e. s.str.) by Than et al. (2008b), before Shivas & Tan (2009) recognised that the two taxa are not conspecific. In this paper C. simmondsii is accepted in a more restricted sense. According to the TUB2 phylogeny in Shivas & Tan (2009, see Fig. 2 of that paper), C. simmondsii includes strain BRIP 4684 from Capsicum, here identified as C. brisbanense, and sequences from GenBank belonging to strains of C. laticiphilum (AY376556) and C. nymphaeae (AY376551, AJ748607), as well as some strains from Litchi and Persea that could represent further segregate species of the C. acutatum species complex.
Conidial measurements of the type of C. simmondsii by Shivas & Tan (2009) are considerably larger (10–16 × 3.5–4.5 μm) than ours. It is possible that this discrepancy could be due to the different growth medium that they used (PDA) or the age of the culture. Measurements of all other strains studied in culture, including strain CBS 294.67, also from papaya in Australia, more closely approximate to the measurements for C. simmondsii given by Shivas & Tan (2009).
The ex-holotype strain (CBS 122122) of C. simmondsii has restricted growth; all other isolates studied are much faster growing, especially CBS 294.67 on OA. Than et al. (2008b) also remarked on the slow growth rate of CBS 122122 (as BRIP 28519), giving measurements of 2.3–2.6 mm (presumably per day). CBS 111531 also differs, showing buff to olivaceous pigmentation on OA, and white aerial mycelium.
Pigments produced in PDA cultures may differ among species in the C. acutatum complex. According to Shivas & Tan (2009) the reverse of C. acutatum cultures are intensely carmine-red without flecking, while those of C. fioriniae pale pink with flecking. Reverses in C. simmondsii appear pale orange or yellow, without flecking. We did not use PDA as a diagnostic growth medium, so a direct comparison cannot be made among studies, but we did not observe substantial differences in colony reverse colours in OA cultures. It appears that culture pigmentation may change with extended storage or subculturing, and we would be cautious about using these characters as diagnostic tools. In a study on C. acutatum s. lat. from grape in Australia, Whitelaw-Weckert et al. (2007) established a further molecular group beyond those recognised by Sreenivasaprasad & Talhinhas (2005), designated as A9. We have not examined their cultures, and the TUB2 sequences generated in Whitelaw-Weckert et al. (2007) are from a different region of the gene and could therefore not be compared with our TUB2 sequence data, but we suspect that their strains may be referable to C. simmondsii. The TUB2 sequence of the ex-type strain of C. simmondsii, CBS 122122, is identical with that of strain DAR32068 (group A9 in Whitelaw-Weckert et al. 2007) from strawberry in Australia as sequenced by Debode et al. (2009, EU635505), which supports this hypothesis.
Colletotrichum simmondsii is separable from other species by GAPDH and TUB2 sequencing, with TUB2 more strongly diagnostic, while ACT, HIS3 and CHS-1 sequences are the same as those of C. paxtonii. A blastn search with the TUB2 sequence of CBS 122122 resulted in 100 % matches with a number of different sequences, including some from the main clade of C. simmondsii seen in the phylogeny of Shivas & Tan (2009, see fig. 2 of that paper), HE573031 from strain ITEM 13492 from Arbutus unedo in Italy (Polizzi et al. 2011), AJ748635 from strain PD 89/582 (= CBS 126524) from Cyclamen sp. Netherlands (Talhinhas et al. 2005), and FJ907443 from strain BRIP 28519 (= CBS 122122, ex-holotype) as generated by Prihastuti et al. (2009).
Colletotrichum sloanei Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800515. Fig. 30.
Fig. 30.
Colletotrichum sloanei (from ex-holotype strain IMI 364297). A–B. Conidiomata. C–J. Conidiophores. K–P. Appressoria. Q–R. Conidia. A, C–E, Q. from Anthriscus stem. B, F–P, R. from SNA. A–B. DM, C–R. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–R.
Etymology: Named after Sir Hans Sloane (1660–1753), physician and noted natural history collector. His specimens formed a major part of the original collections of the Natural History Museum in London, his Jamaican material of the host plant became Linnaeus’s type of Theobroma cacao, and his recipe for a milk chocolate drink was commercialised by the Cadbury brothers (Natural History Museum, 2011).
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–8.5 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, simple or septate and branched. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical to conical, sometimes lacking a basal septum and continuous with the conidiophore, polyphialides also sometimes observed, discrete phialides measuring 8–24 × 2–3.5 μm, opening 1 μm diam, collarette 1–1.5 μm long, periclinal thickening visible. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to clavate with one end round and one end ± acute, sometimes both ends ± acute, (8.5–)12–17(–22) × (3–)3.5–4(–4.5) μm, mean 14.4 ± SD = 3.7 ± 2.5 × ± 0.3 μm, L/W ratio = 3.9. Appressoria single or in loose groups, medium brown, smooth-walled, elliptical, subglobose to clavate in outline, entire, the edge undulate or lobate, (4–)5–11(–17.5) × (4–)4.5–6.5(–8) μm, mean ± SD = 8.0 ± 3.0 × 5.4 ± 0.9 μm, L/W ratio = 1.5.
Asexual morph on Anthriscus stem. Conidiomata either not developed, conidiophores formed directly on hyphae or formed on a cushion of pale brown, angular, basal cells 2.5–6 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 40 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ± inflated, 9–18 × 2.5–4 μm, opening 1–1.5 μm diam, collarette 1–1.5 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, fusiform to cylindrical with both ends acute, (9–)11.5–15.5(–19.5) × (3–)3.5–4(–4.5) μm, mean ± SD = 13.4 ± 1.8 × 3.9 ± 0.3 μm, L/W ratio = 3.5.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline buff to pale honey, on filter paper and Anthriscus stem partly pale olivaceous grey to olivaceous grey, the medium, filter paper and Anthriscus stem partly covered with thin white aerial mycelium, reverse same colours; growth rate 21–24 mm in 7 d (31–34 mm in 10 d). Colonies on OA flat with entire margin; surface iron-grey to black with a buff margin, partly covered with thin felty white aerial mycelium and orange acervuli arranged in a few rings at the margin, reverse olivaceous grey with a buff margin; growth rate 21–22.5 mm in 7 d (31–32.5 mm in 10 d). Conidia in mass salmon to orange.
Material examined: Malaysia, Borneo, Sabah, Tuaran, from leaf of Theobroma cacao, 1994, A.R. Rossman and C.L. Bong, (IMI 364297 holotype, CBS H-20796 isotype, culture ex-type IMI 364297).
Notes: A representative of the C. acutatum species complex does not previously appear to have been associated with Theobroma cacao. Three species from the C. gloeosporioides species complex, C. ignotum, C. theobromicola and C. tropicale were recognised as endophytes of T. cacao by Rojas et al. (2010). Two of these species were considered to have potential to protect host plants from Phytophthora diseases. All have been reviewed by Weir et al. (2012, this issue).
Further Colletotrichum species have been described from T. cacao, including C. brachytrichum from leaves of T. cacao in Trinidad. This species has conidia that are ovoid-cylindrical with an attenuated base and a round apex, measuring 10–13.5 × 3–3.7 μm; it produces sparse setae that are dark brown, aseptate, slightly flexuous and 40 × 3.5 μm, as well as conidiogenous cells measuring 4 × 2 μm (Saccardo 1906). In contrast, C. sloanei forms larger conidia averaging 14.4 × 3.7 μm on SNA and 13.4 × 3.9 μm on Anthriscus stem. No setae were found in cultures of C. sloanei (though these may only form on host material) and its conidiogenous cells are much longer than those of C. brachytrichum.
Colletotrichum cradwickii, described from branches of T. cacao in Jamaica, forms conidia that are hyaline (red in mass), elongate, constricted in the middle, and 14–17 × 5 μm, with setae that are straight, rigid, acute, 2–3-septate, purplish and 70–100 × 4–6 μm in size (Saccardo & Trotter 1913). Colletotrichum luxificum was collected from branches, buds and fruits of T. cacao in Surinam and Demerara (now Guyana). It formed ovoid-oblong conidia, sometimes slightly constricted in the centre, with both sides rounded, smooth, and 13–19 × 4–5 μm. Setae were formed that were described as 2–4-septate, 50–120 × 3.5–4.5 μm (Saccardo & Trotter 1913). Although the larger size is discrepant, the constriction of the conidia and the formation of setae described for these two species is reminiscent of species in the C. gloeosporioides complex.
Colletotrichum theobromae forms oblong, straight conidia with obtuse ends, measuring 9–12 × 3–5 μm, and dark-brown, pluriseptate, acute setae measuring 60–75 × 3 μm (Saccardo 1906). It was found on fruits of T. cacao in Cameroon, and also does not agree in character with C. sloanei. Gloeosporium theobromicola [as “theobromicolum”], from fruits of T. cacao in Brazil, forms conidia that are hyaline, fusoid and 6–9 × 2–2.5 μm, (Saccardo et al. 1931). These are considerably smaller than those of C. sloanei. This organism may not be a species of Colletotrichum.
None of the species previously described on T. cacao originates from Asia, and all known species from other parts of the world differ from C. sloanei. Rojas et al. (2010) noted several unidentified taxa amongst their collections from T. cacao from Panama, but based on ITS sequence data, none of them belongs to the C. acutatum species complex. They also isolated C. gloeosporioides s. lat. and a strain belonging to the C. boninense species complex (CBS 124951); the latter was identified as C. karstii by Damm et al. (2012, this issue).
Colletotrichum sloanei may be separated from other species by TUB2, ACT, GAPDH and HIS3 sequences. It is most easily distinguished with TUB2, HIS3 and ACT. With GAPDH there is only one bp difference from C. paxtonii, while the CHS-1 sequence is the same as that of C. walleri. Closest matches in a blastn search with the TUB2 sequence of strain IMI 364297 (with 99 % identity, 2 or 3 bp differences) were GU183300, GU183299 and GU183295 from C. simmondsii strains from Litchi chinensis in Australia (Shivas & Tan 2009). There are no strains from Litchi included in our analyses, but according to the TUB2 tree in Shivas & Tan (2009), they probably belong to C. simmondsii s. str. The closest match with the GAPDH sequence of strain IMI 364297 covering ± the full length sequence (with 98 % identity, 6 bp differences) was HQ846719 from an unnamed plant, probably from India (Chowdappa P, Chethana CS, Madhura S, unpubl. data). Closest matches with the ITS sequence (with 99 % identity, 1 bp difference) were 25 sequences, that are not listed here.
Colletotrichum tamarilloi Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800516. Fig. 31.
Fig. 31.
