Abstract
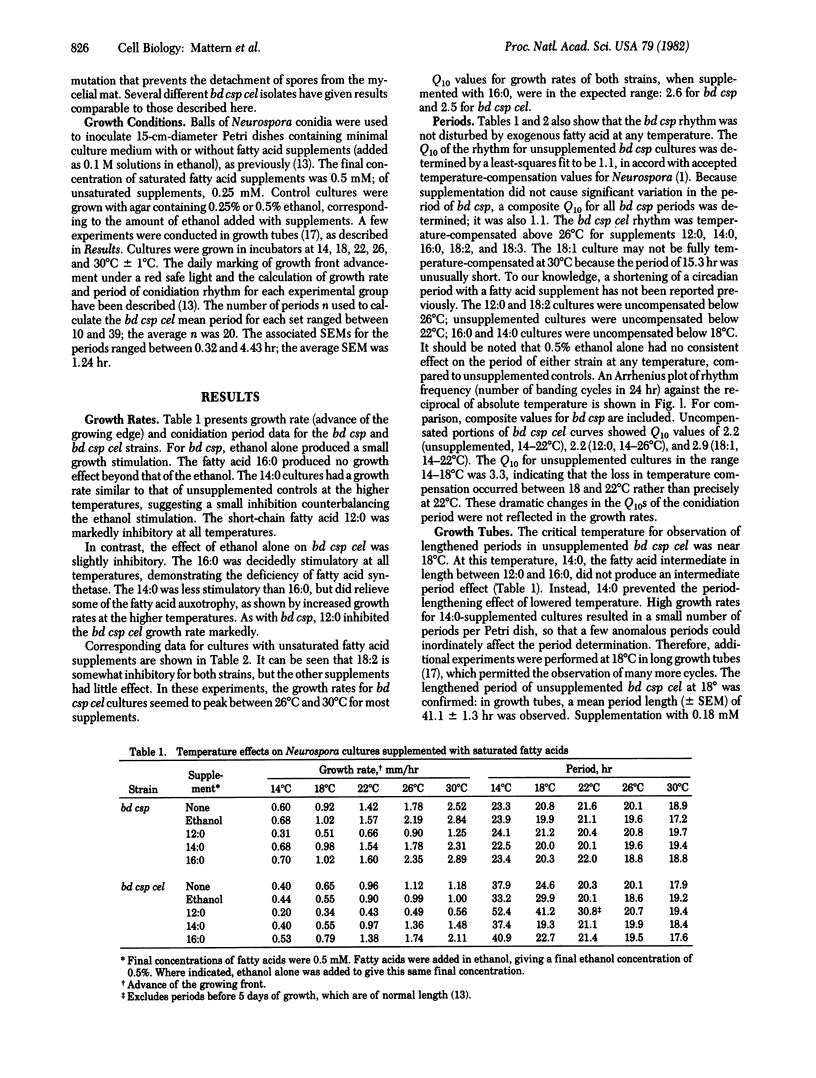
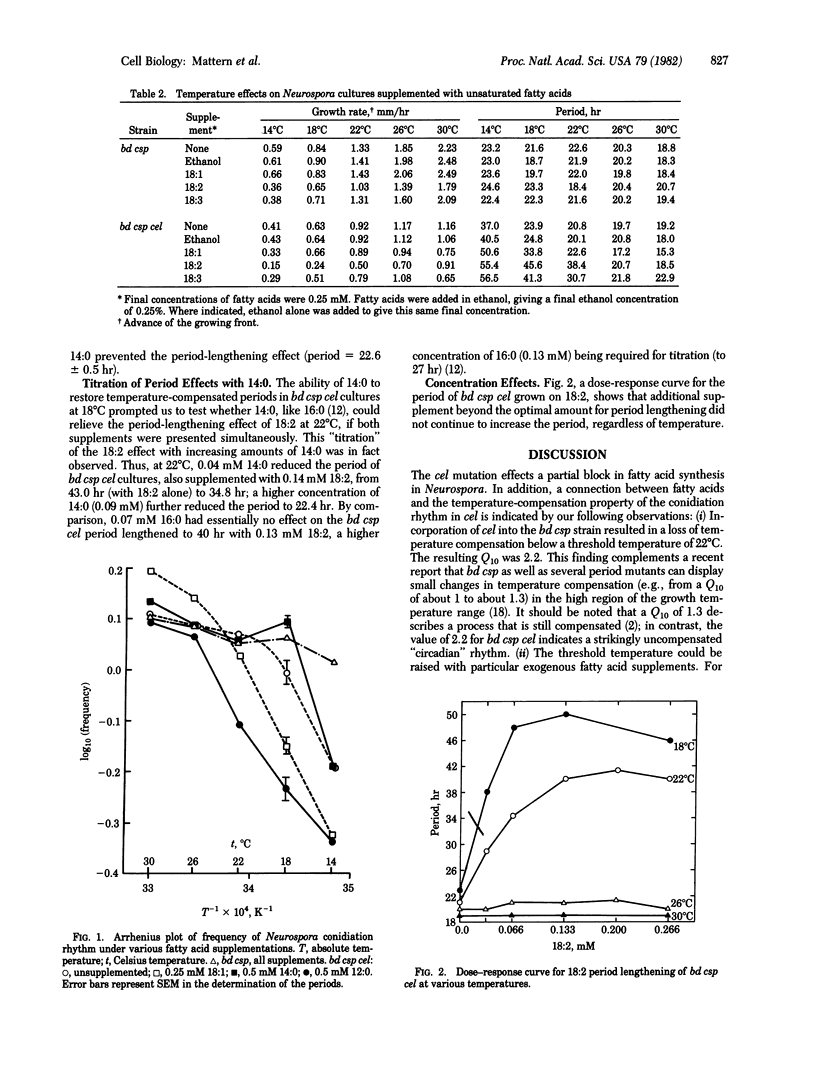
The circadian rhythm of conidiation (spore formation) in Neurospora crassa is known to be temperature compensated, that is, the period is only slightly affected by the incubation temperature. Thus, the Q10 (the relative rate enhancement corresponding to a 10 degrees C rise in temperature) of the rhythm of the bd csp strain from 14 to 30 degrees C was 1.1, whereas the Q10 of the uncompensated growth rate in the same interval was 2.4. A mutation at the cel locus resulted in loss of the temperature-compensation property in cultures grown below 22 degrees C. The Q10 of the rhythm below 22 degrees C was 2.2, and periods of about 40 hr were observed. In contrast, the Q10 of the rhythm above 22 degrees C was 1.1, with circadian periods of 18-21 hr. Thus, cel displayed a threshold temperature or "break point" for the temperature compensation of its rhythm. Supplementation of cel strains, which require fatty acids, with unsaturated or short-chain fatty acids raised the threshold temperature to 26 degrees C, whereas supplementation with long-chain saturated fatty acids lowered it to 18 degrees C. These data suggest a role for fatty acids, as liquid components or as cellular metabolites, in the mechanism of temperature compensation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberghina F. A. Growth regulation in Neurospora crassa. Effects of nutrients and of temperature. Arch Mikrobiol. 1973 Feb 5;89(2):83–94. doi: 10.1007/BF00425025. [DOI] [PubMed] [Google Scholar]
- Brain R. D., Freeberg J. A., Weiss C. V., Briggs W. R. Blue light-induced Absorbance Changes in Membrane Fractions from Corn and Neurospora. Plant Physiol. 1977 May;59(5):948–952. doi: 10.1104/pp.59.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S., Martins S. A. Circadian rhythms in Neurospora crassa: effects of unsaturated fatty acids. J Bacteriol. 1979 Feb;137(2):912–915. doi: 10.1128/jb.137.2.912-915.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünning E. Critical remarks concerning the Q10-values in circadian rhythms. Int J Chronobiol. 1974;2(4):343–346. [PubMed] [Google Scholar]
- Diekmann C., Brody S. Circadian rhythms in Neurospora crassa: oligomycin-resistant mutations affect periodicity. Science. 1980 Feb 22;207(4433):896–898. doi: 10.1126/science.6444467. [DOI] [PubMed] [Google Scholar]
