Abstract
Activation of the mammalian target of rapamycin (mTOR) signaling pathway is aberrant in autosomal-dominant polycystic kidney disease (ADPKD). The mTOR inhibitors, such as rapamycin, ameliorate PKD in rodent models, but clinical trials have not shown benefit, possibly as a result of low tissue concentrations of rapamycin at clinically tolerable doses. To overcome this limitation, we synthesized a folate-conjugated form of rapamycin (FC-rapa) that is taken up by folate receptor–mediated endocytosis and cleaved intracellularly to reconstitute the active drug. We found that renal cyst-lining cells highly express the folate receptor in ADPKD and mouse models. In vitro, FC-rapa inhibited mTOR activity in a dose- and folate receptor–dependent manner. Treatment of a PKD mouse model with FC-rapa inhibited mTOR in the target tissue, strongly attenuated proliferation and growth of renal cysts and preserved renal function. Furthermore, FC-rapa inhibited mTOR activity in the kidney but not in other organs. In summary, these results suggest that targeting the kidney using FC-rapa may overcome the significant side effects and lack of renal efficacy observed in clinical trials with mTOR inhibitors in ADPKD.
Autosomal-dominant polycystic kidney disease (ADPKD) is a prevalent genetic disease affecting 12 million people worldwide.1 Mutations in the PKD1 or PKD2 genes lead to excessive proliferation, renal epithelial cyst formation, and fibrosis that cause progressive destruction of normal renal tissue and eventually loss of kidney function.2 Currently, no treatment for ADPKD is available, and most patients eventually require lifelong dialysis or kidney transplantation. We3,4 and others5–11 have demonstrated that the mammalian target of rapamycin (mTOR) pathway is hyperactivated in PKD rodent models and human ADPKD. Furthermore, treatment of PKD rodent models with mTOR inhibitors, such as rapamycin, greatly improves the cystic phenotype.5–11
Because mTOR inhibitors have already been in clinical use as immunosuppressant drugs, these studies rapidly led to several clinical trials to determine whether they are effective in patients with APDKD.12–14 Unfortunately, although not yet fully conclusive, the results of these trials have been largely disappointing. No significant clinical benefits were observed within the duration of treatment.15–20 One likely reason for the negative outcome in clinical trials compared with the strong efficacy in animal models is that the drug doses used clinically were relatively lower than the much higher doses in animals. Evidence suggests that the doses of rapamycin commonly used for immunosuppression are insufficient for significant inhibition of mTOR in the human kidney.21 Because a large fraction of patients with ADPKD in these trials reported significant undesirable systemic side effects, which have long been recognized in transplant patients, treatment with higher doses of mTOR inhibitors is not feasible to achieve efficacy. However, an alternative approach would be to target an mTOR inhibitor to the kidney in order to achieve a tissue-specific effect while avoiding, or significantly reducing, systemic side effects.
We have previously demonstrated the concept of targeted drug delivery by folate receptor (FR)–mediated endocytosis.22,23 In these studies, targeted delivery is achieved due to the high overexpression of the FR in many cancer cells compared with most normal cells, which allows for the delivery of folate-conjugated drugs to cancer cells with minimal effect on other tissues. By chemical synthesis, folate can be coupled to drugs using a cleavable linker. This permits the cellular uptake of folate conjugates by FR-mediated endocytosis followed by subsequent cleavage and cytoplasmic release of the drug cargo. Using this technology, we have engineered several folate-conjugated compounds, some of which are in clinical trials.22 Experiments using a folate-conjugated imaging agent followed by whole-body visualization revealed that the imaging agent was effectively targeted to the kidney in addition to cancer cells,24 an observation supported by the high level of folate receptor expression in renal tissue.25 The FR is known to be highly expressed primarily on the apical membranes of proximal tubule cells, where it functions as a salvage mechanism to prevent the wasteful excretion of filtered folates. Thus, filtered folates are captured by the proximal tubule FRs and then transcytosed to the basolateral membrane, where they are released back into circulation.26,27 These data suggested that folate-conjugated drugs may be useful as kidney-specific therapeutics, especially in the context of renal disorders, such as ADPKD.
To test this hypothesis, we have synthesized a folate-conjugated form of rapamycin designated EC0371, hereafter referred to as FC-rapa, and tested its ability to inhibit mTOR pathway activity and alleviate renal cystic disease. Using in vitro experiments, we demonstrate that FC-rapa inhibits mTOR pathway activity in a dose- and FR-dependent manner. Furthermore, we show that FC-rapa inhibits mTOR activity and significantly improves the renal cystic phenotype in a mouse model of PKD. Finally, we show that in vivo administration of FC-rapa in adult animals results in inhibition of mTOR in the kidney with reduced systemic effects. These results suggest that folate-conjugated drugs may prove useful for targeted drug delivery to the kidney and that FC-rapa may be promising for treatment of patients with ADPKD.