Colletotrichum tamarilloi (from ex-holotype strain CBS 129814). A–B. Conidiomata. C–J. Conidiophores. K–P. Appressoria. Q–R. Conidia. A, C–E, Q. from Anthriscus stem. B, F–P, R. from SNA. A–B. DM, C–R. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–R.
Etymology: Named after the host plant tamarillo (Solanum betaceum).
Sexual morph not observed (structures that are possibly immature ascomata were seen on Anthriscus stem). Asexual morph on SNA. Vegetative hyphae 1–5.5 μm diam, hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched, to 30 μm long. Conidiogenous cells hyaline smooth-walled, cylindrical to ± inflated, often integrated, discrete phialides measure 8–18 × 2.5–3.5 μm, opening 1–1.5 μm diam, collarette distinct, 1–1.5 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends acute, sometimes clavate with one round and one acute end, (8.5–)11.5–14.5(–15) × (2.5–)3–4(–4.5) μm, mean ± SD = 13.0 ± 1.4 × 3.5 ± 0.4 μm, L/W ratio = 3.7. Appressoria single, medium brown, smooth-walled, subglobose, elliptical or clavate, the edge entire, rarely slightly undulate, (4–)5–10.5(–16) × (3.5–)4.5–6.5(–8) μm, mean ± SD = 7.8 ± 2.6 × 5.5 ± 0.9 μm, L/W ratio = 1.4.
Asexual morph on Anthriscus stem. Conidiomata acervular, conidiophores formed on thick-walled, pale brown, angular, basal cells 4–8 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 50 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, sometimes polyphialidic, 10–21 × 2–4 μm, opening 1–1.5 μm diam, collarette distinct, 1–2 μm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends acute, (10.5–)12–16(–22) × (3–)3.5–4.5(–5) μm, mean ± SD = 14.0 ± 1.9 × 4.0 ± 0.4 μm, L/W ratio = 3.5. Conidia of CBS 129955 and CBS 129811 differ in having slightly longer conidia, measuring (11.5–)13.5–17(–18.5) × 3.5–4(–4.5) μm, mean ± SD = 15.3 ± 1.7 × 3.8 ± 0.3 μm, L/W ratio = 4.0.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to pale honey, on filter paper partly pale olivaceous grey to olivaceous grey, filter paper Anthriscus stem and medium partly covered with felty white aerial mycelium (and salmon acervuli), reverse same colours; growth rate 17.5–21.5 mm in 7 d (28.5–31.5 mm in 10 d). Colonies on OA flat with entire margin; surface honey, isabelline to olivaceous, almost entirely covered by felty white to pale olivaceous grey aerial mycelium, reverse buff, olivaceous, pale olivaceous grey, olivaceous grey to iron-grey, growth rate 16–18 mm in 7 d (26–29 mm in 10 d). Conidia in mass salmon.
Material examined: Colombia, Cundinamarca, from fruit anthracnose of Solanum betaceum, 13 Aug. 2010, J. Molina, (CBS H-20726 holotype, culture ex-type CBS 129814 = T.A.6); Cundinamarca, from anthracnose on a fruit of Solanum betaceum, 13 Aug. 2010, J. Molina, culture CBS 129811 = T.A.3; Antioquia, Santa Rosa, from a flower of Solanum betaceum, 1998, collector unknown, CBS H-20728, culture CBS 129955 = Tom-12.
Notes: Afanador-Kafuri et al. (2003) identified several strains from tamarillo in Colombia as C. acutatum, three of which are included in this study. Sreenivasaprasad & Talhinhas (2005) recognised these strains as a separate molecular group, A8, closely related to A1 (C. lupini).
Colletotrichum tamarilloi can be separated from other species using CHS-1, HIS3, TUB2 and GAPDH sequences, most effectively with GAPDH, and forms a uniform cluster even with six genes (Fig. 1). Afanador-Kafuri et al. (2003) observed uniformity of banding patterns with apPCR, RAPD-PCR and A+T-rich DNA analyses of the strains they studied. They speculated that selection for clonality and homogeneity had occurred among the isolates, all of which were collected in one region in Colombia where only one cultivar of the host was cultivated. Conidia of C. tamarilloi are uniformly fusiform on SNA, and almost so on Anthriscus stem, while C. lupini forms conidia that are usually clavate on SNA and cylindrical on the stems. Additionally, we found that appressoria of C. lupini have an undulate to lobate margin, while those of C. tamarilloi have an entire or rarely slightly undulate edge.
This species is only known on Solanum betaceum in Colombia. There are no previously described species associated with this host. Three Colletotrichum species are reported from tamarillo in the USDA fungal databases (Farr & Rossman 2012): C. acutatum (Guerber et al. 2003, Gadgil 2005) and C. gloeosporioides (Gadgil 2005) in New Zealand and C. simmondsii in Australia (Shivas & Tan 2009). None of these species/groups is identical with C. tamarilloi. While C. lupini and C. tamarilloi form well-supported clusters, there are several additional species and unnamed strains from various hosts in Central and South America, as well as in Florida that are closely related to C. lupini and C. tamarilloi. One of these is from tamarillo in the same locality in Colombia (CBS 129810).
A recently reported anthracnose pathogen of tamarillo in the USA (Jones & Perez 2012) probably belongs to C. fioriniae according to its ITS sequence (JN863589). The Colletotrichum strains available to us from tamarillo in Colombia and New Zealand belong to C. godetiae, C. tamarilloi and an unnamed strain related to C. tamarilloi (this study), as well as C. boninense, C. constrictum and C. karstii belonging to the C. boninense species complex (Damm et al. 2012, this issue). Yearsley et al. (1988) report C. acutatum (s. lat.) infections of tamarillo in New Zealand; however none of our tamarillo strains isolated from New Zealand belongs to the C. acutatum group. The strains from this host included in Guerber et al. (2003) and assigned to group F2 formed a clade with strains described as C. johnstonii in this study. We did not find any species on tamarillo occurring in both Colombia and New Zealand.
Falconi & van Heusden (2011) studied Colletotrichum isolates collected from Lupinus mutabilis and tamarillo in the Ecuadorian Andes. They formed two different subgroups within C. acutatum based on ITS sequence data. The isolates from lupins were pathogenic to tamarillo and vice versa, but lupin and tamarillo isolates were each more virulent to their own hosts. ITS sequence of the ex-type strain of C. tamarilloi, CBS 129814, matched with 100 % identity with JN543070 from isolate Tam7 from tamarillo, as well as JN543066 from isolate Lup28 from L. mutabilis in Ecuador (Falconi et al. 2012).
The closest TUB2 blastn matches for CBS 129814 (with 99 % identity, 4 bp differences) were FN611029 and FN611028 from isolates DPI and CS-1 from Citrus aurantifolia and Citrus sinensis from USA, Florida (Ramos et al. 2006). The closest GAPDH matches (with 97 % identity) were EU647323 from leatherleaf fern and EU168905, EU647318 and EU647319 from sweet orange isolates, all from Florida, USA (Peres et al. 2008, MacKenzie et al. 2009).
Colletotrichum walleri Damm, P.F. Cannon & Crous, sp. nov. MycoBank MB800517. Fig. 32.
Fig. 32.
Colletotrichum walleri (from ex-holotype strain CBS 125472). A–B. Conidiomata. C–H. Conidiophores. I–N. Appressoria. O–P. Conidia. A, C–E, O. from Anthriscus stem. B, F–N, P. from SNA. A–B. DM, C–P. DIC, Scale bars: A = 100 μm, C = 10 μm. Scale bar of A applies to A–B. Scale bar of C applies to C–P.
Etymology: Named after J.M. Waller, tropical pathologist extra-ordinaire and a key worker on the most important Colletotrichum pathogen of coffee.
Sexual morph not observed. Asexual morph on SNA. Vegetative hyphae 1–6 μm diam, hyaline, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata not developed, conidiophores formed directly on hyphae. Setae not observed. Conidiophores hyaline, smooth-walled, septate, branched, to 70 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical to ampulliform, 10–14 × 3–4 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, straight, cylindrical to fusiform with both ends slightly acute or one end round, (6–10.5)15.5–(–19.5) × (3–)3.5–4.5(–5.5) μm, mean ± SD = 13.0 ± 2.7 × 4.0 ± 0.5 μm, L/W ratio = 3.3. Appressoria single, medium brown, smooth-walled, elliptical, clavate, sometimes irregularly shaped, the edge entire or undulate, (4.5–)5.5–12.5(–18.5) × (3.5–)4.5–7.5(–10.5) μm, mean ± SD = 9.0 ± 3.3 × 5.9 ± 1.4 μm, L/W ratio = 1.5.
Asexual morph on Anthriscus stem. Conidiomata either not developed, conidiophores formed directly on hyphae, or acervular, conidiophores formed on pale brown, angular, basal cells 3.5–7 μm diam. Setae not observed. Conidiophores hyaline to pale brown, smooth-walled, septate, branched, to 70 μm long. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, 12–23 × 2.5–3 μm, opening 1–1.5 μm diam, collarette 0.5–1 μm long, periclinal thickening visible to distinct. Conidia hyaline, smooth-walled, aseptate, straight, sometimes slightly curved, cylindrical to fusiform with both ends ± acute or one end round, (10.5–)12–16(–18.5) × 3.5–4(–4.5) μm, mean ± SD = 13.9 ± 1.8 × 4.0 ± 0.3 μm, L/W ratio = 3.5.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline, filter paper pale olivaceous grey, medium, filter paper and Anthriscus stem covert with fely white aerial mycelium, reverse same colours; 21–24 mm in 7 d (31–34 mm in 10 d). Colonies on OA flat with entire margin; surface covert with felty or short floccose white to pale olivaceous grey aerial mycelium, reverse olivaceous grey to iron grey, olivaceous in the centre and white towards the margin; 20–26 mm in 7 d (30.5–37.5 mm in 10 d). Conidia in mass salmon.
Material examined: Vietnam, Buon Ma Thuot-Dak Lac, from leaf tissue of Coffea arabica, unknown collection date, H. Nguyen, (CBS H-20795 holotype, culture ex-type CBS 125472 = BMT(HL)19).
Notes: Species of the C. gloeosporioides species complex are well-known as pathogens of Coffea, especially the African coffee berry disease pathogen C. kahawae (Waller et al. 1993). Additional Coffea-associated components of this species complex from Vietnam and Thailand have been studied by Nguyen et al. (2009) and Prihastuti et al. (2009); see Weir et al. (2012, this issue) for further review.
