Abstract
Gain-of-function mutations in the gene encoding the V2 vasopressin receptor (V2R) cause nephrogenic syndrome of inappropriate antidiuresis. To date, reported mutations lead to the substitution of arginine 137 by either a cysteine or leucine (R137C/L). Here, we describe a 3-month-old hyponatremic infant found to have a phenylalanine 229 to valine (F229V) substitution in V2R. Characterization of this substitution in vitro revealed that it leads to high constitutive activity of the receptor, compatible with spontaneous antidiuresis. In contrast to R137C/L mutant receptors, F229V receptors do not undergo spontaneous desensitization, which results in sustained, high basal activity. Notably, the V2R-selective inverse agonists tolvaptan and satavaptan completely silenced the constitutive signaling activity of the F229V mutant receptor, indicating that this substitution does not lock the receptor in an irreversible active state. Thus, inverse agonists might prove to be effective therapies for treating patients with this or other spontaneously activating mutations that do not lock the V2R in its active state. These results emphasize the importance of genetic testing and the functional characterization of mutant receptors for patients with nephrogenic syndrome of inappropriate antidiuresis because the results might inform treatment decisions.
The vasopressin type 2 receptor (V2R) plays a central role in the control of water homeostasis by the kidney. Its activation by arginine-vasopressin (AVP) leads to water reabsorption, an event requiring V2R-promoted cAMP production.1 Inactivating mutations in the V2R gene (AVPR2) cause nephrogenic diabetes insipidus, an X-linked disease characterized by polyuria and polydipsia,2 whereas activating AVP2R mutations are responsible for the nephrogenic syndrome of inappropriate antidiuresis (NSIAD). Patients with NSIAD have reduced free water excretion and concentrated urine despite hyponatremia and low or undetectable circulating AVP levels.3 Such low AVP levels distinguish NSIAD from the syndrome of inappropriate antidiuretic hormone secretion (SIADH), which is usually associated with elevated serum AVP levels.3 In all previously described NSIAD cases, substitution of arginine-137, located at the bottom of transmembrane domain 3 (TM3) (Figure 1A), by either a cysteine or a leucine (R137C/L) induces the spontaneous activation of the V2R3–5 that is responsible for the inappropriate antidiuresis in the absence of elevated AVP. The increased V2R activity is reflected by elevated basal cAMP levels observed in cells expressing the NSIAD mutants compared with the wild-type (WT) receptor.3,4,6
Figure 1.
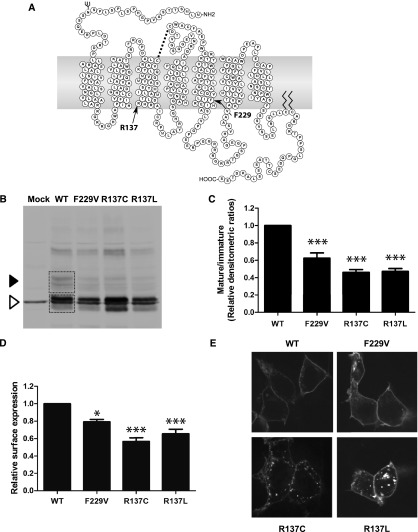
Surface expression and maturation profile of F229V-V2R. (A) Snake plot representation of the human V2R indicating the positions of residue R137 and F229. The residues composing the different transmembrane domains (denoted TMs) were determined using the method described by Abrol et al.13 (B) Western blot analysis performed on total cell lysates using an anti-myc antibody. The black arrowhead indicates the mature forms of the V2R, whereas the empty arrowhead shows the immature forms. (C) Densitometric ratios of mature/immature forms of the receptors obtained via Western blots. The areas used for densitometric quantification of the different bands are depicted with boxes on the WT lane in B. (D) Relative cell surface expression monitored by whole cell ELISA using an anti-myc antibody. (E) Representative confocal microscopy images of cells transfected with the indicated YFP-tagged receptors. The Western blot result shown in B is representative of six independent experiments, and the densitometric ratios in C are the mean ± SEM of three to six independent experiments. Data shown in D are the mean ± SEM of three independent experiments. *P<0.05; ***P<0.001.
