Abstract
Formation of a functional renal network requires the interconnection of two epithelial tubes: the nephron, which arises from kidney mesenchyme, and the collecting system, which originates from the branching ureteric epithelium. How this connection occurs, however, is incompletely understood. Here, we used high-resolution image analysis in conjunction with genetic labeling of epithelia to visualize and characterize this process. Although the focal absence of basal lamina from renal vesicle stages ensures that both epithelial networks are closely apposed, we found that a patent luminal interconnection is not established until S-shaped body stages. Precursor cells of the distal nephron in the interconnection zone exhibit a characteristic morphology consisting of ill-defined epithelial junctional complexes but without expression of mesenchymal markers such as vimentin and Snai2. Live-cell imaging revealed that before luminal interconnection, distal cells break into the lumen of the collecting duct epithelium, suggesting that an invasive behavior is a key step in the interconnection process. Furthermore, loss of distal cell identity, which we induced by activating the Notch pathway, prevented luminal interconnection. Taken together, these data support a model in which establishing the distal identity of nephron precursor cells closest to the nascent collecting duct epithelium leads to an active cell invasion, which in turn contributes to a patent tubular interconnection between the nephron and collecting duct epithelia.
Interconnecting tubular networks is an important event during animal organ morphogenesis. Luminal interconnections made by epithelial cells allow the flow of fluids ensuring gaseous exchange, nutrient supply, and an appropriate homeostatic environment for function of internal organ systems. The interconnection of epithelia enables feeding, reproduction, and removal of waste products.
Different cellular mechanisms have been linked to distinct tubular networks in animal development. In the fruit fly Drosophila melanogaster, the specification of distinct fusogenic cell types at the tips of the developing respiratory system enables the fusion of tracheal branches and the establishment of a tracheal network for gas exchange.1 In the hermaphrodite worm Caenorhabditis elegans, the invasive anchor cell breaks down the basal lamina and fuses with neighboring vulva cells to form the uterine-seam cell that connects the vulva to the uterus.2–5 In the formation of the primary mouth in Xenopus laevis and the foregut of C. elegans, apoptosis6,7 and reorientation of epithelial junction complexes,8 respectively, are critical cellular strategies. Analysis of these distinct developmental events has highlighted the range of cellular processes that establish a patent luminal interconnection between epithelial systems.
The formation of a functional mammalian urinary system requires the interconnection of nephrons with the collecting duct network in the kidney, and the fusion of the ureteric extension of the collecting duct network with the bladder. Obstruction of fluid transfer in the kidney may result in cases of diffuse renal dysplasia or hydronephrosis. Ret and retinoic acid signaling play a key role in connecting the nephric duct with the urogenital sinus, and remodeling the ureter extension in the bladder wall.9,10 In the nephron to collecting duct connection, Wnt9b induces the epithelial transition of a mesenchymal Six2+ nephron progenitor establishing the renal vesicle (RV), the precursor for the nephron. Establishment of patent luminal connections between derivatives of the RV and the ureteric tree enables the passage of fluid from the glomerulus to the bladder but the mechanisms are unclear.
When first visible, the RV has a marked proximodistal axis of polarity relative to the future glomerular collecting duct axis and ureteric epithelium.11,12 Distal cells of the future nephron are thought to lie immediately adjacent to the ureteric epithelium, whereas proximally fated cells are not in close apposition. The emergence of mature proximal and distal epithelia are presaged by distinct gene regulatory programs in proximal and distal regions of the newly formed RV11,12; in particular, Notch signaling plays a central role in establishing proximal cell fates.13–16
At the morphologic level, the RV undergoes a series of cellular rearrangements that accompany the progressive regional differentiation of the developing nephron. At an early stage of the nephron program, an outpouching of distal cells emerges from the RV derivative forming a close association with the ureteric epithelium that is enhanced by the absence of basement membrane in this region11; however, histologic analysis has not described a patent luminal interconnection before S-shaped body stages.17–19
To obtain a more comprehensive insight into these processes, we used high-resolution imaging to examine interconnection events. Through the analysis of apical and tight junction-associated membrane complexes, we show that a continuous lumen is established between the nephron and collecting duct at late S-shaped bodied stages. Before connection, distal cells acquire an active mesenchymal-like invasive property penetrating the ureteric epithelium and entering the lumen of the nascent collecting duct network. Thus, a distinct distal cell phenotype is a key feature of the interconnection process. Interconnection also appears to be a specific property of distal cell fates; proximalized RVs fail to fuse with the ureteric epithelium. This study provides new insights into an important, understudied area of renal development.