Masaba & Waller (1992) commented that strains identified as C. acutatum may cause minor disease of ripening coffee berries. Kenny et al. (2006) and Nguyen et al. (2010) respectively isolated, in Papua New Guinea and Vietnam, taxa in this species complex from coffee leaves, twigs and fruits. None of the Vietnamese isolates could infect undamaged coffee berries (Nguyen et al. 2010). One of the C. acutatum cultures studied by Nguyen et al. (BMT(HL)19) was sent to CBS and a dried sample of this strain is here designated as holotype of C. walleri. In this study, this is the only coffee isolate from Asia, while six other isolates from coffee, originating from Africa and Central America, belong to three other species within the C. acutatum species complex (C. fioriniae, C. acutatum s. str. and C. costaricense). Two of these strains were included in the study by Waller et al. (1993).
Colletotrichum walleri is separated from other species by almost all genes. It is most easily distinguished using HIS3 and ITS sequences, while sequences of other genes differ by only one bp from those of other species. The CHS-1 sequence is the same as that of C. sloanei. The closest TUB2 blastn match for CBS 125472 (with 99 % identity, 5 bp differences) was GU246633 from isolate R14 from Capsicum annuum from South Korea (Sang et al. 2011). The closest GAPDH match for a sequence covering ± the full gene length (with 98 % identity, 4 bp differences) was HQ846724 from isolate OBP6 from an unnamed plant, probably from India (Chowdappa P, Chethana CS, Madhura S, unpubl. data). The only 100 % match with the ITS sequence was FJ968601, the sequence of the same isolate previously sequenced by Nguyen et al. (2009).
DISCUSSION
Colletotrichum acutatum (in the broad sense) was originally distinguished using morphological characteristics. The primary diagnostic feature was given as the possession of fusiform conidia with acute ends (Simmonds 1965). More detailed research has however shown that this characteristic is not absolute; while most strains of species within the C. acutatum complex have at least a proportion of conidia with at least one acute end, it is common to find significant variation in conidial shape within species and even within individual strains. Conidia that are more or less cylindrical are frequently encountered. The variation may have multiple causes; in some circumstances it seems that secondary conidia are formed directly from the germ tube of a germinating primary conidium, and these are smaller and more irregular in form than those from which they are derived (Buddie et al. 1999). Additionally, older strains, especially if they have been frequently subcultured, may have conidia that are more variable in appearance than those derived from recent stock. Nirenberg et al. (2002) observed that shapes differed among conidia formed in acervuli and in the aerial mycelium. The variation in conidial shape has led to species within the C. acutatum complex being incorrectly placed into synonymy with other Colletotrichum species, primarily C. gloeosporioides – a legacy of the revision of the genus by von Arx (1957). His work marked a new era in the understanding of Colletotrichum systematics (Cannon et al. 2012, this issue), but many of the synonymies proposed were inaccurate.
In this study we found many species that had been considered as synonyms of C. gloeosporioides by von Arx (1957) actually belong to the C. acutatum species complex, including C. mahoniae, C. godetiae, Gm. phormii, Gm. lycopersici, and Gm. limetticola. Glomerella miyabeana, here treated as a synonym of the older C. salicis, was regarded as a forma specialis of Ga. cingulata by von Arx (1957). Species treated as synonyms of C. gloeosporioides by von Arx (1957) have also been found in the C. boninense species complex (Damm et al. 2012, this issue). There were 39 strains included in this study that had previously been identified as C. gloeosporioides or Ga. cingulata, based on morphology. These strains in fact belong to 14 species in or closely related to the C. acutatum species complex, including C. acutatum, C. australe, C. cosmi, C. costaricense, C. fioriniae, C. godetiae, C. limetticola, C. lupini, C. melonis, C. nymphaeae, C. phormii, C. rhombiforme, C. salicis and C. orchidophilum.
Not all species of Colletotrichum with acute-ended conidia belong to the C. acutatum complex. There are species with falcate conidia that belong to the C. graminicola species complex (Crouch et al. 2009). Also outside the C. acutatum complex are species from herbaceous hosts with more or less curved conidia; these were previously regarded as C. dematium (Damm et al. 2009). In addition, the newly described C. pseudoacutatum forms straight conidia with acute ends but appears not to belong to the C. acutatum species complex (Cannon et al. 2012, this issue). Conidial shape is therefore not a uniform feature of the C. acutatum species complex.
Bearing in mind the frequency with which strains from the C. acutatum species complex are encountered and their pathogenicity to a wide range of crop plants, it would be surprising if earlier names for C. acutatum did not exist. Walker et al. (1991) found that C. xanthii (Halsted 1893) was synonymous with C. acutatum based on morphological criteria, but no authentic sequences are available and it is not clear at present in which clade this species fits. There was no interest at the time amongst Colletotrichum researchers to replace the name C. acutatum, and now the name is so widely used that a name change would be unlikely to gain recognition. Other older taxa have been recognised as belonging to the C. acutatum complex, but as close relatives rather than formal synonyms. Colletotrichum lupini was found to be an independent taxon within the C. acutatum complex by Nirenberg et al. (2002), rather than belonging to the C. gloeosporioides aggregate as assumed by Yang & Sweetingham (1998) and Elmer et al. (2001). Farr et al. (2006) found C. phormii (based on Fusarium phormii, Hennings 1898) to be closely related to C. acutatum and stated that older reports of C. gloeosporioides as pathogens of Phormium could actually refer to C. phormii as well.
Sreenivasaprasad & Talhinhas (2005) distinguished eight distinct molecular groups within C. acutatum, A1–A8 (based on ITS and TUB2 DNA sequences), each of which was recognised here as comprising one or more separate species. These authors listed previously described groups that corresponded to their own groups, including the seven groups recognised by Lardner et al. (1999), A, B, C, D, E, Ga. miyabeana and C. acutatum f. pineum. The Lardner et al. groups were mainly distinguished by morphology, partial LSU sequences and RAPD banding patterns. Some of the strains from Lardner et al. (1999) are included in the present study and we found that only some of them corresponded with the groups adopted by Sreenivasaprasad & Talhinhas (2005). Colletotrichum acutatum group A from Lardner et al. (1999) was regarded as corresponding to group A5, but in our study, three of the four included strains of C. acutatum group A – ICMP 1701, ICMP 12923 and ICMP 17991 – belonged to C. fioriniae (= group A3) and only one, ICMP 17992, belonged to C. acutatum (= group A5). At the same time, the three strains of C. acutatum group C, which was supposed to correspond to Sreenivasaprasad & Talhinhas group A3, were shown to belong to C. johnstonii, in the case of ICMP 12926 and IMI 357027, and to C. pyricola in the case of ICMP 12924. Colletotrichum acutatum group B was listed as corresponding to group A4, but the only strain included here, ICMP 12921, is now the ex-type of C. acerbum. ICMP strains regarded as Ga. miyabeana, that is ICMP 12954–12957, were confirmed here as C. salicis (= Ga. miyabeana). Our phylogenetic tree (see Fig. 1) attempts to portray the groupings of some of these earlier studies, mapped on to our own phylogeny. This illustrates the problems encountered when one compares groups established by different studies using different criteria for characterisation.
Differences in pathogenicity of strains from different hosts have been observed in several studies. Some fruit diseases caused by the C. acutatum complex have been shown to be caused by distinct phylogenetic lineages (Peres et al. 2008), and strawberry fruit rot in particular was rarely found to be caused by isolates from heterogeneous hosts (Mackenzie et al. 2009). Cross-infection potential was tested by, to give a few examples, Bernstein et al. (1995), Freeman and Shabi (1996), Freeman et al. (2001b) and Mackenzie et al. (2007). Cross-infection may occur in the field as well as in the laboratory (Afanador-Kafuri et al. 2003). Freeman et al. (2001a) found that C. acutatum from strawberry is able to cause lesions on various fruits, both when the fruits are wounded and when they are intact. In vitro infection studies by Whitelaw-Weckert et al. (2007) revealed low host specificity among isolates that can be assigned here to C. acutatum, C. simmondsii and C. fioriniae.
The lack of perceived host specificity in the C. acutatum complex probably has multiple causes, but much of the difficulty rests with poor identification practice in pathology studies. Many investigations even now avoid the inclusion of sequence-based evidence, or only use ITS sequences, and only a few deposit adequate voucher material. This means that name use is much less rigorous than it should be, leading to misleading results and poor comparability between studies. That said, it has to be acknowledged that many, if not most of the species we now recognise via multigene analyses appear not to be restricted to particular plants.
One factor making interpretation of pathogenicity data difficult may be incomplete or misleading information on pathology for the strains we have studied. The stated plant/fungus association does not necessarily involve a pathogenic relationship: strains could be isolated as benign endophytes or as secondary pathogens. There is much further work needed on the mechanisms of pathogenicity and on evolution at the population level, but it does appear that many Colletotrichum species are unusually successful in overcoming multiple host barriers.
There is limited evidence of restricted geographical range for some of the species we accept here. For most species, the number of strains available is too small to allow us to draw definite conclusions. For example, except for C. lupini, all isolates of clade 1 (some of which are not recognised as separate species) appear to have an origin restricted to Central and South America and the southern USA. The globalisation of agriculture has in all probability led to frequent unrecognised introductions to new regions. The baseline information we have on native versus exotic taxa is inadequate to allow introductions to be mapped. However, some of the apparently specific host-fungus connections could be supported further by tracing more strains from the respective hosts in future blastn searches, e.g. for C. scovillei and C. limetticola.
Colletotrichum acutatum has been regarded as a pathogen of countless host plant species, and also as occurring everywhere. Sreenivasaprasad & Talhinhas (2005) discovered that C. acutatum group A5, here accepted as C. acutatum s. str., occurs only on certain hosts, mostly in the southern hemisphere. This study confirms that C. acutatum s. str. does in fact have multiple hosts, but the known host spectrum is much smaller than previously accepted.
Some host plants appear to be particularly susceptible to infection by multiple Colletotrichum taxa. Occurrence of species on strawberry has been particularly well researched due to the former status of C. acutatum as a regulated quarantine pathogen. We have found that strains from this host belong to six different clades within the C. acutatum species complex, namely C. simmondsii (three strains from Australia), C. nymphaeae (38 strains, mostly from Europe and the USA), C. fioriniae (seven strains from New Zealand, UK and USA), C. godetiae (10 strains, all from Europe), C. acutatum s. str. (one strain from Australia) and C. salicis (four strains from New Zealand). In a study by MacKenzie et al. (2009), strains from strawberry were shown to be more aggressive to strawberry than strains from Vaccinium. Based on TUB2 sequences generated by those authors, the strains from strawberry were assigned to C. nymphaeae, and the strains from Vaccinium to C. fioriniae. Possibly the reason for apparent differences in pathogenicity, lie not in the different hosts, but in the fact that the strains studied belong to different species. To our knowledge, C. acutatum s. str. has rarely been found in Europe, and then mostly on ornamental plants. So far, it has been isolated from strawberry only in Australia. In pathogenicity tests by Talhinhas et al. (2005), an isolate of C. acutatum s. str. from olive caused anthracnose symptoms on strawberry fruits; the virulence of this isolate was not different from that of of group A2 (C. nymphaeae and related species), A3 (C. fioriniae) or A4 (C. godetiae). If further quarantine regulation is to take place, other than generalised prohibition of contamination by any and all members of the C. acutatum species complex, then more rigorous diagnostic methods will be needed.
