Abstract
Heart failure is a common consequence of CKD, and it portends high risk for mortality. However, among patients without known heart failure, the associations of different stages of estimated GFR (eGFR) with changes in cardiac structure and function are not well described. Here, we performed a cross-sectional analysis to study these associations among 3487 participants of the Chronic Renal Insufficiency Cohort Study. We estimated GFR using cystatin C. The prevalence of left ventricular hypertrophy (LVH) assessed by echocardiography was 32%, 48%, 57%, and 75% for eGFR categories ≥60, 45–59, 30–44, and <30 ml/min per 1.73 m2, respectively. In fully adjusted multivariable analyses, subjects with eGFR levels of <30 ml/min per 1.73 m2 had twofold higher odds of LVH (OR=2.20, 95% CI=1.40–3.40; P<0.001) relative to subjects with eGFR≥60 ml/min per 1.73 m2. This reduction in kidney function also significantly associated with abnormal LV geometry but not diastolic or systolic dysfunction. An eGFR of 30–44 ml/min per 1.73 m2 also significantly associated with LVH and abnormal LV geometry compared with eGFR≥60 ml/min per 1.73 m2. In summary, in this large CKD cohort, reduced kidney function associated with abnormal cardiac structure. We did not detect significant associations between kidney function and systolic or diastolic function after adjusting for potential confounding variables.
CKD is associated with increased cardiovascular risk and mortality1 and increased incidence of heart failure (HF).2 Even a small reduction in kidney function is associated with higher risk of cardiovascular events, and the strongest association is typically with HF.3 Recent studies have shown an association between kidney function and HF risk across a wide spectrum of disease stages from preclinical kidney disease to advanced CKD.4,5 To evaluate kidney function, these studies used cystatin C, an alternative marker of kidney function that is not affected by muscle mass.6
The pathogenesis of HF in persons with CKD has not been well characterized but likely relates to a combination of cardiac abnormalities and volume handling.7 Among patients without HF, kidney function has been linked with structural cardiac changes, including left ventricular hypertrophy (LVH).8,9 Previous studies have evaluated the association of kidney function with LVH in patients with advanced disease approaching and requiring dialysis10,11 as well as patients with earlier stages of disease8; LVH has been reported in over one-third of persons with CKD.9 Diastolic dysfunction has also been studied in small cohorts of CKD patients.12,13 However, no study has characterized the associations of different stages of estimated GFR (eGFR) with the range of changes in cardiac structure and function in a large cohort of patients with established CKD. This study is an important first step in testing the hypothesis that cardiac structural changes are a key factor in the pathogenesis leading from reduced kidney function to accelerating HF risk. We hypothesize that subclinical cardiac changes start in the early stages of CKD and that this association strengthens with more advanced kidney disease.
In the Chronic Renal Insufficiency Cohort (CRIC) Study, we conducted a cross-sectional analysis of echocardiogram findings among subjects with established CKD and no known HF. Our primary objective was to characterize and compare the associations of kidney function with LV mass, LVH, LV geometry, diastolic dysfunction, and systolic dysfunction using measurements of eGFR by both cystatin C (eGFRcys) and creatinine (eGFRcr).
Results
Participant Characteristics
Among the 3487 participants with serum cystatin C measurements and without HF, mean age was 59±11 years; 45% of the cohort were women, and 40% were black (Table 1). Mean ± SD eGFRcys was 50±20 ml/min per 1.73 m2, and mean eGFRcr was 41±15 ml/min per 1.73 m2. Compared with participants with eGFRcys≥60 ml/min per 1.73 m2, participants with the lowest level of kidney function by cystatin C were more likely to have diabetes and hypertension and be smokers. They also had, on average, higher body mass index (BMI), systolic BP, triglycerides, and albuminuria and lower HDL cholesterol and hemoglobin levels.
Table 1.
