Abstract
Commensal bacteria play an important role in formation of the immune system, but the mechanisms involved are incompletely understood. In this study, we analyze CD1d-restricted invariant NKT (iNKT) cells in germfree mice and in two colonies of C57BL/6 mice termed conventional flora and restricted flora (RF), stably bearing commensal microbial communities of diverse but distinct composition. In germfree mice, iNKT cells were moderately reduced, suggesting that commensal microbiota were partially required for the antigenic drive in maintaining systemic iNKT cells. Surprisingly, even greater depletion of iNKT cell population occurred in RF mice. This was in part attributable to reduced RF levels of intestinal microbial taxa (Sphingomonas spp.) known to express antigenic glycosphingolipid products. However, memory and activated CD8+ T cells were also expanded in RF mice, prompting us to test whether CD8+ T cell activity might be further depleting iNKT cells. Indeed, iNKT cell numbers were restored in RF mice bearing the CD8α−/− genotype or in adult wild-type RF mice acutely depleted with anti-CD8 Ab. Moreover, iNKT cells were restored in RF mice bearing the Prf1−/− phenotype, a key component of cytolytic function. These findings indicate that commensal microbiota, through positive (antigenic drive) and negative (cytolytic depletion by CD8+ T cells) mechanisms, profoundly shape the iNKT cell compartment. Because individuals greatly vary in the composition of their microbial communities, enteric microbiota may play an important epigenetic role in the striking differences in iNKT cell abundance in humans and therefore in their potential contribution to host immune status.
The composition of the enteric microbial community is diverse (1, 2) and represents a unique and possibly stable epigenetic trait distinguishing individuals throughout their life (3, 4). Family studies indicate that microbial composition is an important epigenetic trait whose biologic functionalities may be preserved at the biochemical rather than phylogenetic level (5–7). Accordingly, enteric microbial composition represents a potentially important factor in individual physiology and disease susceptibility. Enteric bacteria influence the abundance of immune constitution of intestinal lymphoid follicles and Peyer's patches, as revealed by antibiotic treatment and genetic manipulations such as mice deficient in activation-induced cytidine deaminase, which disables somatic diversification and attendant immune microbial control (8–12). Local and systemic immune cell populations are numerically and functionally attenuated in germfree (GF) mice (13), and this context has permitted the identification of some microbial products that shape normal immune cell formation (6, 14, 15).
Modeling the diverse microbial composition of the human, our laboratory has developed mice stably bearing distinct microbial communities of complex composition, termed conventional flora (CF) and restricted flora (RF). As characterized by conventional microbiologic and molecular phylotyping, both microbial flora include hundreds of phylotypes spanning the major bacterial and fungal taxa (16, 17). However, the RF and CF microbial communities display broad-based compositional differences at the species to family levels: preferentially Bacteroidetes and Firmicutes phylotypes in CF and RF mice, respectively, features that also distinguish enteric microbial communities among humans with obese and lean dietary habits (18). RF mice are strikingly deficient in innate-like immune cell populations, including marginal zone (MZ) B cells and plasmacytoid dendritic cells (pDCs) (16, 19). Moreover, this phenotype is mediated by a CD8+ T cell population, expanded and activated by RF microbiota, that cytolytically targets these innate immune cell types (16, 19). Although the mechanisms linking RF microbiota and this cytolytic T cell population are uncertain, they may involve targeting of a Qa-1–restricted Ag shared by the depleted innate immune cell types (19).
Invariant NKT (iNKT) cells are an important innate-like lymphocyte population programmed for CD1d-restricted recognition of glycolipid Ags of host or microbial origin (20–23). Systemically distributed and notably localized in extra lymphoid sites, such as the liver (24), they play diverse roles in effector and regulatory responses to microbial infection (25–27), chronic inflammatory diseases (28–30), and tumor immunity (31, 32). In this study, we analyzed the effects of commensal microbiota on the iNKT population. First, we found that GF mice were partially decreased in iNKT cell numbers, suggesting that microbiota is a significant source of antigenic glycosphingolipid required for their full systemic expansion. Second, iNKT cells were depleted in mice bearing RF microbiota, a process mediated by cytolytic CD8+ T cells. Thus, the commensal microbial community systemically shaped iNKT cell populations by two distinct mechanisms, one that stimulated, presumably by Ag-driven expansion of iNKT cells, and another that induced a CD8+ T cell population targeting iNKT cells for cytolytic depletion. The implication is that distinct members of the microbial community differentially shape the iNKT cell population, which opens new strategies to identify microbiota epigenetically modulating specific host immune traits.
Materials and Methods
Mice
C57BL/6 mice bearing CF were obtained from The Jackson Laboratory (Bar Harbor, ME) and kept in specific pathogen-free conditions monitored for a panel of pathogenic microorganisms based on serologic and microbiologic screening by the University of California at Los Angeles (UCLA), Division of Laboratory Animal Medicine. C57BL/6 mice bearing a RF were kept in a separate facility maintained by the UCLA Department of Radiation Oncology. RF mice were periodically tested for RF status by aerobic and anaerobic culture of cecal contents and index molecular phylotypes (16). With respect to specific pathogen-free status, both CF and RF mice were monitored for the absence by serology or culture of a panel of viral, fungal, and bacterial pathogenic taxa, including Helicobacter spp.
CD8α−/− (33) and Prf1−/− mice (34) on C57BL/6 background were rederived with RF microflora by cesarean section delivery on RF foster mothers as described recently (16). All the RF mice were housed in enclosed racks with filtered air, autoclaved bedding, food, and water. GF C57BL/6 and Swiss Webster mice were acquired from the National Institutes of Health (NIH) Gnotobiotic Resource at the College of Veterinary Medicine at North Carolina State University (Raleigh, NC) or purchased from Taconic Farms (Hudson, NY), respectively. Sterility of GF mice was documented on a monthly basis by fecal Gram stain, and aerobic and anaerobic cultures of the feces and bedding. For selected mice, sterility of cecal contents was documented by Gram stain and cultures at the time of necropsy. All the mice examined in the study were age and gender matched. The animal procedures were carried out in accordance with the animal research protocols approved by UCLA and La Jolla Institute of Allergy and Immunology institutional animal research committees.
Reagents
Fluorochome Ab conjugates reactive to mouse CD3ε (145-2C11), CD4 (RM4-5), CD8α (53-6.7), CD11c (HL3), CD44, CD62L (MEL-14), CD69, CD122,NK1.1, DX-5, and Ly-6C were purchased from BD Biosciences (San Diego, CA). Anti-mPDCA-1 was obtained from Miltenyi Biotec (Auburn, CA). The α-galactosylceramide (α-GalCer) loaded CD1d tetramers were produced as described previously (35). Mouse CD1d tetramers loaded with PBS-57, an analog of α-GalCer recently developed by Dr. P. Savage and colleagues (36) were obtained from the NIH Tetramer Core Facility located at the Emory Vaccine Center at Yerkes (Atlanta, GA). Monoclonal anti-CD8α and CD8β Abs used for CD8+ T cell depletion were purchased from BD Biosciences and Biolegend (San Diego, CA), respectively.