Other hosts that are attacked by more than one species of the C. acutatum species complex include apple, citrus, olive, cranberry and blueberry. For example the causal organisms of bitter rot of apple in Korea belong to C. acutatum clades 2 (C. nymphaeae and related species) and 3 (C. fioriniae) (Lee et al. 2007). In our study there are strains from Malus belonging to five species, namely C. acerbum (one strain), C. fioriniae (13 strains), C. godetiae (two strains), C. nymphaeae (one strain) and C. salicis (two strains). Talhinhas et al. (2005) found five groups, now recognised as species, within C. acutatum s. lat. occurring on olives in Portugal: A2 (actually C. nymphaeae), A3 (C. fioriniae), A4 (C. godetiae), A5 (C. acutatum) and A6 (C. rhombiforme).
Our study emphasises the complex nature of the evolutionary pathways that have been traversed within the C. acutatum species complex. Speciation has taken place much more prolifically than has been suspected so far. It seems likely that the C. acutatum species complex is still evolving rapidly. The emergence of new species is doubtless encouraged by the opportunities for mixing of gene pools that are provided by modern global agricultural practices combined with imperfect phytosanitary regulation.
Acknowledgments
We thank the curators and staff of the CABI and CBS culture collections as well as Dr Peter Johnston and Dr Bevan Weir (Landcare Research, Auckland, New Zealand), Prof. dr Lilliana M. Hoyos-Carvajal (Faculty of Agronomy, Plant Health Laboratory, Universidad Nacional de Colombia, Bogotá, Colombia), Dr Pedro Talhinhas (Centro de Investigação das Ferrugens do Cafeeiro – IICT, Oeiras, Portugal), Riccardo Baroncelli (Warwick HRI, University of Warwick, UK), Prof. dr Lisa Vaillancourt (Department of Plant Pathology, University of Kentucky, USA), Prof. dr María de Jesús Yáñez-Morales (Instituto de Fitosanidad, Colegio de Postgraduados, Montecillo, Mexico), Prof. dr Annemiek C. Schilder (Department of Plant Pathology, Michigan State University, USA), Dr Sandra Lamprecht (Soilborne Plant Diseases Unit, Agricultural Research Council, Stellenbosch, South Africa), Y.P. Tan and Dr Roger G. Shivas (Plant Biosecurity Science, Ecosciences Precinct, Dutton Park, Queensland 4102, Australia), Dr Alan Wood (Plant Protection Research Institute, Agricultural Research Council, Stellenbosch, South Africa), Dr Carolyn Babcock (curator of the Canadian Collection of Fungal Cultures, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada), Dr Richard A. Humber (curator of the ARS Collection of Entomopathogenic Fungal Cultures, USDA-ARS, Ithaca, NY, USA), Dr Stanley Freeman (Department of Plant Pathology and Weed Research, The Volcani Center, Bet Dagan, Israel), Dr Charles Lane (The Food and Environment Research Agency, Sand Hutton, York, UK), Hans de Gruyter (Plant Protection Service and National Reference Centre, Wageningen, The Netherlands), Dr Ellis T.M. Meekes (Naktuinbouw, Research & Development, Roelofarendsveen, The Netherlands), Dr Jan Dijksterhuis (CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands), Dr Katherine F. LoBuglio (Harvard University Herbaria, Cambridge, Massachusetts, USA), Dr Lizel Mostert (Department of Plant Pathology, University of Stellenbosch, South Africa) and Dr Françoise Munot (Mycothèque de l’Université catholique de Louvain, Unité de Microbiologie, Louvain-la-Neuve, Belgique) for kindly supplying isolates for this study. We kindly thank the curators of the fungaria at the Royal Botanic Gardens in Kew, UK, at the US National Fungus Collections, Beltsville, Maryland, USA, of the Botanic Garden and Botanical Museum Berlin-Dahlem, Freie Universität Berlin, Berlin, Germany and of the Botanische Staatssammlung München (M), Germany for providing access to historical type specimens. This research was supported by the Dutch Ministry of Agriculture, Nature and Food Quality through an endowment of the FES programme “Versterking infrastructuur Plantgezondheid”.
REFERENCES
- Aa HA van der. (1978). A leaf spot of Nymphaea alba in the Netherlands. Netherlands Journal of Plant Pathology 84: 109–115 [Google Scholar]
- Aa HA van der, Noordeloos ME, Gruyter J de. (1990). Species concepts in some larger genera of the coelomycetes. Studies in Mycology 32: 3–19 [Google Scholar]
- Adhikari KN, Thomas G, Buirchell BJ, Sweetingham MW. (2011). Identification of anthracnose resistance in yellow lupin (Lupinus luteus L.) and its incorporation into breeding lines. Plant Breeding 130: 660–664 [Google Scholar]
- Afanador-Kafuri L, Minz D, Maymon M, Freeman S. (2003). Characterization of Colletotrichum isolates from tamarillo, Passiflora, and mango in Colombia and identification of a unique species from the genus. Phytopathology 93: 579–587 [DOI] [PubMed] [Google Scholar]
- Agostini JP, Timmer LW, Mitchell DJ. (1992). Morphological and pathological characteristics of strains of Colletotrichum gloeosporioides from citrus. Phytopathology 82: 1377–1382 [Google Scholar]
- Albrectsen BR, Björkén L, Varad A, Hagner Å, Wedin M, Karlsson J, Jansson S. (2010). Endophytic fungi in European aspen (Populus tremula) leaves-diversity, detection, and a suggested correlation with herbivory resistance. Fungal Diversity 41: 17–28 [Google Scholar]
- Allescher A. (1895). Mykologische Mittheilungen aus Süd-Bayern. Hedwigia 34: 256–290 [Google Scholar]
- Allescher A. (1902). Fungi Imperfecti. In: Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. 2d edn1(7): 385–704 [Google Scholar]
- Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA. (2003). Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Science, USA 100: 15649–15654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arx JA von. (1957). Die Arten der Gattung Colletotrichum Cda. Phytopathologische Zeitschrift 29:413–468 [Google Scholar]
- Arx JA von. (1970). A revision of the fungi classified as Gloeosporium. Bibliotheca Mycologica 24: 1–203 [Google Scholar]
- Arx JA von. (1981). The Genera of Fungi Sporulating in Pure Culture. 3d edn J. Cramer, Vaduz, Lichtenstein: [Google Scholar]
- Arx JA von, Müller E. (1954). Die Gattungen der amerosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11 (1): 1–434 [Google Scholar]
- Arx JA von, Velden FJJA van der. (1961). Das Colletotrichum der Gurkengewächse. Phytopathologische Zeitschrift 41: 228–235 [Google Scholar]
- Baxter AP, Westhuizen GCA van der, Eicker A. (1983). Morphology and taxonomy of South African isolates of Colletotrichum. South African Journal of Botany 2: 259–289 [Google Scholar]
- Berkeley MJ. (1856). Gloeosporium fructigenum. Gardeners’ Chronicle 1856: 245 [Google Scholar]
- Berkeley MJ. (1874). Notices of North American fungi. Grevillea 3(25): 1–17 [Google Scholar]
- Bernstein B, Zehr EI, Dean RA. (1995). Characteristics of Colletotrichum from peach, apple, pecan and other hosts. Plant Disease 79: 478–482 [Google Scholar]
- Bitancourt AA. (1927). Uma doença do eucalipto. Revista de Agricultura (Piracicaba) 2: 32–39 [Google Scholar]
- Bondar G. (1912). Tremoco branco e suas molestias. Boletim de Agricultura São Paulo 13: 427–432 [Google Scholar]
- Bondarzeva-Monteverde VN, Gutner LS, Novoselova ED. (1936). [The parasitic fungi in the greenhouse of the Botanical Institutes of the Academy of Sciences of the USSR]. Acta Instituti Botanici Nomine V.L. Komarovii Academiae Scientiarum Unionis Rerum Publicarum Soveticarum Socialisticarum Series II, Fasc. 3: 715–801 [Google Scholar]
- Brown AE, Soepena H. (1994). Pathogenicity of Colletotrichum acutatum and C. gloeosporioides on leaves of Hevea spp. Mycological Research 98: 264–266 [Google Scholar]
- Brown AE, Sreenivasaprasad S, Timmer LW. (1996). Molecular characterization of slow-growing orange and key lime anthracnose strains of Colletotrichum from citrus as C. acutatum. Phytopathology 86: 523–527 [Google Scholar]
- Butin H. (1960). Die Krankheiten der Weide und deren Erreger. Mitteilungen aus der Biologischen Bundesanstalt 98: 1–46 [Google Scholar]
- Cannon PF. (1991). A monograph of the species of Phyllachora on the host family Leguminosae. Mycological Papers 163: 1–302 [Google Scholar]
- Cannon PF, Damm U, Johnston PR, Weir BS. (2012). Colletotrichum – current status and future directions. Studies in Mycology 73: 181–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Carpenter JB, Stevenson JA. (1954). A secondary leaf spot of the Hevea rubber tree caused by Glomerella cingulata. Plant Disease Reporter 38: 494–499 [Google Scholar]
- Cesnik R, Ferraz JNG. (2000). Orthezia praelonga, 1891 (Hemiptera, Ortheziidae) biologia, controle químico e biológico. Jaguariúna: embrapa meio ambiente. Boletim Pesquisa 9: 27 [Google Scholar]
- Cesnik R, Ferraz JNG, Oliveira RCAL, Arellano F, Maia AH. (1996). Controle de Orthezia praelonga com o fungo Colletotrichum gloeosporioides isolado Orthezia, na regiao de Limeira, SP. Proceedings, 5 Simpósio de Controle Biológico, Foz de Iguaçu, Brazil [Google Scholar]
- Chen HQ, Cao L, Dekkers K, Chung KR. (2005). A gene with domains related to transcription regulation is required for pathogenicity in Colletotrichum acutatum causing Key lime anthracnose. Molecular Plant Pathology 6: 513–525 [DOI] [PubMed] [Google Scholar]
- Chung WH, Ishii H, Nishimura K, Fukaya M, Yano K, Kajitani Y. (2006). Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Disease 90: 506–512 [DOI] [PubMed] [Google Scholar]