Results
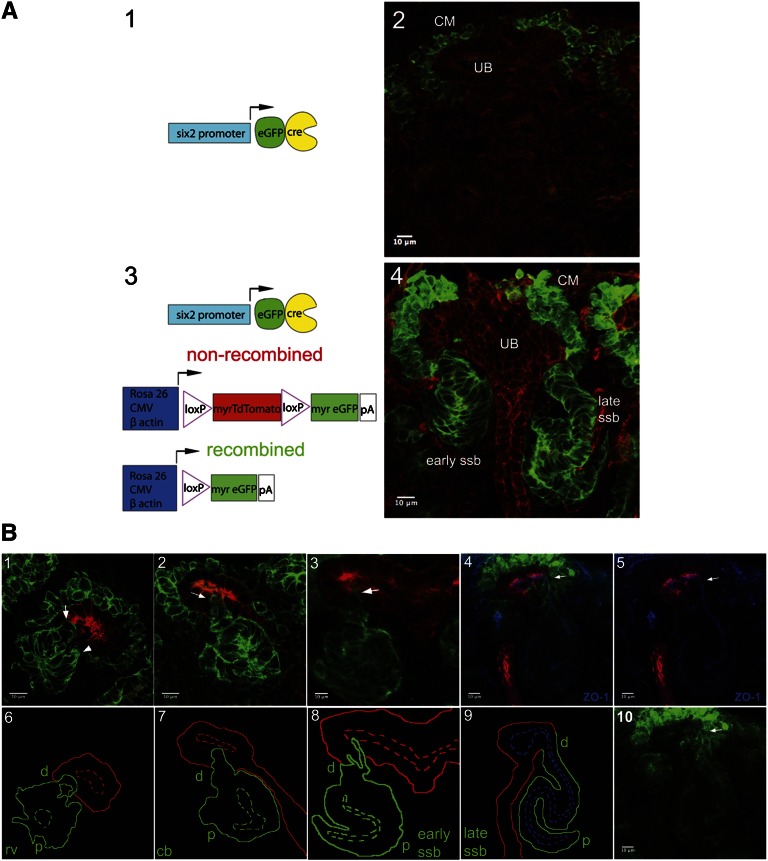
To visualize the cellular behaviors leading up to luminal interconnection, we used a dual global fluorescent genetic system to label nephron progenitors and their RV derivatives. In this system (Figure 1, A1–A4), a male Six2TGC/+ BAC transgenic mouse was crossed to a female homozygous for a Rosa26mTmG allele (subsequently referred to here as mTmG).20 The Six2TGC drives a green fluorescent protein (GFP)::CRE fusion protein exclusively within the Six2 nephron progenitor compartment (Figure 1, A1 and A2). When the mTmG reporter is also present (Figure 1A3), Cre-mediated recombination activates production of a myristoylated form of GFP (mG) labeling the cell membranes of Six2-derived nephrons, whereas all other cell types constitutively produce a myristoylated form of the tandem Tomato fluorescent protein (mT) (Figure 1A4).
Figure 1.
A dual fluorescent membrane labeling system to visualize interactions between nephron and collecting duct networks during mouse development. (A1 and A3) Schematic representation of transgenic alleles and reporter systems. (A2 and A4) GFP and TdTomato activity before and after recombination in sections of E15.5 kidney. (B1–10) Analysis of the interaction between RV derivatives and the ureteric epithelium at the indicated stages of nephrogenesis. Upper panels in B1–B5 and lower panel in B10 show fluorescent labeling of kidney sections at E15.5. The merged image in B4 is also labeled with antibodies to ZO-1 for labeling tight junction complexes (blue), anti-GFP to label Six2+ progenitor cells and RV derivatives (green), and antipan-cytokeratin to label collecting duct epithelium (red). Arrow points to late ssb connected to tip of collecting duct epithelium. (B5) Separated ZO-1 (blue) and pan-cytokeratin (red) from merged image in B4. (B10) Separated GFP-labeled image from B4. Lower panels in B6–B9 are the schematic representations of interactions between the developing nephron and collecting duct networks. Distal, d; proximal, p; rv, RV; cb, comma-shaped body; ssb, S-shaped body.
Figure 1B shows cryosections (Figure 1, B1–B5 and B10) and schematic representations (Figure 1, B6–B9) highlighting the progressive association of RV derivatives and the collecting duct epithelium at different stages of nephrogenesis. At all stages, we observed a close association between the developing nephron and ureteric epithelium, and evidence that distal cells of RV derivatives projected toward (arrow in Figure 1B1) and then inserted into the nascent collecting duct network (arrows in Figure 1, B2 and B3). Examination of the tight junction component zonula occludens-1 (Tjp1/ZO-1, hereafter referred to as ZO-1) highlights a continuous luminal connection established at the late S-shaped body stage of nephron development (Figure 1, B4 and B9).
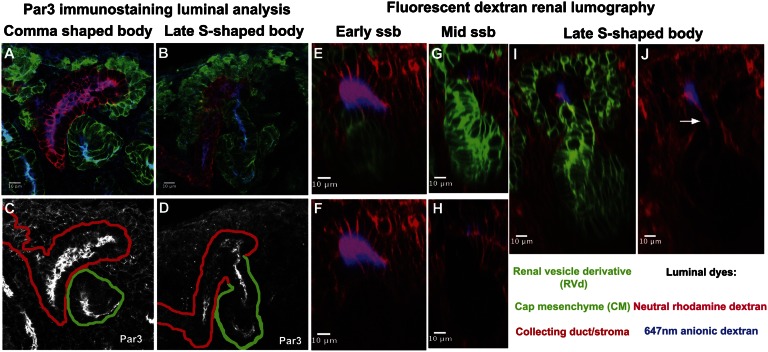
To more precisely time the luminal interconnection event during mouse embryonic kidney development, we examined the distribution of ZO-1 and the apical membrane component Par3 at the interface of the S-shaped body and the ureter.21,22 In addition, we assessed physical connectivity by renal lumography, backfilling the ureteric network via bladder injection of two differentially charged fluorescent dextrans to mark the lumen (neutral rhodamine and anionic infrared Alexa 647 nm dyes). Three-dimensional reconstruction was performed using Fiji/Image J software at E16.5 to distinguish different stages of S-body development through the measurement of the distal straight limb segment (Supplemental Figure 1, A1–D5). Through this approach, we were able to distinguish three substages of S-shaped body development: early, mid, and late S-shaped body stages (Table 1). We failed to observe evidence of a luminal interconnection before the late S-shaped body stage (Figure 2, A, C, and E–H, and Table 2) but at the late S-shaped body stage we observed a continuity in ZO-1 and Par3 distribution between the distal S-shaped body and the ureteric epithelium (Figure 1, B4 and B5, and Figure 2, B and D), and dye was transferred from the lumen of the ureteric tree into the distal limb of the S-shaped body (Figure 2, I and J). Thus, a patent luminal interconnection between nascent nephrons and the collecting duct network occurs at a late stage of S-shaped body development.