Characteristics of CRIC participants without HF by eGFRcys
| eGFRcys≥60 (n=953) | eGFRcys=45–59 (n=952) | eGFRcys=30–44 (n=1073) | eGFRcys<30 (n=509) | P Value | |
|---|---|---|---|---|---|
| Mean age in years (SD) | 56 (11) | 60 (11) | 60 (11) | 60 (11) | <0.001 |
| Female (%) | 40 | 43 | 48 | 53 | <0.001 |
| Race/ethnicity (%) | <0.001 | ||||
| Non-Hispanic white | 48 | 45 | 41 | 35 | |
| Non-Hispanic black | 41 | 40 | 40 | 39 | |
| Hispanic | 7 | 11 | 16 | 23 | |
| Other | 5 | 4.52 | 3 | 4 | |
| Mean BMI (SD) | 30.4 (6.5) | 32.0 (7.9) | 32.5 (8.0) | 33.1 (9.0) | <0.001 |
| Diabetes (%) | 30 | 45 | 53 | 59 | <0.001 |
| Hypertension (%) | 77 | 91 | 93 | 96 | <0.001 |
| Any cardiovascular disease (%) | 19.5 | 27.3 | 33.4 | 36.2 | <0.001 |
| Peripheral vascular disease (%) | 4 | 4 | 9 | 11 | <0.001 |
| Mean hemoglobin (g/dl; SD) | 13.7 (1.6) | 13.0 (1.7) | 12.3 (1.6) | 11.7 (1.8) | <0.001 |
| Mean high-sensitivity CRP (mg/L; SD) | 3.7 (6.0) | 4.7 (6.1) | 6.3 (11.3) | 7.8 (14.4) | <0.001 |
| Mean LDL cholesterol (mg/dl; SD) | 106 (34) | 100 (33) | 99 (34) | 96 (38) | <0.001 |
| Mean HDL cholesterol (mg/dl; SD) | 51 (16) | 49 (16) | 48 (16) | 45 (15) | <0.001 |
| Mean triglycerides (mg/dl; SD) | 140 (103) | 152 (100) | 162 (108) | 169 (108) | <0.001 |
| Mean total cholesterol (mg/dl; SD) | 187 (42) | 183 (42) | 183 (45) | 180 (48) | <0.001 |
| Mean serum albumin (g/dl; SD) | 4.2 (0.4) | 4.0 (0.4) | 4.0 (0.4) | 3.8 (0.5) | <0.001 |
| Median urinary alb/cre ratio (mcg/mg; IQR) | 10.4 (4.2–55.0) | 31.4 (7.5–275.5) | 137.7 (19.6–809.6) | 394.5 (53.2–1604.3) | <0.001 |
| β-Blockers (%) | 34 | 50 | 52 | 60 | <0.001 |
| Calcium channel blockers (%) | 31 | 39 | 45 | 55 | <0.001 |
| Diuretics (%) | 42 | 56 | 63 | 72 | <0.001 |
| ACE inhibitors (includes ARBs; %) | 60 | 74 | 72 | 63 | <0.001 |
| Median FGF-23 (RU/ml; IQR) | 90.7 (67.4–118.5) | 128.1 (92.3–173.9) | 178.8 (121.9–253.3) | 291.0 (183.9–424.6) | <0.001 |
| Median phosphate (mg/dl; IQR) | 3.4 (3.1–3.8) | 3.6 (3.2–3.9) | 3.8 (3.4–4.2) | 4.2 (3.7–4.7) | <0.001 |
| Median calcium (mg/dl; IQR) | 9.2 (9.0–9.5) | 9.2 (8.9–9.5) | 9.2 (8.8–9.5) | 9.0 (8.7–9.4) | <0.001 |
| Median total PTH (pg/ml; IQR) | 36.9 (28.0–50.0) | 46.5 (32.0–70.0) | 65.0 (42.0–103.0) | 103.2 (60.0–172.0) | <0.001 |
| Current smoker (%) | 8 | 13 | 15 | 18 | <0.001 |
| Cocaine (%) | 2.5 | 1.8 | 1.6 | 2.2 | <0.001 |
| Mean alcohol DHQ (g; SD) | 7.4 (19.4) | 6.7 (25.5) | 6.4 (24.3) | 3.3 (13.7) | <0.001 |
| Mean systolic BP (mmHg; SD) | 128.7 (19.0) | 133.5 (19.3) | 135.2 (21.4) | 138.9 (22.3) | <0.001 |
| Mean diastolic BP (mmHg; SD) | 74.8 (12.6) | 75.1 (11.8) | 74.9 (12.2) | 74.7 (12.5) | 0.89 |
P values were obtained using ANOVA or Kruskal–Wallis test for continuous variables and chi-squared test for categorical variables. IQR, interquartile range; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; DHQ, diet history questionnaire.
Kidney Function and Structural Heart Abnormalities
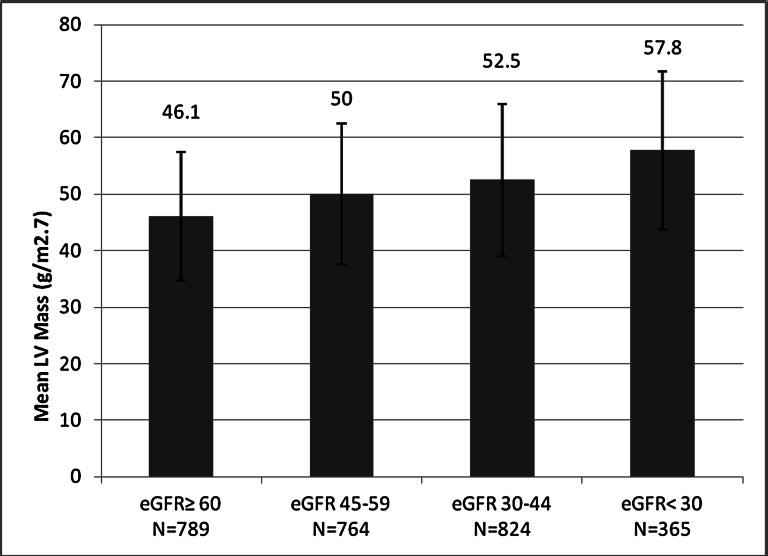
We observed a linear association between categories of progressively worse kidney function and higher LV mass. Mean ± SD LV mass in the lowest category of kidney function (eGFRcys<30 ml/min per 1.73 m2) was 57.8±14.0 g/m2.7 compared with 46.1±11.4 g/m2.7 in the group with eGFRcys≥60 ml/min per 1.73 m2, an average difference of 12 g/m2.7 (Figure 1 and Supplemental Figure 1). Demographically adjusted linear regression models revealed a strong association of all categories of eGFR and LV mass (Table 2). After adjusting for confounders, the association was attenuated by approximately 33% in the eGFRcys<30 category, 44% in the eGFRcys=30–44 category, and lost significance in the eGFRcys=45–59 category (Table 2). After full adjustment, including mediators such as anemia and hypertension and markers of mineral metabolism, there remained a significant strong association between eGFRcys<30 ml/min per 1.73 m2 and LV mass/height2.7.