- Clausen RE. (1912). A new fungus concerned in whiter tip of varieties of Citrus medica. Phytopathology 2: 217–236 [Google Scholar]
- Cooke MC. (1887). New Australian fungi. Grevillea 16(77): 1–6 [Google Scholar]
- Cooke MC. (1891). Australian fungi. Grevillea 19(92): 89–92 [Google Scholar]
- Costa ME, Souza da Camara M de. (1953). Species aliquae mycologicae Lusitaniae. II. Portugaliae Acta Biologica 4, Sér. B (1–2): 162–176 [Google Scholar]
- Crouch JA, Clarke BB, White JF, Hillman BI. (2009). Systematic analysis of the falcate-spored graminicolous Colletotrichum and a description of six new species from warm-season grasses. Mycologia 101: 717–732 [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004a). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Groenewald JZ, Risede JM, Hywel-Jones NL. (2004b). Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkleij GJM, Groenewald JZ, Samson RA. (eds) (2009). Fungal Biodiversity. CBS Laboratory Manual Series 1. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Cunnington JH, Powney RA, Adair RJ, Finlay KJ. (2007). Glomerella miyabeana on willows in Australia. Australasian Mycologist 25: 69–72 [Google Scholar]
- Curzi M. (1927). De novis eumycetibus. Atti dell’Istituto Botanico della Università e Laboratorio Crittogamico di Pavia 3, Ser. 3: 203–208 [Google Scholar]
- Damm U, Crous PW, Fourie PH. (2007). Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora spp. nov. Mycologia 99: 664–680 [DOI] [PubMed] [Google Scholar]
- Damm U, Mostert L, Crous PW, Fourie PH. (2008). Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia 20: 87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Woudenberg JHC, Cannon PF, Crous PW. (2009). Colletotrichum species with curved conidia from herbaceous hosts. Fungal Diversity 39: 45–87 [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, Johnston PR, Weir BS, Tan YP, Shivas RG, Crous PW. (2012). The Colletotrichum boninense species complex. Studies in Mycology 73: 1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JT, Tempelton GE, Smith RJ, Fox WT. (1973). Biological control of northern jointvetch in rice with an endemic fungal disease. Weed Science 21: 303–307 [Google Scholar]
- Debode J, Van Hemelrijck W, Baeyen S, Creemers P, Heungens K, Maes M. (2009). Quantitative detection and monitoring of Colletotrichum acutatum in strawberry leaves using real-time PCR. Plant Pathology 58: 504–514 [Google Scholar]
- Dennis RWG. (1986). Fungi of the Hebrides. Royal Botanic Gardens, Kew, UK: [Google Scholar]
- Dickens JSW, Cook RTA. (1989). Glomerella cingulata on Camellia. Plant Pathology 38: 75–85 [Google Scholar]
- Dingley JM, Gilmour JW. (1972). Colletotrichum acutatum Simmds. f. sp. pineum associated with terminal crook disease of Pinus spp. New Zealand Journal of Forestry Science 2: 192–201 [Google Scholar]
- Dinoor A, Rubin B, Nof E. (2009). Epidemiological aspects of trilateral relationships between higher plants, a parasitic plant and a fungal pathogen. In Gadoury DM, Seem RC, Moyer MM, Fry WE. (eds), Proceedings of the 10th International Epidemiology Workshop Geneva, New York: 38–40 [Google Scholar]
- Ditmore M, Moore JW, TeBeest DO. (2008). Interactions of two selected field isolates of Colletotrichum gloeosporioides f. sp. aeschynomene on Aeschynomene virginica. Biological Control 47: 298–308 [Google Scholar]
- Du M, Schardl CL, Vaillancourt LJ. (2005). Using mating-type gene sequences for improved phylogenetic resolution of Colletotrichum species complexes. Mycologia 97: 641–658 [DOI] [PubMed] [Google Scholar]
- Ellis JB, Everhart BM. (1889). New and rare species of North American fungi (Sphaeropsideae). Journal of Mycology 5(3): 145–157 [Google Scholar]
- Elmer WH, Yang HA, Sweetingham MW. (2001). Characterization of Colletotrichum gloeosporioides isolates from ornamental lupins in Connecticut. Plant Disease 85: 216–219 [DOI] [PubMed] [Google Scholar]
- EPPO (2011). EPPO A1 and A2 Lists of Pests Recommended for Regulation as Quarantine Pests. EPPO, Paris: [Google Scholar]
- Fabricatore JM. (1950). Colletotrichum mahoniae n. sp. parassita su foglie di Mahonia aquifolium. Bollettino della Stazione di Patologia Vegetale Roma Ser. 3, 6: 133–139 [Google Scholar]
- Faedda R, Agosteo GE, Schena L, Mosca S, Frisullo S, Magnano di San Lio G, Cacciola SO. (2011). Colletotrichum clavatum sp. nov. identified as the causal agent of olive anthracnose in Italy. Phytopathologia Mediterranea 50: 283–302 [Google Scholar]
- Falconi CE. (2012). Lupinus mutabilis in Ecuador with special emphasis on anthracnose resistance. Ph.D. dissertation. Department of Plant Breeding, WageningenUniversity, The Netherlands: [Google Scholar]
- Farr DF, Aime MC, Rossman AY, Palm ME. (2006). Species of Colletotrichum on Agavaceae. Mycological Research 110: 1395–1408 [DOI] [PubMed] [Google Scholar]
- Farr DF, Rossman AY. (2012). Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA; Retrieved February 28, 2012, from http://nt.ars-grin.gov/fungaldatabases/ [Google Scholar]
- Freeman S, Horowitz S, Sharon A. (2001a). Pathogenic and non-pathogenic lifestyles in Colletotrichum acutatum from strawberry and other plants. Phytopathology 91: 986–992 [DOI] [PubMed] [Google Scholar]
- Freeman S, Katan T. (1997). Identification of Colletotrichum species responsible for anthracnose and root necrosis of strawberry in Israel. Phytopathology 87: 516–521 [DOI] [PubMed] [Google Scholar]
- Freeman S, Minz D, Maymon M, Zveibil A. (2001b). Genetic diversity within Colletotrichum acutatum sensu Simmonds. Phytopahology 91: 586–592 [DOI] [PubMed] [Google Scholar]
- Freeman S, Shalev Z, Katan T. (2002). Survival in soil of Colletotrichum acutatum and C. gloeosporioides pathogenic on strrawberry. Plant Disease 86: 965–970 [DOI] [PubMed] [Google Scholar]
- Freeman S, Shabi E. (1996). Cross-infection of subtropical and temperate fruits by Colletotrichum species from various hosts. Physiological and Molecular Plant Pathology 49: 395–404 [Google Scholar]
- Fuckel L. (1870). Symbolae mycologicae. Beiträge zur Kenntnis der rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde. 23–24: 1–459 [Google Scholar]
- Fujinaga M, Yamagishi N, Ogiso H, Takeuchi J, Moriwaki J, Sato T. (2011). First report of celery stunt anthracnose caused by Colletotrichum simmondsii in Japan. Journal of General Plant Pathology 77: 243–247 [Google Scholar]
- Fukui T. (1916). On some fungi of the useful plants in Japan. Journal of the Scientific Agricultural Society No. 166: 375–386 (in Japanese) [Google Scholar]
- Fukushi T. (1921). A willow-canker disease caused by Physalospora miyabeana and its conidial form Gloeosporium. Annals of the Phytopathological Society of Japan 1: 1–11 [Google Scholar]
- Gadgil PD. (2005). Fungi on Trees and Shrubs in New Zealand. Fungi of New Zealand Volume 4 Fungal Diversity Press, Hong Kong: Gao ZY, Gan J (1992). Biological control of dodder - a review on research progress of the bioherbicide “Lu Bao No. 1”. Chinese Journal of Biological Control8: 173–175 [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118 [DOI] [PubMed] [Google Scholar]
- Garrido C, Carbú M, Fernández-Acero FJ, Vallejo I, Cantoral JM. (2009). Phylogenetic relationships and genome organisation of Colletotrichum acutatum causing anthracnose in strawberry. European Journal of Plant Pathology 125: 397–411 [Google Scholar]
- Giaretta DR, Bogo A, Coelho CMM, Guidolin AF, Dantas ACM, Gomes EA. (2010). ITS-rDNA phylogeny of Colletotrichum spp., causal agent of apple glomerella leaf spot. Ciência Rural 40: 806–812 [Google Scholar]
- Gielink AJ, Vermeulen H. (1983). The ability of American and African Colletotrichum isolates to cause coffee berry disease symptoms and the association of some isolates with Glomerella cingulata. European Journal of Plant Pathology 89: 188–190 [Google Scholar]
- Glass NL, Donaldson G. (1995). Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González E, Sutton TB, Correll JC. (2006). Clarification of the etiology of Glomerella leaf spot and bitter rot of apple caused by Colletotrichum spp. based on morphology and genetic, molecular and pathogenicity tests. Phytopathology 96: 982–992 [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Fourie A. (2011). Biological control of Hakea sericea Schrad. and J.C.Wendl. and Hakea gibbosa (Sm.) Cav. (Proteaceae) in South Africa. African Entomology 19: 303–314 [Google Scholar]
- Gorter GJMA. (1962). The identity of the fungus causing anthracnose of olives in South Africa. Bothalia 7: 769–778 [Google Scholar]
- Greene HC. (1956). Notes on Wisconsin parasitic fungi. XXII. Transactions of the Wisconsin Academy of Science 45: 177–191 [Google Scholar]
- Greene HC. (1964). Notes on Wisconsin parasitic fungi. XXXI. Transactions of the Wisconsin Academy of Science 53: 197–215 [Google Scholar]
- Guerber JC, Correll JC. (1997). The first report of the teleomorph of Colletotrichum acutatum in the United States. Plant Disease 81: 1334 [DOI] [PubMed] [Google Scholar]
- Guerber JC, Correll JC. (2001). Characterization of Glomerella acutata, the teleomorph of Colletotrichum acutatum. Mycologia 93: 216–229 [Google Scholar]
- Guerber JC, Liu B, Correll JC, Johnston PR. (2003). Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 95: 872–895 [PubMed] [Google Scholar]
- Halsted BD. (1893). Some new weed fungi. Bulletin of the Torrey Botanical Club 20: 250–252 [Google Scholar]
- Hemmi T. (1919). Vorläufige Mitteilung über eine Anthraknose von Carthamus tinctorius. Annals of the Phytopathological Society of Japan 1(2): 1–11 [Google Scholar]