Table 1.
Length of distal segment of RV derivative in addition to the overall morphology of the RV derivative to categorize sub-stages between comma and post-S-shaped RV stages from 30-μm–thick immunostained cryosections
| Renal Vesicle Stage | Total Number of Events | Length of Distal Segment ± SD (μm) |
|---|---|---|
| Early S-shaped body | 19 | 16.23±3.33 |
| Mid S-shaped body | 12 | 24.87±3.25 |
| Late S-shaped body | 19 | 40.57±7.17 |
P<0.05, ANOVA. Significance level was set at 5%.
Figure 2.
Luminal interconnection between the nephron and collecting duct systems. (A–J) Stage-specific analysis of connection events using epithelial markers and dye filling; stages and analysis are indicated on the panels. (A–D) Par3 highlights the apical organization of epithelia; continuous labeling between the RV derivative and ureteric epithelium indicates that a continuous epithelium exists at the late S-shaped body stage (compare A and C with B and D). Backfilling with dye injection into the bladder of an intact urogenital system at stage E16.5 shows that dye labels the distal arm of the developing nephron at the late S-shaped body stage (compare E–H with I and J). ssb, S-shaped body.
Table 2.
Renal lumography assay of connected events in freshly dissected embryonic kidneys (total of 50 events observed)
| Renal Vesicle Stage | Number of Events | Number Connected |
|---|---|---|
| Renal vesicle | 7 | 0 |
| Comma | 2 | 0 |
| Comma-S transition | 5 | 0 |
| Early S-shaped | 10 | 0 |
| Mid S-shaped | 4 | 0 |
| Late S-shaped | 9 | 8 |
| Post S-shaped | 13 | 13 |
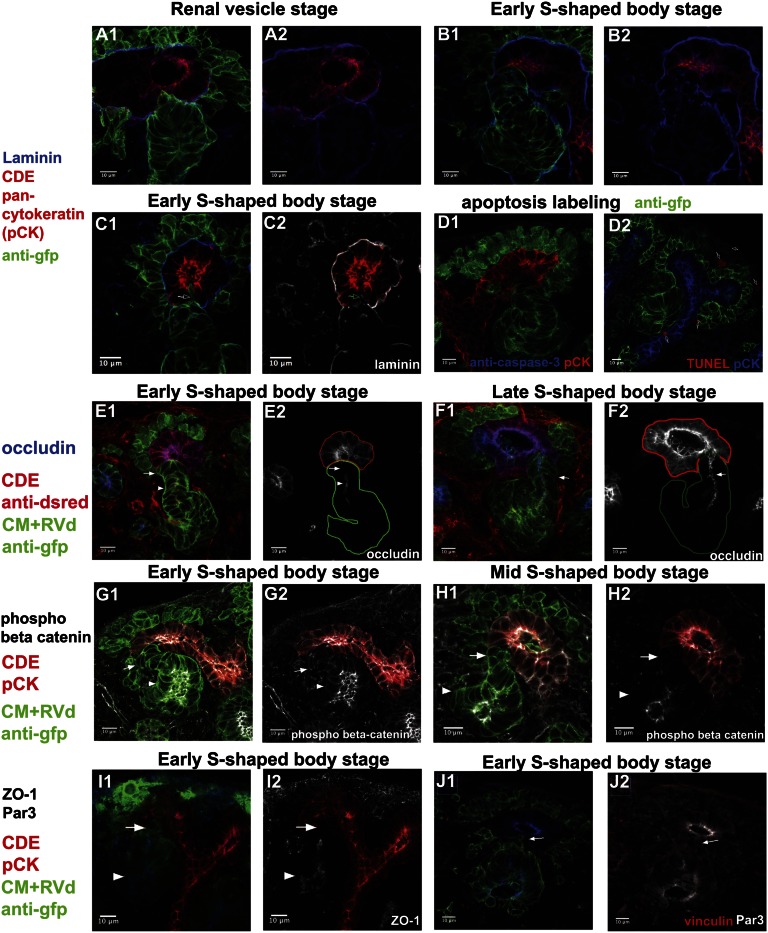
To determine what cellular events couple to luminal interconnection, we first examined the integrity of the basal lamina. Earlier reports have indicated that the basal lamina is discontinuous at sites of apposition between the S-shaped body and the ureter.11,23 In agreement with published data, immunostaining with an anti–pan-laminin antibody demonstrated that laminin was present in a basal lamina associated with the RV and later stages of nephron development. However, the basal lamina was discontinuous where the RV derivative approximates (Figure 3, A and B) or penetrates (Figure 3C) the ureteric epithelium for some time before the establishment of a continuous epithelium. Thus, although basal lamina removal alone is unlikely to control the timing of epithelial interconnection, the absence of a basal lamina may facilitate the entry of RV-derived cells into the ureteric epithelium.