Cell isolation and flow cytometric analysis
Single-cell suspensions were prepared from spleen, thymus, and liver of experimental mice. For spleen and thymus, single-cell suspensions from spleen and thymus were made RBC-free by ammonium chloride lysis. For liver lymphocyte isolation, liver tissue from individual mice was homogenized gently in a 100-μm cell strainer in a petri dish containing 10 to ∼20 ml of DMEM with antibiotics and 10% FCS (Life Technologies, Long Island, NY). Cells were recovered by centrifugation at 2000 rpm for 5 min, RBC lysed, resuspended with 7 ml of 40% Percoll, and layered on 5 ml of 80% Percoll in 15 ml of Corning tubes. Liver lymphocytes were then isolated by Percoll gradient centrifugation at 2000 rpm for 30 min, and cells in the lymphocyte layer were collected for flow cytometric analysis.
Identification of iNKT cells was performed in accord with standard criteria (24), including multiparameter staining for CD1d tetramers loaded with α-GalCer (or PBS-57), anti-CD3ε, anti-TCRβ, and in some cases anti-NK1.1 Abs. Except where specified as PBS-57, CD1d tetramers loaded with α-GalCer were used. Data were collected on a FACSCalibur or LSRII flow cytometer (BD Biosciences) and analyzed using CellQuest (BD Biosciences) or FlowJo (Tree Star, Ashland, OR) software.
DNA extraction from mucosal samples
Intestinal epithelium-associated tissue samples were isolated from small and large intestines of age- and gender-matched CF and RF mice as reported previously (16). DNA was extracted by using the CLS-Y buffer and a 30-s bead-beading step with the FastDNA Spin Kit (MP Biomedicals, Solon, OH) and a FastPrep instrument setting of 5.5, as described by the manufacturer. Purified DNA samples were size fractionated by electrophoresis in 1% agarose gels. DNA >3 kb was excised without exposure to UV or ethidium bromide and recovered using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA), following the manufacturer's instructions, except that the gel pieces were not exposed to heat.
Sphingomonas-selective PCR
Partial Sphingomonas small-subunit rRNA genes were amplified in 20-μl PCRs with the following conditions: 50 mM Tris (pH 8.3), 500 μg/ml BSA, 2.5 mM MgCl2, 250 μM of each deoxynucleotide triphosphate, 400 nM of each primer, 1 μl of mucosal DNA, and 1 U of TaqDNA poly-merase. The Sphingomonas-selective primers were as follows: Sphingo 108f (5′-GCGTAACGCGTGGGAATCTG-3′) and Sphingo 420r (5′-TTACAACCCTAAGGCCTTC-3′) (37). All reagents were combined and heated at 94°C for 5 min. Forty cycles of PCR were then performed at 94°C for 20s, 58°C for 20s, and 72°C for 30s, followed by 72°C for 5 min. PCRs were performed in 200-μl thinned walled plastic tubes using a Rap-idCycler (Idaho Technologies, Salt Lake City, UT). The PCR products were resolved by electrophoresis on 2% agarose gels, stained with ethidium bromide and photographed under UV light.
Intragastric Sphingobium yanoikuyae challenge
Sphingobium yanoikuyae bacteria (number 51230; American Type Culture Collection, Manassas, VA) were expanded overnight in Bacto Tryptic Soy Broth (BD Biosciences) at 30°C and used to challenge the mice. C57BL/6 mice at age of 6–8 wk old (The Jackson Laboratory) were intragastrically challenged with 1 × 1010 S. yanoikuyae bacteria in injection saline buffer, and the changes of iNKT cells in bacteria challenged mice were analyzed 24 h later.
CD8+ T cell depletion by anti-CD8 Ab treatment
Three- to 4-wk-old RF C57BL/6 mice were treated with anti-CD8α or CD8β Ab (250 μg/mouse, i.p.) twice a week for 3 wk. Three days after the last Ab treatment, iNKT cells and T lymphocytes were analyzed. To evaluate the time course of CD8+ T cell depletion and recovery of iNKT cells, mice were treated with anti-CD8α Abs as previously described, and at various time points thereafter, mice were euthanized for analysis of iNKT cells phenotypes and the extent of CD8+ T cell depletion.
Adoptive transfer of RF CD8+ T cells
CD8+ T cells from RF mice were transferred into CF mice to test for short-term in vivo depletion of iNKT cells. Briefly, CD8+ T cells were isolated from spleen of 8-wk-old RF mice using a CD8+ T cell isolation kit (Miltenyi Biotec). Isolated RF CD8+ T cells, with >95% purity, were i.v. injected into CF mice at 4 × 107 cells/mouse. Bacterial lysates prepared from cecal intestinal lumenal contents of RF mice (19) were i.p. injected at 50–100 μg/mouse. Control CF mice were injected with isotype control Ab or the same volume of saline, which for sure has no effect on CD8T cell depletion. Mice were sacrificed 3 d after CD8+ T cell transfer, and splenic MZ B cells and other immune phenotypes were examined by flow cytometry. The splenic pDC population was analyzed as a positive control for depletion. Briefly, the low-density cell fraction was prepared by 450 × g centrifugation on 15% Nycodenz (Accurate Chemical, Westbury, NY) and stained with Abs to identify the CD11c+, mPDCA-1+ pDC population (16).
Statistical analysis
Statistical analysis was performed by using Prism software (www.graphpad.com). The percentages and the absolute number of iNKT cells in different groups were analyzed by using an unpaired two-tailed Student t test for two data sets, or one-way ANOVA for three or more data sets, with a 95% confidence interval. Values of p < 0.05 were considered to be significantly different.
Results
RF mice are deficient for iNKT cells
We recently studied the changes in lymphocyte and dendritic cell (DC) populations and function programmed by divergent resident intestinal microbial communities. Compared with CF mice, RF mice had a profound numerical and functional depletion of pDCs and innate-like (MZ and B-1 B cells) (16, 19). These changes were associated with an expanded CD8+ T cell population (38), whose cytolytic activity was causally involved in the depletion of pDCs and innate-like B cells in RF mice (16, 19). Although conventional (CD4+ and CD8+) T cells were only modestly altered in RF mice (38), we wanted to assess the iNKT cell compartment, another innate-like lymphocyte population whose immune functions have been under extensive study. To determine the status of iNKT cells in RF mice, their abundance was examined by flow cytometry, identifying iNKT cells by conventional multiparameter criteria including binding to glycolipid (α-GalCer) or PBS-57–loaded CD1d tetramers, along with anti-TCRβ and anti-NK1.1 Abs.