- Elovson J. Purification and properties of the fatty acids synthetase complex from Neurospora crassa, and the nature of the fas-mutation. J Bacteriol. 1975 Oct;124(1):524–533. doi: 10.1128/jb.124.1.524-533.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F., Atkinson C. A. Genetic and physiological characteristics of a slow-growing circadian clock mutant of Neurospora crassa. Genetics. 1978 Feb;88(2):255–265. doi: 10.1093/genetics/88.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F., Hoyle M. N. Isolation of circadian clock mutants of Neurospora crassa. Genetics. 1973 Dec;75(4):605–613. doi: 10.1093/genetics/75.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. D., Sargent M. L. Effects of temperature perturbations on circadian conidiation in neurospora. Plant Physiol. 1979 Dec;64(6):1000–1004. doi: 10.1104/pp.64.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman K. J. Role of lipids in the Neurospora crassa membrane. I. Influence of fatty acid composition on membrane lipid phase transitions. J Membr Biol. 1977 Apr 7;32(1-2):33–47. doi: 10.1007/BF01905208. [DOI] [PubMed] [Google Scholar]
- Friedman K. J. Role of lipids in the Neurospora crassa membrane. II. Membrane potential and resistance studies; the effect of altered fatty acid composition on the electrical properties of the cell membrane. J Membr Biol. 1977 Sep 14;36(2-3):175–190. doi: 10.1007/BF01868150. [DOI] [PubMed] [Google Scholar]
- Gardner G. F., Feldman J. F. Temperature Compensation of Circadian Period Length in Clock Mutants of Neurospora crassa. Plant Physiol. 1981 Dec;68(6):1244–1248. doi: 10.1104/pp.68.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W., Sweeney B. M. ON THE MECHANISM OF TEMPERATURE INDEPENDENCE IN A BIOLOGICAL CLOCK. Proc Natl Acad Sci U S A. 1957 Sep 15;43(9):804–811. doi: 10.1073/pnas.43.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel J. R., Prosser C. L. Molecular mechanisms of temperature compensation in poikilotherms. Physiol Rev. 1974 Jul;54(3):620–677. doi: 10.1152/physrev.1974.54.3.620. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Keith A. D. Saturated fatty acid requirer of Neurospora crassa. J Bacteriol. 1971 Apr;106(1):174–182. doi: 10.1128/jb.106.1.174-182.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto J., Raison J. K., Lyons J. M. Temperature "breaks" in Arrhenius plots: a thermodynamic consequence of a phase change. J Theor Biol. 1971 Apr;31(1):47–51. doi: 10.1016/0022-5193(71)90120-2. [DOI] [PubMed] [Google Scholar]