Figure 1.
Mean LV mass (g/m2.7) increases across categories of declining eGFR by cystatin C.
Table 2.
Association of categories of eGFR by cystatin C and LV structure among persons with CKD and without HF
| eGFRcys≥60 (n=953) | eGFRcys=45–59 (n=952) | eGFRcys=30–44 (n=1073) | eGFRcys<30 (n=509) | |
|---|---|---|---|---|
| LV mass absolute change (95% CI) | ||||
| Demographic adjusted only | Reference | 2.50 (1.30–3.80) | 4.80 (3.50–6.00) | 9.10 (7.50–10.70) |
| P<0.001 | P<0.001 | P<0.001 | ||
| Multivariable adjusteda | Reference | 0.80 (−0.30–1.90) | 2.70 (1.50–3.80) | 6.10 (4.60–7.70) |
| P=0.17 | P<0.001 | P<0.001 | ||
| Fully adjustedb | Reference | −0.01 (−1.20–1.20) | 0.90 (−0.30–2.20) | 3.60 (1.90–5.30) |
| P=0.99 | P=0.15 | P<0.001 | ||
| Fully adjusted plus FGF-23, phosphate, calcium, and PTHb | Reference | −0.003 (−1.20–1.20) | 0.70 (−0.70–2.00) | 2.50 (0.60–4.40) |
| P=1.00 | P=0.34 | P=0.008 | ||
| LVH OR (95% CI) | ||||
| Demographically adjusted only | Reference | 1.70 (1.40–2.10) | 2.30 (1.80–2.90) | 4.90 (3.60–6.60) |
| P<0.001 | P<0.001 | P<0.001 | ||
| Multivariable adjustedc | Reference | 1.40 (1.10–1.90) | 1.90 (1.50–2.50) | 4.00 (2.80–5.60) |
| P=0.004 | P<0.001 | P<0.001 | ||
| Fully adjustedb | Reference | 1.30 (1.00–1.70) | 1.40 (1.10–1.90) | 2.70 (1.80–3.90) |
| P=0.06 | P=0.01 | P<0.001 | ||
| Fully adjusted plus FGF-23, phosphate, calcium, and PTHb | Reference | 1.30 (1.00–1.70) | 1.40 (1.00–1.90) | 2.20 (1.40–3.40) |
| P=0.07 | P=0.04 | P<0.001 | ||
| Abnormal LV geometry OR (95% CI) | ||||
| Demographically adjusted only | Reference | 1.70 (1.30–2.10) | 2.30 (1.80–2.90) | 4.90 (3.60–6.70) |
| P<0.001 | P<0.001 | P<0.001 | ||
| Multivariable adjustedc | Reference | 1.40 (1.10–1.90) | 1.90 (1.50–2.50) | 4.10 (2.80–5.80) |
| P=0.005 | P<0.001 | P<0.001 | ||
| Fully adjustedb | Reference | 1.30 (1.00–1.70) | 1.40 (1.10–1.90) | 2.80 (1.80–4.10) |
| P=0.06 | P=0.01 | P<0.001 | ||
| Fully adjusted plus FGF-23, phosphate, calcium, and PTHb | Reference | 1.30 (1.00–1.70) | 1.40 (1.00–1.80) | 2.20 (1.40–3.50) |
| P=0.09 | P=0.05 | P<0.001 |
We do not report estimates for missing values of eGFR. A full list of potential covariates tested in univariate analyses includes age, sex, race, site, hypertension (htn), diabetes mellitus (dm), LDL, HDL, high cholesterol, hemoglobin categories, serum albumin, any cardiovascular (CV) events, peripheral vascular disease (PVD), high-sensitivity CRP (hsCRP), current smoker, cocaine use, BMI categories, and albuminuria categories. Demographically adjusted models are adjusted for age, sex, race, and site. Multivariable-adjusted models include variables with P<0.05 in univariate models; demographics are forced in the model.
Adjusted for age, sex, race, site, htn, dm, LDL, HDL, serum albumin, any CV events, PVD, hsCRP, current smoker, cocaine use, and BMI categories.
Additionally adjusted for albuminuria and hemoglobin categories.
Adjusted for age, sex, race, site, htn, dm, LDL, HDL, high cholesterol, serum albumin, any CV events, PVD, hsCRP, and BMI categories.
