Abstract
Although oncogenic HPV infections have been established as the necessary cause of cervical cancer, most HPV infections are transient and rarely progress to cervical lesions. Current research is focused on identifying factors associated with viral persistence and clearance, such as nutritional status. We evaluated the association between serum antioxidant nutrients (retinol, 10 carotenoids and 3 tocopherols) and type-specific HPV persistence over 4 visits among 405 women participating in the Ludwig-McGill cohort study. We measured circulating carotenoids and tocopherol at 4 different clinical visits for each woman. We report the results from different analytic approaches (a case–control approach at both the woman and viral level, and a prospective approach based on persistent events) that examined the association between these micronutrients and type-specific oncogenic and nononcogenic HPV persistence. In the case–control analysis at the viral level, midcirculating levels of α-tocopherol were inversely associated with nononcogenic HPV persistent infection (adjusted odds ratio (AOR) = 0.28, 95% CI 0.14–0.57), while high levels were marginally associated (AOR = 0.59, 95% CI 0.28–1.19). Similarly, utilizing generalized estimating equation models, circulating levels of α- and δ-tocopherol in the middle or upper tertiles were inversely associated with type-specific nononcogenic HPV persistence (AOR = 0.44, 95% CI 0.19–0.97 and AOR = 0.46, 95% CI 0.19–1.11, respectively). Our study among Brazilian women suggests that serum levels of tocopherols may be protective against nononcogenic HPV persistence. However, we did not find a strong protective effect (as hypothesized) of other serum antioxidant nutrients and type-specific oncogenic HPV persistence measured over 4 clinical visits.
Keywords: human papillomavirus, persistence, carotentoid, tocopherol
Human papillomavirus (HPV) infection has been shown to be the cause of cervical cancer, the second leading cancer among women worldwide.1 Prospective studies have shown that women with persistent HPV infections are more likely to develop cervical lesions compared to women with transient HPV infections.2 Although oncogenic HPV infections have been established as the necessary cause of cervical cancer, most HPV infections are transient and rarely progress to significant cervical lesions.3 Current research is focused on identifying factors that can alter the lifecycle of HPV and, therefore, modulate viral persistence and clearance. Nutritional status may be an important cofactor affecting both HPV persistence and progression of persistent HPV infections to cervical intraepithelial neoplasia (CIN).4,5
The association between nutrient status and cervical carcinogenesis has been extensively investigated as reviewed by Garcia-Closas and collegues.6 In evaluating the associations reported in the past, 2 factors need to be considered: (i) were nutrients assessed as cofactors in HPV-related carcinogenesis (e.g. was there adequate control for HPV status) and (ii) where in this continuum were different classes of nutrients active (e.g. HPV acquisition, HPV persistence, squamous intraepithelial lesions (SIL) progression or invasive cervical cancer).7,8 A majority of case–control studies examine late events in cervical carcinogenesis and did not adequately control for HPV infection.7,8 Epidemiological evidence suggests that there is a possible protective effect of anti-oxidant nutrients (retinol, carotenoids and tocopherols) in HPV persistence6,4,9–11 and cervical cancer.6 These finding are based on relatively few studies that accurately controlled for HPV infection (4 studies of HPV persistence and 7 studies on CIN/invasive cancer). In a prospective study among women attending a family planning clinic for routine care, Sedjo et al.9,12 observed significant inverse associations between dietary intake of lutein, vitamin E, vegetables and serum lycopene levels and HPV persistence.9 While our study used a conservative definition of oncogenic HPV persistence, it was unable to assess type-specific HPV persistence.9 Oncogenic, but not nononcogenic, HPV clearance was also reported to be associated with serum lycopene levels,12 whereas the associations of other antioxidant nutrients assessed did not reach significance. While several studies have found protective effects of antioxidant nutrients on HPV persistence,4,9–11 the studies have had inconsistent results,4,9–11 analyses were conducted on samples sizes of 200 or fewer women, only 1 study had multiple assessments of serum/plasma nutrient levels,11 and none of the studies examined type-specific persistence or nononcogenic types. Therefore, larger prospective studies are needed to further examine the association of individual antioxidant nutrients and the natural history of HPV infection.
The present study was conducted to further evaluate the association between serum antioxidant nutrients (retinol, carotenoids and tocopherols) and type-specific HPV persistence over 4 consecutive study visits in approximately a 12-month period among Brazilian women participating in a prospective study HPV natural history study (Ludwig-McGill cohort study). The study sample size and follow-up period were designed to analyze HPV persistence over the first 4 study visits where circulating nutrients markers were measured at corresponding time points. On the basis of our a priori hypothesis and previous finding that defining HPV persistence as 2 or more consecutively positive tests over the first 4 visits on study is an informative definition of HPV persistence relative to SIL risk and lesion development,13 this present study utilized HPV data from the first 4 study visits as a biomarker of SIL risk. We measured circulating carotenoid and tocopherol concentrations in serum obtained at the same 4 different clinical visits to accurately classify nutrient status.14 Cervical HPV status was assessed by polymerase chain reaction (PCR) at each study visit as well. Using several analytic approaches, we report no association between serum antioxidant nutrients (retionol, carotenoids and tocopherols) and oncogenic HPV persistence.
Material and methods
The Ludwig-McGill cohort study is a prospective study of women attending a comprehensive maternal and child health maintenance program serving low-income families, in São Paulo, Brazil.15 The clinic setting where participants were accrued is part of a network of primary, secondary and tertiary health care institutions maintained by the municipal health department. Cohort participants were recruited between 1993 and 1997 and examined every 4 months in the first year, and twice yearly thereafter for a total of 5 years. At each study visit, participants were interviewed based on a structured questionnaire specific for the current visit.15 The institutional review boards and ethical committees of all institutions with which the authors are affiliated approved these protocols. Each study participant signed an approved informed consent document.
Study sample
Of the 2,528 women in the cohort, we identified a subcohort of women (n = 1,392) entering the study during the first 2 years (1993–1995), had long-term follow-up and complete HPV data and serum available from all of the first 4 visits on study. From this subcohort, HPV-positive women were matched to women who were HPV-negative at all 4 visits on age and month of enrollment. Among the 846 women that were matched, 819 had normal or atypical squamous cells of undetermined significance cytology at baseline, 407 tested positive for HPV at least once during the first 4 visits and 412 women were HPV-negative at all 4 visits. To study the association between HPV persistence and serum carotenoids and tocopherol levels, only the 407 women who tested positive for HPV at any one of the 4 visits were included in this analysis. Women were excluded if they did not have at least 2 serum samples available for nutrient analysis (N = 2), leaving a final sample size of 405 women.
As indicated earlier, the first 4 study visits were scheduled to occur every 4 months in the first year on study. On average, women in this cohort attended their study visits as scheduled, specifically the 405 women included in these analyses were seen at 4.6 ± 2.4 months, 9.0 ± 6.7 months and 13.5 ± 4.1 months after enrollment. Among the first 4 visits, the time between each visit was on average 4.4 ± 2.1 months. The last study visit included in this analysis fell between 11 and 50 months following enrollment.
HPV DNA detection by PCR and genotyping
All HPV analyses were performed at the Ludwig Institute for Cancer Research, São Paulo, Brazil. Ectocervical and endocervical cells were collected with an Accelon biosampler (Medscand, Inc., Hollywood, FL) and immersed in Tris-EDTA buffer at pH 7.4. The deoxyribonucleic acid (DNA) was purified by spin column chromatography and amplified by polymerase chain reaction (PCR) using MY09/11 primers.16,17 Hybridization of the amplified products with oligonucleotide probes and restriction fragment-length polymorphism (RFLP) analyses were used to identify 41 known genital HPV types.18 Amplified products that hybridized only with a generic probe and did not have a discernible pattern in RFLP analysis were considered positive for unknown types. The quality of the DNA specimens was verified by the amplification of a 268-bp region of the human β-globin gene.16 Specimens were tested blindly, and standard precautions were taken to prevent contamination. HPV infections were grouped as oncogenic infections (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 66) or nononcogenic infections (HPV types 6/11, 26, 32, 34, 40, 42, 44, 53, 54, 55, 57, 61, 62, 64, 67, 69, 68, 70, 71, 72, 73, 84, 83, 81, 82, is39, c6108 and unclassified types).
Serum sample processing and storage
All nonfasting blood samples (~10 ml) were collected by venipuncture into vacutainers by a trained nurse at the time of the clinic visit, which occurred between 1993 and 1996. The samples were centrifuged within 6–8 hr of collection (Life Technologies, Paisley, UK), separated into 1 ml aliquots and stored in 1.8 ml Nunc cryovials at −20°C in a nonfrost free freezer until shipped to Craft Technologies (Wilson, NC) for analyses.