Figure 3.
Analysis of cellular organization, cell behavior, and cell death at the site of epithelial interconnection in the mammalian kidney. (A1–C2) Anti-laminin immunostaining shows that the basal lamina is discontinuous at the RV to ureteric epithelial interface at RV (A1 and A2) and early S-shaped body (B1 and B2) stages. Pan-cytokeratin labels the collecting duct epithelium (CDE), whereas anti-GFP labels the cap mesenchyme (CM) and RV derivatives (RVd). At the early S-shaped body stage (C1 and C2), a distal cell invasion of an early S-body–derived cell is observed into the ureteric epithelium in an area displaying a punctate laminin distribution (arrow). (D1 and D2) Anticaspase-3 (D1) and TUNEL (D2) labeling fails to detect cell apoptosis at the S-shaped body/ureter junction although apoptotic figures are observed elsewhere (Supplemental Figure 3). (E1–F2) Occludin labeling of tight junctional complexes supports ZO-1 and Par3 analysis of a patent luminal connection between nephron and ureteric epithelium at late S-shaped body stages. (E2) Arrow indicates distal-most cells of RV derivative do not express occludin, whereas the neighboring RV derivative cells express occludin (arrowhead). (F2) Arrow indicates continuous occludin labeling between two epithelial networks. (G1–I2) The absence of phospho-β-catenin (G1–H2) and ZO-1 (I1 and I2), markers of adherens and tight junctional complexes, respectively, highlights the absence of an epithelial organization to the most distal cells of early and mid S-shaped body stages where they interface with the ureteric epithelium (white arrow). Proximal cells of RV derivative have more mature epithelial junctions (white arrowhead). (J1 and J2) Invading distal cells (arrow) do not express Par3.
Apoptosis has been linked in several systems to connecting discrete epithelial structures. We determined whether apoptotic remodeling may play a role in luminal interconnection by examining cleavage of the proapoptotic enzyme caspase 3, an early event in the apoptotic program, using antibodies specific to the cleaved apoptosis promoting protein, and through terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) of fragmented DNA, a late event in apoptosis.24 Whereas an occasional scattered apoptotic cell was detected (arrows in Figure 3, D1 and D2), none were observed specifically within the distal segment of the RV derivative or the adjacent ureteric epithelium. Thus, apoptosis is unlikely to play a significant role in epithelial interconnection in the renal system.
To look more closely at the cellular organization of the distal RV derivative preceding interconnection, we immunostained tissue sections with antibodies recognizing key components associated with polarized epithelia: occludin and ZO-1 (tight junction), and phospho beta catenin (adherens junctions). Neither tight junction protein was present in the distal-most cells of the early S-shaped body close to, or embedded within, the ureteric epithelium as indicated by the arrow (Figure 3, E1, E2, I1, and I2). Each was detected in more proximal regions of the distal limb (arrowhead), though occludin distribution was more punctate; occludin was also absent from RV derivatives at earlier stages as reported previously.25 These data suggest that there is a progressive maturation of tight junction complexes in the RV derivative that excludes cells at the site of tubule fusion. Phospho-β catenin associates with adherens junctions where epithelial contacts are established. Interestingly, as with the tight junction markers, phospho-β catenin was present within the distal segment of the early and mid-stage S-shaped body (arrowhead), but absent in the distal-most cells (arrow in Figure 3, G1–H2). Furthermore, distal cells invading toward lumen of collecting duct epithelium do not have mature Par3 epithelial junctions (arrow in Figure 3, J1 and J2). Collectively, the data suggest that this population of cells retains or acquires a more mesenchymal organization relative to other regions of the S-shaped body. Furthermore, at early S-shaped RV stages, distal cells of the early S-shaped RV derivative displayed immature Par3 and ZO-1 epithelial junctions, but did not upregulate mesenchymal markers including vimentin and Snai2 (Supplemental Figure 4). Finally, E-cadherin was present at the invasive distal end of the early S-shaped body (Supplemental Figure 5). In summary, the data point to an active invasion of the ureteric epithelium preceding the establishment of an epithelial continuum between nephron and collecting duct networks.
To visualize this event, we developed a live imaging approach on a sylgard membrane that supports development of explanted early stage (E11.5–12.5) kidney rudiments (Supplemental Figure 6, Supplemental Movie 1). To determine whether we could visualize interconnection events in normal kidney explants, we examined later stage (E15.5–16.5) intact kidneys to increase the probability of capturing transient cellular processes and to facilitate imaging given the more peripheral positioning of RV and ureteric bud (UB) tips at these times.
