Abstract
Docosahexenoic acid (DHA, 22:6n-3) plays an important role in development of proper brain function in mammals. We have previously reported that DHA promotes synaptogenesis and synaptic function in hippocampal neurons while DHA-depletion in the brain due to n-3 fatty acid deficiency produces opposite effects. To gain insight into underlying molecular mechanisms, we investigated whether the brain DHA status affects the synaptic plasma membrane (SPM) proteome by using nanoLC/ESI-MS/MS and 16O/18O labeling. The DHA level in mouse brains was lowered by dietary depletion of n-3 fatty acids, and SPM was prepared by differential centrifugation followed by osmotic shock. SPM proteins from DHA-adequate and depleted brains were analyzed by nanoLC/ESI-MS/MS after SDS-PAGE, in-gel digestion and differential O18/O16 labeling. This strategy allowed comparative quantitation of more than 200 distinct membrane or membrane-associated proteins from DHA-adequate or depleted brains. We found that 18 pre- and postsynaptic proteins that are relevant to synaptic physiology were significantly down-regulated in DHA-depleted mouse brains. The protein network analysis suggests involvement of CREB and caspase-3 pathways in the DHA-dependent modulation of synaptic proteome. Reduction of specific synaptic proteins due to brain DHA-depletion may be an important mechanism for the suboptimal brain function associated with n-3 fatty acid deficiency.
Keywords: Synaptic plasma membrane (SPM), synaptic proteins, docosahexaenoic acid (DHA), 18O labeling, nano-LC/ESI-MS/MS, brain
Introduction
Polyunsaturated fatty acids, especially docosahexenoic acid (DHA; 22:6n-3) is highly enriched in the brain and required for normal brain development in mammals. DHA accumulates in brain phospholipids mainly during the third trimester of gestation when neurogenesis and neuritogenesis actively occur1. Deficiency of this fatty acid has been associated to impairments in hippocampal-dependent learning and memory2–4. In contrast, supplementation of DHA in the diet leads to improvement in mental development during infancy in humans5, 6. Previous studies in our laboratory indicated that DHA can promote neurite growth and synaptogenesis in cultured hippocampal neurons7, 8. We have also demonstrated that DHA increased expression of NMDA and glutamate receptors, improving glutamatergic synaptic transmission8, suggesting that the synaptic plasma membrane (SPM) proteome might be under the influence of the DHA status.
The emerging MS-based proteomics has become an important strategy in biological research as it unveils complex networks of a large number of proteins covering diverse biological functions9. Comparative proteome analysis in particular is a useful technique to detect differentially expressed proteins at distinct states such as disease states. Although there are proteomic studies analyzing protein constituents of different organelles and their subcellular complexes, studies focusing on organization of protein networks in the synapses, which represent the principal means of communication among neurons, are still scarce10–12. This is mainly because the central parts of the synaptic protein networks are integral or peripheral membrane proteins like receptors, transporters and channels. Since these proteins are usually hydrophobic and difficult to solubilize, analysis of these proteins remains challenging. Nevertheless, membrane proteins constitute 1/4th to 1/3rd of the mammalian proteome and many of them are important drug targets, stimulating interest towards developing strategies to examine these molecules13.
Recent developments in quantitation techniques using stable isotope labeling are advantageous over 2D-PAGE-based differential proteomics (i.e., 2D-DIGE) by providing better coverage of membrane proteins. Quantitation using stable isotope labeling can be divided into two distinct types: (i) in vivo metabolic labeling in which the cells or organisms of interest are supplied with nutrients highly enriched in stable isotopes, such as stable-isotope labeling by amino acids (SILAC)14; (ii) in vitro stable isotope labeling by modification with chemicals such as iTRAQ or TMT reagents15, 16 or enzymatic incorporation of isotopes into the proteome of interest at the protein and/or peptide level (e.g. 18O labeling with trypsin17). The enzymatic 18O labeling offers the advantage of simplicity with low-cost, particularly for comparative examination of protein expression between two biological sample groups. The technique utilizes a protease typically trypsin and H2O enriched with different isotopic oxygen atoms to produce differentially labeled peptides. In the presence of H218O, trypsin catalyzes the exchange of two 16O atoms for two 18O atoms at the C-terminal carboxyl group of tryptic peptides, resulting in a mass shift of 4 Da in comparison to the case with H216O. The relative abundance of a protein the sample groups can be calculated using the ratio of the peak intensities of the 16O- and 18O-labeled peptides that belong to the same protein18.
In this study, we investigated changes in the synaptic plasma membrane proteome associated with DHA deficiency using 16O/18O labeling along with nano-LC/ESI-MS/MS. The SPM proteins prepared from DHA-adequate or depleted mice brains were subjected to SDS PAGE, and protein bands were digested with trypsin, labeled with 16O or 18O, and analyzed by nano-LC/ESI-MS/MS. Using this approach, more than 180 distinct membrane or membrane-associated proteins were identified from the mouse SPM, including various receptors, kinases and transporter proteins. The 18O/16O labeling of tryptic digests revealed differential expression of SPM proteins involved in synaptic transmission such as munc 18–1, PSD-95, synaptic vesicle protein, synapsin 1a/b, contactin 2, bassoon, GluR2 and NR2 subunits. Protein network analysis suggests that both expression and degradation of these proteins are under significant influence of DHA in the brain.