HPLC analytical procedures for serum retinol, carotenoids and tocopherols
Serum samples from the first 4 study visits were assayed for retinol, carotenoids and tocopherols using a modification of the procedures described by Nomura et al.19 All analytical procedures were performed under lights covered with UV filters to remove the UV light to preserve light sensitive compounds. The percent coefficient of variation was 8% or less for retinol, carotenoids and tocopherols measured. This high pressure liquid chromatography (HPLC) system’s limit of quantification (LOQ) was 0.004 μg/ml for the carotenoids and 0.015 μg/ml for tocopherols. Of the total 1,628 samples analyzed over 4 clinical visits, the following number of samples (percent) were below the LOQ and assigned a value half way between zero and the lower limits of detection: retinol 2 (0.1%), lutein 11 (0.7%), zeaxanthin 21 (1.3%), α-cryptoxanthin 549 (34%), β-cryptoxanthin 168 (10%), trans-lycopene 383 (23%), cis-lycopene 333 (20%), α-carotene 373 (23%), trans-β-carotene 234 (14%), cis-β-carotene 504 (31%), α-tocopherols 18 (1.1%), δ-tocopherol 353 (22%) and γ-tocopherol 22 (1.3%). Serum levels of α-carotene, α-cryptoxanthin and cis-β-carotene were very low, with 33% of the levels falling below the LOQ, which comprised the entire lowest tertile for these nutrients (see statistical analysis for details).
Total serum cholesterol
Carotenoids and tocopherols are fat-soluble nutrients, of which uptake, transport and storage is associated with lipids.20 Therefore, the level of serum lipid, as measured by cholesterol, may influence the level of circulating carotenoids and tocopherols. To account for this effect, total serum cholesterol was assessed arbitrarily at visit 3 to enable adjustment for this potential confounding variable. Total cholesterol was determined by coupled enzymatic, colorimetric assay (Kit No. 401-25P, Sigma-Aldrich, St. Louis, MO) by Craft Technologies (Wilson, NC).
Statistical analysis
Nutrient variables
Preliminary studies indicated high within-person variability for serum carotenoids and tocopherols values at different clinical visits (data not shown). Therefore, to accurately classify serum nutrient status,21 we calculated the mean serum carotenoid and tocopherol value over the 4 time-points for each woman. Using this mean value, we categorized the continuous variable into tertiles based on the distribution of each nutrient among HPV-positive women.
HPV outcome variables and modeling
Our study was designed to examine HPV persistence over the first 4 study visits [(baseline, visit 1 (~month 4), visit 3 (~month 8) and visit 4 (~month 12)]. We examined associations with type-specific HPV persistence using 3 different approaches based on these 4 HPV results: (i) a case–control approach using women as the unit of analysis, (ii) a case–control approach using virus as the unit of analysis and (iii) a prospective approach examining persistent events over time. All outcome variables were defined based on individual type-specific infections and then grouped as oncogenic infections (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 66) or nononcogenic infections (HPV types 6/11, 26, 32, 34, 40, 42, 44, 53, 54, 55, 57, 61, 62, 64, 67, 68, 69, 70, 71, 72, 73, 84, 83, 81, 82, is39, c6108 and unclassified types).
Case–control with woman as unit of analysis
In the case–control approach using women as the unit of analysis, HPV status was based on the HPV evaluations conducted at enrollment and the next 3 visits (4 HPV evaluations). Two groups of women were identified: those transiently positive and those persistently positive for the virus. The transient group (control group) consisted of those who tested HPV-positive in only one of the 4 assessments or were nonconsecutively positive for the same type. The persistent group (case group) consisted of those who tested positive for the same HPV type at 2 or more consecutive visits out of the first 4 visits in the study. In the women level analysis, women infected with single or multiple oncogenic HPV types and women infected with both oncogenic and nononcogenic HPV types were categorized as having an oncogenic infection. Only women infected with a single or multiple nononcogenic types were categorized as having a nononcogenic infection. For this analysis oncogenic and nononcogenic infections were grouped separately resulting in 229 oncogenic HPV infections (141 transient and 88 persistent) and 176 nononcogenic infections (118 transient and 58 persistent). Logistic regression was performed to estimate the odds ratio (OR) and 95% confidence interval (CI) between concentration levels for each serum nutrient and HPV infection persistence. To examine the impact of the definition of persistent HPV infection in our findings, we repeated the above analyses using a more restrictive definition of HPV persistence using women as the unit of analysis. In this approach, persistent infection was defined as those that had 3 or 4 consecutive visits positive for the same type (38 persistent oncogenic and 32 persistent nononcogenic) compared to 2 or more. Women who were nonconsecutively positive for the same type were excluded from the transient group, for a final sample of 137 and 111 women with oncogenic and nononcogenic transient infection, respectively. We re-estimated the ORs and 95% CI between the concentration of serum nutrients and this restricted HPV persistence definition and calculated the percent difference in point estimates generated using both persistence definitions.
Case–control approach using virus as unit of analysis
Overall, at each visit the percentage of women with multiple HPV infections was low (3% of the 819 women and 14% of HPV-positive women). However, when examining women over 4 consecutive study visits, we found that 133 women out of 405 (32%) had more than one HPV type detected (93 were positive for 2 types, 32 for 3 types, 5 for 4 types and 3 women for 5 types). Type-specific HPV persistence was determined for each type as outlined earlier; however in this analysis, all viral infections were utilized. Oncogenic and nononcogenic groupings were conducted for each viral infection, with some women contributing to each group. Of the 589 infections, 277 were oncogenic HPV infections (174 transient and 103 persistent) and 312 nononcogenic infections (225 transient and 87 persistent). Of the coinfections, there were 57 coinfections with only nononcogenic types, 57 coinfections with only oncogenic types and 206 coinfections were mixed oncogenic and nononcogenic types. To analyze the association between serum antioxidants and individual viral persistence, a generalized estimating equation (GEE) model approach was employed22,23 clustered by woman stratified by oncogenic or nononcogenic type. GEE adjusts for the serial correlation within subjects due to the longitudinal nature of the data by modeling the covariance structure within subjects. The dependent variable was the presence or absence of persistent infection; thus, the logit link function was used. The exchangeable working correlation structure with a robust variance estimator to account for within-subject correlation was selected as the best-fitted covariance pattern, using the Quasi-likelihood Information Criterion (QIC).23 Nutrient-HPV type interactions were examined by entereing interaction terms between tertiles of serum nutrients and type of infection (nononcogenic vs oncogenic) followed by stratified analyses.
Prospective approach examining persistent events
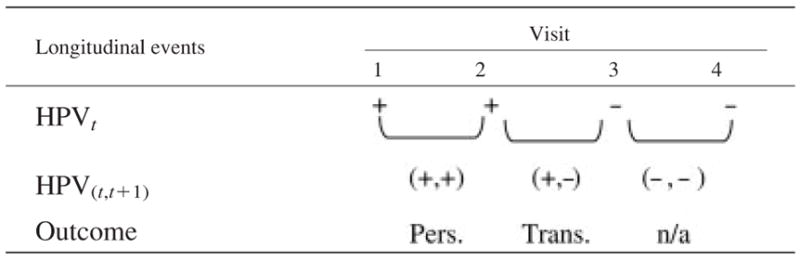
The prospective approach examined HPV persistence defined as type-specific persistent events based on data obtained at enrollment and the next 3 visits (4 HPV evaluations) similar to the approach used by Ho et al.24 (Table I). Women (unit of analysis) who were HPV-positive at a specific visit were considered persistent if their subsequent assessment was also positive for the same HPV type and transient if the subsequent visit was negative (i.e. if they cleared the virus) or a different HPV type infection was detected. We defined persistence status over 4 clinical visits with a maximum total of 3 persistence events possible per woman; therefore, analyses were conducted at the level of individual pairs of HPV observations rather than the individual woman. Of the 405 HPV-positive women included in our study, a total of 1,628 visits formed 1,221 pairs of observations with known HPV status at visits t and t + 1, where t = 1, 2, 3 or 4. The distribution of HPVt, t+1 pairs was 277 with (+,−), 263 with (+,+) and 681 with (−,−) or (−, +). The 540 pairs in the (+,−) and (+,+) categories were contributed by 344 women and included in these analyses. In the prospective approach of HPV persistent events, the multiple HPV measures over time within a woman were correlated and GEE model was used as described earlier. In this analysis, we examined HPV persistence events over time. The unstructured and exchangeable working correlational structures with robust variance estimators were used to account for within-subject correlation for nononcogenic and oncogenic HPV infections respectively. We assumed that HPV persistence depended on the time since the previous measurement; therefore, a time variable (in months) was included as a covariate in all GEE models.