Results
Expression of the FR in Normal and Cystic Renal Tissue
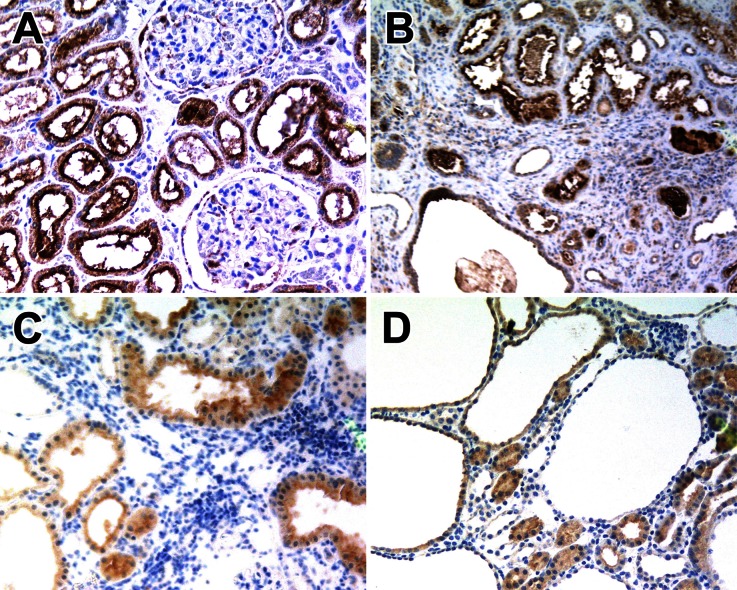
To determine whether FR expression is maintained in polycystic kidneys, we analyzed renal specimens from human ADPKD and two PKD mouse models by immunohistochemistry. As shown in Figure 1, all ADPKD specimens and kidneys from the orpk-rescue and bpk mouse models exhibit strong immunostaining for FR. Both normal tubules and cyst-lining epithelial cells are FR positive. Signal intensities were quantified and found to be similar to those in human serous ovarian cancer specimens (Supplemental Table 1), which has previously been shown to be strongly FR positive.28 These results indicate that FR is highly expressed in renal cysts and thus may allow the targeting of folate-conjugated drugs to the appropriate cells.
Figure 1.
FR expression in PKD. Immunohistochemistry for FR in (A) normal human kidney, (B) human ADPKD, (C) the orpk-rescue mouse model, and (D) the bpk mouse model. Note that both normal tubule epithelial cells and cyst-lining cells are FR positive. Sections were counterstained with hematoxylin. Original magnification: ×100.
Development and Synthesis of FC-Rapa (EC0371)
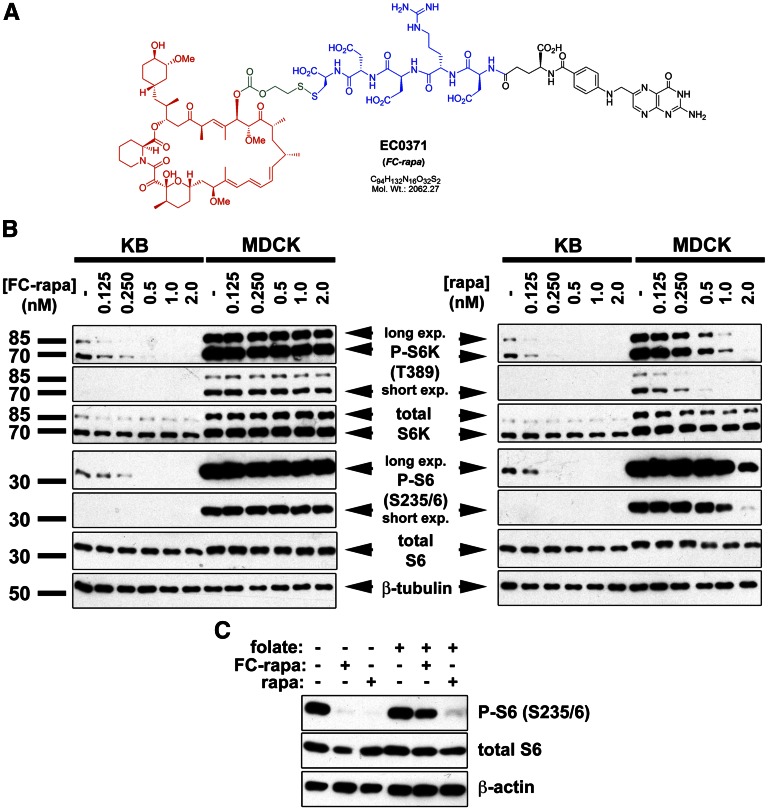
Consistent with previously described folate-drug conjugates,22 FC-rapa was constructed in modular form to contain one folic acid–targeting moiety with a hydrophilic pentapeptide spacer extended off its gamma glutamyl residue, and coupled to the rapamycin drug payload via a disulfide-based, self-immolative linker system (see Figure 2A).
Figure 2.
Folate-conjugated rapamycin (EC0371) inhibits mTOR pathway activity in FR-positive cells. (A) Chemical structure of EC0371 showing the modular components folate (black), hydrophilic spacer (blue), biologically cleavable linker (green), and rapamycin (red). (B) The mTOR pathway is inhibited in FR-positive KB cells and not FR-negative MDCK cells, as assessed by immunoblotting using the surrogate downstream markers P-S6K (Thr389) and P-S6 (Ser235/6). Cells were treated with the indicated concentrations of FC-rapa or unconjugated rapamycin for 16 hours. (C) Pretreatment of KB cells with excess folate prevents FC-rapa, but not unconjugated rapamycin, from inhibiting mTOR pathway activity, as assessed by immunoblotting using a P-S6 (Ser235/6) antibody.