Materials and Methods
Animals and Diets
Pregnant C57/BL6 mice at 2 days of gestation were obtained from Charles River Laboratories (Portage, MI, USA) and fed with either an n-3 fatty acid adequate or deficient diet throughout the pregnancy and lactation period. The offspring mice after weaning were continued on the n-3 fatty acid adequate or deficient diet. Both semi-synthetic pelleted diets were based on the AIN-93G formula19 and were different only in fat composition (Dyets, Bethlehem, PA). The n-3 fatty acid adequate diet consisted of 7.45 %, 1.77 %, 0.48 % and 0.3 % (w/w) of hydrogenated coconut, safflower, flaxseed and DHASCO oil (Martek, Columbia, MD). The deficient diet contained 8.1 % and 1.9 % (w/w) of hydrogenated coconut and safflower oil. The resulting n-3 fatty acid content was 2.5 % (w/w) linolenic acid (LNA) plus 0.9 % DHA (w/w) in the adequate diet, and only 0.09 % (w/w) of LNA in the deficient diet. The procedures employed in this study were approved by the National Institute on Alcohol Abuse and Alcoholism (LMS-HK21). At six months of age, animals were sacrificed by cervical dislocation after exposure to CO2 inhalation and the cortices were rapidly dissected from whole brain for subcellular fractionation.
Preparation of synaptic plasma membranes
Synaptic plasma membranes (SPM) were prepared according to the protocol described by Cottman and Matthews (1971)20 with a slight modification. A total of about 1g of mouse brain cortex was obtained from 3 mice fed on DHA-adequate or deficient diet. The brain cortices were homogenized in 4 mL of 0.32M sucrose solution making 20% (w/v) brain tissue/ sucrose solution. After adding another 4 mL of 0.32M sucrose the homogenate was centrifuged in a Beckman GS-6R centrifuge at 1,000 g at 4 °C for 5 min. The pellet containing nuclei was discarded and the supernatant was centrifuged at 11,000 g for 20 min to obtain the crude mitochondrial pellet. In order to extract plasma membranes from crude mitochondria, density gradient centrifugation was performed using Ficoll. Three fractions were collected following centrifugation at 64,000 g for 45 min using different concentrations of Ficoll (13%, 6% and 4%). Myelin was obtained at the top 4% layer of Ficoll, synaptosomes were collected at the interface between 6% and 13% Ficoll and mitochondrial fraction was collected as pellet at the bottom. All the fractions were washed twice with PBS buffer. SPM were obtained by osmotic shock. The synaptosomes were incubated with 5 mM Tris HCl buffer (pH 7.4) containing 1 mM EDTA at 4°C for one hour with gentle stirring. Suspension was then centrifuged at 20,000 × g for 30 min to obtain SPM pellet.
In-gel tryptic digestion
SPM samples each containing approximate 40 ug proteins were dissolved in lithium dodecyl sulfate (LDS) sample buffer (composition, 10% glycerol, 2% LDS), incubated at 70 °C for 5 min, and loaded onto a 10% Bis-Tris gels (Invitrogen). Electrophoresis was carried out at a constant voltage of 100 V for 2 hours using MOPS SDS running buffer. The separated proteins were visualized with Coomassie blue (SimplyBlue SafeStain, Invitrogen). Gel bands from both samples were cut in-pair into 8–10 segments, destained, dried and subjected to reduction with DTT and alkylation with iodoacetamide. The gel pieces were rehydrated with 12.5 ng/mL trypsin (Promega, Madison, WI) solution in 25 mM ammonium bicarbonate on ice for 30 min and incubated overnight at 37°C in the presence of five standard peptides (at 0.05 ug each) for normalizing 18O/16O ratio in quantitation analysis. These peptides were originated from non-mammalian sources, including PopD from pseudomonas aerigunosa, a peptide from plasmodium falciparum 3D7 (H[313–333]K), a peptide from plasmodium falciparum 3D7 (L[250–269]K), pachytene arrest protein (1–50) from saccharomyces cerevisiae (New England Peptide Company, Gardner, MA) and magnainin spacer peptide (American Peptide Company, Sunnyvale, CA). The resultant tryptic peptides were extracted with 5% formic acid/50% ACN, concentrated with a Speed Vac, and desalted using C-18 ziptip.
18O labeling using immobilized trypsin
18O labeling was performed using a protocol reported previously21. Briefly, the ziptipped peptides were completely dried and reconstituted with 10 μL acetonitrile and 50 μL of 50 mM ammonium bicarbonate in either 16O water (for DHA-adequate sample) or 97% 18O enriched water from Sigma-Aldrich (for DHA-deficient sample), followed by addition of 0.5 μl of 2 M calcium chloride and 2.5 μL immobilized trypsin (Applied Biosystems, Foster City, CA). The mixture was incubated with constant rotation for 36 hours at 30°C. After centrifugation at 15,000 g for 5 min, the supernatant was collected and acidified to pH 3–4 by adding 1–2 ul of 10% TFA. The 16O-labeled DHA-adequate samples were mixed with 18O-labeled DHA-deficient samples from the corresponding gel fractions. The mixture was desalted by C-18 ziptip prior to the analysis by nano-LC/ESI-MS/MS.
Nano-LC/ESI-MS/MS analysis
Nano-LC/ESI-MS/MS was performed on an LTQ-Orbitrap XL mass spectrometer equipped with a nanospray source (Thermo Electron) and an Eksigent nanoLC 1D system. The mobile phases consisted of 0.1% formic acid (solvent A) and 0.1% formic acid in 95% ACN (solvent B). Approximately 1 μg of labeled digests was loaded onto a C18 trap column (Zorbax 5 × 0.3 mm, Agilent) and separated by a 15 cm IntegraFrit column (ProteoPep™, New Objective, Woburn, MA) at a flow rate of 300 nL/min with a gradient from 5–40% solvent B in 150 min. The LC eluant was sprayed into the MS instrument with a glass emitter tip (PicoTip, New Objective) using a spray voltage of 2.0 kV in positive-ion mode. Full scan spectra from m/z 300 to 2000 at 60,000 resolution were acquired by the Orbitrap. Data-dependent MS/MS spectra of ten most intense ions were acquired by the LTQ-XL ion trap using CID with a normalized energy of 35. Dynamic exclusion for the ions for which MS/MS spectra had been already acquired used following parameters: exclusion time 180 s, repeat count 1, repeat duration 30 s, exclusion mass width 10 ppm, and exclusion size 500. Singly charged species were excluded from data-dependent MS/MS analysis.