TABLE I.
TABULAR REPRESENTATION OF THE GEE STATISTICAL APPROACHES UTILIZED TO EXAMINE HPV PERSISTENT EVENTS
|
All approaches imply the same HPV type at each positive visit (+). HPV persistence defined as a repeated event of visit pairs. Women who are HPV positive at a particular measurement are considered persistent if their subsequent measurement is also positive for the same HPV type and transient if their subsequent visit was negative or a different HPV type. HPV, human papillomavirus; Trans, transient HPV group; Pers., persistent HPV group; n/a, not applicable for the analysis.
For all multivariate models, we considered several covariates assessed at baseline including those previously associated with persistent HPV infection in our study population25 and serum carotenoids and tocopherols. The factors considered in univariate analysis were age (categorical), race, income, education, marital status, number in household, smoking, oral contraceptive use, number of pregnancies, age at first sexual intercourse, and number of sexual partners (lifetime, last year or last 5 years). Only those factors that altered the parameter estimate (OR) by ≥10% were retained in the final multivariate model. Tests for linear trend were performed by treating all categorical nutrient variables as continuous in the multivariate models. All statistical tests performed were two-sided and declared at the 5% significance level. Statistical analyses were performed with Intercooled STATA (Stata Statistical Software, Release 8.0; Stata, College Station, TX).
Results
The demographic characteristics of the HPV-positive participants included in these analyses are presented in Table II. The median age for women included in the analysis was 31 years, which was not different from the median age of all HPV-positive women enrolled in the parent cohort study. Most participants were white (61%), married or in a common law marriage (70%), and 24% of the sample continued their education past elementary school. At least 80% of these women reported more than 2 pregnancies and 57% had used oral contraceptives for less than 6 years. A majority of these women were ever smokers (53%), with 36% reporting current smoking. Fifty-six percent of participants reported age of first sexual intercourse less than 18 years and 25% had more than 4 lifetime sexual partners. Table III presents the mean circulating vitamin A, carotenoid and tocopherol values for this sample of 405 women. The most abundant circulating carotenoid was β-cryptoxanthin, followed by lutein, cis-lycopene, trans-β-carotene, trans-lycopene, zeaxanthin, α-carotene, cis-β-carotene and α-cryptoxanthin. The most abundant tocopherol averaged over the 4 visits was α-tocopherol, followed by γ-tocopherol and δ-tocopherol.
TABLE II.
DEMOGRAPHIC CHARACTERISTICS AMONG HPV POSITIVE WOMEN (N = 405)
| N | % | |
|---|---|---|
| Age (years) | ||
| ≤20 | 40 | 9.9 |
| 21–30 | 160 | 39.4 |
| 31–40 | 132 | 32.5 |
| >40 | 73 | 18.2 |
| Ethnicity | ||
| White | 247 | 61.0 |
| Non-white | 158 | 39.0 |
| Marital status | ||
| Single | 76 | 18.8 |
| Married | 154 | 38.0 |
| Widowed/divorced | 46 | 11.4 |
| Common law | 129 | 31.9 |
| Education | ||
| <Elementary | 86 | 21.2 |
| Elementary | 223 | 55.1 |
| <High school | 49 | 12.1 |
| ≥High school | 47 | 11.6 |
| Monthly income (US $) | ||
| <250 | 103 | 26.1 |
| 250–450 | 98 | 24.9 |
| 451–725 | 89 | 22.7 |
| ≥725 | 103 | 26.2 |
| Cigarette smoking | ||
| Never | 190 | 46.9 |
| Current | 148 | 36.5 |
| Former | 67 | 16.5 |
| Oral contraceptive use | ||
| Never | 67 | 16.5 |
| <6 years | 230 | 56.8 |
| ≥6 years | 108 | 26.7 |
| Total no. of pregnancies | ||
| 0–1 | 77 | 19.2 |
| 2–3 | 169 | 42.1 |
| 4–5 | 114 | 28.4 |
| ≥6 | 41 | 10.2 |
| Age at first intercourse | ||
| ≤15 | 122 | 30.1 |
| 16–17 | 104 | 25.7 |
| ≥18 | 179 | 44.2 |
| Lifetime no. of sexual partners | ||
| 0–1 | 146 | 36.1 |
| 2–3 | 157 | 38.8 |
| ≥4 | 102 | 25.2 |
| Total no. of sex partners past 5 years | ||
| 0–1 | 266 | 65.7 |
| ≥2 | 139 | 34.3 |
| Total no. of sexual partners past year | ||
| 0–1 | 362 | 90.3 |
| ≥2 | 39 | 9.7 |
TABLE III.
MEAN (SD)1 OF SERUM CAROTENOIDS AND TOCOPHEROLS AMONG 405 HPV POSITIVE WOMEN
| Serum nutrients | Mean | Standard deviation |
|---|---|---|
| Vitamin A (μg/ml) | ||
| Retinol | 0.458 | 0.142 |
| Carotenoids (μg/ml) | ||
| Lutein | 0.037 | 0.043 |
| Zeaxanthin | 0.016 | 0.007 |
| α-Cryptoxanthin | 0.006 | 0.006 |
| β-Cryptoxanthin | 0.040 | 0.171 |
| trans-Lycopene | 0.017 | 0.015 |
| cis-Lycopene | 0.026 | 0.018 |
| α-Carotene | 0.011 | 0.009 |
| trans-β-Carotene | 0.024 | 0.027 |
| cis-β-Carotene | 0.007 | 0.005 |
| Tocopherols (μg/ml) | ||
| α-Tocopherol | 5.135 | 1.959 |
| δ-Tocopherol | 0.083 | 0.034 |
| γ-Tocopherol | 1.578 | 0.577 |
Mean serum values calculated across 4 visit for each woman and the values presented in the table are the mean nutrients for all 405 women.
Case–control analysis of type-specific HPV persistence, with woman as unit of analysis
The unadjusted and multivariate adjusted odds ratios (AORs) and 95% CIs between individual serum carotenoids and tocopherols and type-specific HPV persistence are reported in Table IV. Higher circulating levels of δ- and γ-tocopherols were marginally inversely associated with nononcogenic HPV persistent infection (p for trend 0.09 and 0.13, respectively), but not oncogenic infection. Midlevels of α-tocopherol were associated with reduced risk of persistent nononcogenic HPV infection (AOR = 0.18; 95% CI 0.06–0.50). No statistically significant associations were observed between serum retinol, lutein, zeaxanthin, α-cryptoxanthin, β-cryptoxanthin, α-carotene, trans- and cis-β carotene and oncogenic or nononcogenic HPV persistence in this analysis. Serum lycopene (cis- and trans-lycopene) was positively associated with type-specific oncogenic HPV persistence (p for trend 0.04 and 0.07, respectively).
TABLE IV.
