Abstract
The long-term outcome of patients with IgA nephropathy who present with normal renal function, microscopic hematuria, and minimal or no proteinuria is not well described. Here, we studied 141 Caucasian patients with biopsy-proven IgA nephropathy who had minor abnormalities at presentation and a median follow-up of 108 months. None of the patients received corticosteroids or immunosuppressants. We reviewed renal biopsies using the Oxford classification criteria. In this sample, 46 (32%) patients had mesangial proliferation, whereas endocapillary proliferation, focal glomerulosclerosis, and tubulointerstitial abnormalities were uncommon. Serum creatinine increases >50% and >100% were observed in five (3.5%) patients and one (0.7%) patient, respectively; no patients developed ESRD. After 10, 15, and 20 years, 96.7%, 91.9%, and 91.9% of patients maintained serum creatinine values less than a 50% increase, respectively. Using Cox proportional hazards regression, the presence of segmental glomerulosclerosis was the only factor that significantly associated with a >50% increase in serum creatinine. Clinical remission occurred in 53 (37.5%) patients after a median of 48 months. Proteinuria>0.5 and >1.0 g/24 h developed in 21 (14.9%) and 6 (4.2%) patients, respectively. Median proteinuria at the end of follow-up was 0.1 g/24 h, with 41 (29.1%) patients having no proteinuria. At presentation, 23 (16.3%) patients were hypertensive compared with 30 (21.3%) patients at the end of follow-up; 59 (41.8%) patients were treated with renin-angiotensin blockers because of hypertension or increasing proteinuria. In summary, the long-term prognosis for Caucasian patients with IgA nephropathy who present with minor urinary abnormalities and normal renal function is excellent.
A number of studies have confirmed the prognostic value of some clinical and biochemical parameters for the long-term outcome of patients with IgA nephropathy (IgAN). Among them, renal function at presentation, hypertension, and proteinuria have conclusively shown their crucial role in the prediction of outcomes in this disease.1–8 In addition, recent clinicopathological collaborations have added new insights into the prognostic value of histologic lesions. The Oxford classification is a new approach to evaluate and systematize histologic findings in IgAN.9–11 Although the results of larger ongoing validation studies are awaited, preliminary investigations have confirmed the prognostic value of Oxford classification.
Nevertheless, information about these clinical and histologic prognostic factors is mostly based on patients in whom diagnostic renal biopsy was performed because of proteinuria higher than 1–2 g/d.1–8 Few nephrologists currently indicate a renal biopsy in patients with isolated microhematuria or proteinuria lower than 0.5 g/d accompanied by microhematuria. However, a substantial proportion of IgAN patients can present with these minor analytical findings.
Few studies have analyzed long-term outcome and prognostic factors in patients with biopsy-proven IgAN, isolated microhematuria, and minimal proteinuria at presentation. This information is important to establish whether IgAN is a progressive disease and seek clinical or histologic findings that could predict a worse long-term outcome. Studies performed in China and Japan have suggested that IgAN presenting with hematuria and minimal proteinuria is usually a progressive disease, and few patients had a complete resolution of hematuria.12–16
We present the largest series of biopsy-proven IgAN presenting with hematuria and minimal or negative proteinuria so far collected; 141 patients with such characteristics were studied by eight Spanish hospitals that had adopted an active policy of renal biopsy in patients with minimal abnormalities in urinalysis some decades ago. Although the majority of these hospitals has currently abandoned this policy, we thought that it could offer an excellent opportunity to review the long-term outcome of IgAN patients with apparently benign presentation and investigate those clinical and analytical findings that could predict long-term prognosis. In addition, because no studies have applied the recently launched Oxford classification to IgAN patients with minimal abnormalities at presentation, we reviewed the renal biopsies of all our patients and graded their lesions according to the criteria proposed by such classification.
Results
Baseline Characteristics
One hundred forty-one patients meeting inclusion/exclusion criteria were collected. Baseline characteristics are shown in Table 1. All patients were Caucasian, and 90 (63.8%) patients were male. Mean age was 23.7 (14.8) years (range=5–71 years). All the patients showed microhematuria. Median proteinuria was 0.2 (0.1–0.4) g/24 h. In 25 (17.7%) patients, proteinuria was negative at baseline. All patients had normal renal function (serum creatinine [SCr]=0.8 [0.2] mg/dl; estimated GFR [eGFR]=111 [31.0] ml/min per 1.73 m2); 23 (16.3%) patients were hypertensive at baseline, and 22 (15.6%) patients were smokers. Mean body mass index (BMI) was 21.9 (4) kg/m2. Overweight (BMI>25 kg/m2) was observed in 31 (22%) patients, and obesity (BMI>30 kg/m2) was observed in 5 (3.5%) patients.