- Hemmi T. (1920). Kurze Mitteilung über drei Fälle von Anthracnose auf Pflanzen. Annals of the Phytopathological Society of Japan 1(3): 13–21 [Google Scholar]
- Hemmi T, Kawase Y. (1954). On a new anthracnose of water-lily caused by Gloeosporium nymphaeae sp.n. Bulletin of Naniwa University Ser. B 4: 1–6 [Google Scholar]
- Hennings P. (1895). Fungi goyazenses. Hedwigia 34: 88–116 [Google Scholar]
- Hennings P. (1898). Die in den Gewächshäusern des Berliner Botanischen Gartens beobachteten Pilze. Verhandlungen des Botanischen Vereins der Provinz Brandenburg 40: 109–177 [Google Scholar]
- Hillis DM, Bull JJ. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192 [Google Scholar]
- Hindorf H. (1973). Colletotrichum-Population auf Coffea arabica L. in Kenia II. Qualitative und quantitative Unterschiede in der Colletotrichum-Population. Phytopathologische Zeitschrift 77: 216–234 [Google Scholar]
- Holm L, Holm K. (1994). Svalbard Pyrenomycetes. An annotated checklist. Karstenia 34: 65–78 [Google Scholar]
- Holm L, Ryman S. (2000). Fungi exsiccati suecici. Fascicle 73 (Nos 3601-3650). Thunbergia 30: 1–21 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998). Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Hyde KD, Cai L, Cannon PC, Crouch JA, Crous PW, et al. (2009). Colletotrichum – names in current use. Fungal Diversity 39: 147–182 [Google Scholar]
- Jayasinghe CK, Fernando THPS, Priyanka UMS. (1997). Colletotrichum acutatum is the main cause of Colletotrichum leaf disease of rubber in Sri Lanka. Mycopathologia 137: 53–56 [DOI] [PubMed] [Google Scholar]
- Jenkins SF, Winstead NN. (1964). Glomerella magna, cause of a new anthracnose of cucurbits. Phytopathology 54: 452–454 [Google Scholar]
- Johnson DA, Carris LM, Rogers JD. (1997). Morphological and molecular characterization of Colletotrichum nymphaeae and C. nupharicola sp. nov. on water-lilies (Nymphaea and Nuphar). Mycological Research 101: 641–649 [Google Scholar]
- Johnston P, Dodd S, Park D, Massey B, Charuchinda B, et al. (2008). Are stable, consistant, reliable, and useful species names possible within Colletotrichum? In: Colletotrichum Diseases of Fruit Crops (Peres NA, Timmer LW, eds). Pre-Congress workshop, ICPP 2008, August 24, Torino, Italy: 1–7 [Google Scholar]
- Johnston PR, Jones D. (1997). Relationships among Colletotrichum isolates from fruit-rots assessed using rDNA sequences. Mycologia 89: 420–430 [Google Scholar]
- Jones RW, Perez FG. (2012). First report of anthracnose caused by Colletotrichum acutatum on tamarillo in the United States. Plant Disease 96: 587 [DOI] [PubMed] [Google Scholar]
- Joshee S, Paulus BC, Park D, Johnston PR. (2009). Diversity and distribution of fungal foliar endophytes in New Zealand Podocarpaceae. Mycological Research 113: 1003–1015 [DOI] [PubMed] [Google Scholar]
- Kadow KJ. (1935). The raspberry white-bud disease and its relation to bitter rot of apple. Phytopathology 25: 91–104 [Google Scholar]
- Kelkar PV. (1972). A new species of Colletotrichum Corda from Maharashtra, India. Maharashtra Vidnyan Mandir Patrika 7: 47–48 [Google Scholar]
- Kenny MK, Galea VJ, Scott PT, Price TV. (2006). A comparison of Colletotrichum species associated with berry diseases of Coffea arabica L. pest and disease incursion: risk, threats and management in Papua New Guinea. ACIAR Technical Reports 62: 192–199 [Google Scholar]
- Kim WG, Moon YG, Cho WD, Park SD. (1999). Anthracnose of safflower caused by Colletotrichum acutatum. Plant Pathology Journal 15: 62–67 [Google Scholar]
- Kinghorn WO. (1936). Glomerella phacidiomorpha (Ces.) Pet. on Phormium tenax in Britain. Annals of Applied Biology 23: 30–44 [Google Scholar]
- Krüger F. (1913). Beiträge zur Kenntnis einiger Gloeosporien. Arbeiten aus der Kaiserlichen Biologischen Anstalt für Land- u. Forstwirtschaft 9: 233–323 [Google Scholar]
- Kumari PS, Nair MC. (1981). Some new host records for Colletotrichum gloeosporioides in India. Indian Phytopathology 34: 402–403 [Google Scholar]
- Kurlovich BS. (2002). Lupins. Geography, classification, genetic resources and breeding. St. Petersburg: Intan; [Google Scholar]
- Kwon J-H, Kang S-W, Son KE, Park C-S. (1999). Anthracnose of cosmos caused by Colletotrichum acutatum in Korea. The Plant Pathology Journal 15: 172–174 [Google Scholar]
- Lardner R, Johnston PR, Plummer KM, Pearson MN. (1999). Morphological and molecular analysis of Colletotrichum acutatum sensu lato. Mycological Research 103, 275–285 [Google Scholar]
- Leach CM. (1958). A disease of dodder caused by the fungus Colletotrichum destructivum. Plant Disease Reporter 42: 827–829 [Google Scholar]
- Leandro LFS, Gleason ML, Nutter FW, Jr., Wegulo SN, Dixon PM. (2001). Germination and sporulation of Colletotrichum acutatum on symptomless strawberry leaves. Phytopathology 91: 659–664 [DOI] [PubMed] [Google Scholar]
- Leandro LFS, Gleason ML, Nutter FW, Jr, Wegulo SN, Dixon PM. (2003). Strawberry plant extracts stimulate secondary conidiation by Colletotrichum acutatum on symptomless leaves. Phytopathology 93: 1285–1291 [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim DH, Jeon YA, Uhm JY, Hong SB. (2007). Molecular and cultural characterization of Colletotrichum spp. causing bitter rot of apples in Korea. Plant Pathology Journal 23: 37–44 [Google Scholar]
- Liao CY, Chen MY, Chen YK, Wang TC, Sheu ZM, Kuo KC, Chang PFL, Chung KR, Lee MH. (2012). Characterization of three Colletotrichum acutatum isolates from Capsicum spp. European Journal of Plant Pathology 133: 599–608 [Google Scholar]
- Liu F, Hyde KD, Cai L. (2011). Neotypification of Colletotrichum coccodes, the causal agent of potato black dot disease and tomato anthracnose. Mycology 2: 248–254 [Google Scholar]
- LoBuglio KF, Pfister DH. (2008). A Glomerella species phylogenetically related to Colletotrichum acutatum on Norway maple in Massachusetts. Mycologia 100: 710–715 [DOI] [PubMed] [Google Scholar]
- Lotter HC, Berger DK. (2005). Anthracnose of lupins in South Africa is caused by Colletotrichum lupini var. setosum. Australasian Plant Pathology 34: 385–392 [Google Scholar]
- Lubbe CM, Denman S, Cannon PF, Groenewald JZ, Lamprecht SC, Crous PW. (2004). Characterization of Colletotrichum species associated with diseases of Proteaceae. Mycologia 96: 1268–1279 [PubMed] [Google Scholar]
- McInnes TB, Black LL, Gatti JM., Jr. (1992). Disease-free plants for management of strawberry anthracnose crown rot disease. Plant Disease 76: 260–264 [Google Scholar]
- MacKenzie SJ, Peres NA, Barquero MP, Arauz LF, Timmer LW. (2009). Host range and genetic relatedness of Colletotrichum acutatum isolates from fruit crops and leatherleaf fern in Florida. Phytopathology 99: 620–631 [DOI] [PubMed] [Google Scholar]
- MacKenzie SJ, Seijo TE, Legard DE, Timmer LW, Peres NA. (2007). Selection for pathogenicity to strawberry in populations of Colletotrichum gloeosporioides from native plants. Phytopathology 97: 1130–1140 [DOI] [PubMed] [Google Scholar]
- Manire CA, Rhinehart HL, Sutton DA, Thompson EH, Rinaldi MG, Buck JD, Jacobson E. (2002). Disseminated Mycotic Infection Caused by Colletotrichum acutatum in a Kemp’s Ridley Sea Turtle (Lepidochelys kempi). Journal of Clinical Microbiology 40: 4273–4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino J, Giordano R, Gouli S, Gouli V, Parker BL, Skinner M, TeBeest D, Cesnik R. (2008). Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 100: 353–374 [DOI] [PubMed] [Google Scholar]
- Marcelino JAP, Gouli S, Parker BL, Skinner M, Schwarzberg L, Giordano R. (2009). Host plant associations of an entomopathogenic variety of the fungus, Colletotrichum acutatum, recovered from the elongate hemlock scale, Fiorinia externa. 11 pp. Journal of Insect Science 9: 25, available online: insectscience.org/9.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Culebras PV, Querol AA, Suarez-Fernandez MB, Garcia-Lopez MD, Barrio E. (2003). Phylogenetic relationship among Colletotrichum pathogens of stawberry and design of PCR primers for their identification. Journal of Phytopathology 151: 135–143 [Google Scholar]
- Masaba DM, Waller JM. (1992). Coffee berry disease: The current status. In Bailey JA, Jeger MJ. (Eds.), Colletotrichum: Biology, Pathology and Control: 237–249 Wallingford: CAB International; [Google Scholar]
- Mason-Gamer RJ, Kellogg EA. (1996). Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545 [Google Scholar]
- Mejía LC, Enith I, Rojas EI, Maynard Z, Van Bael S, Arnold AE, Hebbar P, Samuels GJ, Robbins N, Herre EA. (2008). Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biological Control 46: 4–14 [Google Scholar]
- Misra AK. (2004). Guava diseases – their symptoms, causes and management. In: Diseases of fruits and vegetables. Diagnosis and management Volume 2. (Naqvi SAMH. ed.). Kluwer Academic Publishers, Dordrecht: 81–119 [Google Scholar]
- Morris MJ. (1982). Biological control of Hakea by a fungus.Veld & Flora 68: 51–52 [Google Scholar]
- Mułenko W, Majewski T, Ruszkiewicz-Michalska M. (2008). A preliminary checklist of micromycetes in Poland. W. Szafer Institute of Botany. Polish Academy of Sciences 9: 1–752 [Google Scholar]
- Nakaune R, Nakano M. (2007). Benomyl resistance of Colletotrichum acutatum is caused by enhanced expression of beta-tubulin 1 gene regulated by putative leucine zipper protein CaBEN1. Fungal Genetics and Biology 44: 1324–1335 [DOI] [PubMed] [Google Scholar]