Overall, there was a high prevalence of LVH (50% for total cohort, with 32%, 49%, 57%, and 75% in each category from highest to lowest kidney function). In a demographically adjusted model, associations of eGFRcys with LVH were significant at all levels of kidney function, with fivefold odds for the lowest eGFR group (Table 2). After multivariable adjustment, all categories of eGFRcys remained strongly associated with LVH. After full multivariable adjustment, eGFRcys categories=30–44 and <30 remained associated with LVH. Additional adjustment by mediators, including hypertension and hemoglobin, slightly attenuated the association for eGFRcys<30 ml/min per 1.73 m2 with LVH, but a significant threefold odds remained after full adjustment. Adjustment for markers of mineral metabolism, including fibroblast growth factor (FGF)-23, resulted in minimal additional attenuation (Table 2). These relationships were similar for whites (odds ratio [OR]=1.80, 95% confidence interval [CI]=0.90–3.60; P=0.08) and blacks (OR=2.20, 95% CI=1.10–4.50; P=0.03).
In this cohort, 21% of patients had normal LV geometry, 30% of patients had concentric remodeling, 13% of patients had eccentric hypertrophy, and 36% of patients had concentric hypertrophy. The lowest category of eGFR had the most concentric hypertrophy (Figure 2). Similar to the LVH findings, there was a significant association of all levels of kidney function with abnormal LV geometry after demographic adjustment (Table 2). After multivariable analysis, associations of all eGFRcys categories with abnormal LV geometry were significant, and after full adjustment, eGFRcys=30–44 and <30 ml/min per 1.73 m2 remained associated with a 1.4-fold and 2.2-fold odds of abnormal LV geometry, respectively.
Figure 2.
Categories of LV geometry by level of eGFRcys. Prevalence of normal geometry declines and of concentric hypertrophy increases across categories of declining eGFR. P value for trend is <0.001.
Kidney Function and Functional Heart Abnormalities
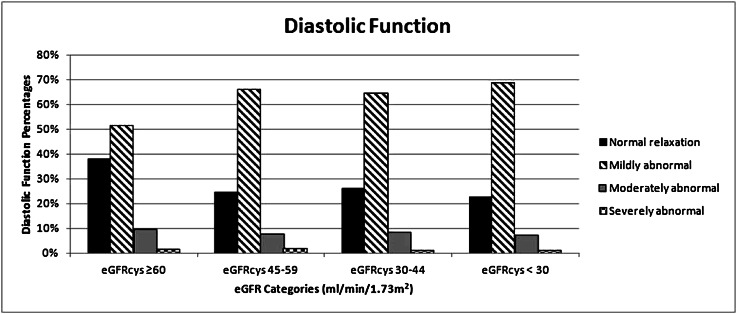
Diastolic function was normal in only 29% of the total cohort (with a distribution of 38%, 25%, 26%, and 23% of participants with eGFR≥60, 45–59, 30–44, and <30 ml/min per 1.73 m2, respectively) (Figure 3). The majority of the cohort had mildly abnormal diastolic relaxation (62%), with a minority categorized as moderately (8%) or severely abnormal (1%). LVH was correlated with diastolic dysfunction and systolic dysfunction, but diastolic dysfunction and systolic dysfunction were not correlated. Because the Brant test (postordinal logit model test) showed that the proportional odds assumption was violated, we examined the association between eGFR and dichotomized diastolic dysfunction. All levels of eGFR were associated with LV diastolic dysfunction after demographic adjustment (Table 3). After multivariable adjustment, there was a significant association in the category of 45–59 only. After full adjustment, we found significantly increased odds (1.5-fold) of diastolic dysfunction in the lowest category of eGFR that was attenuated by adjustment for parathyroid hormone (PTH), which was the only marker of mineral metabolism to enter into the model. A sensitivity analysis comparing the overall cohort to those patients excluded from the cohort because of missing diastolic function measurements showed no difference between these groups.
Figure 3.
Categories of diastolic function by level of eGFRcys. The majority has mildly abnormal diastolic relaxation. P value for trend is <0.001.
Table 3.