FC-Rapa Inhibits mTOR Pathway Activity in FR-Positive, but Not FR-Negative, Cells
To determine whether FC-rapa is capable of inhibiting the mTOR pathway in vitro, we used folate-adapted KB cells, which express high cell surface levels of FR.25 In contrast, MDCK cells do not express the FR29 (Endocyte, Inc. Unpublished observations). Untreated cells exhibit phosphorylation of S6 kinase and its downstream substrate, S6, which are well accepted surrogate markers of mTOR pathway activity (Figure 2B). As expected, treatment of both KB and MDCK cells with unconjugated rapamycin inhibits phosphorylation of S6 kinase and S6. Similarly, incubation of KB cells with FC-rapa also inhibits phosphorylation of S6 kinase and S6 in a dose-dependent manner. In contrast, mTOR pathway activity was unaffected by FC-rapa in MDCK cells, even at the highest dose tested (Figure 2B).
To further test whether the effect of FC-rapa depends on FR-mediated endocytosis, we conducted folate competition experiments. Treatment with 10 nM FC-rapamycin and 10 nM rapamycin results in similar levels of mTOR inhibition in KB cells over the same period (Supplemental Figure 1). Pretreatment of KB cells with 100 μM folate prevents mTOR pathway inhibition by FC-rapa, but not by unconjugated rapamycin (Figure 2C and Supplemental Figure 2). Altogether, these results indicate that FC-rapa is specifically taken up by FR-mediated endocytosis and that FC-rapa is converted intracellularly to the active drug, which achieves effective mTOR inhibition.
FC-Rapa Alleviates the Renal Cystic Phenotype in a Mouse Model of PKD
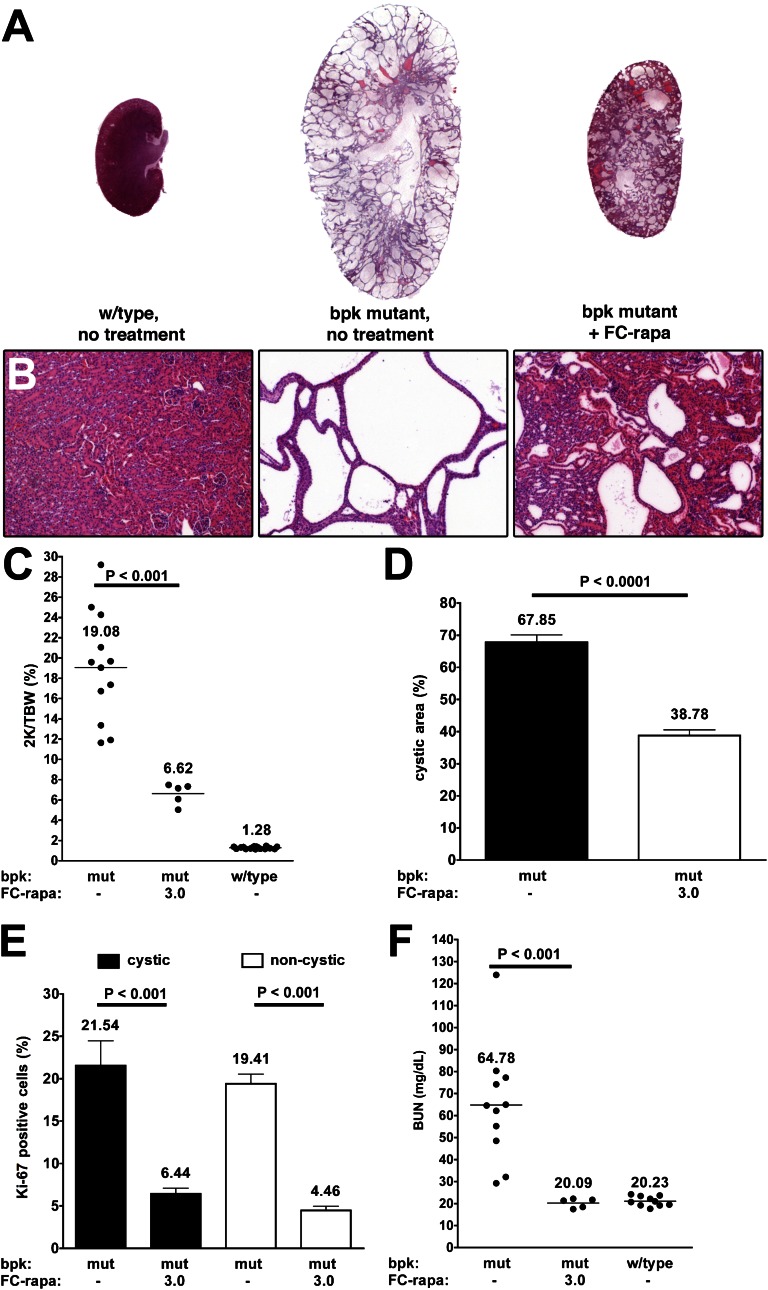
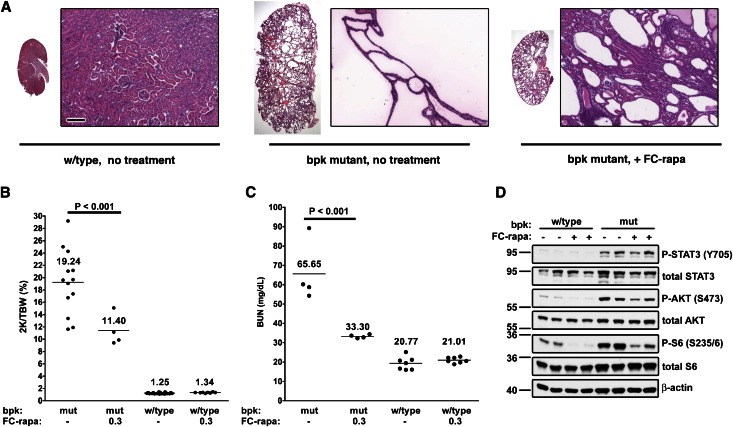
We have shown previously that treatment of bpk mutant mice with rapamycin results in significant alleviation of the renal cystic phenotype.3 Therefore, we used this PKD mouse model to test the possible in vivo efficacy of FC-rapa. Animals were treated from postnatal days 7 to 21 with 3 μmol of FC-rapa per kg per day or vehicle. Administration of FC-rapa significantly inhibits renal enlargement (Figure 3A) and cyst growth (Figure 3B) in bpk mutant animals and results in large decreases in kidney weight (Figure 3C), cystic area (Figure 3D), and cell proliferation (Figure 3E). Analysis with nephron segment markers revealed that