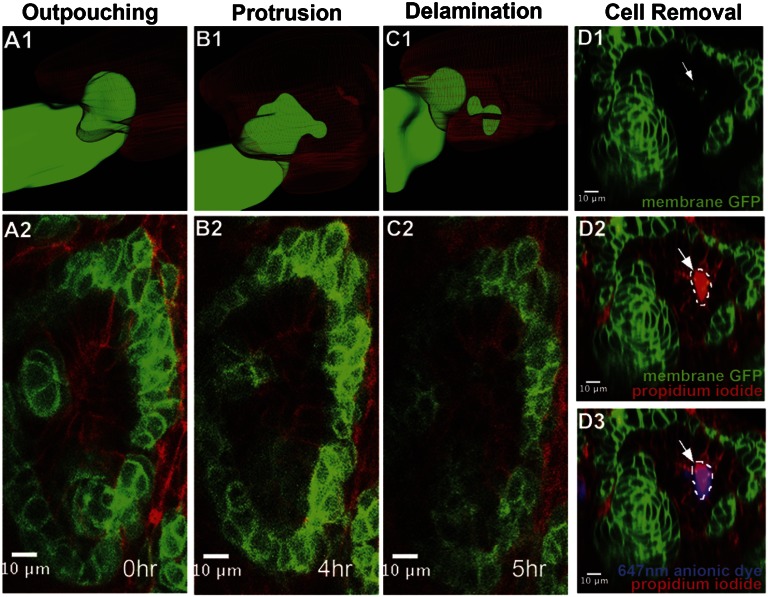
High-resolution spatial analysis with single and two-photon microscopy captured invasive cells delaminating into the lumen of the branched ureter tip (Figure 4, A–C and Supplemental Movies 2 and 3; GFP channels are shown in Supplemental Movies 4 and 5). A time course of events in optical slices of live image stacks from the events in Supplemental Movie 2 is shown in Figure 4, A2, B2, and C2. In addition, three-dimensional reconstruction of these events is also shown in Figure 4, A1, B1, and C1. A distal outpouching of five cells occurs closest to the collecting duct tip with an intense focal point of membrane constriction toward the tip of the collecting duct epithelium (Figure 4, A1 and A2, and Supplemental Movie 2; GFP channel is shown in Supplemental Movie 4). The leading distal cells migrate through the basolateral surface of the neighboring collecting duct epithelium ending up in the lumen of the collecting duct epithelium (Figure 4, A2–C2 and Supplemental Movies 2 and 3; GFP channels shown in Supplemental Movies 4 and 5). From these two movies, the period from distal outpouching to distal delamination takes between 5 and 7 hours. Using renal lumography as demonstrated in Figure 2, propidium iodide labeling of GFP+ cells in the lumen of the ureteric epithelium suggests that migrating cells die post-invasion (Figure 4, D1 and D2).
Figure 4.
Distal cell invasion of RV-derived cells into the lumen of the CDE. (A1– C2) 3D reconstruction (A1–C1) and still confocal optical cross-sectional images (A2–C2) from live imaging of the E15.5 stage mouse kidney (Supplemental Movie 2) highlighting the invasion of the ureteric epithelium by distal cells from the developing RV derivative. (D1–D3) Optical section analysis shows that a Six2-derived cell (arrowed in D1, green) within the lumen of the developing CDE (highlighted by anionic-dye backfilling in D3, blue) is focally labeled by propidium iodide (D2 and D3, magenta), indicating that the cell membrane is not intact and the cell is likely dead or dying.
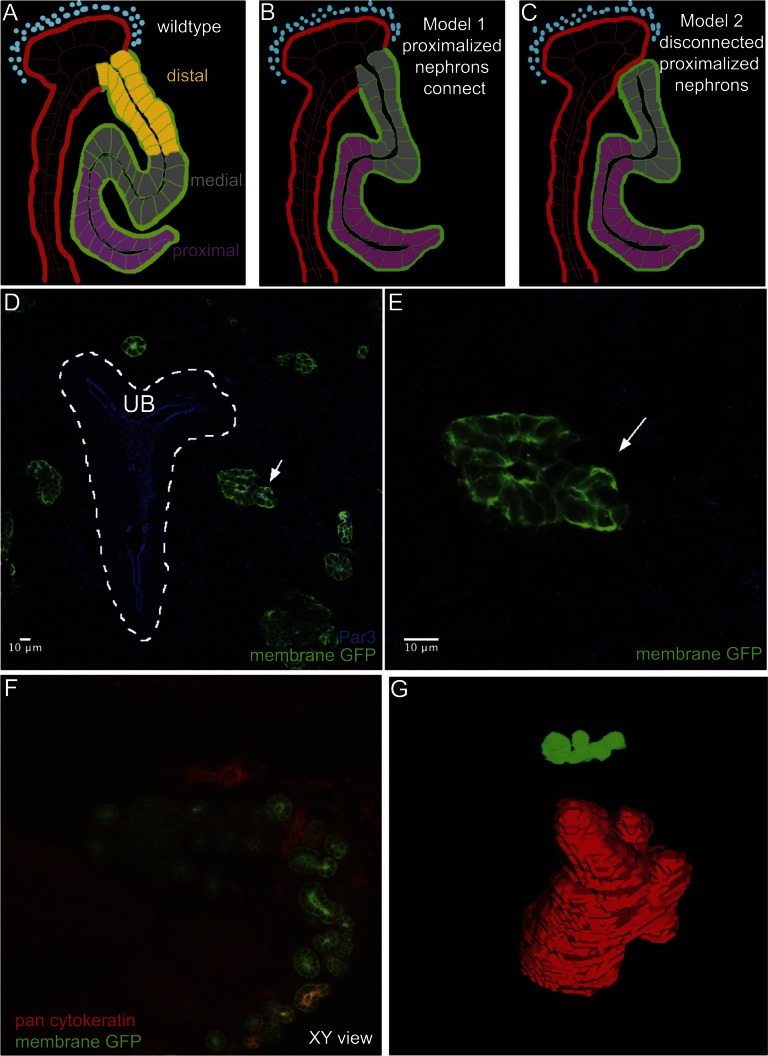
To determine whether connection of the nephron to the collecting duct is a specific property of distal cell types, we proximalized nephron structures through the activation of a GFP tagged Notch intracellular domain (NICD) (Figure 5, A–C).13 At E13.5, early S-shaped RV derivative were not juxtaposed to collecting duct epithelium from immunostained cryosections (Figure 5, D and E). Notch gain-of-function kidneys at E18.5 were immunostained in whole mount with pan-cytokeratin antibodies to visualize the collecting duct epithelium, anti-gfp antibody to identify proximalized nephrons, and either anti-Par3 or anti-ZO-1 to mark epithelia. The immunostained kidneys were then cleared in a special water-soluble called ScaleA2 (Atsushi Miyawaki, personal communication; Figure 5, F and G).33 Reconstruction of proximalized nephrons was then performed using Image J/Fiji software. No evidence of a nephron connection to the collecting duct was observed in any nephron in four independent kidney samples (Figure 5G and Supplemental Figure 7). Taken together, these data support a model wherein the loss of distal cell identity results in failure to form luminal interconnections in the developing kidney.
Figure 5.