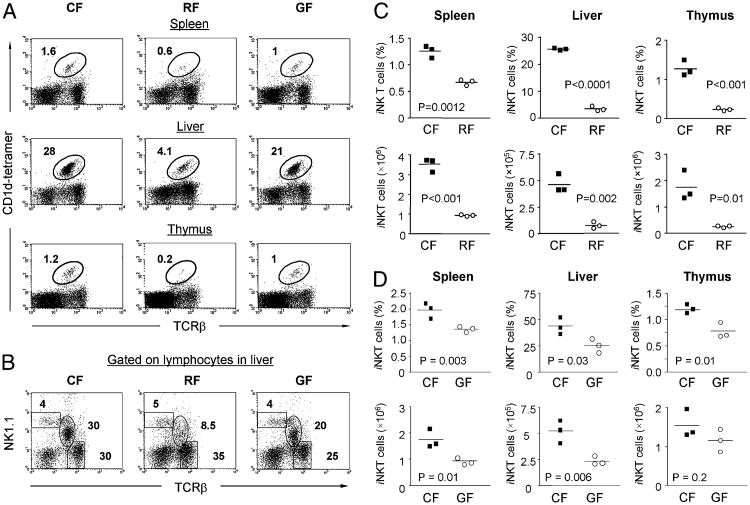
As exemplified in representative individuals, we initially observed that iNKT cells were significantly reduced in RF mice in all evaluated compartments, including spleen, liver, and thymus (Fig. 1). We further tested whether the absence of intestinal bacteria in GF mice might also affect iNKT cells. As shown in Fig. 1, the amount of iNKT cells in GF mice were significantly decreased as compared with age- and gender-matched CF control mice. However, much more profound deficiency of iNKT cells was observed in RF mice (Fig. 1). Analysis of NK1.1+ lymphocytes of the liver in CF, RF, and GF mice confirmed the selective depletion of iNKT cells (NK1.1 intermediate and TCRβ+) versus conventional NK cells (NK1.1 high and TCRβ-neg) in GF and RF mice and the much more profound depletion in RF mice (Fig. 1B). Quantitatively, iNKT cell numbers in GF mice were only moderately reduced, at 50–75% of CF levels for both percentage and absolute numbers in spleen, liver and thymus (Fig. 1D). Similar results were obtained from >24 mice belong to two different GF colonies (Materials and Methods). In contrast, iNKT cells in RF mice were reduced >2-fold in the spleen and >5-fold in the liver and thymus. Thus, RF mice displayed a systemic decrease of iNKT cell number, most strikingly in the liver where they are most prevalent.
Figure 1.
Reduced iNKT cells in RF mice and GF mice compared with CF mice. Lymphocytes isolated from spleen, liver, and thymus of age- and gender-matched CF, RF, or GF C57BL/6 mice were analyzed for the presence of iNKT cells by flow cytometry. A, Relative percentage of iNKT cells in spleen, liver, and thymus were identified by positive staining with α-GalCer–loaded CD1d tetramers and anti-mouse TCRβ. Percentage of iNKT cells in the lymphocyte gate from a representative mouse of each group is indicated. PBS-57–loaded CD1d tetramer yielded similar results (data not shown). B, Analysis of NKT cells defined by NK1.1 and TCRβ expression on total liver lymphocytes from CF, GF, and RF mice. Note the partial depletion (GF) and complete deficiency (RF) of NKT cells (intermediate level of NK1.1 and TCRβ) but the preservation of conventional NK cells (high level of NK1.1). C and D, Tabulation of percentages and absolute numbers of α-GalCer tetramer+ iNKT cells in spleen, liver, and thymus of CF versus RF mice (C) and CF versus GF mice (D). The data of GF mice shown are derived from one experiment representative of the results obtained by examining two GF colonies, GF = Swiss Webster mice (Taconic Farms) and GF = C57BL/6 mice (North Carolina State University) in at least three individual experiments with a total of >24 mice per group.
These findings indicated that commensal intestinal microbiota were not strictly required for iNKT cell formation but that they contributed to the higher systemic levels of iNKT cells achieved in mice bearing CF microflora. However, the modest deficiency of iNKT cells in GF mice was notable for two reasons. First, it indicated that the intestinal microbial products were not absolutely required, some host autonomous processes were sufficient for substantial generation of the iNKT cell subset. Second, it suggested that the profound depletion of iNKT cells in RF mice involved a mi-crobial-induced process distinct from microbial Ag drive.
The role of enteric bacteria in iNKT cells formation
Antigenic drive is an important factor in iNKT cell expansion and the constitutive activation state of these cells. Although an important role for the still undefined cellular Ag(s) is likely, the pertinent glycolipid Ags also may be of microbial origin (20–24). Accordingly, a simple explanation for the deficiency of iNKT cells in RF mice is the absence of microbial species producing relevant microbial glycolipid Ags.
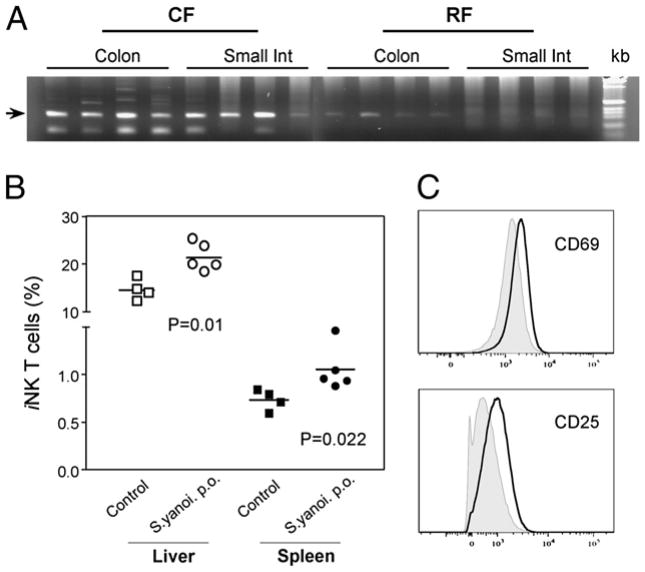
Previously, we observed extensive differences in the composition of intestinal enteric microbiota between CF and RF mice (16, 17). Glycosylceramides from the cell wall of Sphingomonas have been identified as the CD1d ligands stimulating iNKT cells (20, 23). Therefore, we wondered whether the reduced iNKT cell levels in RF mice might be associated with reduced levels of Sphingomonas in the RF versus CF intestinal microbial community. When Sphingomonas was detected in the epithelium-associated samples by specific 16S rRNA PCR, levels of Sphingomonas spp were significantly decreased in the small and large intestines of RF mice as compared with CF mice (Fig. 2A). This result suggests that the absence of stimulating microbial Ags in the intestinal microbiota might partially attribute to the iNKT cell deficiency in RF mice.
Figure 2.
The effects of Sphingomonas spp. in RF intestinal enteric microbiota on iNKT cells. The colonization of Sphingomonas spp. in CF versus RF mice was assayed by PCR for Sphingomonas 16r DNA, and the effects of intestinal inoculation of Sphingomonas on iNKT cells were evaluated in GF mice. A, Sphingomonas-selective PCR of samples from the small and large intestinal mucosal compartments of CF and RF mice. Gel images show the detection of Sphingomonas spp. from four CF versus four RF mice. Arrow indicates amplicons of the anticipated size (303 bp). kb = 1 Kb DNA Ladder (Invitrogen, Carlsbad, CA). Int, intestine. B, Effect of intragastic challenge with S. yanoikuyae on iNKT cells in GF mice. Relative iNKT cell numbers in indicated organs are shown at 24 h after intragastic treatment with PBS (control, squares, n = 4) or S. yanoikuyae (S.yanoi, circles, n = 5). p(liver) = 0.01, p (spleen) = 0.022. C, Expression of CD69 or CD25 on liver iNKT cells from control (tined) or S. yanoikuyae-challenged mice (bold).