Data analysis
The acquired data were searched against the NCBInr database with Mascot (v2.3, Matrix Sciex) for protein identification. Search parameters were set as follows: enzyme, trypsin; precursor ion mass tolerance, 0.3 Da; fragment ion mass tolerance, 0.3 Da; maximum missed cleavages allowed 2; carbamidomethyl of cysteine residues for fixed modification; oxidation of methionine for variable modification. Only the proteins with at least 2 distinct peptides and a Mascot score more than 50 were considered for positive identification. All proteins identified by LC-MS/MS and Mascot were uploaded to the Database for Annotation, Visualization and Integrated Discovery (DAVID) to determine subcellular localization based on gene ontology (GO). Quantitation of 18O/16O ratio was performed by Mascot Distiller (version 2.3) with the 18O-corrected method due to the use of 97% enriched 18O water. Peptide peaks were rejected for quantitation if peak correlation was below 0.7, standard error was greater than 0.1, or area fraction was below 0.5. Protein abundance ratio was presented by the average 18O/16O ratio of at least two peptides and normalized to the average 18O/16O value from five standard peptides.
Western Blot Analysis
Proteins from whole homogenates or subcellular fractions were electrophoresed in 4–12% Bis-Tris gels, transferred to a PVDF membrane, and then blocked for 1 hr at room temperature with 5% milk in TBS containing 0.1% Tween 20 (TBS-T). Blots were incubated with primary antibody overnight at 4°C and washed three times with TBS-T. Blots were then incubated with peroxidase-conjugated secondary antibody for one hour at room temperature, washed three times with TBS-T, incubated with ECL detection reagent, and imaged with a Kodak Gel Logic 440 Imaging System. The primary antibodies used in this study were anti-cadherin, anti-VDAC, anti-calnexin, anti-PSD-95, anti-synapsin-1 (Cell Signaling, Billerica, MA), anti bassoon, anti-dynamin-1, anti-contactin 2, anti-SV2A & SV2B, anti-syntaxin 1A (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-munc18 (Abcam, Cambridge, MA).
Protein Network Analysis
Differentially regulated proteins identified by nano-LC/ESI-MS/MS were further analyzed by pathway analysis using the network building tool MetaCore (GeneGo, St. Joseph, MI). MetaCore pathway analysis is based upon protein interaction networks that are manually annotated from regularly updated databases. For the network analysis, we imported the differentially regulated proteins observed by nano-LC/ESI-MS/MS into the MetaCore. Based upon the shortest paths algorithm, one of the several algorithms integrated within MetaCore, hypothetical networks of proteins from our experiment and proteins from the MetaCore database were built. To simplify the complex networks generated, the proteins that were unrelated to any of the proteins in our study were excluded from the network. Out of the 7 different pathways generated, only the pathways that included a minimum of 5 proteins involved in our study were considered.
Results
Quantitative SPM proteomic analysis using 18O labeling
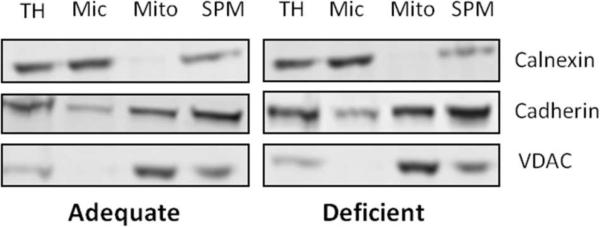
Mouse brains became DHA-depleted (more than 80% depletion of DHA in comparison to the n-3 fatty acid adequate case) after six months of feeding with an n-3 fatty acid deficient diet, similar to the results reported previously8. We prepared the SPM fractions from the DHA-deficient and adequate mouse cortices by differential centrifugation. Pan-Cadherin Antibody was used as a plasma membrane marker. Western blot analysis indicated that cadherin was enriched in the SPM fraction (Fig. 1). The levels of calnexin and VDAC, the microsomal and mitochondrial membrane markers, respectively, were low in the SPM fraction, indicating that SPM was prepared with minimal contamination.
Fig.1.
Enrichment of SPM using subcellular fractionation. Western blot analysis of isolated fractions indicating enrichment of SPM. Cadherin, calnexin VDAC are plasma, microsomal and mitochondrial membrane protein markers, respectively. TH: total homogenate, Mic: microsomal, Mito: Mitochondria, SPM: synaptic plasma membrane.
