Abstract
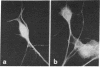
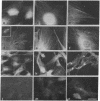
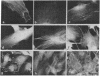
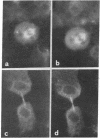
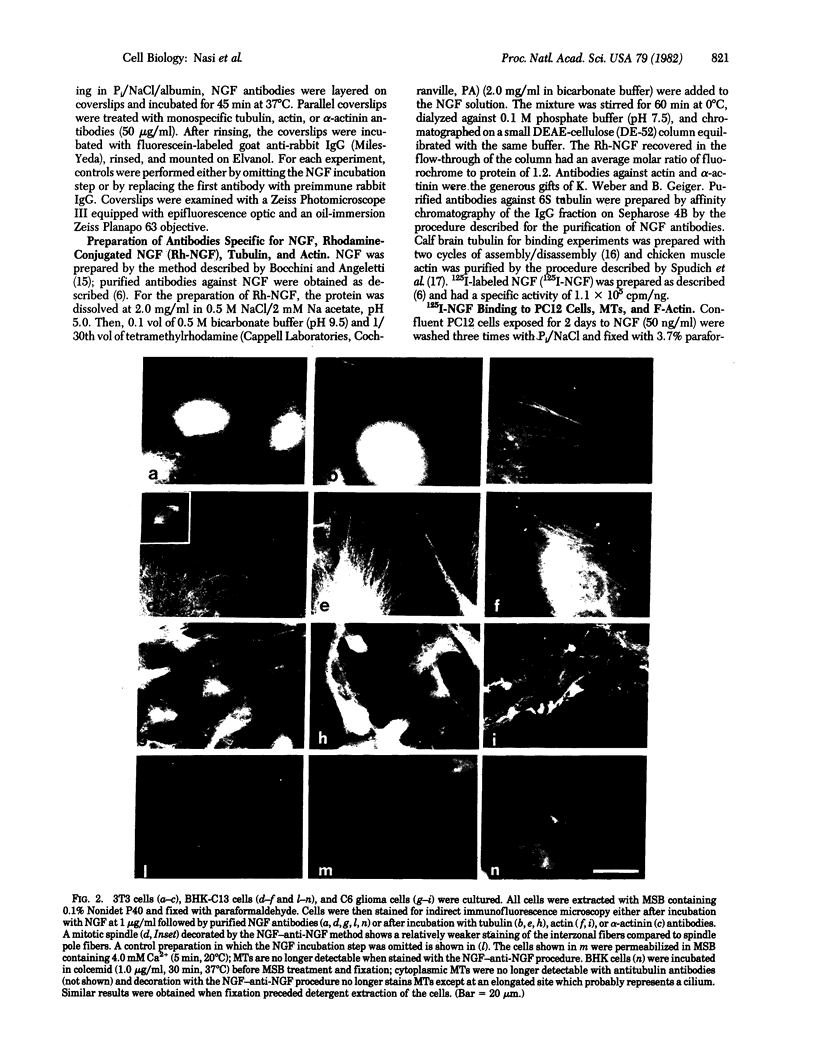
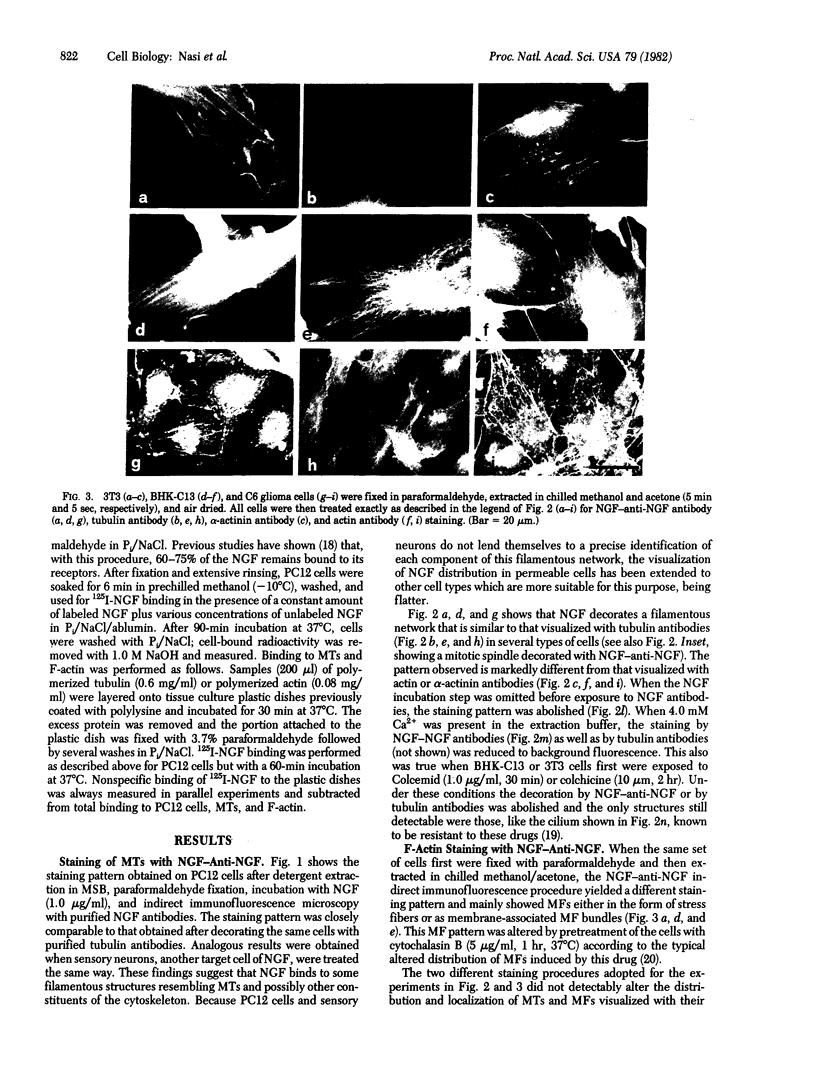
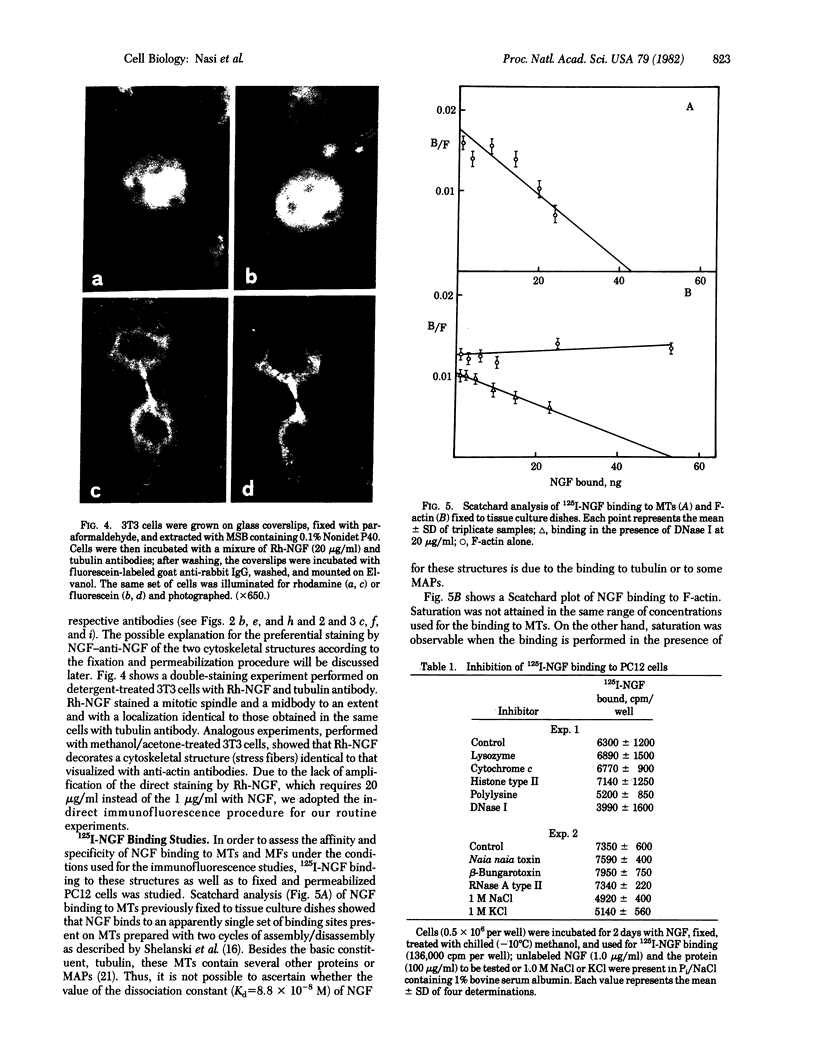
A specific antibody against nerve growth factor (NGF) and indirect immunofluorescence microscopy have been used to follow the in vitro binding of NGF to cells made permeable to large molecules. All cells tested, both target (sensory neurons and PCI2 cells) and nontarget (3T3, BKH 2I, C6 glioma cells), revealed a decoration of cytoskeletal structures which on the basis of their form, reactivity with antibodies, and sensitivity to specific drugs may be identified as microtubules (MTs) and microfilaments (MFs). The decoration of either structure depends on the fixation and permeabilization conditions: MFs, in the form of stress fibers, are stained by NGF when the plasma membrane is permeabilized with methanol/acetone; MTs become intensely stained when the plasma membrane is solubilized with a nonionic detergent in the presence of a MT-stabilizing medium. The two procedures do not affect the staining of these structures with specific antibodies. Binding of 125I-labeled NGF to PCI2 cells was not competitively inhibited by a 100-fold excess of several positively charged proteins but it was markedly decreased in the presence