FC-rapa treatment primarily inhibits the growth of cysts of distal and collecting tubule origin in bpk mutant animals (Supplemental Figure 3). BUN levels of FC-rapa–treated bpk mutant animals were greatly reduced to levels identical to those of wild-type controls, indicating that kidney function was fully preserved (Figure 3F). These results suggest that FC-rapa is as effective as unconjugated rapamycin in inhibiting proliferation and renal cyst growth in this PKD mouse model.
Figure 3.
FC-rapa significantly improves the renal cystic phenotype in the bpk mouse model of PKD. Daily intraperitoneal treatment of bpk mutant mice with FC-rapa, 3 μmol/kg per day, between days 7 and 21 significantly improves the renal polycystic phenotype, as assessed by (A) hematoxylin-eosin staining of whole kidney and (B) high-powered sections (original magnification: ×100), (C) two kidney-to-total body weight ratio (horizontal bar = average 2K/TBW value), and (D) a significant reduction in cystic area (error bars = SEM) compared with nontreated bpk mutant animals. (E) Quantification of Ki-67 immunofluorescence staining revealed a substantial decrease in the proliferation of both cystic and noncystic renal epithelial cells in treated versus nontreated bpk mutant animals (error bars = SEM). (F) BUN values are reduced to wild-type levels in FC-rapa–treated bpk mutant mice (horizontal bar = average BUN value).
FC-Rapa Inhibits Renal mTOR Activity
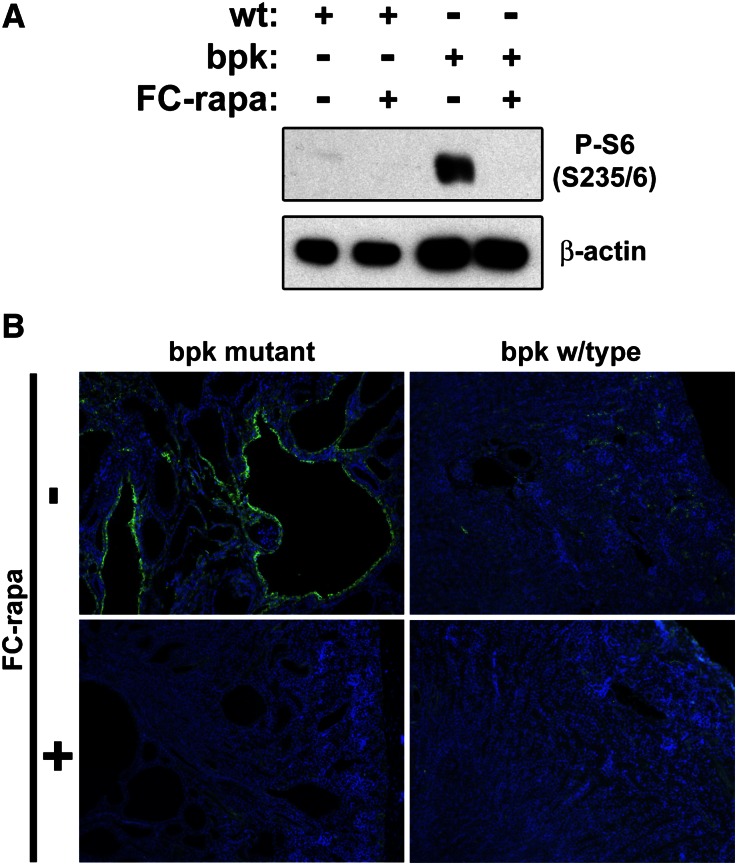
To examine whether the observed strong beneficial effect of FC-rapa is due to inhibition of renal mTOR pathway activity, we determined the level of phospho-S6 (P-S6), a surrogate marker of mTOR activity. P-S6 is highly upregulated in bpk mutant compared with wild-type kidneys, and treatment of bpk mutant mice with FC-rapa results in nearly complete inhibition of P-S6 (Figure 4A). Immunofluorescence microscopy revealed that P-S6 is highly activated in cyst-lining epithelial cells, whereas wild-type renal epithelial cells exhibit minimal staining (Figure 4B). FC-rapa treatment nearly completely eliminates any detectable P-S6 staining in remaining cysts of bpk mutant kidneys (Figure 4B). These results suggest that FC-rapa is mediating its therapeutic effect via inhibition of mTOR activity in cyst-lining epithelial cells.
