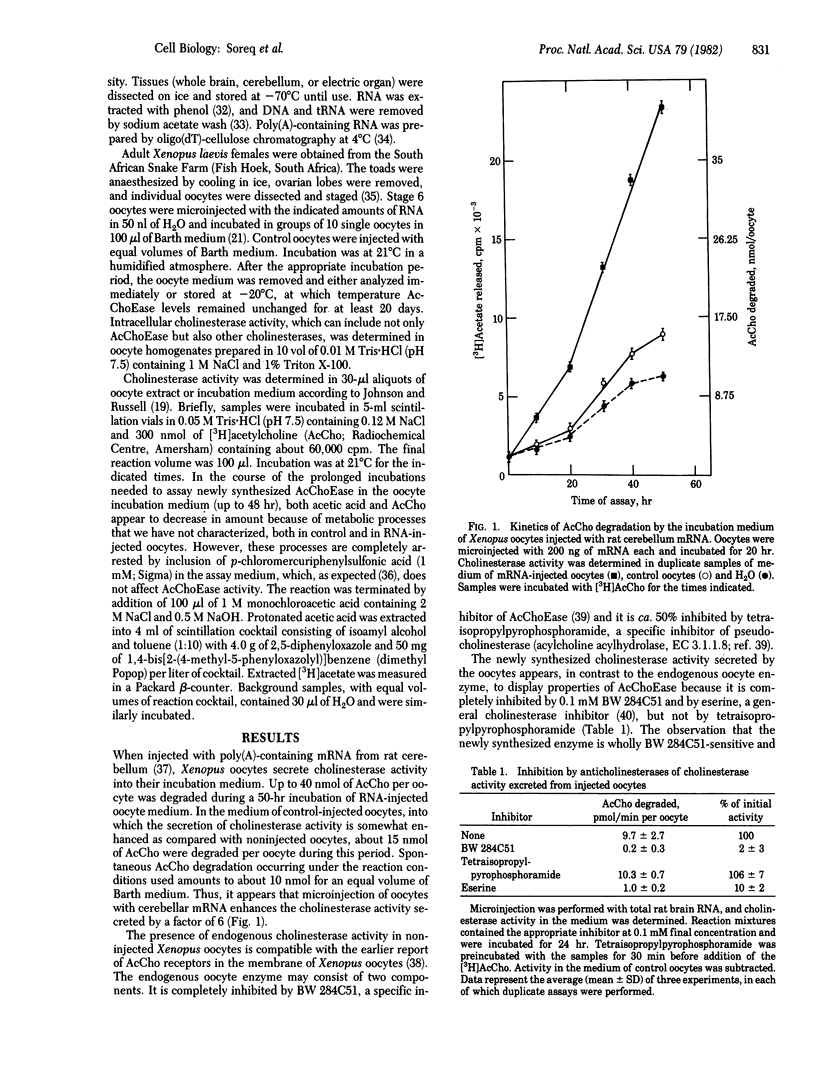
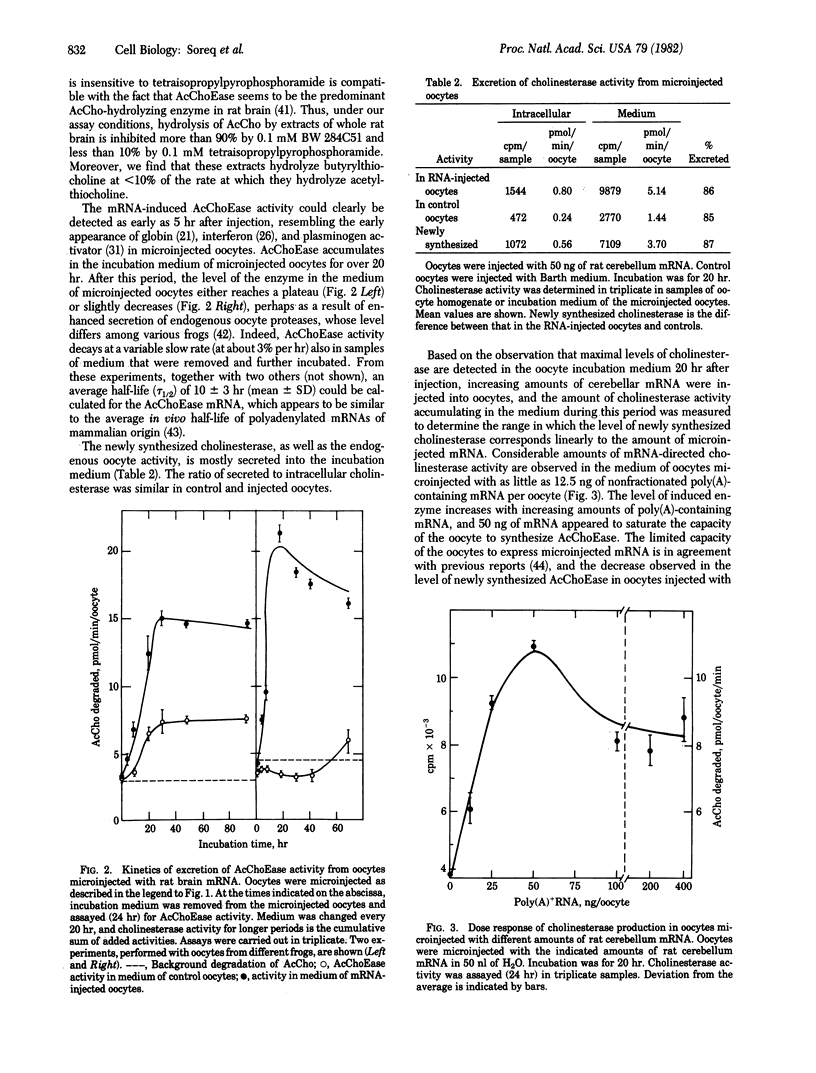
Abstract
A novel technique was developed for monitoring the level of the mRNA species that direct the synthesis of acetylcholinesterase (AcChoEase; acetylcholine acetylhydrolase, EC 3.1.1.7), using microinjected Xenopus oocytes as a translation system. When injected with poly(A)-containing RNA from whole rat brain or rat cerebellum and from electric organ of Torpedo ocellata, Xenopus oocytes synthesize and secrete catalytically active cholinesterase. The newly synthesized enzyme, which is mostly secreted into the oocytes incubation medium, appears to be primarily AcChoEase because it is inhibited by the specific inhibitor BW 284C51. The new enzymatic activity can be detected after injection of as little as 12.5 ng of poly(A)-containing RNA per oocyte, and there is a linear dependence of the oocytes' ability to form AcChoEase on the amount of injected RNA. The AcChoEase mRNA displays a tau 1/2 of about 10 +/- 3 hr in injected oocytes. The abundance of AcChoEase mRNA in the total nonfractionated mRNA injected was calculated to be ca. 1 x 10(-5), a value similar to the level of AcChoEase protein determined in rat brain. The combination of the high turnover number of AcChoEase, the efficiency of the oocyte system, and the sensitivity of the assay used thus permit the accurate monitoring of the scarce mRNA species that direct the synthesis of this enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN L., BERRY W. K. Two selective inhibitors of cholinesterase. Biochem J. 1953 Jul;54(4):695–700. [PMC free article] [PubMed] [Google Scholar]
- Allende C. C., Allende J. E., Firtel R. A. The degradation of ribonucleic acids injected into Xenopus laevis oocytes. Cell. 1974 Jul;2(3):189–196. doi: 10.1016/0092-8674(74)90093-2. [DOI] [PubMed] [Google Scholar]
- Bennett E. L., Diamond M. C., Morimoto H., Hebert M. Acetylcholinesterase activity and weight measures in fifteen brain areas from six lines of rats. J Neurochem. 1966 Jul;13(7):563–572. doi: 10.1111/j.1471-4159.1966.tb11952.x. [DOI] [PubMed] [Google Scholar]
- Betz H., Bourgeois J. P., Changeux J. P. Evolution of cholinergic proteins in developing slow and fast skeletal muscles in chick embryo. J Physiol. 1980 May;302:197–218. doi: 10.1113/jphysiol.1980.sp013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon S., Massoulié J. Collagen-tailed and hydrophobic components of acetylcholinesterase in Torpedo marmorata electric organ. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4464–4468. doi: 10.1073/pnas.77.8.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L., Kohler P. O., O'Malley B. W. Translation of ovalbumin mRNA in Xenopus laevis oocytes. Characterization of the system and effects of estrogen on injected mRNA populations. J Clin Invest. 