Association of categories of eGFR by cystatin C with diastolic and systolic dysfunction among persons with CKD and without HF
| eGFRcys≥60 | eGFRcys=45–59 | eGFRcys=30–44 | eGFRcys<30 | |
|---|---|---|---|---|
| Diastolic dysfunction odds ratio (95% CI) dichotomized outcome (normal versus other) | ||||
| n | 782 | 813 | 887 | 406 |
| Demographically adjusted | Reference | 1.50 (1.20–1.90) | 1.30 (1.00–1.70) | 1.50 (1.10–2.00) |
| P=0.001 | P=0.02 | P=0.008 | ||
| n | 741 | 765 | 830 | 388 |
| Multivariable adjusteda | Reference | 1.40 (1.10–1.80) | 1.20 (1.00–1.60) | 1.40 (1.00–1.90) |
| P=0.006 | P=0.10 | P=0.07 | ||
| n | 741 | 765 | 830 | 388 |
| Fully adjustedb | Reference | 1.40 (1.10–1.80) | 1.30 (1.00–1.70) | 1.50 (1.00–2.00) |
| P=0.005 | P=0.05 | P=0.03 | ||
| Plus PTHb | Reference | 1.50 (1.10–1.90) | 1.20 (0.90–1.60) | 1.30 (0.90–1.90) |
| P=0.004 | P=0.1 | P=0.15 | ||
| Systolic dysfunction odds ratio (95% CI) | ||||
| n | 878 | 859 | 925 | 423 |
| Demographically adjusted | Reference | 1.00 (0.70–1.50) | 1.00 (0.70–1.40) | 1.30 (0.90–2.00) |
| P=0.85 | P=0.82 | P=0.21 | ||
| n | 835 | 807 | 869 | 404 |
| Multivariable adjustedc | Reference | 1.00 (0.70–1.40) | 0.90 (0.60–1.40) | 1.20 (0.80–1.90) |
| P=0.83 | P=0.69 | P=0.40 | ||
| n | 795 | 772 | 822 | 372 |
| Fully adjustedd | Reference | 0.90 (0.60–1.40) | 0.90 (0.60–1.30) | 1.00 (0.60–1.70) |
| P=0.74 | P=0.47 | P=0.96 | ||
| Plus calciumd | Reference | 1.00 (0.60–1.40) | 0.90 (0.60–1.30) | 1.00 (0.60–1.60) |
| P=0.81 | P=0.49 | P=0.88 |
Demographically adjusted models are adjusted for age, sex, race, and site. Multivariable-adjusted models include variables with P<0.05 in univariate models; demographics are forced in the model.
Adjusted for age, sex, race, site, hypertension, diabetes, LDL, high cholesterol, serum albumin, and BMI.
Additionally adjusted for hemoglobin categories.
Adjusted for age, sex, race, site, HDL, serum albumin, any CV disease, and cocaine.
Additionally adjusted for albuminuria categories.
Systolic dysfunction (defined as ejection fraction [EF]<45%) was present in 8% of the cohort. The majority (82%) had an EF>50%; only 10% of patients had EF=46%–50%, 6% of patients had 36%–45%, and 2% had ≤35%. There was no association between kidney function and systolic dysfunction in demographic, multivariate, or fully adjusted models.
Kidney Function by Serum Creatinine
The associations between categories of eGFR measured by serum creatinine and cardiac structural changes were weaker in both demographic and multivariable-adjusted models (Supplemental Table 1). Demographically adjusted relationships remained significant between eGFRcr<30 ml/min per 1.73 m2 and LV mass, LVH, and LV geometry. Adjusted models were significant for associations between eGFRcr<30 ml/min per 1.73 m2 and LVH and abnormal LV geometry, but they were attenuated by additional adjustment for mineral metabolism. There were no independent associations for diastolic dysfunction after multivariable analysis (Supplemental Table 2).
Discussion
CKD is a major risk factor for the development of HF.14,15 Prevalence of LVH in CKD patients has previously been reported to range from 40% to 78%, and it reaches 75% at the time of initiation of dialysis.8,16 Among individuals with CKD and without clinical HF, we found an overall prevalence of LVH of 50% ranging from 32% in those patients with eGFRcys≥60 ml/min per 1.73 m2 to 75% in those patients with eGFRcys<30 ml/min per 1.73 m2. There was a strong association between kidney function and LV mass adjusting for demographic characteristics including age, sex, and race, with greater strength of association at lower levels of kidney function. An eGFRcys<30 ml/min per 1.73 m2 was strongly associated with higher LV mass, increased LVH, and abnormal LV geometry. These relationships were attenuated by adjustment for comorbid conditions, including diabetes and hypertension, and mediators, such as anemia, albuminuria, and markers of mineral metabolism, but the findings remained strong and independent. In contrast, the association with diastolic dysfunction is strongest in the lowest category of eGFRcys but does not follow a graded pattern. Similar to previous smaller studies, reduced kidney function was not significantly associated with reduced systolic function.17 Thus, our findings provide a comprehensive survey of cardiac structural and functional abnormalities across a range of eGFR in a large cohort of CKD patients and reveal that abnormalities in LV structure but not function precede the onset of clinical HF.
The high prevalence of abnormalities of LV mass and geometry in CKD patients without HF is striking. Although we observed associations between eGFR<30 and higher LV mass and between eGFR categories 30–44 and <30 and abnormal LV geometry, this risk threshold would likely be higher if we had a healthy, age-matched control group. These changes may be the critical precursors of clinical HF, because CKD patients are more likely to have HF in the absence of decreased LVEF.18 We observed only a minimal association between kidney function and diastolic dysfunction, which was surprising in light of the fact that this pattern is most likely the predominant pattern of HF in CKD. However, the high prevalence of mild diastolic dysfunction in this cohort may have impeded our ability to detect graded associations. The absence of associations with systolic dysfunction was not surprising given the exclusion of patients with clinical HF by design and the presence of systolic dysfunction in only 8% of the cohort.