We further tested whether the intestinal colonization of certain species of bacteria, bearing known iNKT cell Ags, could affect iNKT cells in the periphery. For this purpose, S. yanoikuyae bacteria (23) were gastrically inoculated in GF mice to observe their effects on iNKT cells. Indeed, intragastric application of S. yanoikuyae to C57BL/6 GF mice led to a significant increase in relative iNKT cell numbers in liver and spleen within a day (Fig. 2B). Furthermore, the activation markers CD69 and CD25 were upregulated on iNKT cells in liver (Fig. 2C) and spleen (data not shown), indicating that enteric bacteria bearing iNKT cell Ags systemically affected iNKT cells.
Expanded CD8+ T cells in RF mice display the memory and cytolytic phenotypes
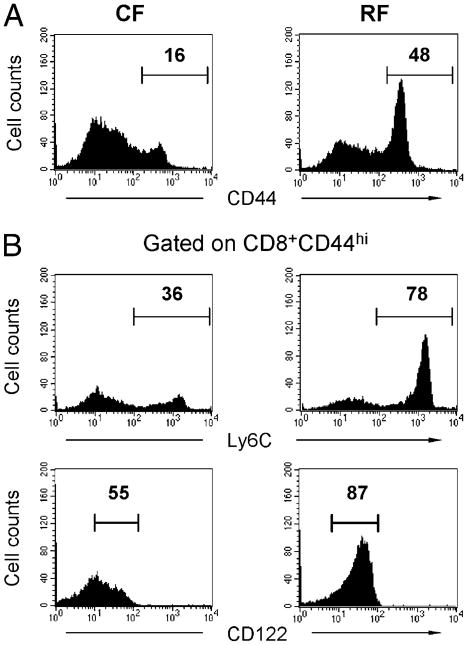
RF mice are distinguished by an expansion of CD8+ T cells (38), which play a direct role in depleting innate or innate-like immune cell populations including pDCs and MZ B cells (16, 19). We therefore asked whether the distinctive reduction of iNKT cells in RF mice are also involved in this expanded CD8+ T cell population. To further define the phenotype of the expanded CD8+ T cell population, we evaluated several markers of T cell activation and memory in CF and RF mice (Fig. 3). In contrast to CF mice, most RF CD8+ T cells expressed a high level of CD44, as reported previously (38) (Fig. 3A). This suggested that the expanded CD8+ T cells in RF mice were part of a memory cell population. Multiparameter analysis of CD8+CD44hi T cells was therefore performed for expression of Ly-6C and CD122, useful in distinguishing effector and central memory T cells (39, 40). As shown in Fig. 3B, CD8+CD44hi T cells in RF mice were increased for the Ly-6C+CD122+ subpopulation. Accordingly, the CD8+ T cell expansion in RF mice primarily represented expansion of a central memory population. Because Ly-6C and CD122 expression were correlated with IFN-γ production and cytolytic activity, these findings were in accord with the increase in these effector functions, such as the increased IFN-γ production by CD8+ T cells in RF mice (16).
Figure 3.
Expanded CD8+ T cells in RF mice display the memory phenotypes. Splenic CD8α+ T cells were analyzed in age- and gender-matched CF and RF mice. A, CD44 expression in CD3ε+CD8α+ lymphocytes. B, CD8α+CD44hi T lymphocytes were gated and analyzed for expression of Ly-6C and CD122. The results are representative of more than three experiments with 24 mice per group.
Effect of CD8+ T cells on the depletion of iNKT cells in RF mice
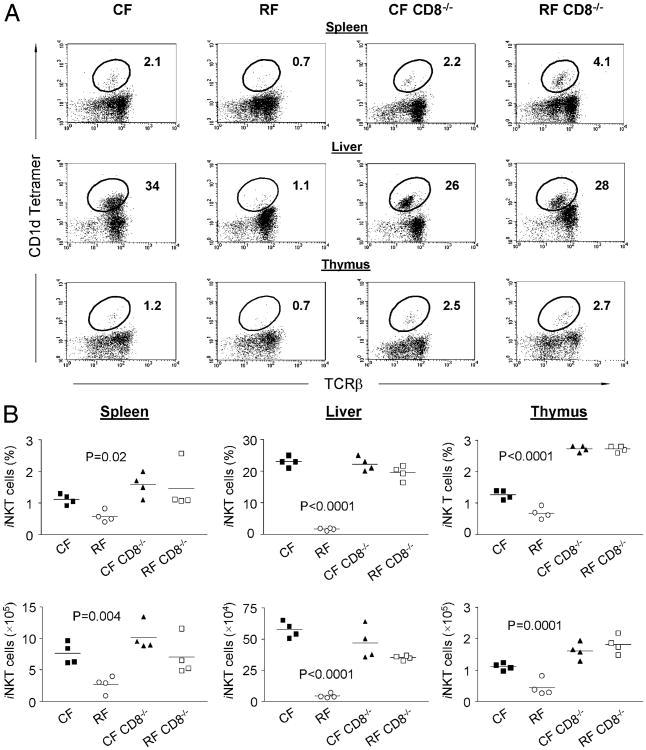
To determine whether CD8+ T cells in RF mice contributed to the decrease in iNKT cell numbers, we rederived CD8α−/− CF mice with RF microbiota and examined whether the genetically determined absence of CD8+ T cells would reverse the RF-associated deficit of iNKT cells. The CD8−/− state indeed caused an increase in iNKT cell numbers in RF mice, including the spleen, liver, and thymus (Fig. 4A). In contrast, under normal CF condition, CD8−/− mice showed normal iNKT cells that were similar with wild-type (WT) CF mice. Comparing groups of individuals, the mean percentages and absolute numbers of iNKT cells in spleen, liver, and thymus of the RF CD8α−/− mice (Fig. 4B) were even higher than those in WT CF mice.
Figure 4.
Normal iNKT cell numbers in CD8α−/− mice. The development of iNKT cells in spleen, liver and thymus of CF and RF CD8α−/− mice was examined and compared with age- and gender-matched CF and RF mice. A, Representative FACS data for the relative percentage of iNKT cells (gated on CD3+ T cells) in indicated mouse strains, showing normal development of iNKT cells with increased number and percentage in CF and RF CD8α−/− mice. B, Absolute number and percentage of the cells positive for CD1d tetramer loaded with α-GalCer in total lymphocytes from WT RF mice and CD8α−/− CF or RF mice were compared by using one-way ANOVA method. Values of p for the comparison of RF versus CF and RF CD8α−/− mice are indicated in the respective graphs. No significant differences were observed between CF CD8α−/− mice and RF CD8α−/− mice (p > 0.05).