- Nattrass RM. (1928). The Physalospora disease of the basket willow. Transactions of the British Mycological Society 13: 286–304 [Google Scholar]
- Natural History Museum (2011). http://www.nhm.ac.uk/research-curation/research/projects/sloane-herbarium/hanssloane.htm, accessed 21/12/2011
- Neergaard P. (1943). 8. Aarsberetning fra J. E. Ohlens Enkes Plantepatologiske Laboratorium 1. April 1942–31. Marts 1943. J. D. Qvist & Komp. Bogtrykkeri Akts., København, Danmark: [Google Scholar]
- Neergaard P. (1950). Mycological Notes III. 7. Colletotrichum godetiae Neerg. 8. Phoma bellidis Neerg. 9. Zygosporium parasiticum (Grove) Bunting & Mason. 10. Peronospora dianthicola Barthelet. Friesia 4: 72–80 [Google Scholar]
- Nguyen PTH, Vinnere Pettersson O, Olsson P, Liljeroth E. (2010). Identification of Colletotrichum species associated with anthracnose disease of coffee in Vietnam. European Journal of Plant Pathology 127: 73–87 [Google Scholar]
- Nguyen THP, Säll T, Bryngelsson T, Liljeroth E. (2009). Variation among Colletotrichum gloeosporioides isolates from infected coffee berries at different locations in Vietnam. Plant Pathology 58: 898–909 [Google Scholar]
- Nirenberg HI. (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1–117 [Google Scholar]
- Nirenberg HI, Feiler U, Hagedorn G. (2002). Description of Colletotrichum lupini comb. nov. in modern terms. Mycologia 94: 307–320 [PubMed] [Google Scholar]
- Nirenberg HI, Gerlach W. (2000). Bestimmung und Pathogenitätsnachweis des Erregers der Anthraknose an Bergenien. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 52: 1–4 [Google Scholar]
- Nylander JAA. (2004). MrModeltest v2. Program distributed by the author Evolutionary Biology Centre, Uppsala University; [Google Scholar]
- O’Donnell K, Cigelnik E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7:103–116 [DOI] [PubMed] [Google Scholar]
- Parikka P, Pääskynkivi E, Lemmetty A. (2006). Colletotrichum acutatum: survival in plant debris and infection on alternate hosts. NJF Report 2(10): 37 [Google Scholar]
- Pennycook SR. (1989). Plant diseases recorded in New Zealand Vol. 3, Plant Diseases Division, DSIR, Auckland, New Zealand: [Google Scholar]
- Peredo HL, Osorio M, Santamaría A. (1979). Colletotrichum acutatum f. sp.pinea, a new pathogen of Pinus radiata on nurseries in Chile. Plant Disease Reporter 63: 121–122 [Google Scholar]
- Peres NAR, Kuramae EE, Dias MSC, Souza NL de. (2002). Identification and characterization of Colletotrichum spp. affecting fruit after harvest in Brazil. Journal of Phytopathology 150: 128–134 [Google Scholar]
- Peres NA, MacKenzie SJ, Peever TL, Timmer LW. (2008). Postbloom fruit drop of citrus and Key lime anthracnose are caused by distinct populations of Colletotrichum acutatum. Phytopathology 98: 345–352 [DOI] [PubMed] [Google Scholar]
- Peres NA, Timmer LW, Adaskaveg JE, Correll JC. (2005). Life styles of Colletotrichum acutatum. Plant Disease 89: 784–796 [DOI] [PubMed] [Google Scholar]
- Petch T. (1906). Descriptions of new Ceylon fungi. Annals of the Royal Botanical Gardens, Peradeniya 3(1): 1–10 [Google Scholar]
- Petch T. (1917). Additions to Ceylon fungi. Annals of the Royal Botanic Gardens, Peradeniya 6(3): 195–256 [Google Scholar]
- Petch T. (1927). Diseases and Pests of the Rubber Tree. Macmillan, London, UK: [Google Scholar]
- Petrak F. (1925). Mykologische Notizen. VIII. Annales Mycologici 23: 1–143 [Google Scholar]
- Phoulivong S, Cai L, Chen H, McKenzie EHC, Abdelsalam K, Hyde KD, Chukeatirote E. (2010). Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Diversity 44: 33–43 [Google Scholar]
- Polashock JJ, Oudemans PV, Caruso FL, Mcmanus P, Crouch J. (2009). Population structure of the North American cranberry fruit rot complex. Plant Pathology 58: 1116–1127 [Google Scholar]
- Polizzi G, Aiello D, Guarnaccia V, Vitale A, Perrone G, Stea G. (2011). First report of damping-off on strawberry tree caused by Colletotrichum acutatum and C. simmondsii in Italy. Plant Disease 95: 1588 [DOI] [PubMed] [Google Scholar]
- Prihastuti H, Cai L, Chen H, McKenzie EHC, Hyde KD. (2009). Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity 39: 89–109 [Google Scholar]
- Rahman M, Louws FJ. (2008). Colletotrichum gloeosporioides on strawberry: From nursery to fruiting field. In: Colletotrichum Diseases of Fruit Crops (Peres NA, Timmer LW, eds). Pre-Congress workshop, ICPP 2008, August 24, Torino, Italy: 54 [Google Scholar]
- Rambaut A. (2002). Sequence Alignment Editor. Version 2.0. University of Oxford, Oxford, UK: [Google Scholar]
- Ramos AP, Merali Z, Talhinhas P, Sreenivasaprasad S, Oliveira H. (2006). Molecular and morphological characterisation of Colletotrichum species involved in citrus anthracnose in Portugal. IOBC/WPRS Bulletin 29: 317–326 [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. Commonwealth Mycological Institute, Kew, UK: [Google Scholar]
- Robideau GP, Caruso FL, Oudemans PV, McManus PS, Renaud M-A, Auclair M-E, Bilodeau GJ, Yee D, Désaulniers NL, DeVerna JW, Lévesque CA. (2008). Detection of cranberry fruit rot fungi using DNA array hybridization. Canadian Journal of Plant Pathology 30: 226–240 [Google Scholar]
- Rojas EI, Rehner SA, Samuels GJ, Van Bael SA, Herre EA, Cannon PF, Chen R, Pang J-f, Wang R-w, Zhang Y-p, Sha T. (2010). Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panama: multilocus phylogenies distinguish pathogen and endophyte clades. Mycologia 102: 1318–1338 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Rostrup FGE. (1891). Bidrag til kundskaben om Norges soparter. II. Ascomycetes fra Dovre. Christiana Videnskabs-Selskabs Forhandlinger 9: 1–14 [Google Scholar]
- Saccardo PA. (1884). Sylloge Fungorum vol. 3 Padova, Italy: [Google Scholar]
- Saccardo PA. (1896). Fungi aliquot Brasilienses phyllogeni. Bulletin de la Société Royale de Botanique Belgique 35: 127–132 [Google Scholar]
- Saccardo PA. (1899). Sylloge Fungorum vol. 14 Padova, Italy: [Google Scholar]
- Saccardo PA. (1906). Sylloge Fungorum vol. 18 Padova, Italy: [Google Scholar]
- Saccardo PA, Trotter A. (1913). Sylloge Fungorum vol. 22 Padova, Italy: [Google Scholar]
- Saccardo PA, Saccardo D, Traverso JB, Trotter A. (1931). Sylloge Fungorum vol. 25 Padova, Italy: [Google Scholar]
- Saha T, Kumar A, Ravindran M, Jacobs K, Roy B, Nazeer MA. (2002). Identification of Colletotrichum acutatum from rubber using random amplified polymorhic DNAs and ribosomal DNA polymorphisms. Mycological Research 106: 215–221 [Google Scholar]
- Sang MK, Kim JD, Kim BS, Kim KD. (2011). Root treatment with rhizobacteria antagonistic to Phytophthora blight affects anthracnose occurrence, ripening, and yield of pepper fruit in the plastic house and field. Phytopathology 101: 666–678 [DOI] [PubMed] [Google Scholar]
- Sawada K. (1943). Descriptive catalogue of the Formosan fungi. Part VIII. Report of the Department of Agriculture, Government Research Institute of Formosa 85: 1–130 [Google Scholar]
- Sawada K. (1959). Descriptive catalogue of Taiwan (Formosan) fungi. XI. Special Publication, College of Agriculture, National Taiwan University 8: 1–268 [Google Scholar]
- Schiller M, Lübeck M, Sundelin T, Meléndez LFC, Danielsen S, Jensen DF, Ordeñana KM. (2006). Two subpopulations of Colletotrichum acutatum are responsible for anthracnose in strawberry and leatherleaf fern in Costa Rica. European Journal of Plant Pathology 116: 107–118 [Google Scholar]
- Schrenk H von, Spaulding P. (1903a). The bitter rot fungus. Science n.s. 17: 750–751 [DOI] [PubMed] [Google Scholar]
- Schrenk H von, Spaulding P. (1903b). The bitter rot of apples. United States Department of Agriculture Bureau of Plant Industry Bulletin 44: 1–54, 9 plates [Google Scholar]
- Schroeter J. (1894). Die Pilze Schlesiens. In: Kryptogamen-Flora von Schlesien 2 (Cohn F, ed). J.U. Kern’s Verlag, Breslau: 257–384 [Google Scholar]
- Sette LD, Passarini MRZ, Delarmelina C, Salati F, Duarte MCT. (2006). Molecular characterization and antimicrobial activity of endophytic fungi from coffee plants. World Journal of Microbiology and Biotechnology 11: 1185–1195 [Google Scholar]
- Shear CL. (1907). New species of fungi. Bullentin of the Torrey Botanical Club 34 (6): 305–317 [Google Scholar]
- Sherriff C, Whelan MJ, Arnold GM, Lafay JF, Brygoo Y, Bailey JA. (1994). Ribosomal DNA sequence analysis reveals new species groupings in the genus Colletotrichum. Experimental Mycology 18: 121–138 [Google Scholar]
- Shivas RG, Tan YP. (2009). A taxonomic re-assessment of Colletotrichum acutatum, introducing C. fioriniae comb. et stat. nov. and C. simmondsii sp. nov. Fungal Diversity 39: 111–122 [Google Scholar]
- Simmonds JH. (1965). A study of the species of Colletotrichum causing ripe fruit rots in Queensland. Queensland Journal of Agricultural and Animal Science 22: 437–459 [Google Scholar]
- Simmonds JH. (1968). Type specimens of Colletotrichum gloeosporioides var. minor and Colletotrichum acutatum. Queensland Journal of Agricultural and Animal Science 25: 178A [Google Scholar]
- Smith BJ, Magee JB, Gupton CL. (1996). Susceptibility of rabbiteye blueberry cultivars to postharvest diseases. Plant Disease 80: 215–218 [Google Scholar]
- Sreenivasaprasad S, Mills PR, Brown AE. (1994). Nucleotide sequence of the rDNA spacer 1 enables identification of isolates of Colletotrichum as C. acutatum. Mycological Research 98: 186–188 [Google Scholar]
