Abstract
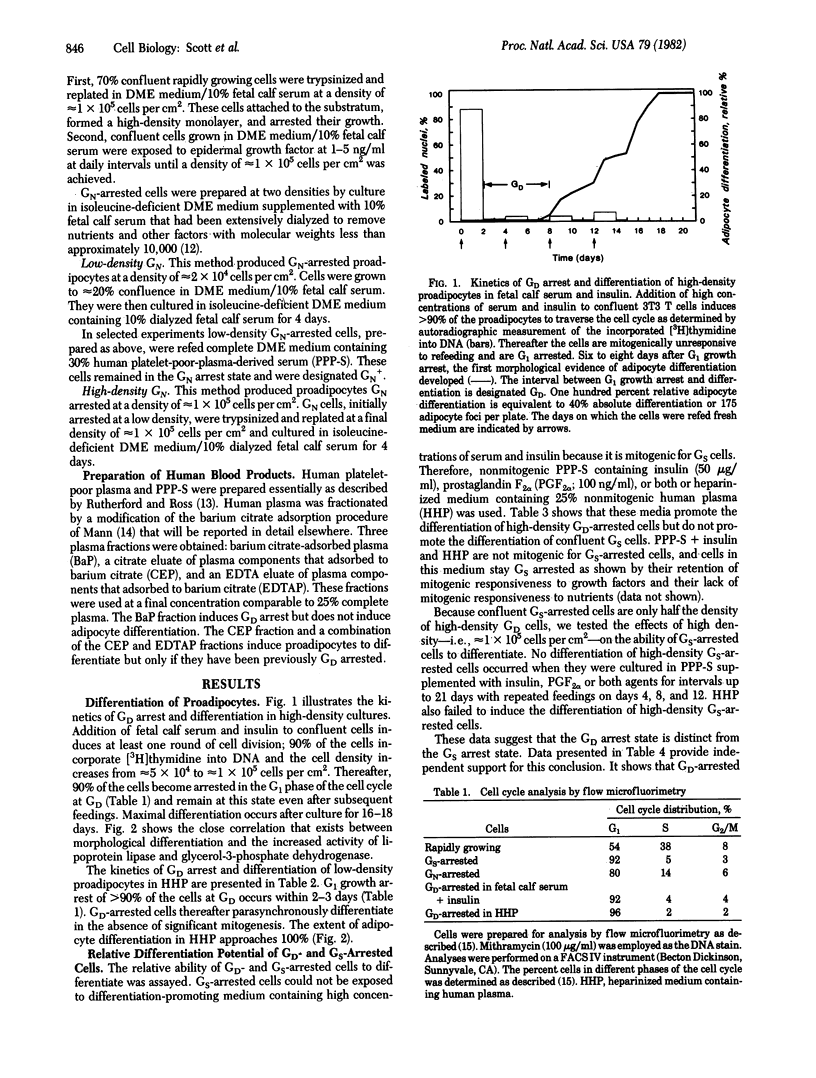
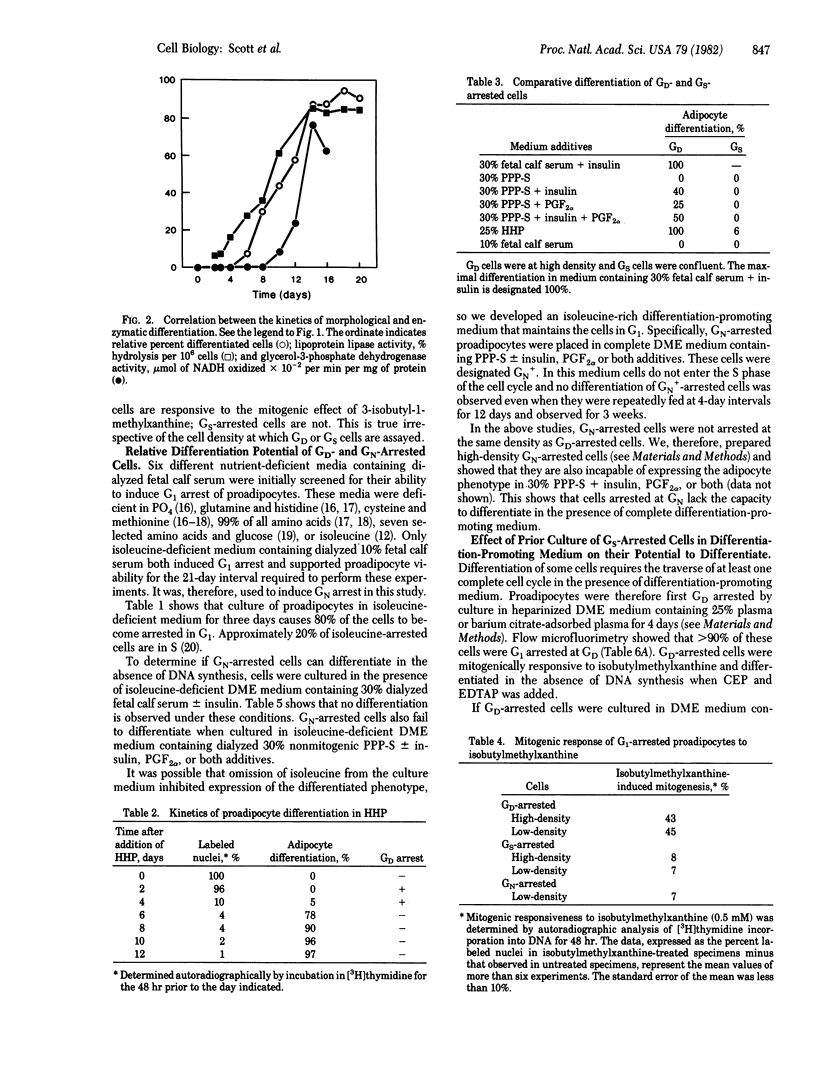
The differentiation of most mammalian cells is preceded by growth arrest in the G1 phase of the cell cycle, but the characteristics of this state have not been established. We now report that the growth arrest that precedes the differentiation of BALB/c 3T3 T mouse proadipocytes must occur at a distinct state in G1 designated GD. GD-arrested cells are characterized by their ability to differentiate in the absence of DNA synthesis and by their unique sensitivity to the mitogenic effect of isobutylmethylxanthine. Proadipocytes induced to become G1 growth arrested at other states by culture in medium deficient in growth factor or nutrients, by contrast, are unable to differentiate in the absence of DNA synthesis and are not stimulated to proliferate by isobutylmethylxanthine even when they are exposed to differentiation-promoting medium prior to arrest. These data support the conclusion that, prior to the expression of a differentiated phenotype, proadipocytes must arrest their growth at a distinct state in the G1 phase of the cell cycle, GD. These data also provide the basis for the hypothesis that carcinogenesis is associated with defects in the coupling of growth arrest and differentiation at the GD state.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz E. W., Jr, Getz M. J., Wells D. J., Moses H. L. Nuclear RNA polymerase activities and poly(A)-containing mRNA accumulation in cultured AKR mouse embryo cells stimulated to proliferate. Exp Cell Res. 1977 Aug;108(1):157–165. [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- Fielding C. J. Validation of a procedure for exogenous isotopic labeling of lipoprotein triglyceride with radioactive triolein. Biochim Biophys Acta. 1979 May 25;573(2):255–265. doi: 10.1016/0005-2760(79)90059-6. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Armour R., Baldwin J. H. Density-dependent regulation of growth of BSC-1 cells in cell culture: control of growth by low molecular weight nutrients. Proc Natl Acad Sci U S A. 1978 Jan;75(1):339–341. doi: 10.1073/pnas.75.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamely D., Rudland P. Nutrient-dependent arrest of fibroblast growth is partially reversed by insulin but not fibroblast growth factor. Nature. 1976 Mar 4;260(5546):51–53. doi: 10.1038/260051a0. [DOI] [PubMed] [Google Scholar]
- Kozak L. P., Jensen J. T. Genetic and developmental control of multiple forms of L-glycerol 3-phosphate dehydrogenase. J Biol Chem. 1974 Dec 25;249(24):7775–7781. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mann K. G. Prothrombin. Methods Enzymol. 1976;45:123–156. doi: 10.1016/s0076-6879(76)45016-4. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Moses H. L., Proper J. A., Volkenant M. E., Wells D. J., Getz M. J. Mechanism of growth arrest of chemically transformed cells in culture. Cancer Res. 1978 Sep;38(9):2807–2812. [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Boman B. M., Swartzendruber D. E., Zschunke M. A., Hoerl B. J. Cell cycle-associated modulation in cAMP-dependent plasma membrane phosphorylation. Exp Cell Res. 1981 May;133(1):73–82. doi: 10.1016/0014-4827(81)90358-x. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey R. A., Ley K. D. Isoleucine-mediated regulation of genome repliction in various mammalian cell lines. Cancer Res. 1971 Jan;31(1):46–51. [PubMed] [Google Scholar]
- Wise L. S., Green H. Studies of lipoprotein lipase during the adipose conversion of 3T3 cells. Cell. 1978 Feb;13(2):233–242. doi: 10.1016/0092-8674(78)90192-7. [DOI] [PubMed] [Google Scholar]
- Yen A., Pardee A. B. Arrested states produced by isoleucine deprivation and their relationship to the low serum produced arrested state in Swiss 3T3 cells. Exp Cell Res. 1978 Jul;114(2):389–395. doi: 10.1016/0014-4827(78)90497-4. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]