Figure 4.
FC-rapa treatment of bpk mice inhibits mTOR pathway activity in renal epithelial cells. Treated bpk mutant mice exhibit almost complete inhibition of mTOR pathway activity in renal epithelial cells, as assessed by (A) immunoblotting and (B) immunofluorescence analyses using an antibody against P-S6 (Ser235/6) (green). Nuclear counter-stain, 4',6-diamidino-2-phenylindole, blue. wt, wild type. Original magnification: ×100.
Establishment of an Effective and Tissue-Specific Dose of FC-Rapa
Although treatment of bpk mutant mice with FC-rapa was highly effective in reducing renal cyst growth and preserving renal function, this dose also caused significant retardation of body growth and thymus weight in both mutant and wild-type animals (Supplemental Figure 4, A and B). The bpk mouse model is notably an early-onset PKD model, and these mice are actively growing during the treatment period (postnatal days 7–21). It is therefore likely that tissue FR expression is much more widespread in these young, growing animals than in adult animals.30 To further test this hypothesis, we determined the activation status of the mTOR pathway in tissue lysates by Western blotting. Liver and spleen of both mutant and wild-type mice treated with FC-rapa, 3 μmol/kg per day, exhibited a significant reduction in P-S6 levels compared with nontreated controls (Supplemental Figure 4D). These results suggest that FC-rapa is taken up in tissues other than the kidneys in these young, growing animals.
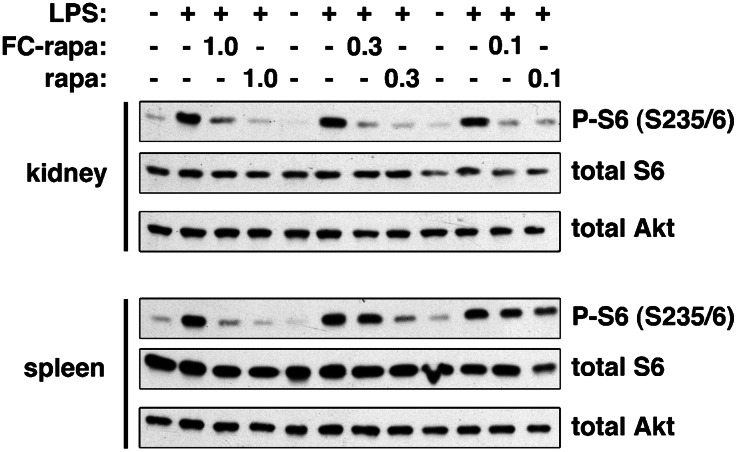
Previous studies using the folate-conjugated imaging agents 99mTc-EC2024 and 111In-DTPA-folate31 have demonstrated that they are significantly taken up only in kidneys of normal adult mice, not in other tissues and organs. Because any therapeutic approach for patients with ADPKD would be relevant only for adult patients, we next tested the ability of FC-rapa to inhibit mTOR pathway activity in different tissues in adult mice. Since mTOR activity is typically low in most normal adult tissues, we used short-term LPS treatment32 as a method to stimulate mTOR activity (Supplemental Figure 5). Animals were pretreated with different doses of FC-rapa or unconjugated rapamycin, followed by short-term LPS treatment. As shown in Figure 5, an FC-rapa dose of 0.3 μmol/kg per day inhibited LPS-induced mTOR pathway activity in the kidney but not the spleen. In contrast, the same dose of unconjugated rapamycin inhibited mTOR activity in both the kidney and the spleen. These results suggest that treatment with this dose of FC-rapa leads to preferential uptake in the kidneys of adult mice compared with the systemic effect of unconjugated rapamycin.
Figure 5.
Low-dose FC-rapa treatment causes tissue-specific mTOR inhibition in the kidney. Adult wild-type mice were treated for 3 days with the indicated doses (in μmol/kg per day) of FC-rapa or unconjugated rapamycin followed by immunoblotting of total kidney or spleen tissues. Treatment with FC-rapa, 0.3 μmol/kg per day, results in specific mTOR pathway inhibition in the kidney but not the spleen.