of DNase I. 125I-Labeled NGF interacted with MTs and F-actin (fixed with paraformaldehyde) in a range of concentrations similar to that used for their cellular localization with NGF-anti-NGF. Our studies show that the specificity and affinity of NGF binding to MTs and MFs is in the range of that of antibodies against tubulin and actin. The possible relevance of these findings to the mechanism of action of NGF in target cells is discussed.
Full text
PDF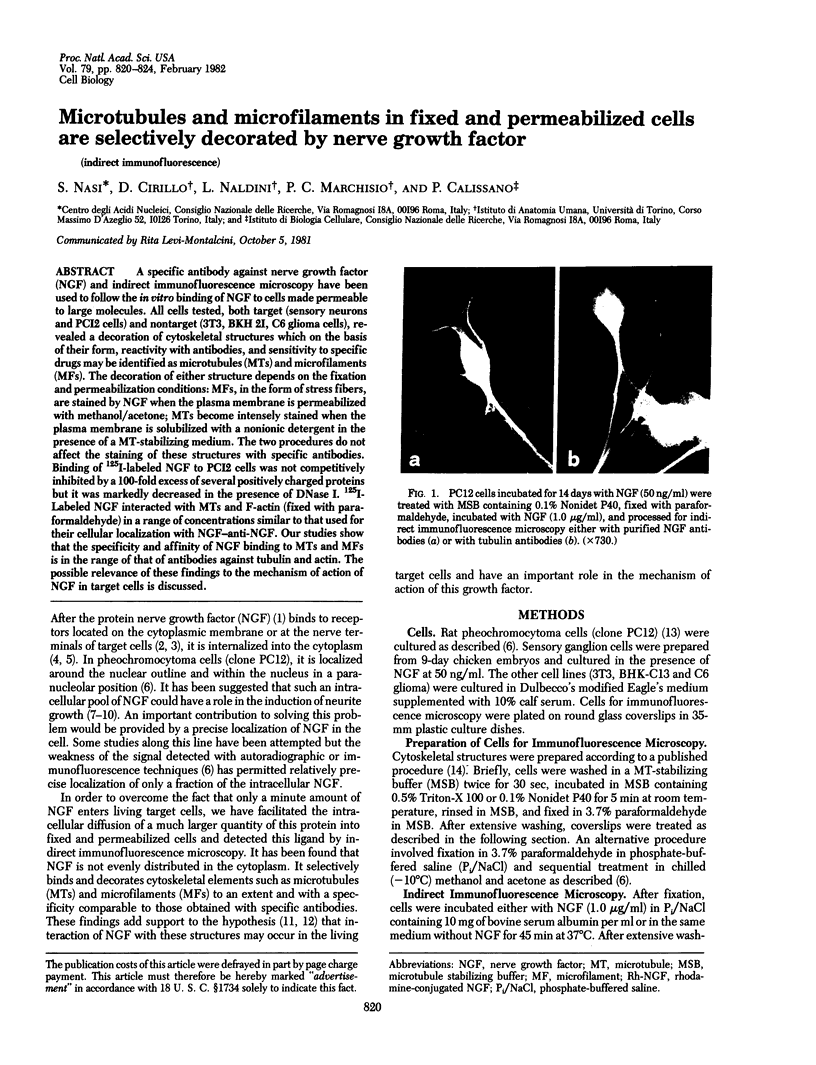

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biocca S., Levi A., Calissano P. Cell density modulates receptor-mediated internalization of nerve growth factor in pheochromocytoma cells. J Recept Res. 1980;1(3):373–387. doi: 10.3109/10799898009038788. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Calissano P., Cozzari C. Interaction of nerve growth factor with the mouse-brain neurotubule protein(s). Proc Natl Acad Sci U S A. 1974 May;71(5):2131–2135. doi: 10.1073/pnas.71.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calissano P., Monaco G., Castellani L., Mercanti D., Levi A. Nerve growth factor potentiates actomyosin adenosinetriphosphatase. Proc Natl Acad Sci U S A. 1978 May;75(5):2210–2214. doi: 10.1073/pnas.75.5.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calissano P., Shelanski M. L. Interaction of nerve growth factor with pheochromocytoma cells. Evidence for tight binding and sequestration. Neuroscience. 