Proximalized nephrons fail to connect to collecting duct network. (A–C) Shows a schematic representation of possible outcomes in an experimental test whether interconnection is dependent on distal nephrons fates. Ectopic activation of the NICD activates Notch signaling throughout the developing nephron leading to the loss of distal cell fates.13 (D) Cryosections from E13.5 Notch gain-of-function kidneys show developing RV derivative (arrow) that are not closely juxtaposed to the collecting duct (dotted white line, UB). (E) Close-up view of highlighted RV derivative in panel D. (F) ScaleA2 cleared kidneys at E18.5 stained in whole mount with pan-cytokeratin to visualize the ureteric epithelium. GFP highlights developing proximalized nephrons. (G) Example of a nephron reconstruction, no interconnection is observed between the nephron and ureteric epithelium.
Discussion
We identified a novel cell invasive property exhibited by distal cells of the developing nephron that precedes the interconnection of nephron and collecting duct epithelia. In this, the distal-most cells of the nephron primordium adjacent to the ureteric epithelium retain or assume a more mesenchymal phenotype than their proximal neighbors, and migrate through the ureteric epithelium into the lumen of the developing collecting duct network. These findings raise interesting questions about how distinct cellular phenotypes are regulated in the developing nephron, and the potential role of transepithelial migration in connecting epithelial networks.
The nephron originates from the RV: a precursor structure generated through a mesenchymal to epithelial transition of nephron progenitors. Although the RV is described as epithelial, the absence of N-cadherin (A-CAM or Cadherin-2),27 and mature tight junction complexes (previous studies and data herein)11,27 at the early stages of distal segment suggest that full epithelial properties are more gradually acquired over RV morphogenesis. The observation that distal cells of the RV derivative, the region most closely associated with the ureteric epithelium, exhibit this cellular behavior suggests that the ureteric epithelium may regulate this property. Furthermore, a distinct cellular organization is underpinned by an early proximal-distal polarity in the expression of regulatory factors in the RV and its derivatives.11,12 Thus, a distinct molecular subdivision of the early nephron primordium may regulate both the differentiation and cellular rearrangements generating a functional renal network. Proximal-distal differences in expression of cell cycle regulators (e.g., cyclinD1) and differential secretion or degradation of the basal lamina indicate that other cell behaviors are polarized along the axis of renal tubule maturation and connectivity.11,26 Interstitial/stromal cell types also surround the nephron progenitor and may also regulate epithelial interconnection.
Whether distal cell properties are established in the same way in the absence of the ureteric epithelium remains to be determined. However, when distal cell fates are blocked on activation of Notch signaling, interconnection between the nephron and collecting duct fails. This is consistent with the requirement of distal cell fates to establish a luminal connection between the nephron and collecting duct. However, given that Notch activation alters normal morphogenesis, we cannot rule out an indirect role in which a tight juxtaposition of nephron and ureteric epithelium fails to be established.