To test whether the 10 times lower dose of FC-rapa (i.e., 0.3 μmol/kg per day) is still effective in inhibiting renal cyst growth, we treated bpk animals with this dose. As shown in Figure 6, the therapeutic efficacy of this dose was somewhat less than that of treatment with the higher dose. However, the lower dose still significantly reduced renal cyst growth (Figure 6A) and renal weight (Figure 6B), and it largely preserved renal function as measured by BUN (Figure 6C) compared with nontreated mutant animals. FC-rapa at this low dose effectively inhibited renal mTOR pathway activity in bpk mutant mice to levels similar to those in wild-type mice (Figure 6D). In contrast, this dose of FC-rapa did not significantly affect the body weight or thymus weight (Supplemental Figure 6). Altogether, these results suggest that treatment with the appropriate dose of FC-rapa can result in kidney-specific inhibition of the mTOR pathway, leading to strong reduction in renal cyst growth and preservation of renal function.
Figure 6.
Low-dose FC-rapa treatment effectively improves renal cystic phenotype. Bpk mutant or wild-type mice were treated with FC-rapa, 0.3 μmol/kg per day, from postnatal days 7 to 21. Treatment of bpk mutant mice results in significant reduction of renal cyst growth (hematoxylin and eosin sections) (high-power original magnification: ×100) (A), two-kidney weight as a percentage of total body weight (2K/TBW) (horizontal line = average 2K/TBW value) (B), and BUN (horizontal line = average BUN value) (C), and it inhibits mTOR pathway activation in the kidney (D).
Discussion
Although mTOR inhibitors have probably been the most robust and effective therapeutics in preclinical trials using PKD rodent models,33 recent results from clinical trials have been disappointing. The mTOR inhibitor rapamycin is an exquisitely specific drug that does not appear to affect molecular targets other than mTOR.34 Any effects and side effects of rapamycin are probably due to mTOR inhibition in various tissues. For example, the immunosuppressive effect is thought to be due to mTOR inhibition in T and B cells, presumably in the thymus, spleen, lymphatic system, and other tissues. Immunosuppression, inhibition of wound healing, and other side effects are highly undesirable in any mTOR inhibitor–based therapy for ADPKD. Because treatment with tolerable doses of mTOR inhibitors appears to be largely ineffective in patients with ADPKD, and because doses cannot feasibly be increased, the only way forward would be to target the drug specifically to the affected tissue.
Our results suggest that targeting of rapamycin to the FR is a promising avenue to specifically affect the pathology of PKD. Both polycystic and normal kidneys express FR at high levels, and both sites appear to preferentially accumulate FC-rapa. One reason that drug targeting via the FR provides a high level of tissue specificity is that folate uptake is accomplished not only by FR but also by a folate carrier. In contrast to the more restricted expression of FR, the folate carrier is widely expressed and does not take up folate-conjugated drugs.23 Therefore, FC-rapa is not expected to affect cells that depend on the folate carrier. Tissues with the highest expression levels of FR are various tumors, kidneys, and (in this study) polycystic kidneys. Therefore, these tissues and organs are expected to be most sensitive to FC-rapa. In addition to this layer of specificity, it is important to point out that any uptake of FC-rapa by normal renal tubule cells in polycystic kidneys may be expected to be largely inconsequential. The reason is that normal renal tubule cells exhibit extremely low or undetectable mTOR activity (see Figure 4)3,4 and that treatment of normal animals even with high doses of rapamycin or for very long duratio does not appear to affect normal renal structure or function.3,4,35–38 We showed previously that rapamycin treatment in PKD mouse models led to apoptosis of cyst-lining cells but not of adjacent, normal tubule cells.3,4 Therefore, even if FC-rapa were equally targeted to both cystic and normal renal epithelial cells, one would expect a specific effect only against the cystic cells.
Unconjugated rapamycin and FC-rapa appear to be similarly efficacious with regard to their beneficial effect in the same PKD mouse model. We have previously treated bpk mice with rapamycin, 5.5 or 1.8 μmol/kg per day (i.e., 5 or 1.67 mg/kg per day),3 using otherwise identical timing and injection site, as in the present study. These doses of rapamycin, and both the 3- and 0.3-μmol/kg per day doses of FC-rapa, are clearly significantly effective. By their chemical nature, rapamycin and FC-rapa are very different and are expected to exhibit rather different pharmacologic properties. We therefore cannot directly compare their efficacy on a mole-by-mole basis.
This study suggests that one limitation of drug targeting by folate-conjugated compounds may be that young, growing mice may take up the compounds relatively nonspecifically, possibly resulting in multiple effects on nontarget tissues and organs. This is not unexpected because folate is absolutely required for DNA synthesis by rapidly dividing cells.30,39 Although it is unclear how these preclinical observations might translate to infants and children, most patients with ADPKD would not require treatment with FC-rapa until well into adulthood; therefore, any assumed limitation would probably be irrelevant. Of note, many previous studies with other folate-conjugated compounds have shown that their effects are rather specific to FR-expressing diseased tissues without exerting appreciable off-target effects.22,40
Our findings suggest that, in addition to ADPKD, the targeting of folate-conjugated compounds to kidneys may be a useful approach for numerous other renal epithelial disorders. As long as the high expression level of the FR is maintained in the diseased target cells, it may be possible to achieve specific drug delivery.