Table 1.
Clinical characteristics at baseline (renal biopsy)
| Variable | Valuesa |
|---|---|
| Age (years) | 23.7 (14.8; 5–71) |
| Males (%) | 63.8 |
| BMI (kg/m2) | 21.9 (4; 14.9–31.7) |
| Systolic BP (mmHg) | 117 (16.9; 80–190) |
| Diastolic BP (mmHg) | 70.6 (13.6; 35–130) |
| MAP (mmHg) | 86.2 (13.9; 50–143.3) |
| SCr (mg/dl) | 0.8 (0.2; 0.7–1.1) |
| eGFR (ml/min per 1.73 m2) | 111.7 (31.6; 65–228) |
| Proteinuria (g/24 h) | 0.2 (0.1–0.4) |
| Microhematuria | 141 (100%) |
| Smokers | 22 (15.6%) |
| Follow-up (months) | 108 (60–180) |
MAP, mean arterial pressure.
For quantitative variables, values are expressed as mean (SD; range), except for proteinuria and follow-up, which are expressed as median value and interquartile range. For qualitative variables, values are expressed as n (%).
Histologic Findings
Mesangial proliferation present in less than 50% of glomeruli (M0) was observed in 95 (67.4%) patients, and mesangial proliferation detected in more than 50% of glomeruli (M1) was observed in 46 (32.6%) patients. Present endocapillary proliferation (E1) was observed in 12 (8.5%) patients. Present focal and segmental glomerulosclerosis (S1) was found in 22 (15.6%) patients. Absent/mild tubulointerstitial fibrosis was found in a majority of patients (134; 95%), whereas the remaining seven (5%) patients showed moderate fibrosis.
A significant correlation between the presence of S1 and both the amount of proteinuria and the presence of hypertension at baseline was found; 7 of 23 patients with hypertension (30%) had lesions of S1, and the amount of proteinuria was significantly higher among patients with these lesions: 0.3 (0.1–0.4) compared with 0.1 (0–0.3) g/24 h among patients without S1 (P<0.005).
Follow-Up and Treatment
Median follow-up was 108 months (range=60–180). No patient received immunosuppressive treatments, including corticosteroids, or fish oil. No patient was submitted to tonsillectomy. A total of 59 (41.8%) patients received treatment with renin-angiotensin system (RAS) blockers, either angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor antagonists (ARBs); 18 (12.8%) patients started treatment with RAS blockers at baseline because of hypertension. Another 41 patients started ACEI/ARB treatment during follow-up because of hypertension (6 patients), increase in proteinuria (32 patients), and hypertension plus increase in proteinuria (3 patients).
Primary Outcomes
Only five (3.5%) patients showed a >50% increase of baseline SCr. Their main clinical characteristics are summarized in Table 2. Four patients were treated with RAS blockers, but final proteinuria was >1 g/24 h in three patients (Table 2, patients 3–5). Doubling of SCr was observed in only one patient (Table 2, patient 1). She was a 38-year-old woman whose renal biopsy showed M1 in more than 50% of the glomeruli, S1, and E1. RAS blockers were not prescribed; renal function and BP were normal, and proteinuria remained lower than 0.1–0.2 g/24 h during follow-up. Six years after diagnosis and coincident with an otherwise normal pregnancy, renal function showed an abrupt and irreversible decline to SCr values of 1.7 mg/dl that remained stable after delivery.
Table 2.