Because we have not been able to directly observe the epithelial interconnection events that follow invasion of mesenchymal cell types, it is unclear how invasion may facilitate this process. One attractive possibility is that cell invasion breaks epithelial contacts within the ureteric epithelium while juxtaposing epithelial regions of the distal S-shaped body enabling these two distinct epithelia to contact, realign, and establish new intertubular junctional complexes. Interestingly, cells at the tip of the nephric duct show protrusive activity during migration of the nephric duct to its epithelial target, the urogenital sinus.10 Whether this activity is required to also connect the epithelia has not been explored. In formation of the Drosophila excretory system, stellate cells migrate, polarize, and intercalate to form the nephric tubule suggesting that cell invasion may be a broader mechanistic theme in development of excretory networks.28,29 Future study of epithelial interconnection in the urinary system will benefit from ex vivo and in vitro models that enable rigorous characterization of the dynamic cellular events and novel strategies in which cellular activities can be manipulated in the interconnection zone. Our study also warrants consideration of the potential role that a tubule interconnection deficiency could play in renal disease.
Concise Methods
Mouse Lines for Visualizing Cell Morphology and Genetic Experiments
To label RV derivatives, male Six2-tet-eGFP-cre recombinase (Six2TGCtg/+) heterozygotes were crossed to female Rosa26-floxed myristoylated TdTomato-stop-myristylated eGFP (mT/mG) homozygotes.20,30 To activate Notch signaling in Six2-derivatives, Six2TGCtg/+;mTmG compound heterozygous male mice were crossed to female RosaNICD/NICD mice, which are homozygous for a Cre-dependent allele of the Notch intracellular domain whose activity leads to transcriptional activation of Notch target genes.31 For branching morphogenesis culture control experiments, the HoxB7cretg/+ heterozygote male was crossed to mT/mG homozygous female mice to generate HoxB7cretg/+; mT/mG fluorescently labeled kidneys.32 Staging was based on the time of mating; E 0.5 was mid-day on the day of detection of the vaginal plug. Mouse lines were genotyped by PCR.
Immunostaining, TUNEL, and Fluorescent Dextran Backfilling Analyses on Kidney Sections
E15.5 Six2TGCtg/+;mTmG kidneys were dissected in cold non-HEPES-buffered medium called CO2-independent media (Invitrogen, #18045–088), and fixed in freshly made 4% paraformaldehyde/1×PBS on ice for one hour. Subsequent steps and details on primary and secondary antibodies are described in the Supplemental Material. Cryosections were mounted with Immumount (Thermo Scientific) and placed with No. 1.5 glass coverslips for imaging or stored at 4°C. For TUNEL analysis, E15.5 Six2TGCtg/+;mTmG kidneys were cryosectioned (as stated above) and stained using a TUNEL mix according to the manufacturer’s protocol (Roche). For fluorescent dextran backfilling, a ventral incision was made in the bladder at E16.5 and a solution mixture containing 50 µg/ml of propidium iodide, 100 µg/ml of anionic 647 nm, and 50 µg/ml rhodamine dextrans were injected into the bladder with a 27.5 gauge needle. See the Supplemental Material for detailed procedures before confocal imaging. Imaging was carried out using two-photon confocal microscopy and orthogonal and planar optical slices were analyzed. Optical stacks were taken according to Nyquist criterion.
Whole Mount Imaging at Various Stages of Mouse Kidney Development
Two types of whole mount imaging were used. Kidneys were dissected at E15.5–18.5 and immobilized in 1% agarose/1×PBS in a 35-mm filled plastic dish. Fluorescently labeled Six2TGCtg/+;mTmG kidneys were imaged using Nyquist criterion using single or two-photon confocal microscopy. Second, for evaluating luminal interconnections in the Six2TGCtg/+;mTmG; RosaNICD/+ kidneys, kidneys were removed at E18.5 or postnatal day 1, stained with anti-GFP, anti–pan-cytokeratin, and anti-ZO-1, and cleared and visualized in ScaleA2 clearing agent.33
Mouse Kidney Culture Live Imaging System
An upright single-photon confocal microscopy with a temperature-controlled incubator was used to verify distal invasion events. An optically transparent sylgard membrane with polycarbonate film was used to immobilize the kidney explant with culture medium surrounding the specimen. Frame rates were set for either 15- or 20-minute intervals and the temperature was maintained constant at 37°C. Fluorescently labeled Six2TGCtg/+;mTmG kidneys were placed in medium on a plastic support in a transwell culture dish with a sylgard membrane on top of the kidney; cultures were housed within a humidified and temperature-controlled imaging incubator (Supplemental Figure 2). Image stacks were collected using a water-dipping objective on a confocal microscope at a frame rate of 20 minutes for a period of up to 12 hours, and images were processed using Image J/Fiji software.