Altogether, we have combined rapamycin, a drug with exquisite molecular target specificity, with a delivery method that provides specificity for the target tissue. Our results suggest that this approach may help overcome the problems encountered in clinical trials with mTOR inhibitors for the treatment of ADPKD. Compared with unconjugated rapamycin, FC-rapa is expected to exhibit reduced off-site toxicity without loss of efficacy in the targeted organ.
Concise Methods
EC0371 Synthesis
EC0371 was synthesized by following known protocols, as described in an earlier publication.41 In brief, commercially available rapamycin was first converted into a pyridinyldisulfanyl-activated derivative while reacted with the heterobifunctional cross-linker 2-[benzotriazole-1-yl-(oxycarbonyloxy)-ethyldisulfanyl]-pyridine. The product was then reacted with the peptidic-based “folate-spacer” unit, pteroyl-γGlu-Asp-Arg-Asp-Asp-Cys. The resulting conjugate was purified by preparative HPLC. Clean product fractions were combined, lyophilized, and characterized by 1H-nuclear magnetic resonance and liquid chromatography/mass spectrometry.
Immunohistochemistry
Standard paraffin sections were subjected to immunostaining using a monoclonal antibody against human FRα (mAb-343/3D2; Endocyte, Inc.) or an affinity-purified, rabbit polyclonal antibody raised against bovine milk folate binding protein (PU-17; Endocyte Inc.). A common scoring system was used, where 0 is the staining equivalent to background (relative to isotype-stained controls) and 3+ is the most intensive staining.
Antibodies
All primary antibodies used in this study were obtained from Cell Signaling Technologies with the following exceptions: mAb-343/3D2 (Endocyte, Inc.), PU-17 (Endoctye, Inc.), rhodamine-conjugated Dolichos biflorus agglutinin (VectorLabs), and biotin-conjugated Lotus tetragonolobus lectin (VectorLabs). HRP- and fluorescent-conjugated secondary antibodies were obtained from Jackson ImmunoResearch and Thermo Scientific, respectively, with the exception of fluorescein-conjugated streptavidin (VectorLabs).
Cell Culture
KB cells are human nasopharyngeal carcinoma cells containing HeLa markers and were purchased from American Type Culture Collection. These cells were adapted to low folate–containing conditions and maintained in folate-free RPMI (Life Technologies) supplemented with 10% heat-inactivated FBS (HyClone). MDCK type II cells were obtained from Keith Mostov (University of California San Francisco), and were grown and maintained in MEM (Life Technologies) supplemented with 5% FBS (Atlantic Biologicals).
Folate Competition Assays
KB cells were pretreated with 100 μM folic acid for 30 minutes, followed by a 5-hour incubation with 100 μM folic acid and 10 nM of FC-rapa or unconjugated rapamycin. Cells were subsequently washed with folate- and drug-free RPMI media and then chased for an additional 19 hours before analysis.
Animal Studies
Animal studies were approved by the Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The bpk animal model of PKD has been described previously.3,42,43 Briefly, mice were treated with various doses of FC-rapamycin or rapamycin, administered via intraperitoneal injections, from day 7 to day 21 before animal euthanasia and analysis.
Disclosures
C.P.L. and I.R.V. are employees of Endocyte, Inc.
Supplementary Material
Acknowledgments
We thank Erin Olsan for help with experiments.
This study was supported by grants from the Department of Defense (Peer Reviewed Medical Research Program, W81XWH-07-1-0509) and the National Institutes of Health (R01-DK078043) to T.W.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012040367/-/DCSupplemental.