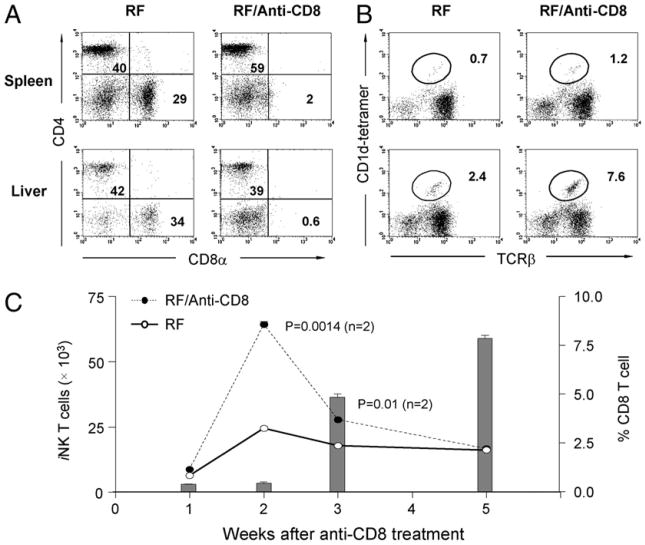
CD8α is expressed on a variety of cell types in addition to conventional CD8αβ T cells, and the absence of these populations during ontogeny may impact iNKT cell differentiation. To assess the effect of CD8+ cells in the adult mouse on iNKT cells in the absence of a developmental defect, RF mice were acutely depleted of these cells by anti-CD8α or anti-CD8β Ab treatment and analyzed 3 d after the last treatment. As expected, CD8+ T cells were profoundly decreased after anti-CD8 treatment (Fig. 5A). When iNKT cells were analyzed, a striking restoration of iNKT cells was observed after 3 wk of continuous CD8+ T cell depletion (Fig. 5B). It should be noted that after anti-CD8 treatment, the residual iNKT T cell population may have been altered with respect the levels of its CD4 and CD8 single- and double-positive components. To determine the timing of iNKT cell recovery in relation to the CD8+ T cell depletion, a time course was performed in mice receiving anti-CD8 Ab treatment (Fig. 5C). This revealed that iNKT cell levels began to recover 2 wk after CD8+ T cell depletion. However, thereafter iNKT cells again declined. The reduction was not surprising, because CD8+ T cell numbers begin to recover after 2 wk because of clearance of the Ab to sub-therapeutic levels (bar graph in Fig. 5C). Taken together, these observations indicated that CD8+ T cells actively mediate depletion of iNKT cells in adult RF mice.
Figure 5.
Depletion of CD8+ T cells in RF mice increases iNKT cell numbers. A and B, RF mice were treated with anti-CD8α (or saline, the vehicle control) for 3 wk and analyzed by FACS at 3 d after the last treatment for levels of CD4+ and CD8+ T cells (A) or iNKT cells (B). C, Dynamic change of absolute number of iNKT cells (curves) and percentage of CD8+ T cells (bar graphs) in liver of anti-CD8α Ab-treated RF mice and RF control at indicated time points. Similar results were obtained using an anti-CD8β Ab (data not shown). Absolute numbers of iNKT cells in RF and control RF mice were compared at week 2 and week 3 after anti-CD8α treatment; p values were analyzed by Student t test. Data are representative of three individual experiments and >24 mice per group.
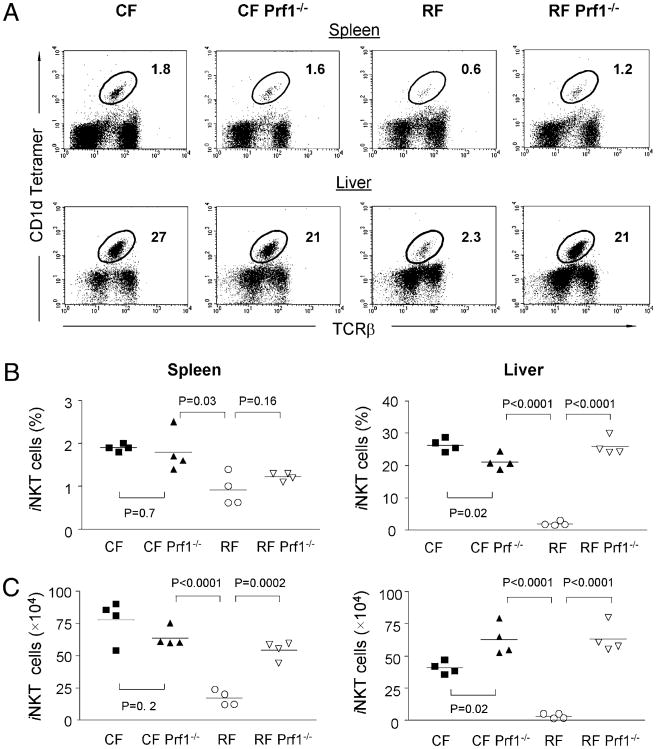
Perforin is a membrane-disruptive protein required for cytolysis by CD8+ T cells and NK cells. To determine whether CD8+T cells in RF mice suppress iNKT cells through CTL-mediated cytotoxicity, we established a colony of RF Prf1−/− mice by cesarean re-derivation. As shown in Fig. 6A, the Prf1−/− genotype restored iNKT cells in RF mice to the normal amount in CF mice. Compared with age- and gender-matched Prf1+/+ RF mice, CF Prf1−/− and RF Prf1−/− mice displayed normal or even increased iNKT cells (Fig. 6B), considered both as percentage and absolute numbers. These results demonstrated that perforin-mediated cytotoxicity plays a role in the depletion of iNKT cells in RF mice. However, we note that these data leave open the possibility that NK cells may also be involved in iNKT cell deficiency in RF mice.
Figure 6.
Perforin (Prf1)−/− mice bearing RF microflora have normal iNKT cell numbers. Re-derived Prf1−/− mice bearing RF microflora were examined for the presence of iNKT cells. A, The percentage of PBS-57–loaded CD1d tetramer+ iNKT cells within lymphocytes from spleen and liver of CF, CF Prf1−/−, RF, and RF Prf1−/− mice are indicated. B, Percentages and absolute number (C) of iNKT cells in CF, CF Prf1−/−, RF, and RF Prf1−/− mice in lymphocytes of indicated organs. Statistical analysis of p values using Student t test comparedWTCFversusCFPrf−/−mice,CFPrf−/− versus RF WT, and RF WT versus RF Prf−/− mice were indicated. The data are representative of at least three individual experiments with >12 mice in each group.