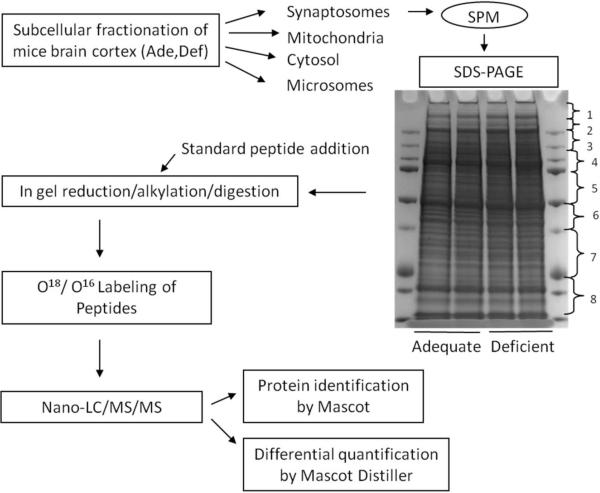
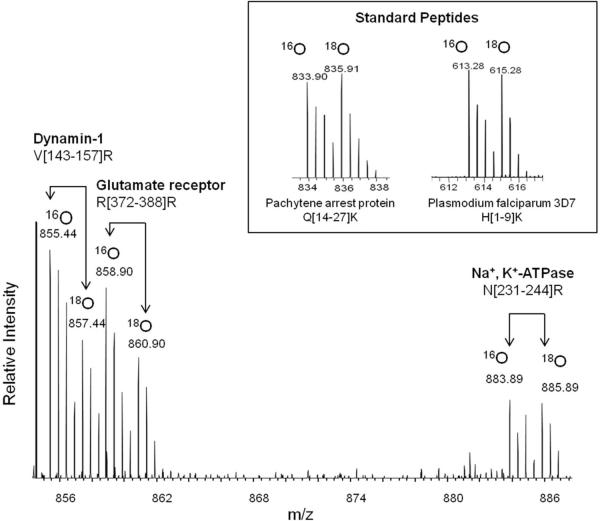
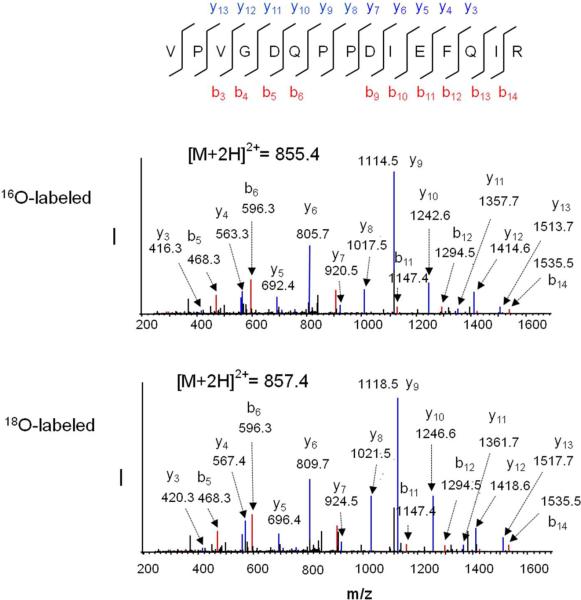
To examine the effect of the DHA status on the SPM proteome, we performed differential proteomic analysis using the strategy outlined in Fig. 2. To increase protein coverage, protein bands in the SDS-PAGE gel were divided into 8 fractions, and subjected to in-gel digestion, 18O labeling and LC-ESI-MS/MS analysis. Proteins were identified using Mascot search engine using the criteria of the Mascot score above 50 and at least two distinct peptides identified, and quantified with Mascot Distiller. This approach allowed the identification of 384 distinct proteins from the SPM fraction, including 196 plasma membrane, 21 mitochondrial membrane and 11 membrane-associated proteins (Supplementary Table 3). The Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 was used for retrieving the subcellular localization of the identified proteins. A key step in determining relative changes of protein abundance between biological groups is normalization of the protein levels to compensate for the loss during sample preparation including in-gel digestion and 18O labeling processes. We spiked the samples with the mixture of five non-mammalian synthetic peptide standards prior to in-gel digestion. Since the majority of the proteins were not altered, the overall 18O/16O ratio may also serve as the reference. In fact, the average 18O/16O ratio of the standard peptides was in good agreement with the overall 18O/16O ratio. However, the coefficient of variation of the 18O/16O ratio from five standard peptides added to each gel band was less than 6%, allowing more reliable normalization for the relative comparison of protein levels. Figure 3 shows the mass spectrum of differentially labeled tryptic peptide mixture derived from the gel slice 4 in Fig.2. The peptides from DHA-adequate and DHA-deficient samples were 16O- and 18O-labeled, respectively. The 18O/16O ratios calculated from Mascot Distiller were 0.61, 0.60 and 0.98 for peptides with m/z 855.44, 858.90 and 883.89, respectively. The identity of the peptides were revealed to be V[143–157]R of dynamin-1, G[350–371]R of AMPA2 receptor, and N[231–245]R of Na+/K+-ATPase by database search based on MS/MS data. For example, Fig. 4 shows the MS/MS spectra of the peptide with m/z of 855.4 (16O labeled) and 857.4 (18O labeled). The MS/MS data matched well with the peptide V[143–157]R from dynamin-1 with good coverage of y and b fragment ions. Note that the m/z values for y ions shifted 4 units while b ions remain unchanged with 18O labeling. The average 18O/16O ratio for the five standard peptides was 1.01 (Fig. 3 inset). These data indicated that the first two peptides were down-regulated in the DHA-deficient SPM sample while the peptide N[231–244]R from Na+/K+-ATPase was unchanged. Protein abundance ratio was calculated by averaging 18O/16O ratio of at least two peptides belonging to the same protein, with a standard error less than 0.1, peak correlation greater than 0.7, and area fraction greater than 0.5. The protein abundance ratio was then normalized by average 18O/16O ratio of the standards. Average normalized 18O/16O ratios of the proteins from three independent experiments revealed differential expression of 18 synaptic proteins in DHA-adequate and depleted brains (Table 1). These proteins showed the Mascot score greater than 100 as indicated in Supplemental Table 1. The 18O/16O ratios of individual peptides belonging to the 18 proteins from a representative experiment have been shown in Supplemental Table 3. All 18 proteins, most of which are known to play important roles in synaptic neurotransmission, were found to be down-regulated in the DHA-depleted brains.