1980;5(6):1033–1039. doi: 10.1016/0306-4522(80)90184-0. [DOI] [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry I. A., Stöckel K., Thoenen H., Iversen L. L. The retrograde axonal transport of nerve growth factor. Brain Res. 1974 Mar 15;68(1):103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Hendry I. A. The effect of the retrograde axonal transport of nerve growth factor on the morphology of adrenergic neurones. Brain Res. 1977 Oct 7;134(2):213–223. doi: 10.1016/0006-8993(77)91068-x. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: its mode of action on sensory and sympathetic nerve cells. Harvey Lect. 1966;60:217–259. [PubMed] [Google Scholar]
- Levi A., Cimino M., Mercanti D., Chen J. S., Calissano P. Interaction of nerve growth factor with tubulin. Studies on binding and induced polymerization. Biochim Biophys Acta. 1975 Jul 14;399(1):50–60. doi: 10.1016/0304-4165(75)90210-x. [DOI] [PubMed] [Google Scholar]
- Levi A., Shechter Y., Neufeld E. J., Schlessinger J. Mobility, clustering, and transport of nerve growth factor in embryonal sensory cells and in a sympathetic neuronal cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3469–3473. doi: 10.1073/pnas.77.6.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Naldini L., Calissano P. Intracellular distribution of nerve growth factor in rat pheochromocytoma PC12 cells: evidence for a perinuclear and intranuclear location. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1656–1660. doi: 10.1073/pnas.77.3.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKanna J. A., Haigler H. T., Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco G., Calissano P., Mercanti D. Effect of NGF on in vitro preformed microtubules. Evidence for a protective action against vinblastine. Brain Res. 1977 Jul 1;129(2):265–274. doi: 10.1016/0006-8993(77)90006-3. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. The display of microtubules in transformed cells. Cell. 1977 Nov;12(3):561–571. doi: 10.1016/0092-8674(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Schwab M., Thoenen H. Selective trans-synaptic migration of tetanus toxin after retrograde axonal transport in peripheral sympathetic nerves: a comparison with nerve growth factor. Brain Res. 1977 Feb 25;122(3):459–474. doi: 10.1016/0006-8993(77)90457-7. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Binding of deoxyribonuclease I to actin: a new way to visualize microfilament bundles in nonmuscle cells. J Histochem Cytochem. 1978 Sep;26(9):745–749. doi: 10.1177/26.9.361884. [DOI] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Shooter E. M. Nerve growth factor in the nucleus: interaction with receptors on the nuclear membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1269–1273. doi: 10.1073/pnas.76.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]