In this study, we report the case of a 3-month-old male infant that presented with an episode of apnea associated with a 1 week history of an upper respiratory infection due to respiratory syncytial virus. Initial evaluation revealed hyponatremia (120 mEq/L) with undetectable vasopressin level and evidence of euvolemia (Table 1). He was initially treated with intravenous fluids (D5 0.45 normal saline), but had no improvement in his serum sodium and his BP became mildly elevated. His serum sodium and hypertension improved with fluid restriction. Despite the low level of circulating AVP, the patient was diagnosed with SIADH secondary to bronchiolitis.7 At 6 months, the patient had a second apneic episode, but no laboratory tests were performed. A third apneic episode occurred at 9 months of age, prompting a second hospitalization with additional laboratory evidences of euvolemic hyponatremia (plasma renin activity <20 ng/dl per hour) and again undetectable vasopressin level (Table 1). The patient was diagnosed with NSIAD and the sequencing of his AVPR2 gene revealed a T to G substitution at nucleotide 1046, causing a change from phenylalanine to valine at amino acid position 229 (F229V) located near the bottom of TM5 (Figure 1A).
Table 1.
Patient laboratory results at 3 and 9 months
| Laboratory Result | Aged 3 mo | Aged 9 mo |
|---|---|---|
| Sodium (mEq/L) | 120 | 124 |
| Vasopressin (normal: 0.5–4.7 pg/ml) | <0.5 | <0.5 |
| Creatinine (mg/dl) | 0.2 | 0.2 |
| BUN (mg/dl) | 3 | 2 |
| Urine osmolality (mOsm/L) | 351 | 544 |
| Urine sodium (mEq/L) | 107 | 177 |
To determine how the F229V substitution could contribute to the pathogenesis of NSIAD, a biochemical and functional characterization of F229V-V2R was carried out in HEK293T cells and compared with the previously described R137C/L-V2R. For the three mutants, Western blot analyses showed a reduced proportion of the mature fully glycosylated (45–55 kD) versus the immature core-glycosylated and deglycosylated (40 and 37 kD, respectively) receptor forms compared with the WT-V2R (Figure 1, B and C). Consistent with the reduced maturation observed for the mutant receptors, surface ELISA revealed a reduced expression level for F229V (80% of WT), albeit to a lesser extent than what was observed for R137L (65% of WT) and R137C (57% of WT) (Figure 1D). Visual assessment by confocal fluorescence microscopy (Figure 1E) revealed a similar labeling for the F229V- and WT-V2R, whereas R137C- and R137L-V2R showed an endosomal-like punctate labeling, consistent with their previously described increased constitutive endocytosis.4,8 Taken together, these results indicate that F229V-V2R has a reduced cell surface expression, resulting from an impaired maturation and, most likely, not from increased endocytosis. However, the reduced maturation efficiency and cell surface targeting of the F229V-V2R cannot account for NSIAD because decreased cell surface expression is expected to lead to a loss of function disease (nephrogenic diabetes insipidus).