Clinical characteristics of patients showing >50% increase of baseline SCr values
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age (years) | 38 | 12 | 7 | 42 | 32 |
| Sex | Female | Male | Female | Male | Male |
| Baseline SCr (mg/dl) | 0.8 | 0.8 | 0.7 | 0.9 | 0.9 |
| Baseline eGFR (ml/min per 1.73 m2) | 117 | 99 | 101 | 86.4 | 95.5 |
| Baseline proteinuria (g/24 h) | 0.3 | 0.1 | 0.3 | 0.5 | 0.3 |
| Oxford classification score | M1E1S1T0 | M0E0S0T0 | M0E0S1T0 | M1E0S1T0 | M1E0S0T0 |
| Final SCr (mg/dl) | 1.7 | 1.3 | 1.4 | 1.4 | 1.4 |
| Final eGFR (ml/min per 1.73 m2) | 34 | 76 | 68 | 42 | 56 |
| Final proteinuria (g/24 h) | 0.1 | 0.6 | 2.5 | 1.1 | 1.6 |
| TA proteinuria (g/24 h) | 0.4 | 0.3 | 0.7 | 0.7 | 0.9 |
| ACEI/ARB treatment | No | Yes | Yes | Yes | Yes |
| Follow-up (months) | 84 | 108 | 180 | 96 | 204 |
T0, absent/mild tubulointerstitial fibrosis; E0, absent endocapillary proliferation; S0, absent focal and segmental glomerulosclerosis.
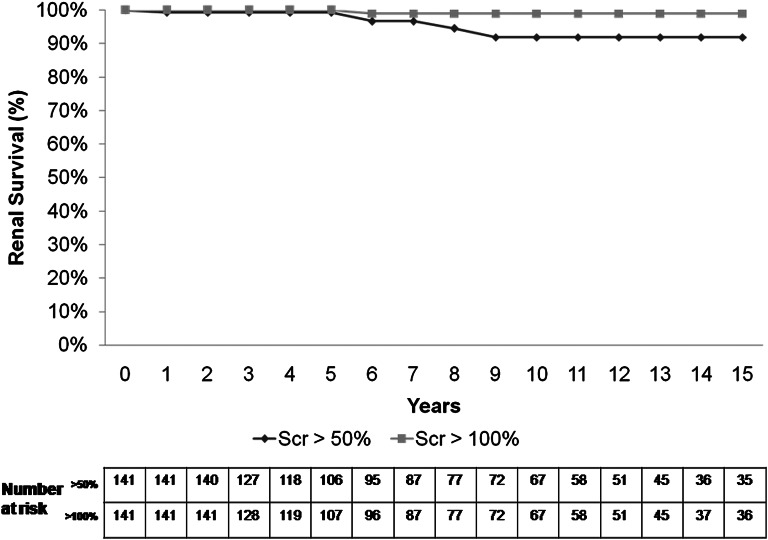
No patient developed ESRD. As shown in Figure 1, renal survival (status free of >50% SCr increase) was 96.7%, 91.9%, and 91.9% after 10, 15, and 20 years, respectively, of follow-up. When renal survival was defined by a status free of >100% SCr increase, it was 99% after 10, 15, and 20 years of follow-up.
Figure 1.
Renal survival (defined by a status free of >50% and >100% baseline SCr increase).
By univariate analysis, time-average (TA) proteinuria>0.5 g/24 h and the presence of S1 were factors significantly associated with an SCr increase>50%. In the multivariable model, only the presence of S1 (hazard ratio=6.86; 95% confidence interval=1.08–43.55; P=0.04) remained as an independent predictor of SCr increase (Table 3).
Table 3.
Univariate and multivariate analyses of independent prognosis factors for renal survival (>50% baseline SCr increase)
| Factor | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| TA proteinuria>0.5 g/24 h | 7.89 (1.32–47.30) | 0.03 | 5.30 (0.84–33.36) | 0.07 |
| S1 (Oxford classification) | 9.78 (1.63–58.81) | 0.02 | 6.86 (1.08–43.55) | 0.04 |
95% CI, 95% confidence interval.
Clinical remission of the disease was observed in 53 (37.5%) patients. Mean time to remission was 60.2 (42.8) months (median=48 months; range=24–85 month); 41 of 53 patients who achieved remission did not receive any treatment during follow-up. RAS blockers were prescribed in the remaining 12 patients because of increasing proteinuria (7 patients) or hypertension (5 patients). The main clinical and analytical findings in patients with and without remission are shown in Table 4. The only significant differences were a higher proteinuria at baseline and the presence of S1 among patients who did not remit. RAS blockers were more frequently prescribed in nonremitting patients, but this finding likely reflects the higher proteinuria values of these patients compared with patients who developed remission. As shown in Table 5, smoking, presence of M1, and baseline proteinuria were the factors significantly associated with the absence of remission by univariate analysis. By multivariable analysis, only baseline proteinuria and presence of M1 showed a significant association.