Our findings were more significant when eGFR was measured by cystatin C than serum creatinine. This finding suggests that cystatin C may distinguish kidney function stages better than creatinine and allow for detection of associations between more subtle gradations in kidney function and subclinical cardiac structural and functional changes.19 Our analyses were based on creatinine values from year 1, which would be expected to have a stronger association with echocardiographic findings performed concurrently at year 1. However, the range of eGFR spanned by cystatin C measurement is larger than the range of eGFR estimated by creatinine, which may also facilitate the increased strength of associations of kidney function defined by eGFRcys. Entry into CRIC was determined by Modification of Diet in Renal Disease (MDRD) -calculated eGFR from serum creatinine, which constrains its range of values. Thus, the design of CRIC may have inadvertently biased our analyses in favor of cystatin C having a stronger association with these outcomes than creatinine.
This study has multiple strengths, including a large sample size and representation across different stages of CKD. Compared with previous studies, our study also includes a high percentage of black individuals (40%) and persons with diabetes. Our findings complement the findings of other studies within CRIC, in which a large prevalence of LVH was observed,20 and offer additional insight into the severity of cardiac disease with lower levels of kidney function. Our cross-sectional findings suggest that FGF-23 and other markers of mineral metabolism, including calcium, phosphorus, and PTH, are partly but not wholly responsible for this relationship.21 Our study also has important limitations. The absence of a healthy control group impeded our finding associations for the intermediate eGFR categories by decreasing the gradient between abnormal and referent groups. In addition, the associations may differ between blacks and whites, but we were limited in power to further explore this association. Other limitations include some missing echocardiographic measurements, which did not seem to introduce systematic bias according to a sensitivity analysis. Some echocardiographic parameters that have become more widely used since the CRIC echo protocol was first developed (e.g., longitudinal strain) could not be evaluated because of cost restrictions, but these parameters are not readily applicable to general populations. In addition, alternative methods of assessment of LV abnormalities, such as cardiac magnetic resonance imaging, may be more accurate and cost-effective. Because this study is cross-sectional, causality and mechanistic pathways of HF pathogenesis could not be evaluated. As is common in cohort studies, duration of comorbid conditions, such as hypertension and diabetes, could not be ascertained. This consideration is important given the relationship between BP and LVH;22 although we adjusted for baseline BP differences, treatment bias cannot be fully eliminated. Also, CRIC did not routinely biopsy participants, and the etiology of CKD is, thus, not confirmed pathologically, although this lack of confirmation is consistent with general clinical practice. Because patients with HF were excluded from our analysis, our findings with respect to systolic dysfunction may not be representative of the entire CKD population. Some individuals with undiagnosed HF may remain in the analysis, because inclusion in CRIC and additional exclusion by our study design were assessed by symptoms and self-report, which may be difficult to characterize in this population.23 Finally, measurement of EF by echocardiogram may not fully characterize systolic function because of the intrinsic variability of EF, which relates to volume status and inotropic state. Future studies may consider performing additional assessments of systolic function on stored echocardiographic images.
In a large cohort of patients with CKD and without clinical HF, we have established a remarkably high prevalence of structural cardiac abnormalities and diastolic dysfunction, which may represent the precursor structural abnormalities underlying the development of clinical HF in this population. In contrast, systolic dysfunction was far less common and not associated with eGFR. Future studies in CRIC will evaluate the extent to which each of these subclinical structural and functional abnormalities predicts the onset of clinical HF.
Concise Methods
Subjects
The National Institute of Diabetes and Digestive and Kidney Diseases established CRIC in 2001 as an observational study to evaluate the determinants of progression to ESRD and cardiovascular disease among persons with CKD.24,25 Participants were recruited from seven clinical centers between April of 2003 and September of 2008. Inclusion criteria were eGFR=20–70 ml/min per 1.73 m2 for persons ages 21–44 years, 20–60 ml/min per 1.73 m2 for persons ages 45–64 years, and 20–50 ml/min per 1.73 m2 persons ages 65–74 years based on MDRD creatinine-based eGFR. Exclusion criteria included prior transplantation, polycystic kidney disease, multiple myeloma, use of immunosuppression, and severe comorbid illnesses, such as cirrhosis, HIV disease, and severe (New York Heart Association class III or IV) HF. Of the 3939 participants in CRIC, we additionally excluded 443 patients, because they had HF at baseline by self-report of physician diagnosis. This exclusion was to further ensure that our population was free of HF at baseline. Comorbid conditions were defined at time of enrollment by medical history.24 Assessments of heart structure and function were performed by echocardiography 1 year after enrollment according to the American Society of Echocardiography guidelines,26 and the data were sent to a core echocardiography laboratory for measurement and analysis (University of Pennsylvania). Echocardiogram is an important method for assessment of cardiac mass and function in individuals with CKD.21,27,28 Multiple reproducibility, inter-reader reliability, intrareader reliability, and reader drift analyses were performed throughout the course of this large-scale prospective cohort study on a 2% random sample of the entire cohort each year. The intraclass correlation coefficients (and κ statistics) for the echocardiographic measures are LVH=0.759 (κ=0.61), diastolic dysfunction=0.848 (κ=0.75), and LV ejection fraction=0.854 (n/a).