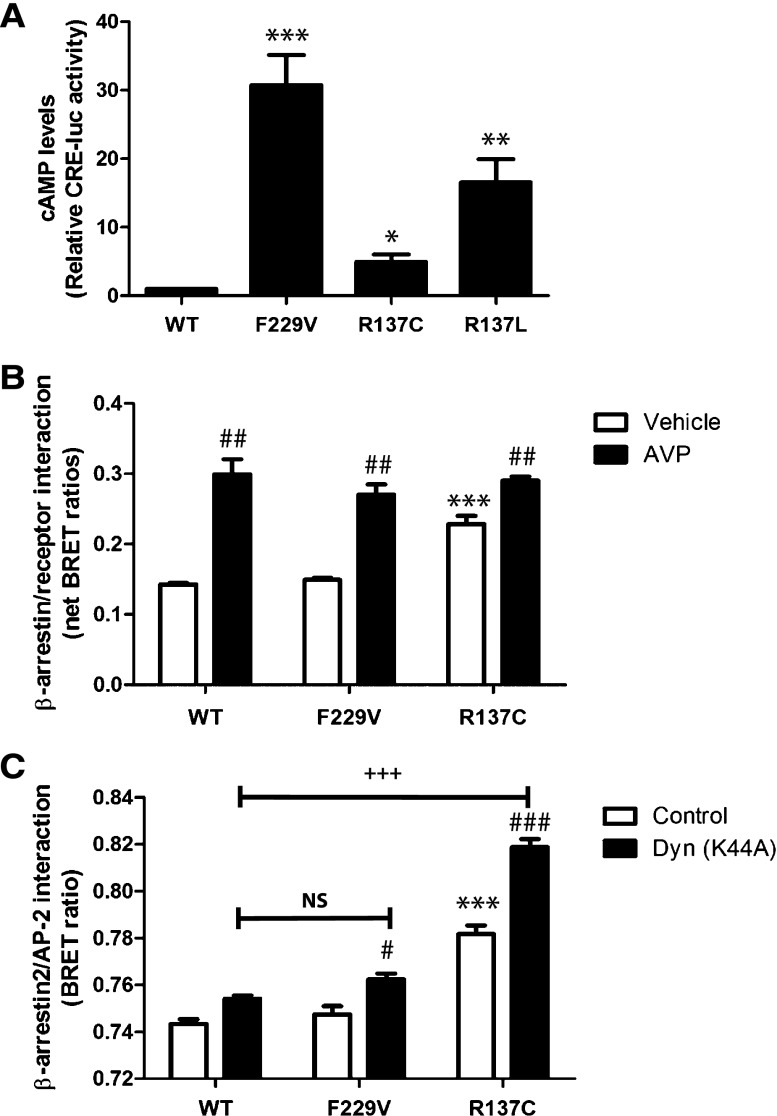
The signaling activity of F229V-V2R was assessed by measuring cAMP levels in cells expressing either WT-, F229V-, R137C-, or R137L-V2R using a gene reporter assay (CRE-luciferase; Figure 2A). The F229V-V2R promoted a significantly higher level of basal cAMP level than the WT-V2R, demonstrating that the mutation increases constitutive activity. The constitutive F229V-V2R basal cAMP production was approximately 31-fold greater than WT level, compared with only 5- and 17-fold for R137C- and R137L-V2R, respectively (Figure 2A).
Figure 2.
Constitutive activity and desensitization of the F229V-V2R. (A) Basal cAMP measured by the CRE-luciferase reporter assay in cells expressing the indicated V2R. (B) BRET-based monitoring of the interaction between β-arrestin2-RLuc and the indicated YFP-tagged V2R, under basal condition (vehicle) or upon stimulation with 1 μM AVP for 15 minutes. (C) BRET-based monitoring of the basal interaction between β-arrestin2-RLuc and AP2-YFP in cells expressing WT- or the indicated mutant V2R in the presence or absence (control) of Dyn(K44A). Receptor amounts were quantified in this experiment by saturation binding, yielding an average of approximately 52,000, 35,400, and 15,600 receptors per cell for WT, F229V, and R137C, respectively. Data are the mean ±SEM of three independent experiments. *Comparison of mutant receptors with WT; #comparison of treated samples with control; +comparison of treated mutants with treated WT receptor. *,#P<0.05; **,##P<0.01; ***,###,+++P<0.001. NS, nonsignificant.