ODD RATIO AND 95% CONFIDENCE INTERVAL (CI) FOR THE ASSOCIATIONS BETWEEN NONONCOGENIC AND ONCOGENIC HPV PERSISTENCE AND SERUM ANTIOXIDANT NUTRIENT LEVEL: CASE–CONTROL APPROACH WITH WOMEN AS UNIT OF ANALYSIS1
| Nononcogenic HPV types
|
Oncogenic HPV types
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trans. (N)2 | Pers. (N) | Crude OR | Adjusted3 |
Trans. (N) | Pers. (N) | Crude OR | Adjusted3 |
|||
| OR | 95% CI | OR | 95% CI | |||||||
| Vitamin A (μg/ml) | ||||||||||
| Retinol | ||||||||||
| ≤0.380 | 39 | 18 | 1.00 | 1.00 | Referent | 48 | 27 | 1.00 | 1.00 | Referent |
| 0.381–0.453 | 42 | 21 | 1.08 | 0.86 | (0.37–1.49) | 44 | 29 | 1.17 | 1.33 | (0.64–2.77) |
| 0.434–1.900 | 37 | 19 | 1.11 | 0.86 | (0.35–2.17) | 49 | 32 | 1.17 | 1.19 | (0.58–2.48) |
| p-trend | 0.76 | 0.64 | ||||||||
| Carotenoids (μg/ml) | ||||||||||
| Lutein | ||||||||||
| ≤0.0292 | 38 | 15 | 1.00 | 1.00 | Referent | 48 | 31 | 1.00 | 1.00 | Referent |
| 0.0293–0.0415 | 39 | 23 | 1.49 | 1.57 | (0.67–3.69) | 48 | 23 | 0.74 | 0.71 | (0.34–1.51) |
| 0.042–0.807 | 41 | 20 | 1.24 | 1.27 | (0.52–3.11) | 43 | 24 | 1.17 | 1.12 | (0.55–2.27) |
| p-trend | 0.63 | 0.72 | ||||||||
| Zeaxanthin | ||||||||||
| ≤0.0125 | 37 | 16 | 1.00 | 1.00 | Referent | 49 | 30 | 1.00 | 1.00 | Referent |
| 0.0126–0.0202 | 42 | 17 | 0.93 | 1.05 | (0.42–2.56) | 46 | 25 | 0.81 | 0.80 | (0.44–1.80) |
| 0.0203–0.054 | 39 | 25 | 1.48 | 1.51 | (0.64–3.56) | 46 | 20 | 1.17 | 1.12 | (0.56–2.26) |
| p-trend | 0.33 | 0.75 | ||||||||
| α-Cryptoxanthin | ||||||||||
| ≤0.005 | 43 | 25 | 1.00 | 1.00 | Referent | 49 | 34 | 1.00 | 1.00 | Referent |
| 0.006–0.0075 | 36 | 16 | 0.76 | 0.76 | (0.33–1.73) | 47 | 23 | 0.68 | 0.75 | (0.37–1.54) |
| 0.0076–0.088 | 39 | 17 | 0.75 | 0.71 | (0.31–1.59) | 45 | 30 | 0.93 | 0.88 | (0.43–1.81) |
| p-trend | 0.38 | 0.73 | ||||||||
| β-Cryptoxanthin | ||||||||||
| ≤0.0207 | 41 | 18 | 1.00 | 1.00 | Referent | 47 | 34 | 1.00 | 1.00 | Referent |
| 0.0208–0.0395 | 39 | 18 | 1.05 | 1.04 | (0.44–2.48) | 44 | 17 | 0.56 | 0.67 | (0.31–1.44) |
| 0.040–2.20 | 38 | 23 | 1.32 | 1.05 | (0.45–2.46) | 48 | 37 | 1.11 | 1.00 | (0.49–2.04) |
| p-trend | 0.92 | 0.99 | ||||||||
| trans-Lycopene | ||||||||||
| ≤0.013 | 35 | 20 | 1.00 | 1.00 | Referent | 54 | 23 | 1.00 | 1.00 | Referent |
| 0.0135–0.0271 | 40 | 19 | 0.83 | 0.84 | (0.36–1.95) | 43 | 29 | 1.58 | 1.78 | (0.84–3.73) |
| 0.0272–0.120 | 43 | 19 | 0.77 | 0.86 | (0.32–2.06) | 44 | 36 | 1.92 | 2.04 | (0.95–4.39) |
| p-trend | 0.74 | 0.07 | ||||||||
| cis-Lycopene | ||||||||||
| ≤0.0210 | 37 | 17 | 1.00 | 1.00 | Referent | 49 | 23 | 1.00 | 1.00 | Referent |
| 0.0211–0.0397 | 41 | 27 | 1.43 | 1.34 | (0.60–3.24) | 46 | 24 | 1.11 | 1.48 | (0.68–3.24) |
| 0.0398–0.1633 | 40 | 14 | 0.76 | 0.70 | (0.27–1.83) | 46 | 41 | 1.90 | 2.21 | (1.04–4.67) |
| p-trend | 0.46 | 0.04 | ||||||||
| α-Carotene | ||||||||||
| ≤0.0087 | 46 | 18 | 1.00 | 1.00 | Referent | 41 | 28 | 1.00 | 1.00 | Referent |
| 0.0088–0.016 | 30 | 22 | 1.82 | 1.63 | (0.68–3.89) | 55 | 24 | 0.64 | 0.70 | (0.36–1.46) |
| 0.0165–0.065 | 42 | 18 | 1.09 | 1.12 | (0.48–2.61) | 45 | 36 | 1.17 | 1.18 | (0.58–2.51) |
| p-trend | 0.83 | 0.60 | ||||||||
| trans-β-Carotene | ||||||||||
| ≤0.0184 | 40 | 22 | 1.00 | 1.00 | Referent | 47 | 29 | 1.00 | 1.00 | Referent |
| 0.0185–0.036 | 35 | 19 | 0.98 | 0.99 | (0.47–2.54) | 52 | 26 | 0.86 | 0.95 | (0.47–1.97) |
| 0.037–0.238 | 43 | 17 | 0.72 | 0.75 | (0.32–1.74) | 42 | 33 | 1.27 | 1.13 | (0.54–2.39) |
| p-trend | 0.51 | 0.74 | ||||||||
| cis-β-Carotene | ||||||||||
| ≤0.005 | 43 | 20 | 1.00 | 1.00 | Referent | 52 | 30 | 1.00 | 1.00 | Referent |
| 0.006–0.008 | 41 | 25 | 1.43 | 1.01 | (0.46–2.26) | 41 | 26 | 1.09 | 0.88 | (0.47–1.95) |
| 0.008–0.165 | 34 | 13 | 0.97 | 0.69 | (0.29–1.72) | 48 | 33 | 1.16 | 1.02 | (0.51–2.03) |
| p-trend | 0.32 | 0.95 | ||||||||
| Tocopherols | ||||||||||
| α-Tocopherol | ||||||||||
| ≤4.27 | 32 | 21 | 1.00 | 1.00 | Referent | 57 | 29 | 1.00 | 1.00 | Referent |
| 4.28–6.03 | 48 | 14 | 0.43 | 0.18 | (0.06–0.50) | 33 | 27 | 1.60 | 1.68 | (0.80–3.51) |
| 6.04–17.81 | 36 | 21 | 0.97 | 0.49 | (0.17–1.40) | 51 | 32 | 1.23 | 1.12 | (0.53–2.34) |
| p-trend | 0.32 | 0.71 | ||||||||
| δ-Tocopherol | ||||||||||
| ≤1.36 | 37 | 21 | 1.00 | 1.00 | Referent | 53 | 30 | 1.00 | 1.00 | Referent |
| 1.37–1.77 | 40 | 22 | 0.97 | 0.83 | (0.37–1.87) | 42 | 28 | 1.18 | 1.06 | (0.53–2.19) |
| 1.78–4.85 | 41 | 15 | 0.64 | 0.45 | (0.18–1.13) | 46 | 30 | 1.15 | 1.27 | (0.63–2.55) |
| p-trend | 0.09 | 0.27 | ||||||||
| γ-Tocopherol | ||||||||||
| ≤1.36 | 33 | 20 | 1.00 | 1.00 | Referent | 53 | 27 | 1.00 | 1.00 | Referent |
| 1.37–1.77 | 45 | 20 | 0.81 | 0.67 | (0.28–1.60) | 46 | 32 | 1.46 | 1.61 | (0.79–3.26) |
| 1.78–4.85 | 40 | 20 | 0.92 | 0.50 | (0.10–1.32) | 45 | 29 | 1.27 | 1.38 | (0.66–2.86) |
| p-trend | 0.13 | 0.37 | ||||||||
HPV, human papillomavirus; Trans, transient HPV group; Pers., persistent HPV group; OR, odds ratio; CI, confidence interval.
Results obtained from logistic regression with binary outcome of transient or persistent HPV infection.–
Total number of observations did not add up due to missing data.–
Adjusted for serum cholesterol, age, ethnicity, number of sexual partners (1 year), income and number in household.
To further examine the associations among serum antioxidants and type-specific HPV infection, we repeated these analyses using a restricted definition of HPV persistence. These results were compared to the risk estimates presented in Table IV. In this sensitivity analysis, the risk estimates presented in Table IV for α-, δ- and γ-tocopherols and nononcogenic HPV infections were strengthened (data not shown). Specifically using this restricted definition, circulating levels of α-tocopherol in the middle and upper tertiles were inversely associated with type-specific nononcogenic persistence HPV (AOR = 0.16, 95% CI 0.04–0.59 and AOR = 0.20, 95% CI 0.05–0.79, respectively) compared to levels in the lowest tertile. Consistent associations were also observed among levels of δ- and γ-tocopherols and nononcogenic HPV persistence. Alternatively, the AORs for the association between mid to high levels of cis-lycopene and oncogenic HPV persistence changed from 1.40 and 2.32 in the analyses presented in Table IV to 1.28 and 1.53, respectively. The conclusions presented in Table IV did not alter when assessing the associations between the remaining carotenoids and oncogenic or nononcogenic HPV persistence (data not shown) using the restrictive definition.
