Abstract
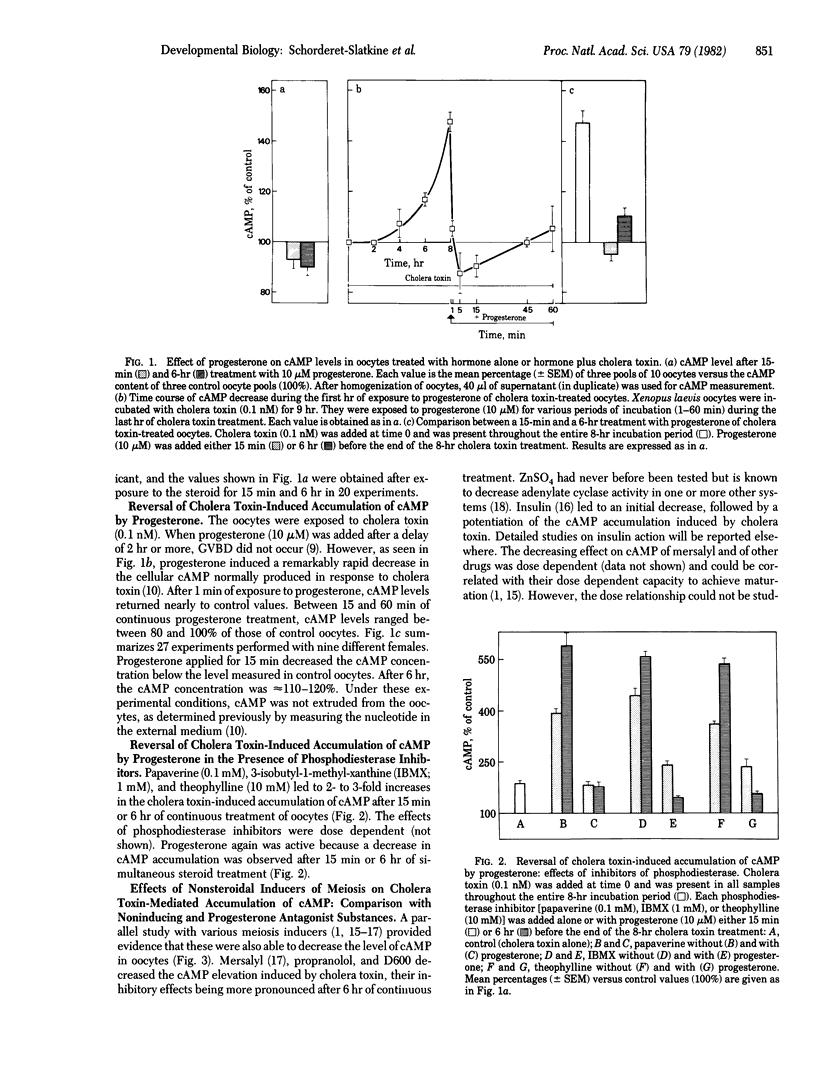
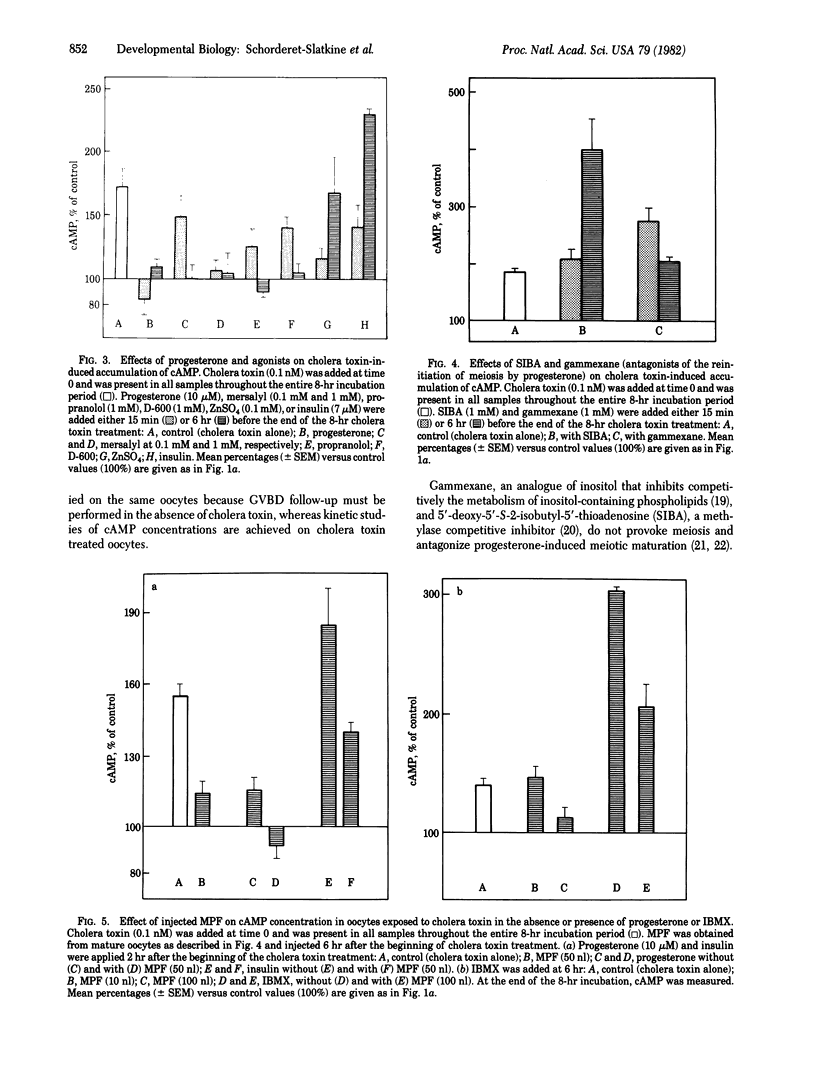
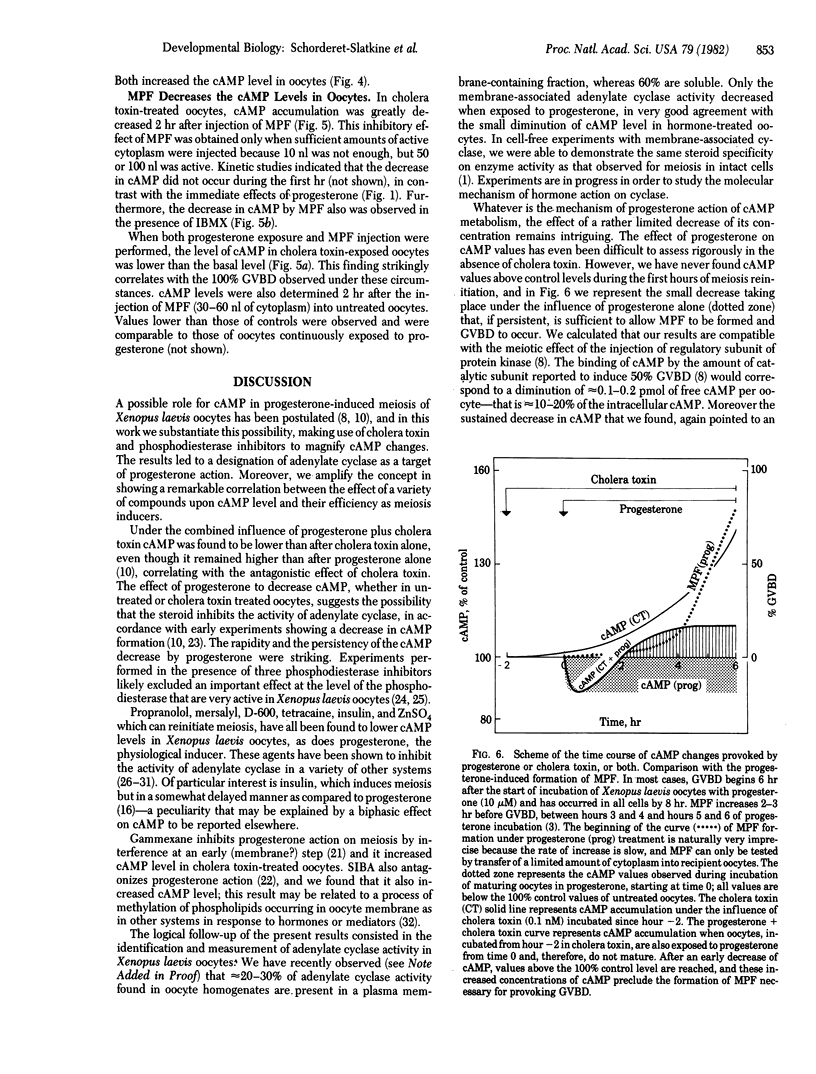
Progesterone depressed rapidly (50% at 1 min) and persistently cyclic AMP (cAMP) concentration that had been elevated by cholera toxin in Xenopus laevis oocytes. cAMP remained below 1 pmol per oocyte (mean basal level) for approximately 1 hr and thereafter rose to approximately 120% of control values, while germinal vesicle (nucleus) breakdown did not occur. In the absence of cholera toxin, progesterone treatment for 6 hr maintained cAMP concentration below the basal level (but not lower than 80%), and germinal vesicle breakdown occurred. Experiments in the presence of phosphodiesterase inhibitors suggested that progesterone modulates adenylate cyclase activity. The maturation promoting factor, which is formed after 3-5 hr of progesterone treatment and provokes germinal vesicle breakdown after its injection into untreated oocytes, also decreased cAMP concentration, an observation that may explain its "autoamplification." Nonsteroidal inducers of meiosis reinitiation (e.g., propranolol, methoxyverapamil, mersalyl) diminished the cholera toxin-mediated accumulation of cAMP, in contrast to compounds devoid of meiotic-inducing capacity and antagonist to progesterone action, such as gammexane (an inositol analogue) and 5'-deoxy-S-(2-methylpropyl)-5'-thioadenosine (a methylase inhibitor), that increased the nucleotide level. The fine control, suggested by the effects of small changes in cAMP levels, gives evidence of great sensitivity to a critical determinant governing meiotic cell division.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende C. C., Bravo R., Allende J. E. Comparison of in vivo and in vitro properties of cyclic adenosine 3':5'-monophosphate phosphodiesterase of amphibian oocytes. J Biol Chem. 1977 Jul 10;252(13):4662–4666. [PubMed] [Google Scholar]