Previous studies have shown that the activating R137L/C substitutions lead to constitutive desensitization and endocytosis of the receptor via an increase in spontaneous recruitment of the regulatory protein β-arrestin4–6 and subsequent interaction with the clathrin adaptor protein AP-2.4 Using a bioluminescence resonance energy transfer (BRET)–based assay, no increased constitutive recruitment of β-arrestin2 was observed for F229V, which is in contrast to the results obtained for R137C (Figure 2B). Similar results were obtained when monitoring receptor-promoted β-arrestin2/AP-2 assembly by BRET (Figure 2C). Such increased β-arrestin and AP-2 engagement were observed despite a significantly lower expression level of R137C-V2R compared with both WT- and F229V-V2R (Figure 2, legend). Inhibition of endocytosis with a dominant-negative mutant of dynamin-2 (DynK44A) potentiated the basal BRET signal between β-arrestin2 and AP-2, emphasizing the occurrence of constitutive endocytosis.9 The DynK44A-promoted increased BRET signal observed for R137C-V2R was much greater than for the WT receptor, whereas no such difference was observed for F229V-V2R (Figure 2C), indicating that the F229V substitution does not increase constitutive endocytosis. Collectively, these results show that F229V-V2R does not undergo elevated constitutive desensitization, thus providing an explanation for the much higher basal cAMP level observed in cells expressing F229V-V2R compared with R137C/L-V2R (Figure 2A). The reduced constitutive β-arrestin recruitment and AP-2 engagement could not arise from an intrinsic defect of F229V-V2R to recruit β-arrestin2 because AVP stimulation increased interactions between β-arrestin2 and F229V-V2R, similar to the WT receptor (Figure 2B). Thus, the F229V substitution promotes a receptor state that possesses high constitutive activity toward adenylyl cyclase while not affecting β-arrestin recruitment. Such a biased effect of a mutation is consistent with the notion that these two pathways can be controlled independently by distinct receptor conformational changes.10–12 To provide a structural basis for constitutive activation mechanism, we used a de novo structure prediction methodology called GEnSeMBLE (GPCR Ensemble of Structures in Membrane BiLayer Environment)13 to predict the most stable conformations for the WT, F229V and R137C receptors. As shown in Supplemental Figure 1, the modeling predicted differences between the lowest energy structures obtained for the three receptors. Most notably, the F229V mutation resulted in a significant outward movement of TM6 away from TM3 and TM5 (compared with WT-V2R), which is classically associated with GPCR activation.14 Despite an uncoupling of TM6 from TM3, no such obvious movement was observed for the R137C-V2R structure. Instead an inward movement of TM2 was observed. Additional studies will be needed to assess the contribution of these structural differences to the different constitutive activation profiles of the receptor.
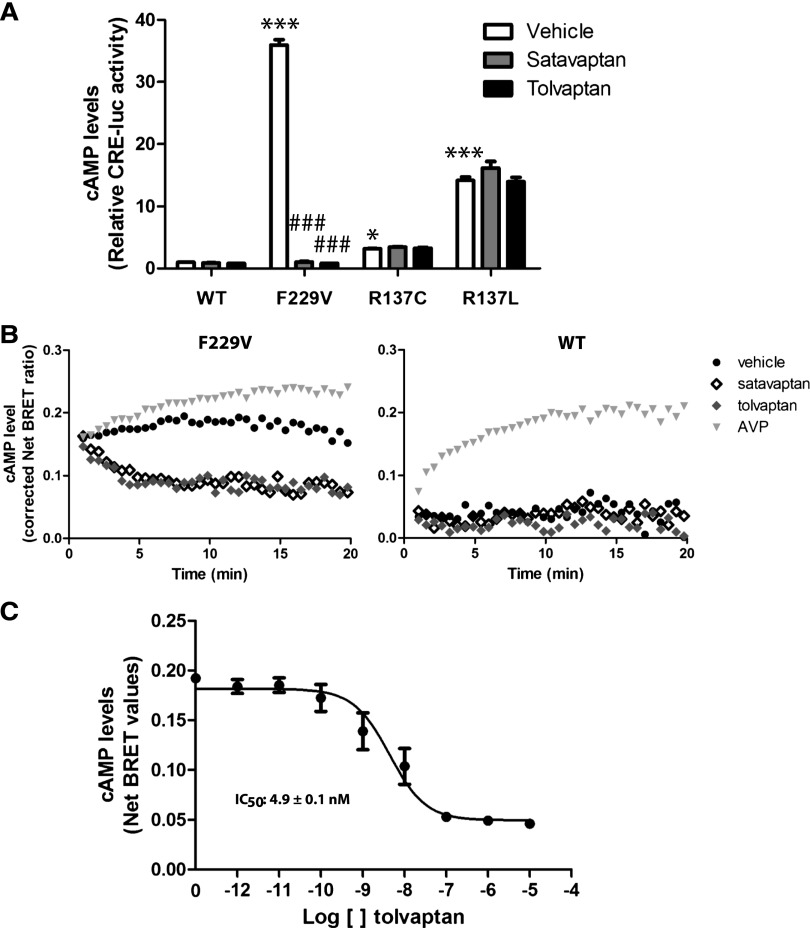