Table 4.
Clinical characteristics at baseline of patients with and without clinical remission of the disease during follow-up
| Variablea | Patients with Remission (n=53) | Patients without Remission (n=88) | P Value |
|---|---|---|---|
| Age (years) | 21.6 (13.7) | 25 (15.4) | 0.20 |
| Males (%) | 73.6 | 59.1 | 0.08 |
| BMI (kg/m2) | 22.2 (4.3) | 21.9 (3.9) | 0.60 |
| Mean BP (mmHg) | 84.1 (15.3) | 87.5 (12.8) | 0.20 |
| SCr (mg/dl) | 0.8 (0.2) | 0.8 (0.2) | 0.80 |
| eGFR (ml/min per 1.73 m2) | 114.2 (32) | 110.3 (31.4) | 0.50 |
| Proteinuria (g/24 h) | 0.1 (0.1) | 0.3 (0.2) | <0.001 |
| ACEI/ARB treatment | 12 (22.6%) | 47 (53.4%) | <0.001 |
| Smokers | 4 (7.5%) | 18 (20.5%) | 0.04 |
| Follow-up (months) | 102 (52–156) | 120 (66–180) | 0.20 |
| M1 Oxford classification | 13 (24.5%) | 33 (37.5%) | 0.10 |
| E1 Oxford classification | 2 (3.8%) | 10 (11.4%) | 0.10 |
| S1 Oxford classification | 4 (7.5%) | 18 (20.5%) | 0.04 |
For quantitative variables, values are expressed as mean (SD) or mean (range). For qualitative variables, values are expressed as n (%).
Table 5.
Results of univariate and multivariate analyses of independent prognosis factors for the appearance of remission
| Factor | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Smoker (no versus yes) | 2.62 (0.95–7.26) | 0.03 | — | NS |
| Baseline proteinuria | 0.01 (0.00–0.009) | <0.01 | 0.01 (0.00–0.07) | <0.001 |
| M0 versus M1 Oxford classification | 2.06 (1.10–3.86) | 0.02 | 2.04 (1.07–2.89) | 0.02 |
95% CI, 95% confidence interval.
Secondary Outcomes
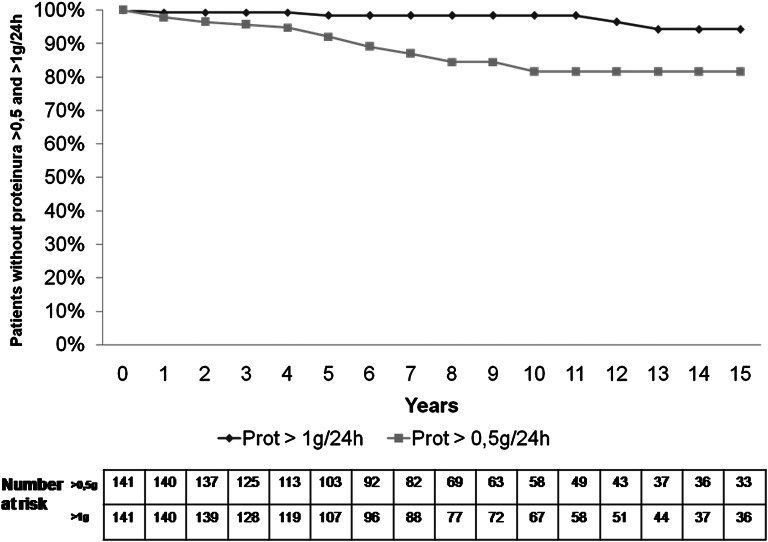
Proteinuria>0.5 g/24 h developed in 21 (14.9%) patients, and 19 of these patients started treatment with RAS blockers (Figure 2). The only significant histologic difference between patients who developed proteinuria>0.5 g/24 h and patients who did not was a significantly higher presence of S1: 7 (33.3%) versus 14 (66.7%) patients (P<0.001). Despite RAS blockers, proteinuria continued to increase to >1 g/24 h in six patients, and three of these patients showed a >50% increase in SCr (corresponding to patients 3–5 in Table 2). At the end of follow-up, median proteinuria was 0.1 (0–0.4) g/24 h, and 41 (29.1%) patients showed a negative proteinuria.
Figure 2.
Probability of absence of proteinuria>0.5 and >1.0 g/24 h during follow-up.