Fig.2.
Experimental strategy for investigating the differential expression of synaptic plasma membrane proteins from DHA-adequate and deficient mice using 18O labeling technique.
Fig. 3.
Representative mass spectrum from 16O-labeled DHA-adequate sample mixed with 18O labeled DHA-deficient sample. The 18O/16O peak intensity ratio was used for quantitation of the peptides. Inset, 18O or 16O-peak of a standard peptides used for normalization purpose.
Fig.4.
MS/MS spectra of the doubly charged ions at m/z 855.44 and 857.44 from 16O-labeled DHA-adequate sample mixed with 18O-labeled DHA-deficient sample. The sequence of the peptide was assigned with single letter abbreviation based on the fragment ions observed for the peptide segments. N-terminal b ions and C-terminal y ions resulting from the amide bond cleavage are labeled. The MS/MS spectra revealed that the peaks were originated from the peptide segment V[143–157]R of dynamin-1.
Table 1.
Synaptic plasma membrane proteins down-regulated in DHA-depleted mouse brainsa
| Protein | Accession number | Gene symbol | MW (kDa) | No. of Peptides quantified | 18O/16O |
|---|---|---|---|---|---|
| bassoon | 12217 | Bsn | 420 | 23 | 0.57 ± 0.02 |
| spectrin beta 2 | 20742 | Spnb2 | 275 | 5 | 0.56 ± 0.04 |
| NMDA receptor, NR2B | 14812 | Grin2b | 168 | 4 | 0.59 ± 0.06 |
| synaptojanin-1 | 104015 | Synj1 | 146 | 4 | 0.56 ± 0.11 |
| contactin associated protein 1 | 53321 | Cntnap1 | 158 | 3 | 0.54 ± 0.05 |
| contactin 2 | 21367 | Cntn2 | 135 | 3 | 0.61 ± 0.13 |
| dynamin-1 | 13429 | Dnm1 | 96 | 13 | 0.66 ± 0.06 |
| intercellular adhesion molecule 5 | 15898 | Icam5 | 98 | 2 | 0.60 ± 0.04 |
| PSD-95 | 13385 | Dlg4 | 95 | 5 | 0.59 ± 0.07 |
| AMPA2 receptor | 14800 | Gria2 | 104 | 4 | 0.57 ± 0.03 |
| synaptic vesicle glycoprotein 2 a | 64051 | Sv2a | 83 | 4 | 0.62 ± 0.01 |
| synaptic vesicle glycoprotein 2 b | 64176 | Sv2b | 78 | 3 | 0.46 ± 0.02 |
| PSD-93 | 23859 | Dlg2 | 95 | 3 | 0.59 ± 0.05 |
| synapsin I | 20964 | Syn1 | 74 | 17 | 0.65 ± 0.07 |
| synaptopodin | 104027 | Synpo | 74 | 3 | 0.52 ± 0.03 |
| synaptotagmin I | 20979 | Syt1 | 48 | 4 | 0.60 ± 0.07 |
| munc 18-1 | 20910 | Stxbp1 | 68 | 12 | 0.47 ± 0.06 |
| syntaxin 1A | 20907 | Stx1a | 40 | 2 | 0.49 ± 0.05 |
A list of proteins with their accession number, gene symbol, molecular weight (MW), number of peptides quantified and 18O/16O ratio that is normalized to average 18O/16O of standard peptides. 18O/16O has been calculated from three independent experiments. These proteins met the criteria for identification described in the experimental section.
Validation of MS-based protein quantitation by western blot analysis
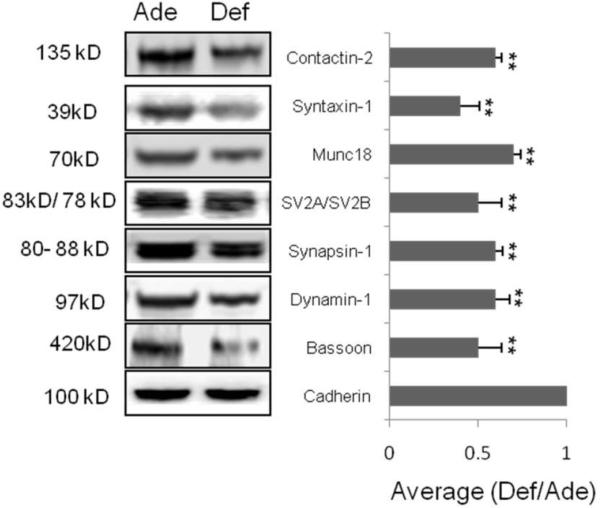
To confirm the MS-based quantitative data obtained from the 18O/16O ratio (Table 1), western blot analysis was performed for some of the differentially expressed proteins. Out of 18 SPM proteins found to be down-regulated in the DHA-depleted brain, analyzed were eight proteins for which antibodies are available, namely, bassoon, dynamin-1, contactin 2, synapsin I, SV2A, SV2B, munc18 and syntaxin 1A. Cadherin, a plasma membrane marker, was used for the protein loading control. The western blot analysis clearly indicated that DHA-depleted brains contained lower level of all the above mentioned proteins, as shown in Fig. 5. The good agreements between MS and western blot data validated the quantitative proteomics data using 18O labeling and confirmed that DHA-depletion in the mouse brain leads to the reduction of specific synaptic protein levels.
Fig.5.
Western blot validation of proteins down-regulated in the DHA-deficient mouse brain. Extracts from DHA-adequate and deficient brains containing equal amounts of protein (15 μg) were loaded onto a 4–12 % SDS gel and subjected to western blotting against the antibodies indicated. Quantitative analysis of the Western blot data was based on three biological replicates. The band intensity was normalized to cadherin. The data are expressed as mean ± standard deviation.