Predictors
The primary predictor was kidney function determined using eGFR by cystatin C. Cystatin C was measured using a Siemens BNII nephelometer at the CRIC central laboratory with a coefficient of variation of 4.9%. An internal standardization was implemented to correct for drift over time when using different calibrator and reagent lots manufactured by Siemens. Specifically, all cystatin C values were calibrated to the combination of calibrator lot 51 and reagent lot 40. Serum creatinine measurements were performed in the CRIC central laboratory at the University of Pennsylvania on the Hitachi Vitros 950 AT (coefficient of variation=1.1%) and calibrated to the Cleveland Clinic Roche laboratory values, which are traceable to isotope dilution mass spectrometry.29
eGFRcys was calculated by the CKD-Epi equation using baseline measurements of cystatin C with the equation GFR=76.7×(cystatin C)−1.19.6 eGFRcr was determined using the year 1 measurements of serum creatinine with the abbreviated MDRD equation.30 We used cystatin C as the primary predictor, because the range of kidney function captured by cystatin C is larger and thus, more suited to examining the range of change in cardiac function in this population.31
Covariates included demographic variables (age, sex, race, and site), lifestyle factors (smoking, alcohol use, and illicit drug use), comorbid conditions (diabetes, coronary heart disease, stroke, hypertension, peripheral vascular disease, hyperlipidemia, and cardiovascular events), urine protein/creatinine ratio, hemoglobin, baseline C-reactive protein (CRP), FGF-23, and BMI categories.
Outcomes
LVH and Geometry
LV mass was calculated using the area–length method and indexed to height2.7.26 LVH was defined as LV mass/height2.7≥47 g/m2.7 in women and ≥50 g/m2.7 in men.32 This definition was chosen in contrast to left ventricular mass index (g/m2) because of CRIC consensus.33 Relative wall thickness (RWT) was calculated as two times posterior wall thickness/LV internal linear dimension in diastole. RWT was considered to be increased if ≥0.45. LV mass and RWT were used to categorize LV geometry: normal (normal LV mass and normal RWT), concentric remodeling (normal LV mass and increased RWT), eccentric hypertrophy (increased LV mass and normal RWT), and concentric hypertrophy (increased LV mass and increased RWT).
LV Diastolic Function
Mitral inflow E- and A-wave velocities, E-wave deceleration time, and pulmonary venous reverse A-wave duration were used to categorize LV diastolic function into normal or mildly, moderately, or severely abnormal (corresponding to normal and grades 1, 2, and 3 diastolic dysfunction).34 Because one center was unable to evaluate these parameters of diastolic function due to equipment limitations, these measures were unavailable in 564 participants. In addition, a small number of patients with atrial fibrillation (<1%) could not have measurements of diastolic function.
LV Systolic Function
LV end-diastolic and end-systolic volumes were calculated using the modified biplane method, and EF was calculated as (end-diastolic volume−end-systolic volume)/end-diastolic volume. LV systolic dysfunction was defined as an EF<45%.35–38
Analyses
We first described the baseline characteristics of CRIC participants without prevalent HF across eGFRcys categories of ≥60, 45–59, 30–44, and <30 ml/min per 1.73 m2. Continuous variables were analyzed by ANOVA and nonparametric methods for skewed variables; categorical variables were analyzed by the chi-squared test.
Associations of kidney function categories with LV mass were assessed by multivariable linear regression. Because LVH and systolic dysfunction were dichotomized, we used multivariable logistic regression for these outcomes. Because the four categories of LV geometry were not clearly ranked in severity, we used multivariable nominal logistic regression and dichotomized the outcome between normal and concentric remodeling versus concentric and eccentric hypertrophy. We dichotomized diastolic dysfunction at normal versus mild, moderate, and severely abnormal, and we modeled these four levels of severity using ordered logistic regression and tested the proportional odds assumption using the Brant test.
For all regression models, candidate variables for multivariable analysis included the covariates listed previously; these covariates were evaluated using a stepwise selection procedure, with a P value=0.20 as the threshold for entry into the model and a P value=0.05 as criterion for retention (with demographic characteristics included in all models). We performed demographic adjustment (age, sex, race and site) and two sequential multivariable analyses for each outcome based on our assessment of the covariates’ likelihood of being a confounder or mediator in the relationship between kidney and heart disease. Confounders were defined by their likelihood of preceding or contributing to the development of reduced kidney function and included hypertension, diabetes, history of cardiovascular events, peripheral vascular disease, BMI categories, LDL cholesterol, HDL cholesterol, serum albumin, high-sensitivity CRP, smoking, and cocaine use. The fully adjusted model included all of the above variables plus albuminuria and hemoglobin levels, which are likely to be either mediators of the relationship between kidney disease and cardiac function and structure or additional indicators of kidney disease severity. The final model included the above mediators and markers of mineral metabolism, including FGF-23, calcium, phosphorus, and PTH. Hemoglobin levels were categorized as >14.0, 13.1–14.0, 12.1–13.0, 11.1–12.0, and ≤11.0 g/dl. All associations were tested by both eGFRcys and eGFRcr. We adjusted for sites in all models.
Disclosures
None.