1976 Mar;57(3):576–585. doi: 10.1172/JCI108313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Morser J. Export of proteins from oocytes of Xenopus laevis. Cell. 1979 Jul;17(3):517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Fernandez H. L., Duell M. J., Festoff B. W. Neurotrophic control of 16S acetylcholinesterase at the vertebrate neuromuscular junction. J Neurobiol. 1979 Sep;10(5):441–454. doi: 10.1002/neu.480100503. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Hubert E., Trávnícek M., Bolognesi D. P., Burny A., Cleuter Y., Huez G., Kettmann R., Marbaix G., Portetelle D. Frog oocytes synthesize and completely process the precursor polypeptide to virion structural proteins after microinjection of avian myeloblastosis virus RNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3230–3234. doi: 10.1073/pnas.74.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisiger V. A specific form of acetylcholinesterase is secreted by rat sympathetic ganglia. FEBS Lett. 1977 Dec 15;84(2):253–256. doi: 10.1016/0014-5793(77)80700-x. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hall Z. W. Multiple forms of acetylcholinesterase and their distribution in endplate and non-endplate regions of rat diaphragm muscle. J Neurobiol. 1973;4(4):343–361. doi: 10.1002/neu.480040404. [DOI] [PubMed] [Google Scholar]
- Jedrzejczyk J., Silman I., Lyles J. M., Barnard E. A. Molecular forms of the cholinesterases inside and outside muscle endplates. Biosci Rep. 1981 Jan;1(1):45–51. doi: 10.1007/BF01115148. [DOI] [PubMed] [Google Scholar]
- Johnson C. D., Russell R. L. A rapid, simple radiometric assay for cholinesterase, suitable for multiple determinations. Anal Biochem. 1975 Mar;64(1):229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- Kaplan B. B., Schachter B. S., Osterburg H. H., de Vellis J. S., Finch C. E. Sequence complexity of polyadenylated RNA obtained from rat brain regions and cultured rat cells of neural origin. Biochemistry. 1978 Dec 12;17(25):5516–5524. doi: 10.1021/bi00618a029. [DOI] [PubMed] [Google Scholar]
- Karlin A. Chemical distinctions between acetylcholinesterase and the acetylcholine receptor. Biochim Biophys Acta. 1967 Jul 11;139(2):358–362. doi: 10.1016/0005-2744(67)90039-3. [DOI] [PubMed] [Google Scholar]
- Kimhi Y., Mahler A., Saya D. Acetylcholinesterase in mouse neuroblastoma cells: intracellular and released enzyme. J Neurochem. 1980 Mar;34(3):554–559. doi: 10.1111/j.1471-4159.1980.tb11180.x. [DOI] [PubMed] [Google Scholar]
- Koenig J., Vigny M. Neural induction of the 16S acetylcholinesterase in muscle cell cultures. Nature. 1978 Jan 5;271(5640):75–77. doi: 10.1038/271075a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. MRNA-directed synthesis of catalytically active mouse beta-glucuronidase in Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4462–4465. doi: 10.1073/pnas.74.10.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J. C., Leung T. K., Lim L. Brain regional distribution of glutamic acid decarboxylase, choline acetyltransferase, and acetylcholinesterase in the rat: effects of chronic manganese chloride administration after two years. J Neurochem. 