References
- 1.Harris PC, Torres VE: Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher AR, Germino GG, Somlo S: Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shillingford JM, Piontek KB, Germino GG, Weimbs T: Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 21: 489–497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, Patel V, Igarashi P, Alvord WG, Leighty R, Yao M, Bernardo M, Ileva L, Choyke P, Warren MB, Zbar B, Linehan WM, Schmidt LS: Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst 100: 140–154, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Futami K, Petillo D, Peng J, Wang P, Knol J, Li Y, Khoo SK, Huang D, Qian CN, Zhao P, Dykema K, Zhang R, Cao B, Yang XJ, Furge K, Williams BO, Teh BT: Deficiency of FLCN in mouse kidney led to development of polycystic kidneys and renal neoplasia. PLoS ONE 3: e3581, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao Y, Kim J, Schrier RW, Edelstein CL: Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP: Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 21: 598–604, 2006 [DOI] [PubMed]
- 9.Wu M, Wahl PR, Le Hir M, Wackerle-Men Y, Wuthrich RP, Serra AL: Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res 30: 253–259, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Zafar I, Belibi FA, He Z, Edelstein CL: Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD). Nephrol Dial Transplant 24: 2349–2353, 2009 [DOI] [PMC free article] [PubMed]
- 11.Zafar I, Ravichandran K, Belibi FA, Doctor RB, Edelstein CL: Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int 78: 754–761, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt K-U: Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, Ondei P, Rubis N, Diadei O, Gherardi G, Prandini S, Panozo A, Bravo RF, Carminati S, De Leon FR, Gaspari F, Cortinovis M, Motterlini N, Ene-Iordache B, Remuzzi A, Remuzzi G: Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol 21: 1031–1040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponticelli C, Locatelli F: Autosomal dominant polycystic kidney disease and mTOR inhibitors: The narrow road between hope and disappointment. Nephrol Dial Transplant 25: 3809–3812, 2010 [DOI] [PubMed]
- 16.Watnick T, Germino GG: mTOR inhibitors in polycystic kidney disease. N Engl J Med 363: 879–881, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Huber TB, Walz G, Kuehn EW: mTOR and rapamycin in the kidney: Signaling and therapeutic implications beyond immunosuppression. Kidney Int 79: 502–511, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Ibraghimov-Beskrovnaya O, Natoli TA: mTOR signaling in polycystic kidney disease. Trends Mol Med 17: 625–633, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Wüthrich RP, Kistler AD, Serra AL: Impact of mammalian target of rapamycin inhibition on autosomal-dominant polycystic kidney disease. Transplant Proc 42[Suppl]: S44–S46, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Perico N, Remuzzi G: Do mTOR inhibitors still have a future in ADPKD? Nat Rev Nephrol 6: 696–698, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Canaud G, Knebelmann B, Harris PC, Vrtovsnik F, Correas JM, Pallet N, Heyer CM, Letavernier E, Bienaimé F, Thervet E, Martinez F, Terzi F, Legendre C: Therapeutic mTOR inhibition in autosomal dominant polycystic kidney disease: What is the appropriate serum level? Am J Transplant 10: 1701–1706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leamon CP: Folate-targeted drug strategies for the treatment of cancer. Curr Opin Investig Drugs 9: 1277–1286, 2008 [PubMed] [Google Scholar]
- 23.Leamon CP, Jackman AL: Exploitation of the folate receptor in the management of cancer and inflammatory disease. Vitam Horm 79: 203–233, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Leamon CP, Parker MA, Vlahov IR, Xu L-C, Reddy JA, Vetzel M, Douglas N: Synthesis and biological evaluation of EC20: A new folate-derived, (99m)Tc-based radiopharmaceutical. Bioconjug Chem 13: 1200–1210, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP: Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 338: 284–293, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Birn H, Nielsen S, Christensen EI: Internalization and apical-to-basolateral transport of folate in rat kidney proximal tubule. Am J Physiol 272: F70–F78, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Birn H, Selhub J, Christensen EI: Internalization and intracellular transport of folate-binding protein in rat kidney proximal tubule. Am J Physiol 264: C302–C310, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M: Overexpression of folate binding protein in ovarian cancers. Int J Cancer 74: 193–198, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Kim CH, Park YS, Chung KN, Elwood PC: Sorting of the human folate receptor in MDCK cells. J Biochem Mol Biol 37: 362–369, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RG, Finnell RH: Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet 23: 228–232, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS: Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol 66: 1406–1414, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Lee PS, Wilhelmson AS, Hubner AP, Reynolds SB, Gallacchi DA, Chiou TT, Kwiatkowski DJ: mTORC1-S6K activation by endotoxin contributes to cytokine up-regulation and early lethality in animals. PLoS ONE 5: e14399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres VE, Boletta A, Chapman A, Gattone V, Pei Y, Qian Q, Wallace DP, Weimbs T, Wüthrich RP: Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin J Am Soc Nephrol 5: 1312–1329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris TE, Lawrence JC, Jr: TOR signaling. Sci STKE 2003: re15, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Lieberthal W, Fuhro R, Andry CC, Rennke H, Abernathy VE, Koh JS, Valeri R, Levine JS: Rapamycin impairs recovery from acute renal failure: Role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol 281: F693–F706, 2001 [DOI] [PubMed] [Google Scholar]
- 36.DiJoseph JF, Sharma RN, Chang JY: The effect of rapamycin on kidney function in the Sprague-Dawley rat. Transplantation 53: 507–513, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Di Joseph JF, Sehgal SN: Functional and histopathologic effects of rapamycin on mouse kidney. Immunopharmacol Immunotoxicol 15: 45–56, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA: Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegelstein O, Mitchell LE, Merriweather MY, Wicker NJ, Zhang Q, Lammer EJ, Finnell RH: Embryonic development of folate binding protein-1 (Folbp1) knockout mice: Effects of the chemical form, dose, and timing of maternal folate supplementation. Dev Dyn 231: 221–231, 2004 [DOI] [PubMed]
- 40.Lu Y, Stinnette TW, Westrick E, Klein PJ, Gehrke MA, Cross VA, Vlahov IR, Low PS, Leamon CP: Treatment of experimental adjuvant arthritis with a novel folate receptor-targeted folic acid-aminopterin conjugate. Arthritis Res Ther 13: R56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlahov IR, Santhapuram HK, Kleindl PJ, Howard SJ, Stanford KM, Leamon CP: Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part 1: EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorg Med Chem Lett 16: 5093–5096, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T: Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci U S A 108: 18067–18072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, Watnick T, Weimbs T: Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci U S A 108: 7985–7990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.