RF CD8+ T cells do not directly deplete iNKT cells in CF mice
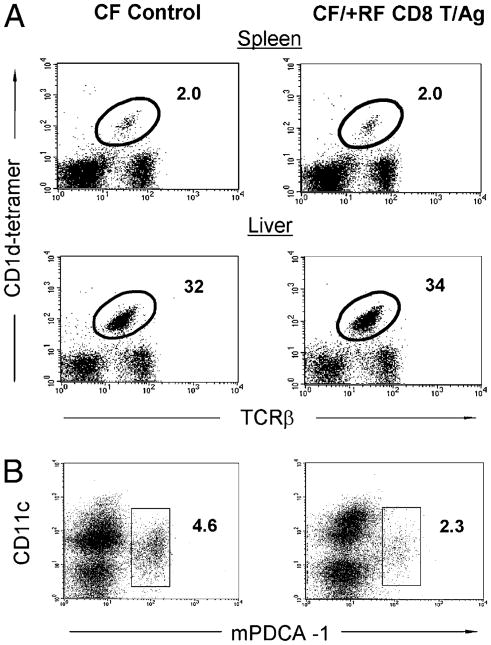
Transfer of RF CD8+ T cells into CF mice acutely depleted pDCs and MZ B cells, providing evidence of in vivo cytolysis by RF CD8+ T cells of these target cell types (16, 19). Using these same conditions, we therefore tested whether RF CD8+ T cell transfer also depleted iNKT cells. Accordingly, RF CD8+ T cells were transferred i.v. into CF mice and, at the same time, stimulated with RF enteric microbial Ags i.p., which is required for optimal CD8+ T cell activity in the CF recipient mice (16, 19). However, RF CD8+ T recipients showed no change in splenic or liver iNKT cell abundance (Fig. 7A). In contrast, as a positive control, splenic pDCs were successfully depleted under these conditions (Fig. 7B). Thus, the predominant population of peripheral iNKT cells is not acutely susceptible to in vivo cytolysis by RF CD8+ T cells.
Figure 7.
Effect of transferred RF CD8+ T cells on iNKT cell numbers in CF mice. Five- to 6-wk-old CF mice were i.v. transferred with 4 × 107 RF CD8+ T cells and i.p. injected with microbial Ags derived from lumen contents of RF mice (or saline alone as the control) and then sacrificed at day 3 after RF CD8+ T cell transfer. A, Relative percentage of iNKT cells in spleen and liver. B, Relative percentages of pDCs (CD11c+, mPDC-1+) in splenocytes. Representative data from three or more experiments with ≥15 mice in each group.
Discussion
The present study uncovered two distinct effects of the intestinal microbial community on iNKT cells. The iNKT cell population was moderately reduced in GF mice, suggesting that the commensal microbiota positively modulates the iNKT cell population. Conversely, in mice bearing RF microbiota, iNKT cells were greatly reduced, a process dependent on active depletion by CD8+ T cells and cytolytic function mediated by perforin. This discussion addresses the potential mechanisms underlying these different modes of interaction between the iNKT cell population and the host commensal microbial community.
The expansion of the iNKT cell population is highly dependent on interaction with cognate glycolipid/CD1d complexes in the thymus (24, 41). Some CD1d ligands are presumed to be of self-origin as exemplified by the discovery of an endogenous lysosomal glycosphingolipid, iGb3, as a potential CD1d ligand (20, 21). Although the characterization of the full scope of endogenous ligands remains an active research area, peripheral encounter with self- or foreign Ags presented by CD1d in principle could modulate or fine-tune the iNKT cell population, in terms of number, activation state, or expression of maturation markers such as NK1.1 (24, 31, 42–45).
Although an earlier study did not find any difference in iNKT cells in GF as compared with conventional mice (46), in this study, we report a significant decrease in the frequency of iNKT cells. The divergent results can be attributed to differences in the GF colonies or to α-GalCer–loaded CD1d tetramers [used in the current study, but not the study of Park et al. (46)], which provides the most reliable numeration of iNKT cells. Given our results, one may speculate as to the source and identity of the pertinent natural glycosphingolipid driving iNKT cell homeostasis. It is noteworthy that the greatest effect on iNKT cells was observed in the liver, which is directly supplied with enteric microbial Ag via portal circulation from the intestine (47–49). A variety of microbial taxa have proven to be sources of antigenic glycosphingolipid, which if present at significant levels in the commensal microbial community are plausible sources of endogenous glycolipid Ags (20, 22, 23). Previous studies have indicated that a microbial taxon, known for carrying antigenic glycosphingolipid product, is detectable in the murine and human fecal microbiota (50). In this study, we have further confirmed that the colonization of Sphingomonas species is significantly decreased in the small and large intestines of RF mice as assayed by phylotype-specific 16S rRNA PCR (Fig. 2A). The decreased colonization of Sphingomonas spp. in RF mice may at least partially contribute to the deficiency of iNKT cells, because Sphingomonas spp stimulated the activation and proliferation of iNKT cells in GF mice (Fig. 2B, 2C).
It is notable that substantial levels of iNKT cells were preserved in GF mice. This suggests that other sources may provide an important, alternate source of antigenic glycolipid. One possibility is that dietary Ags derived from food or its microbial contaminants affect the homeostasis of iNKT cells. In addition, mice bearing null mutations of glycolipid biosynthesis (51) and processing, including microsomal triglyceride transfer protein (52) and adaptor protein AP-3 (53), are deficient in iNKT cells. These observations suggest that host production of glycosphingolipid may represent an endogenous mammalian source of antigenic glycolipids. However, these mutations also may affect the processing and intracellular trafficking of exogenous glycolipids (54, 55) including those of commensal microbial origin (56). Thus, the current study can most simply be interpreted as evidence that commensal microbiota are at least partially required for iNKT cell homeostasis.
A second phenotype related to the commensal microbiota was the much more extensive depletion of iNKT cells observed in mice bearing the RF microbiota enriched for Firmicutes spp. Several lines of evidence indicated that this depletion was mediated by CD8+ T cells. First, RF mice bear an expanded memory CD8+ T cell population, and RF mice genetically lacking CD8α displayed normal and even increased levels of iNKT cells. This finding potentially implicated a variety of CD8α+ cell types (e.g., conventional CD8α β T cells and CD8αα T cells or lymphoid DCs) (57). However, treatment of RF mice with anti-CD8α or CD8β Ab substantially restored iNKT cell levels. This rapid replenishment suggested that iNKT cell numbers were suppressed by an active process mediated by CD8+ cells, with replenishment presumably by expansion from new thymic emigrants or residual mature iNKT cells. A defect in the iNKT cell precursor, by contrast, might be less reversible or at least might require a longer time for the restoration of the population. The action of CD8+ cells in this setting could have been indirect, for example, through competition for IL-15 or production of inhibitory products affecting T cell activation and expansion (58, 59). However, RF mice bearing a null mutation for perforin, a critical component of cell-mediated cytolysis, were also restored for iNKT cells. Taken together, these findings strongly suggest the iNKT cell deficiency in RF mice was mediated by the cytolytic action of CD8+ T cells.