- Balakier H., Czolowska R. Cytoplasmic control of nuclear maturation in mouse oocytes. Exp Cell Res. 1977 Dec;110(2):466–469. doi: 10.1016/0014-4827(77)90314-7. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978 Oct 19;275(5681):593–598. doi: 10.1038/275593a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Melmon K. L., Tomkins G. M., Weinstein Y. Genetic analysis of cyclic AMP in a mammalian cell. Adv Cyclic Nucleotide Res. 1975;5:771–786. [PubMed] [Google Scholar]
- Brachet J., Baltus E., De Schutter-Pays A., Hanocq-Quertier J., Hubert E., Steinert G. Induction of maturation (meiosis) in Xenopus laevis oocytes by three organomercurials. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1574–1578. doi: 10.1073/pnas.72.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Otero C., Allende C. C., Allende J. E. Amphibian oocyte maturation and protein synthesis: related inhibition by cyclic AMP, theophylline, and papaverine. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1242–1246. doi: 10.1073/pnas.75.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W. K., Stern S., Biggers J. D. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool. 1974 Mar;187(3):383–386. doi: 10.1002/jez.1401870307. [DOI] [PubMed] [Google Scholar]
- Davies T. F., McLachlan S. M., Povey P. M., Smith B. R., Hall R. The influence of propranolol on the thyrotropin receptor. Endocrinology. 1977 Apr;100(4):974–979. doi: 10.1210/endo-100-4-974. [DOI] [PubMed] [Google Scholar]