Twenty-three (16.3%) patients were hypertensive at baseline. At the end of follow-up, the number of hypertensive patients had increased to 30 (21.3%) patients. Mean BP at the end of follow-up was 90.5 (9.9) mmHg.
Discussion
Information about the long-term outcome of IgAN patients presenting with minor or benign clinical presentations is scarce, because a majority of IgAN diagnosis comes from renal biopsies performed in patients who present with proteinuria higher than 1–2 g/24 h, renal function impairment, or macroscopic hematuria bouts. Few nephrological departments maintain the policy of renal biopsy performance in patients with minor urinary abnormalities (persistent microscopic hematuria with or without minimal proteinuria), although a significant proportion of patients in whom a renal biopsy later establishes the diagnosis of IgAN can present with these minor manifestations. The exception to this current policy comes from some centers (most of them located in China and Japan) that have maintained a policy of renal biopsy performance in patients with persistent microscopic hematuria with or without minimal proteinuria.12–16 According to the experience reported by these centers, 33%–46% of patients with benign clinical characteristics at the time of renal biopsy (normal renal function, proteinuria=0.4 g/24 h or less, and normal BP) developed proteinuria>0.5–1.0 g/24 h, 26%–38% hypertension, and 7%–24% impaired renal function after a median follow-up of 7–11 years. On the contrary, spontaneous remissions of hematuria and proteinuria were uncommon.12,13,15 These results suggest that IgAN is a generally progressive disease, even in patients with the more benign types of clinical presentation.
Our study analyzed the long-term outcome of patients with biopsy-proven IgAN who present with minor abnormalities: normal renal function, microscopic hematuria, and proteinuria≤0.5 g/24 h; 141 patients with these characteristics at presentation were collected from eight Spanish nephrology departments that had maintained a policy of renal biopsy indication in patients with minor urinary abnormalities in the past. Importantly, all included patients were Caucasian. Contrary to previous reports, our results show that long-term prognosis is generally excellent. Only five (3.5%) patients achieved the primary outcome of >50% SCr increase, and only one (0.7%) patient increased her baseline SCr>100%. Renal survival, defined by a status free of SCr increase>50%, was 96.7%, 91.9%, and 91.9% after 10, 15, and 20 years of follow-up, and no patients developed ESRD.
Moreover, in contrast with the aforementioned studies performed in Chinese and Japanese patients,14–16 a significant proportion of our patients (53 patients; 37.5%) showed a clinical remission of the disease defined by a disappearance of microhematuria and proteinuria≤0.2 g/24 h together with normal renal function and normal BP. As shown in Table 4, patients who developed clinical remission had a lower proteinuria at presentation and were less likely to be smokers compared with patients without remission. Spontaneous clinical remission of IgAN is a well known characteristic of the disease,17 although its precise incidence and persistence over time have not been specifically studied. It has been shown that IgA deposits persist in second renal biopsies performed in patients who had achieved clinical remissions.18 Our study shows that clinical remissions are common among IgAN patients with minor abnormalities at presentation.
Our results also show that the amount of proteinuria has a significant influence on long-term renal outcomes, even in patients with the more benign types of presentation. By multivariate analysis, TA proteinuria during follow-up was the only clinical risk factor that independently predicted a >50% SCr increase (Table 3), and baseline proteinuria showed a significant influence on the probability of remission: the lower the amount of proteinuria, the higher the probability of remission (Table 5). These results are in agreement with many observational and prospective radomized controlled trials that have emphasized the crucial role of proteinuria in the long-term outcome of the disease.1–8,19–22 Although overweight and obesity have been identified as independent risk factors for chronic renal failure and increasing proteinuria in IgAN,23,24 we found no influences of BMI on primary or secondary outcomes by univariate or multivariate analysis. This finding would suggest that the harmful effect of obesity on IgAN is restricted to more severe types of the disease.