Protein network analysis
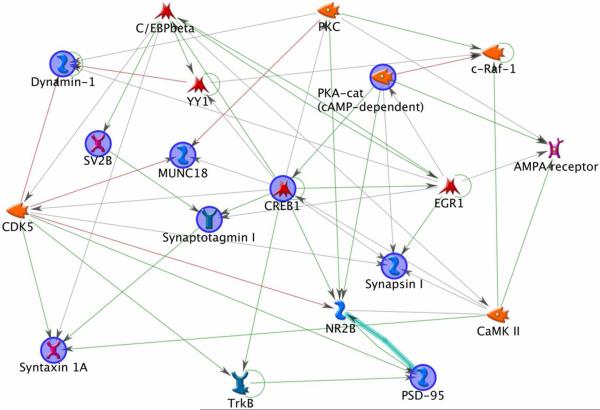
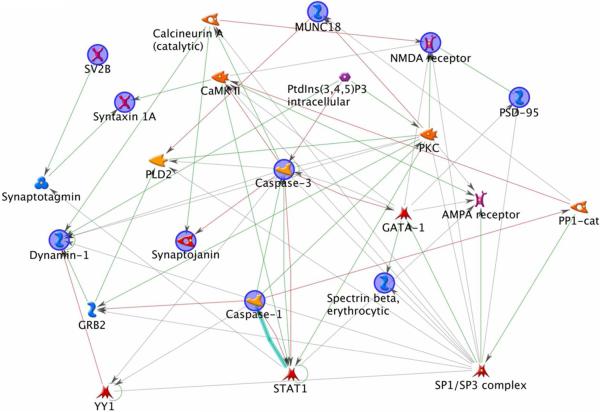
To gain the insight for the protein interaction, a network analysis was performed for the proteins down-regulated by DHA-depletion using the MetaCore software from GeneGo. Two prominent protein interaction networks were identified (Fig. 6a, b). The first pathway suggested importance of CREB1 in DHA-mediated expression of synaptojanin-1, synaptotagmin I, NMDA receptor, syntaxin 1A, AMPA receptor, SV2B, synapsin I, munc 18-1, dynamin-1 and PSD-95. The second network involved the caspase-3 pathway, suggesting a role of caspase-3 activation in reduction of synaptojanin-1 and spectrin beta, protein levels in the DHA-depleted brains.
Fig.6.
Network analyses of synaptic plasma membrane (SPM) proteins down-regulated in the DHA-deficient mouse brain. Involvement of CREB1 signaling pathway (a) or caspase-3 network (b) is indicated. Analysis was performed with GeneGo-MetaCore software.
Discussion
Previous studies in our laboratory have demonstrated that DHA supplementation significantly promotes neurite outgrowth, synaptogenesis and increased the levels of a few pre- and post-synaptic proteins that are involved in synaptic neurotransmission and LTP in hippocampal neuronal cells in cultures and tissues7, 8. Using proteomics approach, we investigated the effect of DHA on global synaptic proteome in this study. We identified 18 proteins that were down-regulated in the DHA-depleted mouse brains, revealing the brain synaptic proteome responding to the DHA status. The list includes bassoon, glutamate receptors NR2B and AMPA2, synaptojanin-1, spectrin beta 2, contactin associated protein 1, contactin 2, intercellular adhesion molecule 5, dynamin-1, PSD-95, PSD-93, synaptic vesicle glycoproteins 2a and 2b, synaptopodin, synaptotagmin I, synapsin I, munc18-1 and syntaxin 1A. Many of these down-regulated proteins belong to the CREB1 and caspase-3 network pathways, suggesting that both transcription and degradation activity is under the control of the DHA status in the brain. No concurrent up-regulation of SPM proteins was apparent in our study, possibly reflecting the diminished synapses under DHA-depleted conditions, which is consistent with the reduced synaptogenesis previously demonstrated in DHA-depleted neurons8.
Most of the synaptic proteins downregulated in the DHA-depleted brains are involved in vesicle trafficking and recycling processes, and neurotransmission. For example, dynamin-1 controls synaptic vesicle recycling via endocytosis to sustain the synaptic vesicle pool in a pre-synaptic cell22, 23. While synaptotagmin I has been proposed as the major Ca2+ sensor for synchronous neurotransmitter release in hippocampal neurons24, munc18-1 (also known as syntaxin binding protein 1) plays an important role in vesicle docking and exocytosis25. By binding to syntaxin 1 munc18-1 enables the formation of SNARE (soluble N-ethylmaleimide sensitive factor attachment protein receptor) complexes and the docking of synaptic vesicles at the plasma membrane26. Synapsin I anchors synaptic vesicles containing neurotransmitters to the cytoskeleton at the resting stage for ready mobilization for neurotransmitter release upon phosphorylation27. Synaptic vesicle glycoprotein 2a (SV2A), the major isoform of SV2, is also required for neurotransmission. The electrophysiological and morphological studies indicated that loss of SV2A leads to a reduction in action potential-dependent γ-aminobutyric acid (GABA)ergic neurotransmission without changing synapse number or structure28. Significant losses of these critical proteins and consequential impairment of exocytosis, neurotransmitter release and vesicle recycling may at least partly explain learning and memory impediment associated with DHA-depletion in the brain caused by dietary n-3 fatty acid deficiency2–4.