The V2R antagonist satavaptan acts as an inverse agonist by inhibiting basal V2R-promoted cAMP production.10,15 However, attempts to reduce the constitutive activity of the NSIAD-causing R137C/L-V2R by vaptans were unsuccessful, either in vitro4 or in a patient harboring the R137C substitution,16 strongly suggesting that these receptors were “locked” in their active state. Consistent with this notion, AVP stimulation did not promote further cAMP production in cells expressing R137C- or R137L-V2R.4 To determine if F229V-V2R is also locked in an active conformation, we assessed the effect of satavaptan and the recently US Food and Drug Administration–approved tolvaptan (OPC-41061).17 An 18-hour treatment with either compound significantly reduced the F229V-V2R–promoted constitutive cAMP production (Figure 3A) while having no effect on the cAMP levels in cells expressing the WT-, R137C-, or R137L-V2R. As shown in Figure 3B, the inhibitory effect of both satavaptan and tolvaptan occurred rapidly after drug addition, reaching their maximal effect within 5 minutes. A concentration-response curve performed for tolvaptan (Figure 3C) revealed an IC50 of 4.9±0.1 nM for its inverse agonist activity on the spontaneous activity of F229V-V2R, a potency most likely compatible with its used in clinical setting. These data indicate that both compounds can silence the high constitutive activity of F229V-V2R, a finding that contrasts with observations made for the two other NSIAD-causing V2R mutants.4 Consistent with the notion that F229V-V2R is not in a locked active conformation, AVP promoted further cAMP increase, (Figure 3B, gray triangles). This indicates that this substitution, while increasing constitutive activity, maintains a receptor conformation that is amenable to pharmacological modulation.
Figure 3.
Silencing of F229V-V2R. (A) cAMP levels assessed using the CRE-luciferase reporter assay in cells expressing WT-, F229V-, R137C-, or R137L-V2R, under basal condition (vehicle) or after an 18-hour treatment with satavaptan or tolvaptan (10 μM). (B) Time-dependent changes in cAMP levels assessed using the BRET-based exchange protein directly activated by cAMP (EPAC) biosensor on cells expressing F229V- (left panel) or WT-V2R (right panel) in the presence of either vehicle, AVP (1 μM), satavaptan, or tolvaptan (10 μM each). (C) Dose-response curve of tolvaptan on cells expressing F229V-V2R using the BRET-based EPAC biosensor. Data are the mean ± SEM of three independent experiments (A and C) or representative of three independent experiments (B). *Comparison of mutant receptors with WT; #comparison of treated samples with control. *P<0.05; ***,###P<0.001.
In addition to unraveling the molecular basis underlying NSIAD resulting from F229V-V2R, our study clearly shows that inverse agonists such as satavaptan and tolvaptan are promising candidates for the treatment of patients carrying this mutation or new constitutively activating mutations that do not maintain the receptor in a locked active state. This is particularly important when considering that 10%–20% of patients diagnosed with SIADH have undetectable serum AVP levels upon water restriction,18 suggesting that some of these patients may in fact have NSIAD due to an activating mutation in their AVPR2 gene.3 Finally, our study demonstrates that a disease resulting from distinct mutations of the same gene may respond differently to a given therapy, highlighting the importance of a clear understanding of the functional consequences of the mutations that will allow appropriate personalized medicine.