The favorable renal outcome of our patients is particularly remarkable when considering that they did not receive immunosuppressive treatments (including corticosteroids) or fish oil. RAS blockade is currently considered as the first-step treatment in IgAN patients who develop proteinuria>0.5–1.0 g/24 h or hypertension.19–22,25–27 Fifty-nine (41.8%) patients received ACEI/ARB treatment because of hypertension or increasing proteinuria. At the end, only 21 (14.9%) patients developed proteinuria>0.5 g/24 h, and only 6 of these 21 patients achieved proteinuria>1.0 g/24 h, the limit above which the risk of ESRD increases significantly.5
Our study also provides interesting data on the histologic characteristics of IgAN in patients presenting with minor abnormalities. The Oxford classification, proposed as a reproducible method for the interpretation and scoring of histologic lesions in IgAN,9–11 has mainly been validated in patients with proteinuria>1 g/24 h. Information about its application in patients with minor abnormalities is very scarce. We found that E1 and S1 were uncommon lesions in our patients (8.5% and 15.6%, respectively) and that a great majority (95%) showed no tubulointerstitial abnormalities. M1 was the most common finding, and it was observed in 46 (32.6%) patients. Interestingly, we found (by multivariate analysis) that the presence of S1 was independently associated with renal survival and that M1 significantly decreased the probability of clinical remission (Tables 3 and 5). However, S1 lesions were more commonly observed among patients with baseline hypertension and showed a significant association with higher amounts of baseline proteinuria and a greater tendency to proteinuria increase over time compared with patients without S1. The only patients who doubled baseline SCr had M1 and E1 along with S1 (Table 2). All these findings support the usefulness of Oxford classification criteria in IgAN with benign clinical presentations.
Our results indicate that IgAN presenting with normal renal function and minimal or negative proteinuria is not a progressive disease in our population, which was composed of patients of Caucasian ancestry. Additional studies will be necessary to explain the apparently discordant prognosis of our patients compared with IgAN patients with similar benign presentations reported in China and Japan,12–16 because apparently, their baseline clinical characteristics were similar. A comparison of histologic findings is not possible, because these studies were preformed before the appearance of Oxford classification. However, in agreement with our results, the percentage of glomerular sclerosis predicted a poorer outcome.12,13 An attractive possibility is that genetic differences can explain the different outcomes of these patients. Recently published studies have provided deep insights into IgAN genetics,28,29 illustrating its complexity and the important variations between patients of different ancestries.
Our study has the limitations inherent to its retrospective character. Another limitation is the absence of intraclass correlation coefficients to assess the reproducibility of histologic evaluation by the different pathologists. However, our study has important implications for the clinical management of patients presenting with minor urinary abnormalities. Differential diagnoses include IgAN and benign familial hematuria (thin basement membrane disease) as the most common causes of persistent microscopic hematuria with minimal or negative proteinuria,30 although urological causes of hematuria should always be ruled out. We think that the performance of a diagnostic renal biopsy might be unnecessary given the excellent prognosis of our patients. However, regular monitoring is recommended to detect proteinuria increase or hypertension. In such cases, RAS blockade should be prescribed. Using this policy, an excellent long-term prognosis could be predicted according to the results of our study. However, the clinical management of Asian patients presenting with similar characteristics might be different considering the worse prognosis and their tendency to progression.12–16
In conclusion, the long-term prognosis of Caucasian IgAN patients presenting with normal renal function, persistent microhematuria, and minimal or negative proteinuria is excellent. Renal function decline was exceptional, whereas sustained clinical remission was observed in more than one-third of the patients. Increasing proteinuria to >1 g/24 h and hypertension were observed in a minority of patients. S1 was the only histologic finding associated with a worse outcome.
Concise Methods
This study was approved and designed by the Scientific Committee of Spanish Group for the Study of Glomerular Diseases. Eight Spanish hospitals agreed to participate, recruiting all patients with biopsy-proven IgAN who met the inclusion and exclusion criteria.
Patients
Inclusion criteria were renal biopsy-proven IgAN, normal renal function (defined by eGFR>60 ml/min per 1.73 m2), and proteinuria≤0.5 g/24 h. Patients with Henoch–Schönlein purpura, liver diseases, diabetes, systemic diseases, and any type of secondary IgAN were excluded; 141 patients met these criteria in the period 1975–2008, and they were included in the study.
Data Recollection and Patient Follow-Up
Patients performed regular visits at intervals of 6–12 months. Baseline data and data during follow-up were collected by means of a standard protocol agreed on by all of the participating centers. The following data were systematically recorded: sex, age at kidney biopsy, weight, BP, SCr, eGFR, serum total proteins, serum albumin, serum total cholesterol and triglycerides, 24-hour proteinuria, and urine sediment. Proteinuria and urine sediment were tested at every visit throughout follow-up, even in those patients in whom hematuria and proteinuria had disappeared. Urine sediment was examined in a centrifuged and concentrated sample of the first urine of the morning using standardized methodology. Proteinuria was quantified in a 24-hour urine collection at every visit using the routine standard method at every participating center. The most common method to measure proteinuria was the sulfosalicylic acid method.