1981 Apr;36(4):1443–1448. doi: 10.1111/j.1471-4159.1981.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Lanks K. W., Dorwin J. M., Papirmeister B. Increased rate of acetylcholinesterase synthesis in differentiating neuroblastoma cells. J Cell Biol. 1974 Dec;63(3):824–830. doi: 10.1083/jcb.63.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Gurdon J. B., Partington G. A. Protein synthesis in oocytes of Xenopus laevis is not regulated by the supply of messenger RNA. Cell. 1977 Jun;11(2):345–351. doi: 10.1016/0092-8674(77)90051-4. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Soreq H. The regulatory function of poly(A) and adjacent 3' sequences in translated RNA. Prog Nucleic Acid Res Mol Biol. 1982;27:53–83. doi: 10.1016/s0079-6603(08)60597-8. [DOI] [PubMed] [Google Scholar]
- Lømo T., Slater C. R. Control of junctional acetylcholinesterase by neural and muscular influences in the rat. J Physiol. 1980 Jun;303:191–202. doi: 10.1113/jphysiol.1980.sp013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meedel T. H., Whittaker J. R. Development of acetylchilinesterase during embryogenesis of the ascidian Ciona intestinalis. J Exp Zool. 1979 Oct;210(1):1–10. doi: 10.1002/jez.1402100102. [DOI] [PubMed] [Google Scholar]
- Miskin R., Soreq H. Microinjected Xenopus oocytes synthesize active human plasminogen activator. Nucleic Acids Res. 1981 Jul 24;9(14):3355–3363. doi: 10.1093/nar/9.14.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Lane C. D., Colman A., Wylie C. C. The secretion of proteins in vitro from Xenopus oocytes and their accessory cells: a biochemical and morphological study. J Embryol Exp Morphol. 1981 Feb;61:367–383. [PubMed] [Google Scholar]
- Nudel U., Soreq H., Littauer U. Z. Globin mRNA species containing poly(A) segments of different lengths. Their functional stability in Xenopus oocytes. Eur J Biochem. 1976 Apr 15;64(1):115–121. doi: 10.1111/j.1432-1033.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Prives J., Silman I., Amsterdam A. Appearance and disappearance of acetycholine receptor during differentiation of chick skeletal muscle in vitro. Cell. 1976 Apr;7(4):543–550. doi: 10.1016/0092-8674(76)90204-x. [DOI] [PubMed] [Google Scholar]
- Rieger F., Faivre-Bauman A., Benda P., Vigny M. Molecular forms of acetylcholinesterase: their de novo synthesis in mouse neuroblastoma cells. J Neurochem. 1976 Nov;27(5):1059–1063. doi: 10.1111/j.1471-4159.1976.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L., Fambrough D. M. Synthesis, transport and fate of acetylcholinesterase in cultured chick embryos muscle cells. Cell. 1980 Nov;22(2 Pt 2):583–594. doi: 10.1016/0092-8674(80)90368-2. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Schuetze S. M., Weill C. L., Fischbach G. D. Regulation of acetylcholinesterase appearance at neuromuscular junctions in vitro. Nature. 1980 Jan 17;283(5744):264–267. doi: 10.1038/283264a0. [DOI] [PubMed] [Google Scholar]