Concise Methods
Microscopy, Cell Surface, and Total Receptor Expression
Microscopy images were taken from cells expressing the different YFP-tagged constructs (WT-V2R-YFP,19 F229V-V2R-YFP [generated by site-directed mutagenesis of the WT-V2R-YFP], R137C-V2R-YFP, and R137L-V2R-YFP4) using a Zeiss LSM 510 Laser Scanning Confocal Microscope (Jena, Germany). Cell surface receptor expression was assessed by surface ELISA detecting the transiently expressed myc-tagged V2R constructs (myc-WT-V2R,20 myc-R137C-V2R, myc-R137L-V2R, and myc-F229V-V2R; generated by site-directed mutagenesis of myc-WT-V2R using the Quick Chang mutation kit from Agilent Technologies, Santa Clara, CA). Functional assays (AVP binding affinity and EC50 of cAMP production) of the YFP- and myc-tagged WT-V2R were performed and results were found similar to the untagged version (Supplemental Table 1). ELISAs were performed as previously described9 using a mouse anti-myc antibody (9E10 clone) produced by our core facility as ascites fluids and an AP-conjugated goat anti-mouse (Bio-Rad, Hercules, CA) secondary antibody. Total receptor expression was assessed by Western blot analysis performed on total lysates of cells expressing the myc-tagged receptors using the mouse anti-myc antibody and a horseradish peroxidase–conjugated rabbit anti-myc secondary antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) as previously described.21
cAMP Measurements
Intracellular cAMP of cells expressing the different myc-tagged V2R constructs was monitored using either the CRE-luciferase reporter assay (pCRE-luciferase) purchased from Clontech (Mountain View, CA) as described4 or the exchange protein directly activated by cAMP protein-based bioluminescence resonance energy transfer biosensor.4
Engagement of the Endocytic Machinery
The β-arrestin2-Rluc/receptor-YFP and β-arrestin2-Rluc/AP-2-YFP interactions were monitored by BRET, as previously described.4 For β-arrestin2-Rluc/receptor interaction, YFP-tagged constructs were cotransfected with the β-arrestin2-Rluc construct9 in HEK293T cells and exposed or not to 1 μM AVP for 15 minutes before BRET reading. For β-arrestin2-Rluc/AP-2-YFP interaction, HEK293T cells stably expressing the AP-2-YFP construct9 were cotransfected with β-arrestin2-Rluc along with the indicated myc-tagged receptor construct, with or without the dominant-negative Dynamin2 (DynK44A) construct.9 BRET measurements were performed 48 hours post-transfection.
Structural Modeling
A de novo structure prediction methodology called GEnSeMBLE13 was used to predict the most stable conformations for WT-, F229V-, and R137C-V2R. The methodology is summarized in the Supplemental Material.
Statistical Analyses
Data are presented as mean ± SEM, and statistical significance of the differences were assessed by ANOVA. Pair-wise comparisons were made by the post hoc Bonferroni multiple comparison test. Differences with P<0.05 were considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to Dr. Serradeil-Le-Gal from Sanofi-Aventis for kindly providing satavaptan (SR121463) and Drs. Komuro and Czerwiec from Otsuka Pharmaceutical for their generous gift of tolvaptan (OPC41061). We also thank Christian Charbonneau for his expertise and help in confocal microscopy and Dr. Monique Lagacé for her critical reading of the manuscript.
This work was supported by grants from the Kidney Foundation of Canada and the Canadian Institutes of Health Research to M.B. E.C was supported by a doctoral studentship from the Fond de la Recherche en Santé du Québec. M.B. holds a Canada Research Chair in Signal Transduction and Molecular Pharmacology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012010077/-/DCSupplemental.