Baseline data were considered to be the data coinciding with the performance of renal biopsy. Baseline proteinuria was established as the mean of the three 24-hour proteinuria measurements before kidney biopsy. For each patient, an average proteinuria was determined for each 6 months during follow-up. Then, we calculated TA proteinuria, which represents an average of the mean of proteinuria measurements in every 6-month period.5 GFR was calculated by the Modification of Diet in Renal Disease abbreviated equation. In the pediatric population, corrections were made according to body surface area. Hypertension was defined as systolic BP≥140 mmHg and/or diastolic BP≥90 mmHg. Mean BP was estimated as the sum of diastolic BP plus one-third of pulse pressure. All the treatments were recorded, including type and dose of antihypertensive drugs and RAS inhibitors (either ACEIs or ARBs).
Follow-up time was considered as the interval between renal biopsy and the last outpatient visit, death, or ESRD (eGFR<15 ml/min per 1.73 m2, onset of chronic dialysis, or renal transplantation).
Renal Biopsy
Diagnosis of IgAN was established when there was a predominant IgA staining by immunofluorescence. The intensity of IgA staining should be more than trace. The distribution of IgA staining should include the mesangium with or without capillary loop staining. IgG, IgM, and C3 can be present but not in greater intensity than IgA. All the biopsies were re-evaluated for the present study by a nephropathologist at each participating center who was unaware of the outcome of the patient. Histologic findings were grouped according to Oxford classification criteria.9,10 Briefly, histologic lesions proposed by this classification are M0 or M1, absent endocapillary proliferation or E1, absent focal and segmental glomerulosclerosis or S1, and mild, moderate, and severe tubulointerstitical involvement.
Outcomes
Primary outcomes were renal survival (defined by a status free of SCr>50% and >100% with respect to baseline value and ESRD) and clinical remission of the disease. Clinical remission was defined when all the following criteria were met: (1) persistent disappearance of microhematuria throughout follow-up, with normal urine sediment at every visit, (2) proteinuria persistently lower than 0.2 g/24 h or negative throughout follow-up, (3) normal renal function, and (4) normal BP.
Secondary outcomes were the presence of a final proteinuria>0.5 and >1.0 g/24 h and the number of hypertensive patients.
Statistical Analyses
Quantitative variables with normal distribution were expressed as mean and SD and were compared using t tests. Asymmetric variables were expressed as median and interquartile range, and they were analyzed with median tests. Qualitative variables were described by frequency distribution and analyzed by Fisher and chi-squared tests.
The cumulative probability of developing a defined clinic event (remission, >50% and 100% increases of baseline SCr, and final proteinuria >0.5 and >1.0 g/24 h) was estimated by the Kaplan–Meier method, and survival curves were compared with log-rank tests. Survival time for each patient was computed from baseline evaluation to last follow-up. Univariable regressions were used to select the variables that could explain end points. Variables showing a P value<0.10 in univariable tests or variables with biologic relevancy were considered for the multivariable model. The Cox proportional hazard model was performed to explore the influence of several variables on the occurrence of remission and >50% increase of baseline SCr. A P value<0.05 was considered statistically significant. Statistical analysis was performed with SPSS software for Windows, version 15 (SPSS, Chicago, IL).
Disclosures
None.
Acknowledgments
The authors thank Cristina Fernández-Pérez (Hospital Clínico, Madrid, Spain) for her help in statistical analysis.
This study was funded by Fondo de Investigaciones Sanitarias Grants FIS 10/02668 and FIS 10/02581 and the Asociación para la Investigación y Tratamiento de la Enfermedad Renal.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “How Benign Is IgA Nephropathy with Minimal Proteinuria?,” on pages 1607–1610.