Certain other immune cell types, including pDCs and MZ B cells, are also selectively depleted by the cytotoxic action of CD8+ T cells in RF mice (16, 19). In contrast, many other cell types, including conventional myeloid DCs, follicular B cells, and memory CD4+ T cells, are preserved in RF mice. The reason why only a few cell types, including iNKT cells, pDCs, and MZ B cells, are coordinately depleted is uncertain. One possibility is that these cell types may share a common target Ag recognized by the relevant CD8+ T cell population. In the case of MZ B cells, there is direct evidence that they are targeted via the MHC class 1b molecule Qa-1 (19). Moreover, the molecular stress response associated with activation via the NF-κB pathway, or the metabolic stress of cellular proliferation (60, 61), includes up-regulation of Qa-1 and a shift in Qa-1 peptide loading to Hsp60-derived pep-tides (62) targeted by Qa-1-restricted CD8+ T cells (63–65). The role of such cytolytic T cells in targeting iNKT cells provides a simple explanation for the systemic depletion of iNKT cells, including those residents in sites remote from the intestine such as in the thymus.
In this regard, it was notable that during the short-term action of in vivo-transferred RF CD8+ T cells, a significant depletion was detectable for pDCs and MZ B cells (16, 19) but not of iNKT cells (this study). Although the reason for this difference is uncertain, it suggests two explanations. First, peripheral pDCs and MZ B cells bear higher amounts of the putative shared target Ag (such as Qa-1/Hsp60 peptide), permitting their more efficient targeting. Alternatively, the target Ag may be predominantly expressed by precursor rather mature iNKT cells. Because the mature iNKT cell population turns over slowly, such precursor cell depletion would not be manifested in the mature iNKT cell population over the short term. These ideas predict that quantitative levels of Qa-1 and Hsp60 expression, or Qa-1/Hsp60 peptide loading, are physiologically elevated in these target populations, which may be experimentally testable predictions.
The mechanism linking commensal RF microbiota with induction of this cytolytic CD8+ T cell population is unknown. Speculatively, we note that both murine Qa-1/Hsp60 and human HLA-E/Hsp60 CD8+ T cell responses are elicited by certain microbiota, including Listeria and Salmonella (63, 66). This cross-reactivity has been linked to microbial GroEL (192–200) and mammalian Hsp60 (216–224), which encode a Qa-1/HLA-E binding nonapeptide with identical residues for TCR contact (−FD–Y-). Thus, we speculate that RF microbiota may be distinguished from CF microbiota either by microbial community members responsible for greater bioavailability of such Hsp60 mimics or by traits that elicit or provide adjuvant activity for the local enteric mucosal stress response, leading to immunogenicity and expansion of an Hsp60/Qa-1 CD8+ T cell population.
The mechanism(s) by which RF microbiota induce the striking RF immunologic phenotype is a complex issue. The data from GF mice indicate that enteric organisms only contribute incrementally to iNKT levels, because the population is largely preserved in GF mice. Conversely, broad-spectrum antibiotic treatment on RF mice produced only modest and inconsistent reversal of the immune phenotypes in RF mice (B. Wei and J. Borneman, unpublished observations). The interpretation of this finding is uncertain, because restoration of depleted immune cell types in RF mice was reliably converted (to CF composition) only when the microbiota were manipulated in the immediate postnatal period (19). Finally, and conversely, RF CD8+ T cells modify the composition of the enteric microbial community (67). Taken together, the interplay of microbiotal composition for such immunologic traits are complex, and its delineation will require fastidious experimental design with respect to host ontogeny, and both selective manipulation and careful longitudinal analysis of microbial and immune effector composition.
The composition of commensal microbial communities is a distinguishing human trait, which is profoundly affected by diet and other lifestyle factors. Because the modern urban lifestyle is associated with striking increases in certain chronic inflammatory and autoimmune diseases, the mechanisms linking lifestyle, commensal microbiota, and such disease susceptibility is of considerable interest. The present study provides evidence for two mechanisms linking the commensal microbiota with the mature iNKT population. Our findings may provide an explanation for the striking difference in iNKT cells number found in the peripheral blood of different individuals (68). Furthermore, considering the pleiotropic roles of iNKT cells in host defense and immunoregulation, these findings suggest potentially relevant processes contributing to the link between the commensal microbiota and the associated profile of disease susceptibilities.
Acknowledgments
We thank the Center for Gastrointestinal Biology and Disease at North Carolina State University for providing the GF C57BL/6 mice, the Jonsson Comprehensive Cancer Center Flow Cytometry Center for assistance in flow cytometry, and the NIH Tetramer Facility in providing CD1d tetramers loaded with PBS-57 for this work.
This work is supported by National Institutes of Health Grants DK69434 (to J.B.), DK46763 (to J.B. and M.K.), AI52031 (to S.B.), GM07185 (to M.M.), AI69296 (to M.K.), CA016042 (Jonsson Comprehensive Cancer Center Flow Cytometry Core), DK349870 (Center for Gastrointestinal Biology and Disease at North Carolina State University germfree animal facility), and an Outgoing International Fellowship by the Marie Curie Actions (to G.W.). B.W. was a fellow of the Crohn's and Colitis Foundation of America.
Abbreviations used in this paper
- α-GalCer
α-galactosylceramide
- CF
conventional flora
- DC
dendritic cell
- GF
germfree
- iNKT
invariant NKT lymphocyte
- Int
intestine
- MZ
marginal zone
- NIH
National Institutes of Health
- pDC
plasmacytoid dendritic cell
- RF
restricted flora
- UCLA
University of California at Los Angeles
- WT
wild-type
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Conway PL. Microbial ecology of the human large intestine. In: Gibson GR, editor. Human Colonic Bacteria: Role in Nutrition, Physiology, And Pathology. CRC Press; Boca Raton, FL: 1995. pp. 1–24. [Google Scholar]
- 3.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 4.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 9.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 10.Shikina T, Hiroi T, Iwatani K, Jang MH, Fukuyama S, Tamura M, Kubo T, Ishikawa H, Kiyono H. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J Immunol. 2004;172:6259–6264. doi: 10.4049/jimmunol.172.10.6259. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin β receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Meek B, Doi Y, Honjo T, Fagarasan S. Two distinctive pathways for recruitment of naive and primed IgM+ B cells to the gut lamina propria. Proc Natl Acad Sci USA. 2005;102:2482–2486. doi: 10.1073/pnas.0409539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bealmear PM, Mirand EA, Holtermann OA. Miscellaneous immune defects in gnotobiotic and SPF mice. Prog Clin Biol Res. 1983;132C:423–432. [PubMed] [Google Scholar]
- 14.Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur J Nutr. 2002;41(Suppl. 1):I32–I37. doi: 10.1007/s00394-002-1105-4. [DOI] [PubMed] [Google Scholar]