Case–control approach using virus as unit of analysis
In the analysis of type-specific persistence using virus as the unit of analysis (589 viral infections), we first examined the main effects of serum nutrient level and any type-specific HPV persistence controlling for oncogenicity and coinfections and the oncogenicity-nutrient interaction. Higher levels of serum α-cryptoxanthin were inversely associated with any type HPV persistence (middle tertile: AOR = 0.55, 95% CI 0.35–0.85 and upper tertile AOR = 0.63, 95% CI 0.49–1.00); however, there was no significant interaction by oncogenic vs. nononcogenic types (data not shown). Significant interactions were found between serum nutrient level and type of HPV infection (nononcogenic vs oncogenic) for lutein, trans-lycopene and α-tocopherol and marginal interactions for retinol, β-cryptoxanthin, cis-lycopene and δ- and γ-tocopherol. Therefore, we present in Table V the stratified unadjusted and multivariate AORs and 95% CIs between individual serum carotenoids and tocopherols and type-specific HPV persistence using virus as the unit of analysis. Levels of α-tocopherol in the second tertile were inversely associated with nononcogenic HPV persistent infection (AOR = 0.28; 95% CI 0.14–0.57), but not oncogenic infection. No statistically significant associations were observed between serum retinol, lutein, zeaxanthin, α-cryptoxanthin, β-cryptoxanthin, α-carotene, trans- and cis-β carotene and oncogenic or nononcogenic HPV persistence in this stratified analysis.
TABLE V.
ODD RATIO AND 95% CONFIDENCE INTERVAL (CI) FOR THE ASSOCIATIONS BETWEEN NONONCOGENIC AND ONCOGENIC HPV PERSISTENCE AND SERUM ANTIOXIDANT NUTRIENT LEVEL: CASE–CONTROL APPROACH WITH VIRUS AS UNIT OF ANALYSIS1
| Nononcogenic HPV types
|
Oncogenic HPV types
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trans (N) | Pers. (N) | Crude OR | Adjusted2 |
Trans (N) | Pers. (N) | Crude OR | Adjusted2 |
|||
| OR | 95% CI | OR | 95% CI | |||||||
| Vitamin A (μg/ml) | ||||||||||
| Retinol | ||||||||||
| <0.380 | 68 | 30 | 1.00 | 1.00 | Referent | 61 | 32 | 1.00 | 1.00 | Referent |
| 0.381–0.453 | 76 | 30 | 0.89 | 0.68 | (0.34–1.27) | 56 | 36 | 1.12 | 1.20 | (0.64–2.54) |
| 0.434–1.900 | 81 | 27 | 0.76 | 0.59 | (0.30–1.15) | 57 | 35 | 1.21 | 1.41 | (0.70–2.85) |
| 0.13 | 0.34 | |||||||||
| Carotenoids (μg/ml) | ||||||||||
| Lutein | ||||||||||
| ≤0.0292 | 74 | 26 | 1.00 | 1.00 | Referent | 56 | 40 | 1.00 | 1.00 | Referent |
| 0.0293–0.0415 | 74 | 31 | 1.17 | 1.27 | (0.65–2.51) | 64 | 25 | 0.63 | 0.52 | (0.25–1.07) |
| 0.042–0.807 | 77 | 30 | 1.10 | 1.09 | (0.57–2.15) | 54 | 38 | 1.00 | 0.90 | (0.50–1.94) |
| p-trend | 0.79 | 0.90 | ||||||||
| Zeaxanthin | ||||||||||
| ≤0.0125 | 73 | 26 | 1.00 | 1.00 | Referent | 58 | 39 | 1.00 | 1.00 | Referent |
| 0.0126–0.0202 | 69 | 27 | 1.09 | 1.13 | (0.58–2.23) | 56 | 30 | 0.87 | 0.81 | (0.41–1.62) |
| 0.0203–0.054 | 83 | 34 | 1.14 | 1.28 | (0.68–2.40) | 60 | 34 | 1.08 | 1.04 | (0.52–2.06) |
| p-trend | 0.45 | 0.92 | ||||||||
| α-Cryptoxanthin | ||||||||||
| ≤0.005 | 80 | 40 | 1.00 | 1.00 | Referent | 54 | 43 | 1.00 | 1.00 | Referent |
| 0.006–0.0075 | 71 | 26 | 0.68 | 0.68 | (0.38–1.22) | 63 | 27 | 0.57 | 0.47 | (0.23–0.97) |
| 0.0076–0.088 | 74 | 23 | 0.62 | 0.68 | (0.36–1.29) | 57 | 33 | 0.84 | 0.58 | (0.28–1.20) |
| p-trend | 0.20 | 0.15 | ||||||||
| β-Cryptoxanthin | ||||||||||
| ≤0.0207 | 77 | 32 | 1.00 | 1.00 | Referent | 58 | 42 | 1.00 | 1.00 | Referent |
| 0.0208–0.0395 | 70 | 25 | 0.86 | 0.90 | (0.49–1.68) | 57 | 21 | 0.53 | 0.49 | (0.22–1.10) |
| 0.040–2.20 | 78 | 30 | 0.92 | 0.94 | (0.40–1.82) | 57 | 40 | 1.05 | 0.80 | (0.40–1.59) |
| p-trend | 0.85 | 0.53 | ||||||||
| trans-Lycopene | ||||||||||
| ≤0.013 | 64 | 33 | 1.00 | 1.00 | Referent | 59 | 29 | 1.00 | 1.00 | Referent |
| 0.0135–0.0271 | 83 | 26 | 0.61 | 0.59 | (0.30–1.07) | 57 | 35 | 1.45 | 1.48 | (0.72–3.05) |
| 0.0272–0.120 | 78 | 28 | 0.69 | 0.84 | (0.38–1.47) | 58 | 37 | 1.69 | 1.42 | (0.66–3.02) |
| p-trend | 0.53 | 0.03 | ||||||||
| cis-Lycopene | ||||||||||
| ≤0.0210 | 65 | 37 | 1.00 | 1.00 | Referent | 53 | 28 | 1.00 | 1.00 | Referent |
| 0.0211–0.0397 | 77 | 36 | 1.13 | 0.99 | (0.54–1.83) | 59 | 31 | 1.04 | 1.26 | (0.56–2.67) |
| 0.0398–0.1633 | 83 | 24 | 0.69 | 0.70 | (0.34–1.42) | 62 | 44 | 1.58 | 1.45 | (0.69–3.07) |
| p-trend | 0.32 | 0.33 | ||||||||
| α-Carotene | ||||||||||
| ≤0.0087 | 73 | 32 | 1.00 | 1.00 | Referent | 50 | 36 | 1.00 | 1.00 | Referent |
| 0.0088–0.016 | 70 | 28 | 0.92 | 0.94 | (0.49–1.79) | 63 | 29 | 0.66 | 0.73 | (0.36–1.51) |
| 0.0165–0.065 | 82 | 27 | 0.74 | 0.82 | (0.42–1.59) | 61 | 38 | 1.02 | 0.92 | (0.45–1.88) |
| p-trend | 0.55 | 0.86 | ||||||||
| trans-β-Carotene | ||||||||||
| ≤0.0184 | 70 | 39 | 1.00 | 1.00 | Referent | 57 | 38 | 1.00 | 1.00 | Referent |
| 0.0185–0.036 | 79 | 29 | 0.75 | 0.78 | (0.42–1.14) | 61 | 30 | 0.78 | 0.89 | (0.45–1.74) |
| 0.037–0.238 | 76 | 24 | 0.64 | 0.78 | (0.41–1.51) | 56 | 35 | 1.14 | 0.87 | (0.41–1.85) |
| p-trend | 0.43 | 0.72 | ||||||||
| cis-β-Carotene | ||||||||||
| ≤0.005 | 84 | 34 | 1.00 | 1.00 | Referent | 64 | 37 | 1.00 | 1.00 | Referent |
| 0.006–0.008 | 71 | 34 | 1.18 | 0.93 | (0.52–1.69) | 49 | 29 | 1.10 | 0.92 | (0.45–1.86) |
| 0.008–0.165 | 70 | 19 | 0.67 | 0.64 | (0.33–1.27) | 61 | 31 | 1.13 | 0.97 | (0.49–1.93) |
| p-trend | 0.22 | 0.93 | ||||||||
| Tocopherols | ||||||||||
| α-Tocopherol | ||||||||||
| ≤4.27 | 65 | 34 | 1.00 | 1.00 | Referent | 70 | 35 | 1.00 | 1.00 | Referent |
| 4.28–6.03 | 74 | 19 | 0.49 | 0.28 | (0.14–0.57) | 37 | 32 | 1.65 | 1.98 | (0.96–4.09) |
| 6.04–17.81 | 86 | 34 | 0.74 | 0.59 | (0.29–1.19) | 67 | 36 | 1.13 | 1.07 | (0.54–2.14) |
| p-trend | 0.17 | 0.76 | ||||||||
| δ-Tocopherol | ||||||||||
| ≤1.36 | 72 | 32 | 1.00 | 1.00 | Referent | 61 | 34 | 1.00 | 1.00 | Referent |
| 1.37–1.77 | 70 | 30 | 0.95 | 0.85 | (0.46–1.58) | 55 | 32 | 1.08 | 0.98 | (0.48–2.01) |
| 1.78–4.85 | 83 | 25 | 0.67 | 0.72 | (0.39–1.35) | 58 | 37 | 1.11 | 1.34 | (0.64–2.69) |
| p-trend | 0.31 | 0.31 | ||||||||
| γ-Tocopherol | ||||||||||
| ≤1.36 | 64 | 26 | 1.00 | 1.00 | Referent | 60 | 32 | 1.00 | 1.00 | Referent |
| 1.37–1.77 | 78 | 32 | 1.00 | 0.94 | (0.30–1.77) | 60 | 38 | 1.29 | 1.28 | (0.64–2.57) |
| 1.78–4.85 | 83 | 29 | 0.85 | 0.72 | (0.35–1.47) | 54 | 32 | 1.22 | 1.59 | (0.79–3.21) |
| p-trend | 0.37 | 0.50 | ||||||||
HPV, human papillomavirus; Trans, transient HPV group; Pers., persistent HPV group; OR, odds ratio; CI, confidence interval.