- Sreenivasaprasad S, Mills PR, Meehan BM, Brown AE. (1996). Phylogeny and systematics of 18 Colletotrichum species based on ribosomal DNA spacer sequences. Genome 39: 499–512 [DOI] [PubMed] [Google Scholar]
- Sreenivasaprasad S, Talhinhas P. (2005). Genotypic and phenotypic diversity in Colletotrichum acutatum, a cosmopolitan pathogen causing anthracnose on a wide range of hosts. Molecular Plant Pathology 6: 361–378 [DOI] [PubMed] [Google Scholar]
- Srivastava RN, Gupta JS. (1981). Seed mycoflora from Indian seed lots of Cosmos bipinnatus and their control. Indian Phytopathology 34: 402–403 [Google Scholar]
- Stevenson JA. (1971). An account of fungus exsiccati containing material from the Americas. Beihefte zur Nova Hedwigia 36: 563 pp [Google Scholar]
- Stoneman B. (1898). A comparative study of the development of some anthracnoses. Botanical Gazette Chicago 26: 69–120 [Google Scholar]
- Su YY, Noireung P, Liu F, Hyde KD, Moslem MA, Bahkali AH, Abd-Elsalam KA, Cai L. (2011). Epitypification of Colletotrichum musae, the causative agent of banana anthracnose. Mycoscience 52: 376–382 [Google Scholar]
- Sun X, Guo L-D, Hyde KD. (2011). Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Diversity 47: 85–95 [Google Scholar]
- Suryanarayanan TS, Senthilarasu G, Muruganandam V. (2000). Endophytic fungi from Cuscuta reflexa and its host plants. Fungal Diversity 4: 117–123 [Google Scholar]
- Swofford DL. (2000). PAUP* 4.0: phylogenetic analysis using parsimony (* and other methods). Sinauer Associates, Sunderland, MA: [Google Scholar]
- Takeuchi J, Horie H. (2006). First report of anthracnose of Phormium tenax and Chimonanthus praecox f. concolor by Colletotrichum gloeosporioides occurring in Japan. Annual Report of the Kanto-Tosan Plant Protection Society 53: 83–85 [Google Scholar]
- Takimoto K. (1924). Anthracnose of crown daisy. Japanese Journal of Horticulture (Nihon-Engei Zasshi) 36(9): 27–29 [Google Scholar]
- Talgø V, Aamot HU, Strømeng GM, Klemsdal SS, Stensvand A. (2007). Glomerella acutata on highbush blueberry (Vaccinium corymbosum L.) in Norway. Online. Plant Health Progress doi:10.1094/PHP-2007-0509-01-RS
- Talhinhas P, Sreenivasaprasad S, Neves-Martins J, Oliveira H. (2002). Genetic and morphological characterization of Colletotrichum acutatum causing anthracnose of lupin. Phytopathology 92: 986–996 [DOI] [PubMed] [Google Scholar]
- Talhinhas P, Sreenivasaprasad S, Neves-Martins J, Oliveira H. (2005). Molecular and phenotypic analyses reveal association of diverse Colletotrichum acutatum groups and a low level of C. gloeosporioides with olive anthracnose. Applied and Environmental Microbiology 71: 2987–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhinhas P, Neves-Martins J, Oliveira H, Sreenivasaprasad S. (2009). The distinctive population structure of Colletotrichum species associated with olive anthracnose in the Algarve region of Portugal reflects a host-pathogen diversity hot spot. FEMS Microbiology Letters 296: 31–38 [DOI] [PubMed] [Google Scholar]
- Talhinhas P, Mota-Capitão C, Martins S, Ramos AP, Neves-Martins J, Guerra-Guimarães L, Várzea V, Silva MC, Sreenivasaprasad S, Oliveira H. (2011). Epidemiology, histopathology and aetiology of olive anthracnose caused by Colletotrichum acutatum and C. gloeosporioides in Portugal. Plant Pathology 60: 483–495 [Google Scholar]
- Tanaka T. (1917). New Japanese fungi. Notes and translations – 1. Mycologia 9: 249–253 [Google Scholar]
- Taylor JE, Hyde KD. (2003). Microfungi of Tropical and Temperate Palms. Fungal Diversity Press, Hong Kong: [Google Scholar]
- TeBeest DO. (1988). Additions to host range of Colletotrichum gloeosporioides f. sp. aeschynomenes. Plant Disease 72: 16–18 [Google Scholar]
- Thambugala TADP, Deshappriya N. (2009). The role of Colletotrichum species on the Colletotrichum leaf disease of Hevea brasiliensis – a preliminary study. Journal of the National Science Foundation Sri Lanka 37: 135–138 [Google Scholar]
- Than PP, Jeewon R, Hyde KD, Pongsupasamit S, Mongkolporn O, Taylor PWJ. (2008a). Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathology 57: 562–572 [Google Scholar]
- Than PP, Shivas RG, Jeewon R, Pongsupasamit S, Marney TS, Taylor PWJ, Hyde KD. (2008b). Epitypification and phylogeny of Colletotrichum acutatum J.H. Simmonds. Fungal Diversity 28: 97–108 [Google Scholar]
- Timmer LW, Brown GE. (2000). Biology and control of anthracnose diseases of citrus. In: Host Specificity, Pathology, and Host-Pathogen Interactions of Colletotrichum (Prusky D, Freeman S, Dickman MB. eds) American Phytopathological Society, St. Paul, MN, USA: 300–316 [Google Scholar]
- Turconi M. (1924). Una moria di giovani piante di Eucalipti. Atti dell’Istituto Botanico della Università e Laboratorio Crittogamico di Pavia 1, Ser. 3: 125–135 [Google Scholar]
- Turner E, Jacobson DJ, Taylor JW. (2010). Biogeography of postmating reproductive isolation barriers is consistent with reinforcement selection in Neurospora, a model microbial eukaryote. Journal of Evolutionary Biology 23: 1642–1656 [DOI] [PubMed] [Google Scholar]
- Turner E, Jacobson DJ, Taylor JW. (2011). Genetic architecture of a reinforced, postmating, reproductive isolation barrier between Neurospora species indicates evolution via natural selection. PLoS Genetics 7: e1002204 doi:10.1371/journal.pgen.1002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Kageyama K, Moriwaki J, Sato T. (2012). Colletotrichum carthami comb nov., an anthracnose pathogen of safflower, garland chrysanthemum and pot marigold, revived by molecular phylogeny with authentic herbarium specimens. Journal of General Plant Pathology. doi: 10.1007/s10327-012-0397-3
- Vichova J, Vejrazka K, Cholastova T, Pokorny R, Hrudova E. (2011). Colletotrichum simmondsii causing anthracnose on safflower in the Czech Republic. Plant Disease 95: 79 [DOI] [PubMed] [Google Scholar]
- Viégas AP. (1946). Alguns fungos do Brasil XII. Fungi imperfecti – Melanconiales. Bragantia 6: 1–37 [Google Scholar]
- Vinnere O. (2004). Approaches to Species Delineation in Anamorphic (Mitosporic) Fungi: A Study on Two Extreme Cases. Ph.D. dissertation. Department of Evolutionary Biology, Uppsala University, Sweden: [Google Scholar]
- Vinnere O, Fatehi J, Wright SAI, Gerhardson B. (2002). The causal agent of anthracnose of Rhododendron in Sweden and Latvia. Mycological Research 106: 60–69 [Google Scholar]
- Walker J, Nikandrow A, Millar GD. (1991). Species of Colletotrichum on Xanthium (Asteraceae) with comments on some taxonomic and nomenclatural problems in the genus Colletotrichum. Mycological Research 95: 1175–1193 [Google Scholar]
- Waller JM, Bridge PD, Black B, Hakiza G. (1993). Characterization of the coffee berry disease pathogen Colletotrichum kahawae sp. nov. Mycological Research 97: 989–994 [Google Scholar]
- Watson AK, Gressel J, Sharon A, Dinoor A. (2000). Colletotrichum strains for weed control. In: Colletotrichum. Host Specificity, Pathology and Host-Pathogen Interaction (Prusky D, Freeman S, Dickman MB, eds). APS Press, St Paul, USA: 245–265 [Google Scholar]
- Weir BS, Johnston PR, Damm U. (2012). The Colletotrichum gloeosporioides species complex. Studies in Mycology 73: 115–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton PS, Diéguez-Uribeondo J. (2004). The biology of Colletotrichum acutatum. Anales del Jardín Botánico de Madrid 61: 3–22 [Google Scholar]
- Wharton PS, Schilder AMC. (2008) Novel infection strategies of Colletotrichum acutatum on ripe blueberry fruit. Plant Pathology 57: 122–134 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, USA: 315–322 [Google Scholar]
- Whitelaw-Weckert MA, Curtin SJ, Huang R, Steel CC, Blanchard CL, Roffey PE. (2007). Phylogenetic relationships and pathogenicity of Colletotrichum acutatum isolates from grape in subtropical Australia. Plant Pathology 56: 448–463 [Google Scholar]
- Wollenweber HW. (1916). Fusaria Autographice Delineata 1, 1 edn., Berlin, Germany: [Google Scholar]
- Woudenberg JHC, Aveskamp MM, Gruyter J de, Spiers AG, Crous PW. (2009). Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 22: 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi Y, Suyama K, Ushiyama K, Kobayashi M, Saito T, Nakamura S. (1996). Anthracnose of Cosmos bipinnatus caused by Colletotrichum acutatum Simmonds ex Simmonds. Annales of the Phytopathological Society of Japan 62: 433–436 [Google Scholar]
- Yang HA, Sweetingham MW. (1998). The taxonomy of Colletotrichum isolates associated with lupin anthracnose. Australian Journal of Agricultural Research 49: 1213–1223 [Google Scholar]
- Yang YL, Cai L, Yu ZN, Liu ZY, Hyde KD. (2011). Colletotrichum species on Orchidaceae in southwest China. Cryptogamie Mycologie 32: 229–253 [Google Scholar]
- Yang YL, Liu ZY, Cai L, Hyde KD, Yu ZN, McKenzie EHC. (2009). Colletotrichum anthracnose of Amaryllidaceae. Fungal Diversity 39: 123–146 [Google Scholar]
- Yearsley CW, Huang BY, McGrath HJ, Fry J, Stec MGH, Dale JR. (1988). Red tamarillos (Cyphomandra betacea): comparison of two postharvest dipping strategies for the control of fungal storage disorders. New Zealand Journal of Experimental Agriculture 16: 359–366 [Google Scholar]
- Yuan ZL, Chen YC, Yang Y. (2009). Diverse non-mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): estimation and characterization. World Journal of Microbiology and Biotechnology 25: 295–303 [Google Scholar]
- Zhang TY. (1985). A forma specialis of Colletotrichum gloeosporioides on Cuscuta spp. Acta Mycologica Sinica 4: 234–239 [Google Scholar]