Confocal Microscopy, Image Analyses, Three-Dimensional Reconstruction, and Statistical Analyses
A Zeiss Meta 510 upright single-photon confocal was used to image three-color fluorescently stained cryosections with a ×40 or ×63 oil immersion objective lens. A single-photon LSM 700 was used for imaging four-color fluorescence-stained cryosections with a ×63 oil objective lens. A Zeiss physiology microscope equipped with both single and two-photon imaging was used for imaging backfilled fluorescent kidneys through a ×20 water-dipping objective lens. For Scale kidney imaging, the same Zeiss physiology microscope using single-photon excitation was used in addition to the Zeiss prototype Scale-dipping objective lens. Nyquist criterion was used for optimal axial sampling. For live kidney explant imaging, we used the Zeiss Meta 510 Pascal upright confocal microscope. Image J/Fiji software was used to analyze molecular and cellular changes at the distal compartment of the RV derivative and for morphometric analysis of RV derivatives. Fisher’s and ANOVA statistical tests were used to determine if there was statistically significant difference between groups (significance level was set at P=0.05).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Jeff Lichtman, Bernhard Götz, and Dave Smith at the Harvard Center for Biological Imaging; Kevin Peterson, Patrick Müller, and Julien Dubrulle for helpful discussions; Thomas Boudier and Gary Chinga for their morphometric Image J plug-ins; and John Russ for conversations on shape descriptors. We thank Tzu-Cheg Kao for statistical consultations and Bob Hard, Colin Izzard, and faculty at the 2008 Optical Microscopy and Imaging in the Biomedical Sciences Marine Biological Laboratory course for instruction, and staff of the Harvard Biology Research Infrastructure facility for mouse husbandry. We also thank the four anonymous reviewers for comments and suggestions. We are grateful to Ken Kronenberg for Schreiner English translation; Bruce Popp for Emery English translation; and Dorothy Barr at the Harvard MCZ library for helping to unearth these articles by Emery and Schreiner.
R.K. was supported by a National Science Foundation graduate research fellowship (Grants DGE-0644491 and DGE-0946799). A.V. was supported by National Institutes of Health (NIH) Grant K08DK082782 and a Harvard Stem Cell Institute Seed Grant. I.A.D. was supported by an NIH grant (RO1 DK053093). Work in A.P.M.’s laboratory was supported by an NIH grant (R37 DK054364).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Connecting the Segments,” on pages 1603–1605.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012030283/-/DCSupplemental.
References
- 1.Samakovlis C, Manning G, Steneberg P, Hacohen N, Cantera R, Krasnow MA: Genetic control of epithelial tube fusion during Drosophila tracheal development. Development 122: 3531–3536, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Sherwood DR: Cell invasion through basement membranes: An anchor of understanding. Trends Cell Biol 16: 250–256, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Sherwood DR, Butler JA, Kramer JM, Sternberg PW: FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell 121: 951–962, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Sherwood DR, Sternberg PW: Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell 5: 21–31, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR: UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol 11: 183–189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickinson AJ, Sive H: Development of the primary mouth in Xenopus laevis. Dev Biol 295: 700–713, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Dickinson AJ, Sive HL: The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development 136: 1071–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portereiko MF, Mango SE: Early morphogenesis of the Caenorhabditis elegans pharynx. Dev Biol 233: 482–494, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, Carroll TJ, Mendelsohn CL: Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 37: 1082–1089, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Chia I, Grote D, Marcotte M, Batourina E, Mendelsohn C, Bouchard M: Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development 138: 2089–2097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP, Little MH: Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332: 273–286, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Mugford JW, Yu J, Kobayashi A, McMahon AP: High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev Biol 333: 312–323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R: Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134: 801–811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng HT, Kopan R: The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int 68: 1951–1952, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R: Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 130: 5031–5042, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Surendran K, Boyle S, Barak H, Kim M, Stomberski C, McCright B, Kopan R: The contribution of Notch1 to nephron segmentation in the developing kidney is revealed in a sensitized Notch2 background and can be augmented by reducing Mint dosage. Dev Biol 337: 386–395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emery C: Rechereches Embryologiques sur le Rein des Mammiferes, Pisa, Italy, University of Pisa, 1883
- 18.Neiss WF: Morphogenesis and histogenesis of the connecting tubule in the rat kidney. Anat Embryol (Berl) 165: 81–95, 1982 [DOI] [PubMed] [Google Scholar]
- 19.Schreiner KE: Uber die Entwicklung der Amniotenniere, London, UK, Natural History Museum Library, 1902, pp 128–135
- 20.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S, Tsukita S: Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell 19: 2465–2475, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE: A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12: 1035–1045, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekblom P, Alitalo K, Vaheri A, Timpl R, Saxén L: Induction of a basement membrane glycoprotein in embryonic kidney: Possible role of laminin in morphogenesis. Proc Natl Acad Sci U S A 77: 485–489, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R, Pascual J, Imamura S, Kishi S, Amatruda JF, Kanki JP, Green DR, D’Andrea AA, Look AT: Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell 133: 864–877, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brakeman PR, Liu KD, Shimizu K, Takai Y, Mostov KE: Nectin proteins are expressed at early stages of nephrogenesis and play a role in renal epithelial cell morphogenesis. Am J Physiol Renal Physiol 296: F564–F574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekblom P: Formation of basement membranes in the embryonic kidney: An immunohistological study. J Cell Biol 91: 1–10, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nouwen EJ, Dauwe S, van der Biest I, De Broe ME: Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int 44: 147–158, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Campbell K, Casanova J, Skaer H: Mesenchymal-to-epithelial transition of intercalating cells in Drosophila renal tubules depends on polarity cues from epithelial neighbours. Mech Dev 127: 345–357, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denholm B, Sudarsan V, Pasalodos-Sanchez S, Artero R, Lawrence P, Maddrell S, Baylies M, Skaer H: Dual origin of the renal tubules in Drosophila: Mesodermal cells integrate and polarize to establish secretory function. Curr Biol 13: 1052–1057, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA: Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100: 14920–14925, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Carroll TJ, McMahon AP: Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H, Fukami K, Sakaue-Sawano A, Miyawaki A: Scale: A chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci 14: 1481–1488, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.