Results obtained from generalized estimating equation with a logit link for binary outcomes clustered by woman ID number.–
Adjusted for serum cholesterol, age, race, number of sexual partners (1 year), income and number in household.
Longitudinal analysis of type-specific HPV persistent events
Circulating levels of α-tocopherol in the middle tertile were inversely associated with type-specific nononcogenic persistent HPV events (AOR = 0.44, 95% CI 0.19–0.97) compared to levels in the lowest tertile (Table VI); however, the significant association was not maintained for the upper tertile. Serum levels of δ-tocopherol were marginally inversely associated with type-specific non-oncogenic persistent HPV events (p for trend = 0.09) compared to levels in the lowest tertile. Serum trans-β-carotene was marginally inversely associated with nononcogenic persistent events (p for trend = 0.09). Midlevels of trans-lycopene were associated with increased risk of oncogenic persistent events; however, no significant linear trend was observed (p trend 0.29) (Table VI).
TABLE VI.
ODD RATIO AND 95% CI FOR THE ASSOCIATIONS BETWEEN NONONCOGENIC AND ONCOGENIC HPV EVENTS AND SERUM ANTIOXIDANT NUTRIENT LEVEL: LONGITUDINAL ANALYSIS OF PERSISTENT EVENTS1
| Nononcogenic HPV types
|
Oncogenic HPV types
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| +,−2 (N)3 | +,+ (N) | Crude OR | Adjusted4 |
+,− (N) | +,+ (N) | Crude OR | Adjusted5 |
|||
| OR | 95% CI | OR | 95% CI | |||||||
| Vitamin A (μg/ml) | ||||||||||
| Retinol | ||||||||||
| ≤0.380 | 43 | 38 | 1.00 | 1.00 | Referent | 51 | 47 | 1.00 | 1.00 | Referent |
| 0.381–0.453 | 50 | 34 | 0.75 | 0.61 | (0.29–1.25) | 46 | 50 | 1.22 | 1.50 | (0.78–2.90) |
| 0.434–1.900 | 35 | 37 | 1.15 | 1.07 | (0.50–2.28) | 52 | 53 | 1.14 | 1.22 | (0.62–2.38) |
| p-trend | 0.94 | 0.56 | ||||||||
| Carotenoids (μg/ml) | ||||||||||
| Lutein | ||||||||||
| ≤0.0292 | 41 | 33 | 1.00 | 1.00 | Referent | 49 | 55 | 1.00 | 1.00 | Referent |
| 0.0293–0.0415 | 44 | 35 | 1.06 | 0.98 | (0.50–2.01) | 48 | 39 | 0.75 | 0.82 | (0.41–1.67) |
| 0.042–0.807 | 42 | 41 | 1.11 | 0.95 | (0.44–2.04) | 52 | 56 | 0.97 | 1.03 | (0.53–1.99) |
| p-trend | 0.50 | 0.91 | ||||||||
| Zeaxanthin | ||||||||||
| ≤0.0125 | 43 | 31 | 1.00 | 1.00 | Referent | 45 | 55 | 1.00 | 1.00 | Referent |
| 0.0126–0.0202 | 43 | 28 | 0.90 | 0.98 | (0.45–2.19) | 48 | 43 | 0.76 | 0.76 | (0.40–1.50) |
| 0.0203–0.054 | 42 | 50 | 1.60 | 1.17 | (0.55–2.50) | 56 | 52 | 0.80 | 0.81 | (0.44–1.55) |
| p-trend | 0.67 | 0.49 | ||||||||
| α-Cryptoxanthin | ||||||||||
| ≤0.005 | 45 | 47 | 1.00 | 1.00 | Referent | 50 | 62 | 1.00 | 1.00 | Referent |
| 0.006–0.0075 | 39 | 27 | 0.66 | 0.80 | (0.40–1.61) | 50 | 42 | 0.66 | 0.64 | (0.33–1.29) |
| 0.0076–0.088 | 44 | 35 | 0.76 | 0.68 | (0.33–1.41) | 49 | 46 | 0.78 | 0.83 | (0.44–1.57) |
| p-trend | 0.29 | 0.76 | ||||||||
| β-Cryptoxanthin | ||||||||||
| ≤0.0207 | 40 | 36 | 1.00 | 1.00 | Referent | 51 | 56 | 1.00 | 1.00 | Referent |
| 0.0208–0.0395 | 49 | 28 | 0.62 | 0.52 | (0.24–1.14) | 45 | 33 | 0.67 | 0.76 | (0.36–1.58) |
| 0.040–2.20 | 39 | 45 | 1.22 | 1.08 | (0.52–2.26) | 53 | 61 | 1.05 | 1.43 | (0.59–2.11) |
| p-trend | 0.89 | 0.73 | ||||||||
| trans-Lycopene | ||||||||||
| ≤0.013 | 38 | 38 | 1.00 | 1.00 | Referent | 51 | 43 | 1.00 | 1.00 | Referent |
| 0.0135–0.0271 | 42 | 36 | 0.83 | 0.69 | (0.33–1.41) | 39 | 50 | 1.61 | 2.11 | (1.03–4.37) |
| 0.0272–0.120 | 48 | 35 | 0.63 | 0.49 | (0.21–1.09) | 59 | 57 | 1.28 | 1.43 | (0.75–2.75) |
| p-trend | 0.09 | 0.29 | ||||||||
| cis-Lycopene | ||||||||||
| ≤0.0210 | 40 | 29 | 1.00 | 1.00 | Referent | 47 | 43 | 1.00 | 1.00 | Referent |
| 0.0211–0.0397 | 50 | 52 | 1.41 | 1.45 | (0.69–3.05) | 40 | 44 | 1.25 | 1.53 | (0.73–3.24) |
| 0.0398–0.1633 | 38 | 28 | 0.87 | 0.72 | (0.28–1.80) | 62 | 63 | 1.28 | 1.45 | (0.75–2.83) |
| p-trend | 0.49 | 0.29 | ||||||||
| α-Carotene | ||||||||||
| ≤0.0087 | 49 | 35 | 1.00 | 1.00 | Referent | 47 | 50 | 1.00 | 1.00 | Referent |
| 0.0088–0.016 | 33 | 43 | 1.86 | 1.53 | (0.72–3.26) | 46 | 40 | 0.84 | 0.85 | (0.41–1.71) |
| 0.0165–0.065 | 46 | 31 | 0.85 | 0.62 | (0.27–1.44) | 56 | 60 | 1.06 | 1.09 | (0.57–2.09) |
| p-trend | 0.22 | 0.76 | ||||||||
| trans-β-Carotene | ||||||||||
| ≤0.0184 | 46 | 41 | 1.00 | 1.00 | Referent | 51 | 50 | 1.00 | 1.00 | Referent |
| 0.0185–0.036 | 37 | 41 | 1.22 | 1.25 | (0.62–2.56) | 45 | 47 | 1.08 | 1.11 | (0.56–2.23) |
| 0.037–0.238 | 45 | 27 | 0.63 | 0.51 | (0.23–1.10) | 53 | 53 | 1.10 | 1.08 | (0.57–2.04) |
| p-trend | 0.09 | 0.81 | ||||||||
| cis-β-Carotene | ||||||||||
| ≤0.005 | 48 | 33 | 1.00 | 1.00 | Referent | 55 | 52 | 1.00 | 1.00 | Referent |
| 0.006–0.008 | 47 | 53 | 1.56 | 1.55 | (0.76–3.09) | 48 | 41 | 0.88 | 0.88 | (0.45–1.71) |
| 0.008–0.165 | 33 | 23 | 0.97 | 0.83 | (0.36–1.91) | 46 | 57 | 1.24 | 1.33 | (0.69–2.52) |
| p-trend | 0.69 | 0.42 | ||||||||
| Tocopherols | ||||||||||
| α-Tocopherol | ||||||||||
| ≤4.27 | 43 | 39 | 1.00 | 1.00 | Referent | 56 | 51 | 1.00 | 1.00 | Referent |
| 4.28–6.03 | 47 | 28 | 0.62 | 0.44 | (0.19–0.97) | 38 | 49 | 1.42 | 1.40 | (0.70–2.82) |
| 6.04–17.81 | 38 | 42 | 1.21 | 0.90 | (0.39–2.11) | 55 | 50 | 1.10 | 1.15 | (0.56–2.27) |
| p-trend | 0.90 | 0.62 | ||||||||
| δ-Tocopherol | ||||||||||
| ≤1.36 | 42 | 39 | 1.00 | 1.00 | Referent | 56 | 46 | 1.00 | 1.00 | Referent |
| 1.37–1.77 | 44 | 41 | 0.99 | 0.82 | (0.38–1.45) | 45 | 54 | 1.42 | 1.48 | (0.76–2.87) |
| 1.78–4.85 | 42 | 29 | 0.73 | 0.46 | (0.19–1.11) | 48 | 50 | 1.27 | 1.41 | (0.73–2.73) |
| p-trend | 0.09 | 0.31 | ||||||||
| γ-Tocopherol | ||||||||||
| ≤1.36 | 37 | 34 | 1.00 | 1.00 | Referent | 51 | 44 | 1.00 | 1.00 | Referent |
| 1.37–1.77 | 50 | 37 | 0.82 | 0.76 | (0.36–1.59) | 52 | 60 | 1.30 | 1.30 | (0.67–2.51) |
| 1.78–4.85 | 41 | 38 | 0.95 | 0.62 | (0.27–1.44) | 46 | 46 | 1.20 | 1.27 | (0.62–2.57) |
| p-trend | 0.26 | 0.51 | ||||||||
HPV, human papillomavirus; OR, odds ratio; CI, confidence interval.