Most of the synaptic proteins reduced in response to DHA-depletion observed in this study are CREB1-downstream proteins (Fig. 6a). According to the protein network analysis, CREB1 regulates the expression of NR2B and synaptotagmin I directly while indirectly syntaxin 1A, synapsin I, PSD-95, dynamin-1, AMPA receptor and SV2B. It has been demonstrated that CREB1-dependent gene expression plays an important role in learning and memory in mammals29. Batteries of behavioral tests have indicated that long-term memory is significantly affected in mice with defective alpha and delta isoforms of CREB30. A recent study with the fresh water pond snail L. stagnalis indicated that long term memory-induced de novo synthesis of synaptic proteins, syntaxin 1 and dynamin-1, requires CREB1-mediated gene expression31. Similarly, long-term potentiation (LTP), a cellular model for learning and memory, was significantly impaired in hippocampal slices of CREB mutant mice32 and DHA-depleted mice8. In the latter case, concomitant loss of synapsin I and NR2B, CREB-1 downstream proteins, was observed in the DHA-depleted mouse hippocampi8, suggesting that CREB1-dependent synaptic protein expression may also be an important mechanism for DHA-enhanced learning and memory function.
Some of the CREB-1 dependent synaptic proteins including dynamin-1, munc 18, syntaxin 1 and PSD-95 also belong to cyclin-dependent kinase 5 (Cdk5) network. Cdk5 is primarily found in developing nervous system and is activated by two non-cyclin partners p35 and p3933. Recent investigations suggests role of Cdk5 in vesicle cycling, ion channel modulation and intracellular signaling implicating Cdk5 in synaptic plasticity and learning and memory. Cdk5 is involved in direct phosphorylation of numerous substrates relevant for synaptic plasticity including munc18/ syntaxin 1A34, dynamin-1/amphiphysin-135, synaptojanin-136, NMDA receptor37, 38, PSD-9539, synapsin I40. Cdk5 is also implicated both in vesicle exocytosis and endocytosis. Munc18 phosphorylation (Thr574) by p35-activated Cdk5 leads to the dissociation of munc18 from syntaxin 1A34, 41 which may allow syntaxin 1A to participate in the formation of SNARE complex required for membrane fusion during exocytosis42. Most of the above mentioned proteins involved in Cdk5 pathway have been found to decrease in our study due to DHA-depletion. Consequential attenuation of Cdk5 signaling may be a contributing factor for the impaired learning and memory function associated with DHA-inadequacy.
The network analysis also indicated that caspase-3 is upstream of synaptojanin-1, syntaxin 1A and beta-spectrin and has a negative/inhibitory relation with these proteins (Fig. 6b). Caspases are a family of cytosolic aspartate-specific cysteine proteases that have essential role in the initiation and execution of apoptotic cell death43. Enhanced degradation of these SPM proteins which are downstream of caspase-3 indicates the activation of caspase-3 under DHA-depleted conditions. This data is consistent with our previous findings that DHA-depleted neurons are more susceptible to cell death while DHA supplementation suppresses caspase-3 activation through the accumulation of phosphatidylserine (PS) in neuronal membranes44, 45. The degradation pathways activated during the DHA deficiency may have resulted in the reduction of some of the synaptic proteins observed in this study which may have in turn contributed negatively to the learning and memory functions.
In summary, using the mass spectrometric proteomics approach in conjunction with subcellular fractionation, SDS-PAGE and quantitative profiling based on O16/O18 labeling, we found significant differences in the synaptic proteome of DHA-adequate and DHA-deficient mouse brains. Some of the proteins that are important for synaptic integrity and synaptic neurotransmission were reduced in the DHA-deficient brains. The network pathway analysis indicated the importance of CREB1 and caspase-3 pathways in the down-regulation of these synaptic proteins, although further studies are needed for detailed molecular mechanisms involved in these processes. The DHA-dependent control of key synaptic proteins that are necessary for synaptic activity provides a molecular basis for the significant impact of nutritional DHA on learning and memory function.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Alcohol Abuse and Alcoholism, National Institutes of Health. The authors thank Karl Kevala and NIAAA animal facility staff for keeping animals on special diets.
Footnotes
Publisher's Disclaimer: “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a free service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are accessible to all readers and citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors or consequences arising from the use of information contained in these “Just Accepted” manuscripts.