References
- 1.Ball SG: Vasopressin and disorders of water balance: The physiology and pathophysiology of vasopressin. Ann Clin Biochem 44: 417–431, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Morello JP, Bichet DG: Nephrogenic diabetes insipidus. Annu Rev Physiol 63: 607–630, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Feldman BJ, Rosenthal SM, Vargas GA, Fenwick RG, Huang EA, Matsuda-Abedini M, Lustig RH, Mathias RS, Portale AA, Miller WL, Gitelman SE: Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med 352: 1884–1890, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochdi MD, Vargas GA, Carpentier E, Oligny-Longpré G, Chen S, Kovoor A, Gitelman SE, Rosenthal SM, von Zastrow M, Bouvier M: Functional characterization of vasopressin type 2 receptor substitutions (R137H/C/L) leading to nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis: implications for treatments. Mol Pharmacol 77: 836–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tenenbaum J, Ayoub MA, Perkovska S, Adra-Delenne AL, Mendre C, Ranchin B, Bricca G, Geelen G, Mouillac B, Durroux T, Morin D: The constitutively active V2 receptor mutants conferring NSIAD are weakly sensitive to agonist and antagonist regulation. PLoS ONE 4: e8383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocan M, See HB, Sampaio NG, Eidne KA, Feldman BJ, Pfleger KD: Agonist-independent interactions between beta-arrestins and mutant vasopressin type II receptors associated with nephrogenic syndrome of inappropriate antidiuresis. Mol Endocrinol 23: 559–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna S, Tibby SM, Durward A, Murdoch IA: Incidence of hyponatraemia and hyponatraemic seizures in severe respiratory syncytial virus bronchiolitis. Acta Paediatr 92: 430–434, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Kocan M, See HB, Sampaio NG, Eidne KA, Feldman BJ, Pfleger KD: Agonist-independent interactions between beta-arrestins and mutant vasopressin type II receptors associated with nephrogenic syndrome of inappropriate antidiuresis. Mol Endocrinol 23: 559–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamdan FF, Rochdi MD, Breton B, Fessart D, Michaud DE, Charest PG, Laporte SA, Bouvier M: Unraveling G protein-coupled receptor endocytosis pathways using real-time monitoring of agonist-promoted interaction between beta-arrestins and AP-2. J Biol Chem 282: 29089–29100, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G: Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci U S A 100: 11406–11411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galandrin S, Oligny-Longpré G, Bouvier M: The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28: 423–430, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Violin JD, Lefkowitz RJ: Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28: 416–422, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Abrol R, Griffith AR, Bray JK, Goddard WA, 3rd: Structure Prediction of G Protein-Coupled Receptors and Their Ensemble of Functionally Important conformations. In: Membrane Protein Structure: Methods and Protocols (Methods in Molecular Biology) Edited by Vaidehi N, Klein-Seetharaman J, New York, Humana, Vol. 914, pp. 237–254 [DOI] [PubMed] [Google Scholar]
- 14.Abrol R, Kim SK, Bray JK, Griffith AR, Goddard WA, 3rd: Characterizing and predicting the functional and conformational diversity of seven-transmembrane proteins. Methods 55: 405–414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin D, Cotte N, Balestre MN, Mouillac B, Manning M, Breton C, Barberis C: The D136A mutation of the V2 vasopressin receptor induces a constitutive activity which permits discrimination between antagonists with partial agonist and inverse agonist activities. FEBS Lett 441: 470–475, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Decaux G, Vandergheynst F, Bouko Y, Parma J, Vassart G, Vilain C: Nephrogenic syndrome of inappropriate antidiuresis in adults: High phenotypic variability in men and women from a large pedigree. J Am Soc Nephrol 18: 606–612, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ghali JK, Hamad B, Yasothan U, Kirkpatrick P: Tolvaptan. Nat Rev Drug Discov 8: 611–612, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Zerbe R, Stropes L, Robertson G: Vasopressin function in the syndrome of inappropriate antidiuresis. Annu Rev Med 31: 315–327, 1980 [DOI] [PubMed] [Google Scholar]
- 19.Charest PG, Bouvier M: Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances beta-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem 278: 41541–41551, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Morello JP, Salahpour A, Laperrière A, Bernier V, Arthus MF, Lonergan M, Petäjä-Repo U, Angers S, Morin D, Bichet DG, Bouvier M: Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest 105: 887–895, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bockenhauer D, Carpentier E, Rochdi D, Van't Hoff W, Breton B, Bernier V, Bouvier M, Bichet DG: Vasopressin type 2 receptor V88M mutation: Molecular basis of partial and complete nephrogenic diabetes insipidus. Nephron Physiol 114: p1–p10, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Breton B, Sauvageau E, Zhou J, Bonin H, Le Gouill C, Bouvier M: Multiplexing of multicolor bioluminescence resonance energy transfer. Biophys J 99: 4037–4046, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.