- Shulman L., Revel M. Interferon-dependent induction of mRNA activity for (2'-5')oligo-isoadenylate synthetase. Nature. 1980 Nov 6;288(5786):98–100. doi: 10.1038/288098a0. [DOI] [PubMed] [Google Scholar]
- Silman I., di Giamberardino L., Lyles L., Couraud J. Y., Barnard E. A. Parallel regulation of acetylcholinesterase and pseudocholinesterase in normal, denervated and dystrophic chicken skeletal muscle. Nature. 1979 Jul 12;280(5718):160–162. doi: 10.1038/280160a0. [DOI] [PubMed] [Google Scholar]
- Soreq H., Harpold M., Wilson M., Darnell J. E., Jr Rate of synthesis and concentration of specific mRNA sequences in cultured Chinese hamster ovary cells compared to liver cells. Biochem Biophys Res Commun. 1980 Jan 29;92(2):485–491. doi: 10.1016/0006-291x(80)90359-9. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R. Secreted proteins in the medium of microinjected Xenopus oocytes are degraded by oocyte proteases. FEBS Lett. 1981 Jun 15;128(2):305–310. doi: 10.1016/0014-5793(81)80104-4. [DOI] [PubMed] [Google Scholar]
- Soreq H., Nudel U., Salomon R., Revel M., Littauer U. Z. In vitro translation of polyadenylic acid-free rabbit globin messenger RNA. J Mol Biol. 1974 Sep 5;88(1):233–245. doi: 10.1016/0022-2836(74)90307-6. [DOI] [PubMed] [Google Scholar]
- Soreq H., Sagar A. D., Sehgal P. B. Translational activity and functional stability of human fibroblast beta 1 and beta 2 interferon mRNAs lacking 3'-terminal RNA sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1741–1745. doi: 10.1073/pnas.78.3.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevor A. J., Gordon M. A., Parker K. K., Chan S. L. Acetylcholinesterases. Life Sci. 1978 Sep 25;23(12):1209–1220. doi: 10.1016/0024-3205(78)90498-8. [DOI] [PubMed] [Google Scholar]
- Valle G., Besley J., Colman A. Synthesis and secretion of mouse immunoglobulin chains from Xenopus oocytes. Nature. 1981 May 28;291(5813):338–340. doi: 10.1038/291338a0. [DOI] [PubMed] [Google Scholar]
- Vigny M., Bon S., Massoulié J., Leterrier F. Active-site catalytic efficiency of acetylcholinesterase molecular forms in Electrophorus, torpedo, rat and chicken. Eur J Biochem. 1978 Apr 17;85(2):317–323. doi: 10.1111/j.1432-1033.1978.tb12241.x. [DOI] [PubMed] [Google Scholar]
- Vijayan V. K., Brownson R. H. Polyacrylamide gel electrophoresis of rat brain acetylcholinesterase: isoenzymes of normal rat brain. J Neurochem. 1974 Jul;23(1):47–53. doi: 10.1111/j.1471-4159.1974.tb06914.x. [DOI] [PubMed] [Google Scholar]
- Walker C. R., Wilson B. W. Regulation of acetylcholinesterase in chick muscle cultures after treatment with diisopropylphosphorofluoridate: ribonucleic acid and protein synthesis. Neuroscience. 1976 Dec;1(6):509–513. doi: 10.1016/0306-4522(76)90103-2. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Walker C. R. Regulation of newly synthesized acetylcholinesterase in muscle cultures treated with diisopropylfluorophosphate. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3194–3198. doi: 10.1073/pnas.71.8.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]