- Martin C. E., Hiramitsu K., Kitajima Y., Nozawa Y., Skriver L., Thompson G. A. Molecular control of membrane properties during temperature acclimation. Fatty acid desaturase regulation of membrane fluidity in acclimating Tetrahymena cells. Biochemistry. 1976 Nov 30;15(24):5218–5227. doi: 10.1021/bi00669a004. [DOI] [PubMed] [Google Scholar]
- Marzuki S., Cobon G. S., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. The effects of altered steady-state membrane lipid composition on mitochondrial-energy metabolism in Saccharomyces cerevisiae. Arch Biochem Biophys. 1975 Aug;169(2):577–590. doi: 10.1016/0003-9861(75)90202-7. [DOI] [PubMed] [Google Scholar]
- Mattern D., Brody S. Circadian rhythms in Neurospora crassa: effects of saturated fatty acids. J Bacteriol. 1979 Sep;139(3):977–983. doi: 10.1128/jb.139.3.977-983.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Petit V. A., Edidin M. Lateral phase separation of lipids in plasma membranes: effect of temperature on the mobility of membrane antigens. Science. 1974 Jun 14;184(4142):1183–1185. doi: 10.1126/science.184.4142.1183. [DOI] [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R., Woodward D. O. Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol. 1966 Oct;41(8):1343–1349. doi: 10.1104/pp.41.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Kaltenborn S. H. Effects of medium composition and carbon dioxide on circadian conidiation in neurospora. Plant Physiol. 1972 Jul;50(1):171–175. doi: 10.1104/pp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitt S. C., Russell P. J. Neurospora crassa cytoplasmic ribosomes: isolation and characterization of a cold-sensitive mutant defective in ribosome biosynthesis. J Bacteriol. 1974 Nov;120(2):666–671. doi: 10.1128/jb.120.2.666-671.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. R., McElhaney R. N. Membrane lipid physical state and modulation of the Na+,Mg2+-ATPase activity in Acholeplasma laidlawii B. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1255–1259. doi: 10.1073/pnas.77.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilo L., Träuble H., Overath P. Mechanistic interpretation of the influence of lipid phase transitions on transport functions. Biochemistry. 1977 Apr 5;16(7):1283–1290. doi: 10.1021/bi00626a007. [DOI] [PubMed] [Google Scholar]