Results obtained from a time-dependent GEE approach with a logit link for binary outcome.–
HPV results of 2 consecutive visits were grouped as a pair, see Table I.–
Total number of observations did not add up to 259 among (+,+) and 277 among (+,−) due to missing data.–
Adjusted for serum cholesterol, time, age, ethnicity, total number of pregnancies, lifetime number of sexual partners and marital status.–
Adjusted for serum cholesterol, time, age, smoking and lifetime number of sexual partners.
Discussion
This investigation focused on early events in cervical carcinogenesis and factors that influence HPV persistence. Specifically, we examined if individual serum antioxidant nutrients were associated with the natural history of type-specific oncogenic or nononcogenic HPV infections. There have not been consistent definitions of HPV persistence26 among the published reports of HPV persistence and nutrients,4,9–11,27,28 as well as in most published reports on HPV persistence. This inconsistency along with methodological issues inherent to nutritional epidemiology studies21 may explain, in part, the lack of consistent findings among previous reports of antioxidant nutrients and HPV persistence.6 Our study took advantage of the repeated measurements available within the large Lud-wig-McGill cohort study to fully evaluate the associations of serum nutrients and HPV infection. HPV was measured at 4 clinical visits using a highly sensitive PCR assay and over 40 individual HPV types were assessed. For this evaluation, we defined HPV persistence using several different analytical strategies to examine associations with HPV infection measured over 4 consecutive visits, including (i) case–control definition based on women as the unit of analysis, (ii) case–control definition at the virus level and (iii) longitudinal analysis of persistence events. This is one of the few studies examining associations with HPV infection to have measurements of serum carotenoids and tocopherols at 4 different time points over approximately a 1-year time period. Multiple measures of serum nutrients provides a more accurate assessment of a woman’s nutrient status over time and reduces the measurement error inherent in nutritional epidemiology studies.21
While designed with statistical power to detect significant associations, we did not find a strong protective effect (as oncogenic hypothesized) of serum antioxidant nutrients and type-specific HPV persistence measured over 4 clinical visits. Our study among Brazilian women suggests that serum levels of tocopherols may be protective against nononcogenic HPV persistence. However, our data do not support previous findings for a protective association between other antioxidant nutrients and HPV persistence, including previous reports of a protective effect of dietary lutein/zeaxanthin9,10 and β-cryptoxanthin intake10 and serum lutein27 and oncogenic HPV persistence. When examining the association of serum carotenoids and tocopherols with HPV positivity ~4 months after the detection of the same HPV type, we found no significant trends across nutrient levels for oncogenic type infections and only marginally significant trends for nononcogenic types. While this GEE approach increased our statistical power to detect differences, it is unclear if our lack of a significant finding among HPV persistent events was due to a true lack of an association or misclassification of the HPV persistent event variable.
This is one of the first investigations to examine whether the associations between serum nutrient levels differ for oncogenic and nononcogenic HPV infection. Of the serum carotenoids and tocopherols assessed, α- and δ-tocopherols appeared to be inversely associated with having a type-specific nononcogenic HPV infections persist over 2–4 consecutive visit for both analyses based on woman and individual virus, however, the same associations were not detected for oncogenic HPV infections. Tocopherols were marginally inversely associated with being HPV-positive with a nononcogenic type an average of 4.4-months after the detection of the same nononcogenic HPV type; however, overall clearance was not associated with tocopherol levels (data not shown). While these associations may be due to chance, it does support the importance of examining HPV persistence stratified by oncogenicity, and if possible based on the level of the virus.
A proposed mechanism of action for serum tocopherols on the natural history of HPV is proposed to occur through the antioxidant activity of these compounds as reviewed by Castle and Giuliano.7 Our data suggest that tocopherols may be effective at reducing nononcogenic HPV positivity over 4 consecutive visits over approximately a 12-month time period. Biologically, both oncogenic and nononcogenic HPV types have the ability to infect cervical epithelial cells and initiate productive viral infections; however, there are differences in the strength with which the viral proteins bind to cellular proteins to induce DNA synthesis and cell cycle progression.29 The continued presence of oncogenic HPV infection is thought to be a result of functional losses within cervical cells that control viral expression30 and a lack of an effective immune response.31 Tocopherols have been postulated to protect against HPV persistence by enhancing immunological functions and by modulating inflammatory response to infection.32 The mechanism by which tocopherols might confer protection against nononcogenic HPV infections but not oncogenic infections, as suggested by these results, remains to be determined.
In our study, lycopene was positively associated with having the same type of oncogenic HPV infection at 2–4 consecutive visits, and marginally associated in a 4-month follow-up interval. Lycopene has biological activities including antioxidant activity, induction of cell–cell communication and growth control, but does not have provitamin A activity.33 These results must be interpreted with caution as this is the first report of an observed positive association with serum or dietary lycopene and HPV persistence and is contrary to previous reports.9,12,27
As with any observational study, there were limitations with our study that need to be addressed. Our definition of HPV persistence included both prevalent and incident HPV infections; therefore, our estimate of duration may not represent the true duration of infection. In general, women found to be positive for oncogenic HPV infection upon enrollment were more likely to have a persistent infection (e.g. 50% infections) compared to incident infections (26% persistent). The small number of incident HPV infections (total 22%) within this subcohort restricted our use of only incident HPV persistence as an outcome. We assessed HPV persistence over the first 4 study visits, which has been previously shown to be an informative timeframe to examine HPV persistence, in terms of SIL risk and lesion development,13 within our study population. Among women with transient infection, 28% were positive for the first time at the last clinical visit examined and potentially could have developed a persistent infection if followed for subsequent visits. Similar to other biological markers, the values of serum nutrients presented in this report might not reflect the absolute value due to losses that might have occurred during storage or in the extraction process. However, we would expect that any changes in micronutrient level would have occurred systematically across all samples and not differ by HPV status. Therefore, the relative levels of each nutrient should not be different; leading to valid associations found in our study with potentially the magnitude of the associations being lower than the true association due to methodological errors. Because of the correlation between individual antioxidant nutrients, we analyzed each nutrient independently. Because of these multiple comparisons, chance cannot be ruled out as an explanation for our significant findings for tocopherols and nononcogenic HPV persistence.
Overall, results from the current study suggest that serum anti-oxidant nutrients may influence the natural history of HPV infections. We found differences in specific nutrient associations between oncogenic and nononcogenic type HPV infections. The use of 3 different analytical approaches allowed for the examination of the association of circulating carotenoids and tocopherols with both short-term and long-term duration of infection. Future research is needed to determine if serum antioxidant nutrients confer protection against longer-term persistent infection (>13 months) and cervical lesion development.