References
- 1.Green P, Yavin EJ. Mechanisms of docosahexaenoic acid accretion in the fetal brain. Neurosci Res. 1998;52(2):129–36. doi: 10.1002/(SICI)1097-4547(19980415)52:2<129::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida S, Yasuda A, Kawazato H, Sakai K, Shimada T, Takeshita M, Yuasa S, Kobayashi T, Watanabe S, Okuyama H. Synaptic vesicle ultrastructural changes in the rat hippocampus induced by a combination of alpha-linolenate deficiency and a learning task. J. Neurochem. 1997;68:1261–68. doi: 10.1046/j.1471-4159.1997.68031261.x. [DOI] [PubMed] [Google Scholar]
- 3.Moriguchi T, Greiner RS, Salem N., Jr. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–73. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 4.Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, Salem N., Jr Cognitive deficits in docosahexaenoic acid-deficient rats. Behav. Neurosci. 2002;116:1022–31. doi: 10.1037//0735-7044.116.6.1022. [DOI] [PubMed] [Google Scholar]
- 5.Willatts P, Forsyth JS, Di Modugno MK, Varma S, Colvin M. Effect of long chain polyunsaturated fatty acids in infant formula at problem solving at 10 months of age. Lancet. 1998;352:688–91. doi: 10.1016/s0140-6736(97)11374-5. [DOI] [PubMed] [Google Scholar]
- 6.Birch EE, Garfield S, Hoffman DR, Uauy RD, Birch DG. A randomized controlled trial of early dietary supply of long chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000;42:174–81. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- 7.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004;90:979–88. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 8.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 2009;111:510–21. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palzkill T. Proteomics. Kluwer Academic Publishers; Dordrecht: 2002. pp. 1–33. [Google Scholar]
- 10.Stevens SM, Jr., Zharikova AD, Prokai L. Proteomic analysis of the synaptic plasma membrane fraction isolated from rat forebrain. Mol. Brain Res. 2003;117:116–28. doi: 10.1016/s0169-328x(03)00282-1. [DOI] [PubMed] [Google Scholar]
- 11.Walikonis RS, Jensen ON, Mann M, Provance DW, Jr., Mercer JA, Kennedy MB. Identification of proteins in the postsynaptic density fraction by mass spectrometry. J. Neurosci. 2000;20:4069–80. doi: 10.1523/JNEUROSCI.20-11-04069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant SGN, Blackstock WP. Proteomics in neuroscience: from protein to network. J. Neurosci. 2001;21(21):8315–18. doi: 10.1523/JNEUROSCI.21-21-08315.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cascio M, Rapaka RS. Structural biology and structural genomics/ proteomics. J. Pept. Res. 2002;60:307–11. doi: 10.1034/j.1399-3011.2002.21071.x. [DOI] [PubMed] [Google Scholar]
- 14.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–86. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 15.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–99. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 16.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–69. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Yao X, Freas A, Ramirez J, Demirev PA, Fenselau C. Proteolytic 18O labeling for comparative proteomics: model studies with two serotypes of adenovirus. Anal Chem. 2001;73:2836–42. doi: 10.1021/ac001404c. [DOI] [PubMed] [Google Scholar]
- 18.Ye X, Luke B, Andresson T, Blonder J. 18O Stable Isotope Labeling in MS-based Proteomics. Brief Funct Genomic Proteomic. 2009;8(2):136–44. doi: 10.1093/bfgp/eln055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 20.Cottman CW, Matthews DA. Synaptic plasma membranes from rat brain synaptosomes: isolation and partial characterization. Biochem. Biophys. Acta. 1971;249:380–94. doi: 10.1016/0005-2736(71)90117-9. [DOI] [PubMed] [Google Scholar]
- 21.Huang BX, Kim HY. Probing Akt-inhibitor interaction by chemical cross-linking and mass spectrometry. J Am Soc Mass Spectrom. 2009;20(8):1504–13. doi: 10.1016/j.jasms.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C.; Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O'toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camilli P. A selective activity-dependent requirement for Dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–74. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 23.Praefcke GJK, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 24.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2C-sensor for transmitter release at a central synapse. Cell. 1994;79:717–27. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 25.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuzi HJ, Südhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–69. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 26.Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, Südhof TC, Neher E, Verhage M. Munc18-1 promotes large dense-core vesicle docking. Neuron. 2001;31:581–91. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 27.Fdez E, Hilfiker S. Vesicle pools and synapsins: new insights into old enigmas. Brain Cell Biol. 2006;35(2–3):107–15. doi: 10.1007/s11068-007-9013-4. [DOI] [PubMed] [Google Scholar]
- 28.Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci U S A. 1999;96:15268–73. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubynina EV, Dolotov OV. The CREB Transcription Factor and Processes of Memory Formation. Neurochemical Journal. 2009;3:155–63. [Google Scholar]
- 30.Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- 31.Guo C, Senzel A, Li K, Feng ZP. De novo protein synthesis of syntaxin-1 and dynamin-1 in long-term memory formation requires CREB1 gene transcription in Lymnaea stagnalis. Behav. Genet. 2010;40:680–93. doi: 10.1007/s10519-010-9374-9. [DOI] [PubMed] [Google Scholar]
- 32.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 33.Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–71. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuang R, Zhang L, Fletcher A, Groblewski GE, Pevsner J, Stuenkel EL. Regulation of Munc-18/syntaxin 1A interaction by cyclindependent kinase 5 in nerve endings. J Biol Chem. 1998;273:4957–66. doi: 10.1074/jbc.273.9.4957. [DOI] [PubMed] [Google Scholar]
- 35.Tomizawa K, Sunada S, Lu YF, Oda Y, Kinuta M, Ohshima T, Saito T, Wei FY, Matsushita M, Li ST, Tsutsui K, Hisanaga S, Mikoshiba K, Takei K, Matsui H. Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol. 2003;163:813–24. doi: 10.1083/jcb.200308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci USA. 2004;101:546–51. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC. Regulation of NMDA receptors by cyclin dependent kinase-5. Proc Natl Acad Sci USA. 2001;98:12742–47. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci. 2003;6:1039–47. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- 39.Morabito MA, Sheng M, Tsai LH. Cyclin-Dependent Kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J. Neurosci. 2004;24(4):865–76. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsubara M, Kusubata M, Ishiguro K, Uchida T, Titani K, Taniguchi H. Site-specific phosphorylation of synapsin I by mitogen-activated protein kinase and Cdk5 and its effects on physiological functions. J Biol Chem. 1996;271:21108–13. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- 41.Fletcher AI, Shuang R, Giovannucci DR, Zhang L, Bittner MA, Stuenkel EL. Regulation of exocytosis by cyclin-dependent kinase 5 via phosphorylation of Munc18. J Biol Chem. 1999;274:4027–35. doi: 10.1074/jbc.274.7.4027. [DOI] [PubMed] [Google Scholar]
- 42.Leenders AG, Sheng ZH. Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacol. Ther. 2005;105:69–84. doi: 10.1016/j.pharmthera.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death and Differentiation. 2007;14(1):44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- 44.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–63. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang BX, Akbar M, Kevala K, Kim HY. Phosphatidylserine is a critical modulator for Akt activation. J. Cell Biol. 2011;192(6):979–92. doi: 10.1083/jcb.201005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.