References
- 1.D’Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F: Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis 18: 12–19, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartosik LP, Lajoie G, Sugar L, Cattran DC: Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry: Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Barratt J, Feehally J: IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM: Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant 17: 1197–1203, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Coppo R, D’Amico G: Factors predicting progression of IgA nephropathies. J Nephrol 18: 503–512, 2005 [PubMed] [Google Scholar]
- 9.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society: The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barrat J, Berthoux F, Bonsib S, Brujin JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Foggo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H: The Oxford Classification of IgA nephropathy: Rationale, clinicopathological correlations, and clasification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, Feehally J, Roberts IS, Amore A, Alpers CE, Barrat J, Berthoux F, Bonsib S, Brujin JA, D’Agati V, D’Amico G, Emancipator SN, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo AB, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H: The Oxford IgA nephropahty clinicopathological classification is valid for children as well as adults. Kidney Int 77: 921–927, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Lai FM, Szeto CC, Choi PC, Li PK, Chan AW, Tang NL, Lui SF, Wang AY, To KF: Characterization of early IgA nephropathy. Am J Kidney Dis 36: 703–708, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Szeto CC, Lai FM, To KF, Wong TY, Chow KM, Choi PC, Lui SF, Li PK: The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med 110: 434–437, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Li PK, Ho KK, Szeto CC, Yu L, Lai FM: Prognostic indicators of IgA nephropathy in the Chinese—clinical and pathological perspectives. Nephrol Dial Transplant 17: 64–69, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Shen P, He L, Huang D: Clinical course and prognostic factors of clinical early IgA nephropathy. Neth J Med 66: 242–247, 2008 [PubMed] [Google Scholar]
- 16.Koyama A, Igarashi M, Kobayashi M; Research Group on Progressive Renal Diseases: Natural history and risk factors for immunoglobulin A nephropathy in Japan. Am J Kidney Dis 29: 526–532, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Costa RS, Droz D, Noel LH: Long-standing spontaneous clinical remission and glomerular improvement in primary IgA nephropathy (Berger’s disease). Am J Nephrol 7: 440–444, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Alamartine E, Sabatier JC, Berthoux FC: Comparison of pathological lesions on repeated renal biopsies in 73 patients with primary IgA glomerulonephritis: Value of quantitative scoring and approach to final prognosis. Clin Nephrol 34: 45–51, 1990 [PubMed] [Google Scholar]
- 19.Praga M, Gutiérrez E, González E, Morales E, Hernández E: Treatment of IgA nephropathy with ACE inhibitors: A randomized and controlled trial. J Am Soc Nephrol 14: 1578–1583, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Praga M, Hernández E, Montoyo C, Andrés A, Ruilope LM, Rodicio JL: Long-term beneficial effects of angiotensin-converting enzyme inhibition in patients with nephrotic proteinuria. Am J Kidney Dis 20: 240–248, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Cattran DC, Greenwood C, Ritchie S: Long-term benefits of angiotensin-converting enzyme inhibitor therapy in patients with severe immunoglobulin a nephropathy: A comparison to patients receiving treatment with other antihypertensive agents and to patients receiving no therapy. Am J Kidney Dis 23: 247–254, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Coppo R, Peruzzi L, Amore A, Piccoli A, Cochat P, Stone R, Kirschstein M, Linné T: IgACE: A placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol 18: 1880–1888, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bonnet F, Deprele C, Sassolas A, Moulin P, Alamartine E, Berthezène F, Berthoux F: Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis 37: 720–727, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka M, Tsujii T, Komiya T, Iwasaki Y, Sugishita T, Yonemoto S, Tsukamoto T, Fukui S, Takasu A, Muso E: Clinicopathological influence of obesity in IgA nephropathy: Comparative study of 74 patients. Contrib Nephrol 157: 90–93, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Appel GB, Waldman M: The IgA nephropathy treatment dilemma. Kidney Int 69: 1939–1944, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Eitner F, Ackermann D, Hilgers RD, Floege J: Supportive Versus Immunosuppressive Therapy of Progressive IgA nephropathy (STOP) IgAN trial: Rationale and study protocol. J Nephrol 21: 284–289, 2008 [PubMed] [Google Scholar]
- 27.Floege J, Eitner F: Current therapy for IgA nephropathy. J Am Soc Nephrol 22: 1785–1794, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, Kumar A, Peden JF, Maxwell PH, Morris DL, Padmanabhan S, Vyse TJ, Zawadzka A, Rees AJ, Lathrop M, Ratcliffe PJ: HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol 21: 1791–1797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP: Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praga M: New insights into familial microhematuria. Curr Opin Nephrol Hypertens 8: 173–177, 1999 [Google Scholar]