Acknowledgments
We are indebted to Ms. Maria L. Baggio and Ms. Lenice Galan for management of the patients and specimen collections and to Ms. Silvaneide Ferreira for data entry, sample retrieval and shipment and laboratory analysis. Our study could not have been conducted without the work of Mr. Joao Simao Sobrinho and Mr. Jose Carlos Mann Prado in the laboratory on the HPV assays and all those that have contributed over the years to the Ludwig-McGill cohort study. We appreciate the thoughtful comments on this manuscript by Drs. Robin Harris, Elena Martinez, Denise Roe and Jesse Martinez. Dr. Eduardo. L. Franco is recipient of a Distinguished Scientist Award from the CIHR. Dr. Erin M. Siegel is recipient of a NCI Cancer Prevention and Control Pre-Doctoral Fellowship.
Grant sponsor: National Cancer Institute; Grant numbers: CA70269, CA81310; Grant sponsor: Canadian Institutes of Health Research (CIHR); Grant numbers: MA-13647, MOP-49396; Grant sponsor: Ludwig Institute for Cancer Research; Grant sponsor: NCI Cancer Prevention and Control Pre-Doctoral Fellowship; Grant number: R25CA078447.
Abbreviations
- AOR
adjusted odds ratio
- CI
confidence intervals
- CIN
cervical intraepithelial neoplasia
- DNA
deoxyribonucleic acid
- GEE
general estimating equation
- HPLC
high pressure liquid chromatography
- HPV
human papillomavirus
- LOQ
limit of quantification
- OR
odds ratio
- PCR
polymerase chain reaction
- QIC
quasi-likelihood information criterion
- RFLP
restriction fragment-length polymorphism
- SIL
squamous intraepithelial lesions
Footnotes
Presented in part at the 21st International Papillomavirus Conference, Mexico City, 2004 (Abstract Number 70).
References
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL, Franco EL. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–14. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 3.Hinchliffe SA, van Velzen D, Korporaal H, Kok PL, Boon ME. Transience of cervical HPV infection in sexually active, young women with normal cervicovaginal cytology. Br J Cancer. 1995;72:943–5. doi: 10.1038/bjc.1995.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano AR, Papenfuss M, Nour M, Canfield LM, Schneider A, Hatch K. Antioxidant nutrients: associations with persistent human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 1997;6:917–23. [PubMed] [Google Scholar]
- 5.Giuliano AR, Gapstur S. Can cervical dysplasia and cancer be prevented with nutrients? Nutr Rev. 1998;56:9–16. doi: 10.1111/j.1753-4887.1998.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Closas R, Castellsague X, Bosch X, Gonzalez CA. The role of diet and nutrition in cervical carcinogenesis: a review of recent evidence. Int J Cancer. 2005;117:629–37. doi: 10.1002/ijc.21193. [DOI] [PubMed] [Google Scholar]
- 7.Castle PE, Giuliano AR. Chapter 4: genital tract infections, cervical inflammation, and antioxidant nutrients–assessing their roles as human papillomavirus cofactors. J Natl Cancer Inst Monogr. 2003:29–34. doi: 10.1093/oxfordjournals.jncimonographs.a003478. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AR. The role of nutrients in the prevention of cervical dysplasia and cancer. Nutrition. 2000;16:570–3. doi: 10.1016/s0899-9007(00)00338-5. [DOI] [PubMed] [Google Scholar]
- 9.Sedjo RL, Roe DJ, Abrahamsen M, Harris RB, Craft N, Baldwin S, Giuliano AR. Vitamin A, carotenoids, and risk of persistent oncogenic human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 2002;11:876–84. [PubMed] [Google Scholar]
- 10.Giuliano AR, Siegel EM, Roe DJ, Ferreira S, Luiza Baggio M, Galan L, Duarte-Franco E, Villa LL, Rohan TE, Marshall JR, Franco EL. Dietary intake and risk of persistent human papillomavirus (HPV) infection: the Ludwig-McGill HPV natural history study. J Infect Dis. 2003;188:1508–16. doi: 10.1086/379197. [DOI] [PubMed] [Google Scholar]
- 11.Palan PR, Chang CJ, Mikhail MS, Ho GY, Basu J, Romney SL. Plasma concentrations of micronutrients during a nine-month clinical trial of β-carotene in women with precursor cervical cancer lesions. Nutr Cancer. 1998;30:46–52. doi: 10.1080/01635589809514639. [DOI] [PubMed] [Google Scholar]
- 12.Sedjo RL, Papenfuss MR, Craft NE, Giuliano AR. Effect of plasma micronutrients on clearance of oncogenic human papillomavirus (HPV) infection (United States) Cancer Causes Control. 2003;14:319–26. doi: 10.1023/a:1023981505268. [DOI] [PubMed] [Google Scholar]
- 13.Schlecht NF, Platt RW, Duarte-Franco E, Costa MC, Sobrinho JP, Prado JC, Ferenczy A, Rohan TE, Villa LL, Franco EL. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003;95:1336–43. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- 14.Cooney RV, Franke AA, Hankin JH, Custer LJ, Wilkens LR, Harwood PJ, Le Marchand L. Seasonal variations in plasma micronutrients and antioxidants. Cancer Epidemiol Biomarkers Prev. 1995;4:207–15. [PubMed] [Google Scholar]
- 15.Franco E, Villa L, Rohan T, Ferenczy A, Petzl-Erler M, Matlashewski G. Design and methods of the Ludwig-McGill longitudinal study of the natural history of human papillomavirus infection and cervical neoplasia in Brazil. Ludwig-McGill study group. Rev Panam Salud Publica. 1999;6:223–33. doi: 10.1590/s1020-49891999000900001. [DOI] [PubMed] [Google Scholar]
- 16.Bauer HM, Ting Y, Greer CE, Chambers JC, Tashiro CJ, Chimera J, Reingold A, Manos MM. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265:472–7. [PubMed] [Google Scholar]
- 17.Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Zhang T, Scott DR, Rush BB, Lawler P, Sherman ME, Kurman RJ, Manos MM. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–40. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 18.Bernard HU, Chan SY, Manos MM, Ong CK, Villa LL, Delius H, Peyton CL, Bauer HM, Wheeler CM. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis. 1994;170:1077–85. doi: 10.1093/infdis/170.5.1077. [DOI] [PubMed] [Google Scholar]
- 19.Nomura AM, Stemmermann GN, Lee J, Craft NE. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev. 1997;6:487–91. [PubMed] [Google Scholar]
- 20.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–8. [PubMed] [Google Scholar]
- 21.Willett WC. Nutritional epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 22.Diggle PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. 1. Oxford: Oxford University Press; 1994. [Google Scholar]
- 23.Hardin J, Hilbe JM. Generalized estimating equationsed. Boca Raton, FL: Chapman & Hall; 2003. [Google Scholar]
- 24.Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, Basu J, Tachezy R, Lewis R, Romney S. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 25.Rousseau MC, Franco EL, Villa LL, Sobrinho JP, Termini L, Prado JM, Rohan TE. A cumulative case-control study of risk factor profiles for oncogenic and nononcogenic cervical human papillomavirus infections. Cancer Epidemiol Biomarkers Prev. 2000;9:469–76. [PubMed] [Google Scholar]
- 26.Schiffman M, Kjaer SK. Chapter 2: natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003:14–9. doi: 10.1093/oxfordjournals.jncimonographs.a003476. [DOI] [PubMed] [Google Scholar]
- 27.Goodman MT, Hernandez B, McDuffie K, Franke A, Wilkens L. Effect of diet and serum micronutrients on clearance of cervical human papillomavirus. Presented at the 22nd International Papillomavirus Conference; 2005. [Google Scholar]
- 28.Goodman M, Cheung L, Hernandez B, McDuffie K, Wilkens L. Serum micronutrient concentrations and cervical human papillomavirus persistence. Presented at the 21st International Papillomavirus Conference; 2004. [Google Scholar]
- 29.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–8. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 30.von Knebel Doeberitz M. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer. 2002;38:2229–42. doi: 10.1016/s0959-8049(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 31.Tendler A, Ho GY, Kadish AS. Do ‘‘high-risk’’ human papillomavirus (HPV) types lead to cancer by evading the immune system? Gynecol Oncol. 1999;73:169–70. doi: 10.1006/gyno.1998.5325. [DOI] [PubMed] [Google Scholar]
- 32.Beck MA. Antioxidants and viral infections: host immune response and viral pathogenicity. J Am Coll Nutr. 2001;20:384S–388S. doi: 10.1080/07315724.2001.10719172. discussion 96S–97S. [DOI] [PubMed] [Google Scholar]
- 33.Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]