- 15.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara D, Wei B, Presley LL, Brewer S, McPherson M, Lewinski MA, Borneman J, Braun J. Systemic control of plasmacytoid dendritic cells by CD8+ T cells and commensal microbiota. J Immunol. 2008;180:5843–5852. doi: 10.4049/jimmunol.180.9.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scupham AJ, Presley LL, Wei B, Bent E, Griffith N, McPherson M, Zhu F, Oluwadara O, Rao N, Braun J, Borneman J. Abundant and diverse fungal microbiota in the murine intestine. Appl Environ Microbiol. 2006;72:793–801. doi: 10.1128/AEM.72.1.793-801.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 19.Wei B, Su TT, Dalwadi H, Stephan RP, Fujiwara D, Huang TT, Brewer S, Chen L, Arditi M, Borneman J, et al. Resident enteric mi-crobiota and CD8+ T cells shape the abundance of marginal zone B cells. Eur J Immunol. 2008;38:3411–3425. doi: 10.1002/eji.200838432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 22.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 23.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 24.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 25.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tupin E, Benhnia MR, Kinjo Y, Patsey R, Lena CJ, Haller MC, Caimano MJ, Imamura M, Wong CH, Crotty S, et al. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc Natl Acad Sci USA. 2008;105:19863–19868. doi: 10.1073/pnas.0810519105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotter H, González-Roldán N, Lindner B, Winau F, Isibasi A, Moreno-Lafont M, Ulmer AJ, Holst O, Tannich E, Jacobs T. Natural killer T cells activated by a lipopeptidophosphoglycan from Entamoeba histolytica are critically important to control amebic liver abscess. PLoS Pathog. 2009;5:e1000434. doi: 10.1371/journal.ppat.1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grajewski RS, Hansen AM, Agarwal RK, Kronenberg M, Sidobre S, Su SB, Silver PB, Tsuji M, Franck RW, Lawton AP, et al. Activation of invariant NKT cells ameliorates experimental ocular autoimmunity by a mechanism involving innate IFN-γ production and dampening of the adaptive Th1 and Th17 responses. J Immunol. 2008;181:4791–4797. doi: 10.4049/jimmunol.181.7.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mars LT, Gautron AS, Novak J, Beaudoin L, Diana J, Liblau RS, Lehuen A. Invariant NKT cells regulate experimental autoimmune en-cephalomyelitis and infiltrate the central nervous system in a CD1d-independent manner. J Immunol. 2008;181:2321–2329. doi: 10.4049/jimmunol.181.4.2321. [DOI] [PubMed] [Google Scholar]
- 30.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreac-tivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 32.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung-Leung WP, Schilham MW, Rahemtulla A, Kündig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 34.van den Broek ME, Kägi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, III, Ravkov EV, Ibegbu CC, Altman JD, et al. A modified α-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Leys NMEJ, Ryngaert A, Bastiaens L, Verstraete W, Top EM, Springael D. Occurrence and phylogenetic diversity of Sphingomonas strains in soils contaminated with polycyclic aromatic hydrocarbons. Appl Environ Microbiol. 2004;70:1944–1955. doi: 10.1128/AEM.70.4.1944-1955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang T, Wei B, Velazquez P, Borneman J, Braun J. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes. Clin Immunol. 2005;117:221–230. doi: 10.1016/j.clim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Tuma RA, Pamer EG. Homeostasis of naive, effector and memory CD8 T cells. Curr Opin Immunol. 2002;14:348–353. doi: 10.1016/s0952-7915(02)00338-2. [DOI] [PubMed] [Google Scholar]
- 40.Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C−) to TCR/CD8 signaling in response to antigen. J Immunol. 1998:160, 3236–3243. [PubMed] [Google Scholar]
- 41.Chiu YH, Park SH, Benlagha K, Forestier C, Jayawardena-Wolf J, Savage PB, Teyton L, Bendelac A. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nat Immunol. 2002;3:55–60. doi: 10.1038/ni740. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald HR. NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 44.McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, Godfrey DI. The influence of CD1d in postselection NKT cell maturation and homeostasis. J Immunol. 2005;175:3762–3768. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 45.Vallabhapurapu S, Powolny-Budnicka I, Riemann M, Schmid RM, Paxian S, Pfeffer K, Körner H, Weih F. Rel/NF-κB family member RelA regulates NK1.1− to NK1.1+ transition as well as IL-15-induced expansion of NKT cells. Eur J Immunol. 2008;38:3508–3519. doi: 10.1002/eji.200737830. [DOI] [PubMed] [Google Scholar]
- 46.Park SH, Benlagha K, Lee D, Balish E, Bendelac A. Unaltered phenotype, tissue distribution and function of Vα14+ NKT cells in germ-free mice. Eur J Immunol. 2000;30:620–625. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 47.Lichtman SN, Keku J, Schwab JH, Sartor RB. Evidence for peptidoglycan absorption in rats with experimental small bowel bacterial overgrowth. Infect Immun. 1991;59:555–562. doi: 10.1128/iai.59.2.555-562.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts EC, Hobson CH, Anderson RP, Chadwick VS. Radio-immunoassay for formyl methionyl leucyl phenylalanine. II. Demonstration of an enterohepatic circulation of immunoreactive bacterial chemotactic peptides in man. J Gastroenterol Hepatol. 1990;5:38–43. doi: 10.1111/j.1440-1746.1990.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 49.Zeuzem S. Gut-liver axis. Int J Colorectal Dis. 2000;15:59–82. doi: 10.1007/s003840050236. [DOI] [PubMed] [Google Scholar]
- 50.Kinjo Y, Pei B, Bufali S, Raju R, Richardson SK, Imamura M, Fujio M, Wu D, Khurana A, Kawahara K, et al. Natural Sphingomonas glyco-lipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15:654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou D, Cantu C, 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, Kaser A, Glickman J, Kuo T, Little A, et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- 53.Elewaut D, Lawton AP, Nagarajan NA, Maverakis E, Khurana A, Honing S, Benedict CA, Sercarz E, Bakke O, Kronenberg M, Prigozy TI. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Vα14i NKT cells. J Exp Med. 2003;198:1133–1146. doi: 10.1084/jem.20030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, Salio M, Denkberg G, Reddington F, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J Exp Med. 2004;199:567–579. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci USA. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8αα. Immunity. 2008;28:149–159. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Niederkorn JY. Emerging concepts in CD8+ T regulatory cells. Curr Opin Immunol. 2008;20:327–331. doi: 10.1016/j.coi.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29:337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, Zheng M, Mani M, Henderson J, Pinkus GS, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11:349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies A, Kalb S, Liang B, Aldrich CJ, Lemonnier FA, Jiang H, Cotter R, Soloski MJ. A peptide from heat shock protein 60 is the dominant peptide bound to Qa-1 in the absence of the MHC class Ia leader sequence peptide Qdm. J Immunol. 2003;170:5027–5033. doi: 10.4049/jimmunol.170.10.5027. [DOI] [PubMed] [Google Scholar]
- 63.Lo WF, Woods AS, DeCloux A, Cotter RJ, Metcalf ES, Soloski MJ. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat Med. 2000;6:215–218. doi: 10.1038/72329. [DOI] [PubMed] [Google Scholar]
- 64.Chen W, Zhang L, Liang B, Saenger Y, Li J, Chess L, Jiang H. Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci USA. 2007;104:20472–20477. doi: 10.1073/pnas.0709878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci USA. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salerno-Gonçalves R, Fernandez-Viña M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173:5852–5862. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 67.Presley LL, Wei B, Braun J, Borneman J. Bacteria associated with immunoregulatory cells in mice. Appl Environ Microbiol. 2009 doi: 10.1128/AEM.01561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, Mikayama T, Van De Water J, Coppel RL, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]