Supplementary Material
Acknowledgments
M.P. was supported by an American Heart Association Western Affiliates Fellowship Grant and is currently supported by National Institutes of Health National Research Service Award Grant F32 DK093231. C.-y.H. was partially funded by Grant K24 DK92291. This project was supported by Grant R01 DK066488 (to M.G.S.). In addition, we would like to acknowledge the Chronic Renal Insufficiency Cohort General Clinical Research Center and Clinical and Translational Science Awards: University of Pennsylvania Grant UL1 RR-024134, Johns Hopkins University Grant UL1 RR-025005, University of Maryland Grant M01 RR-16500, Case Western Reserve University Grant UL1 RR-024989, University of Michigan Grants M01 RR-000042 and UL1 RR-024986, and University of Illinois at Chicago Grant UL1 RR-029879.
M.P. and M.G.S. had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012020145/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J: Reduced kidney function as a risk factor for incident heart failure: The atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 18: 1307–1315, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG, Cardiovascular Health S, Cardiovascular Health Study : Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 142: 497–505, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B: The severe cardiorenal syndrome: ‘Guyton revisited.’ Eur Heart J 26: 11–17, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G: Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 46: 320–327, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O: Left ventricular mass index increase in early renal disease: Impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE: Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47: 186–192, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Nardi E, Palermo A, Mulè G, Cusimano P, Cottone S, Cerasola G: Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens 27: 633–641, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Otsuka T, Suzuki M, Yoshikawa H, Sugi K: Left ventricular diastolic dysfunction in the early stage of chronic kidney disease. J Cardiol 54: 199–204, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Nardi E, Cottone S, Mulè G, Palermo A, Cusimano P, Cerasola G: Influence of chronic renal insufficiency on left ventricular diastolic function in hypertensives without left ventricular hypertrophy. J Nephrol 20: 320–328, 2007 [PubMed] [Google Scholar]
- 14.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, Shlipak MG: Predictors of heart failure among women with coronary disease. Circulation 110: 1424–1430, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Chae CU, Albert CM, Glynn RJ, Guralnik JM, Curhan GC: Mild renal insufficiency and risk of congestive heart failure in men and women > or =70 years of age. Am J Cardiol 92: 682–686, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Middleton RJ, Parfrey PS, Foley RN: Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol 12: 1079–1084, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Edwards NC, Hirth A, Ferro CJ, Townend JN, Steeds RP: Subclinical abnormalities of left ventricular myocardial deformation in early-stage chronic kidney disease: The precursor of uremic cardiomyopathy? J Am Soc Echocardiogr 21: 1293–1298, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, Fabsitz RR, Howard BV: Congestive heart failure despite normal left ventricular systolic function in a population-based sample: The Strong Heart Study. Am J Cardiol 86: 1090–1096, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Peralta CA, Katz R, Sarnak MJ, Ix J, Fried LF, De Boer I, Palmas W, Siscovick D, Levey AS, Shlipak MG: Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol 22: 147–155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricardo AC, Lash JP, Fischer MJ, Lora CM, Budoff M, Keane MG, Kusek JW, Martinez M, Nessel L, Stamos T, Ojo A, Rahman M, Soliman EZ, Yang W, Feldman HI, Go AS, CRIC and HCRIC Investigators : Cardiovascular disease among hispanics and non-hispanics in the chronic renal insufficiency cohort (CRIC) study. Clin J Am Soc Nephrol 6: 2121–2131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauer MS, Anderson KM, Levy D: Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: The Framingham Heart Study. J Am Coll Cardiol 18: 1287–1294, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Shlipak MG, Lash JP, Yang W, Teal V, Keane M, Cappola T, Keller C, Jamerson K, Kusek J, Delafontaine P, He J, Miller ER, 3rd, Schreiber M, Go AS, CRIC Investigators : Symptoms characteristic of heart failure among CKD patients without diagnosed heart failure. J Card Fail 17: 17–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The chronic renal insufficiency cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee. European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE: The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5: 2024–2031, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Yamada S, Ishii H, Takahashi H, Aoyama T, Morita Y, Kasuga H, Kimura K, Ito Y, Takahashi R, Toriyama T, Yasuda Y, Hayashi M, Kamiya H, Yuzawa Y, Maruyama S, Matsuo S, Matsubara T, Murohara T: Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin J Am Soc Nephrol 5: 1793–1798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Variability of creatinine measurements in clinical laboratories: Results from the CRIC study. Am J Nephrol 31: 426–434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation : National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D: Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA 305: 1545–1552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH: Effect of growth on variability of left ventricular mass: Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol 25: 1056–1062, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Lauer MS, Anderson KM, Kannel WB, Levy D: The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA 266: 231–236, 1991 [PubMed] [Google Scholar]
- 34.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A: Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22: 107–133, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I-PRESERVE Investigators : Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359: 2456–2467, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R: Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol 66: 981–986, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Bergström A, Andersson B, Edner M, Nylander E, Persson H, Dahlström U: Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC). Eur J Heart Fail 6: 453–461, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, Yip T, Lau ST, Lau CP, Tang MO, Yu CM, Sanderson JE: The Hong Kong diastolic heart failure study: A randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 94: 573–580, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.