- Drury K. C., Schorderet-Slatkine S. Effects of cycloheximide on the "autocatalytic" nature of the maturation promoting factor (MPF) in oocytes of Xenopus laevis. Cell. 1975 Mar;4(3):269–274. doi: 10.1016/0092-8674(75)90175-0. [DOI] [PubMed] [Google Scholar]
- El-Etr M., Schorderet-Slatkine S., Baulieu E. E. Meiotic maturation in Xenopus laevis oocytes initiated by insulin. Science. 1979 Sep 28;205(4413):1397–1399. doi: 10.1126/science.472755. [DOI] [PubMed] [Google Scholar]
- Enouf J., Lawrence F., Tempete C., Robert-Gero M., Lederer E. Relationship between inhibition of protein methylase I and inhibition of Rous sarcoma virus-induced cell transformation. Cancer Res. 1979 Nov;39(11):4497–4502. [PubMed] [Google Scholar]
- Finidori-Lepicard J., Schorderet-Slatkine S., Hanoune J., Baulieu E. E. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981 Jul 16;292(5820):255–257. doi: 10.1038/292255a0. [DOI] [PubMed] [Google Scholar]
- Godeau F., Boquet P., Schorderet M., Schorderet-Slatkine S., Baulieu E. E. Inhibition par l'entéro-toxine de Vibrio cholerae de la réinitiation méiotique de l'ovocyte de Xenopus laevis induite in vitro par la progestérone. C R Acad Sci Hebd Seances Acad Sci D. 1978 Mar 6;286(9):685–688. [PubMed] [Google Scholar]
- Hancock A. A., Hess M. E. Verapamil-induced changes in myocardial contractile force and cyclic nucleotides in the isolated perfused rat heart. Biochem Pharmacol. 1979 Sep 1;28(17):2601–2606. doi: 10.1016/0006-2952(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Hepp K. D., Rinninger J., Langley J., Renner R. Inhibition of catecholamine-stimulated adenylate cyclase in fat cells by local anaesthetics. FEBS Lett. 1978 Jul 15;91(2):325–328. doi: 10.1016/0014-5793(78)81202-2. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Lambert B., Jacquemin C. Inhibition by insulin of the adrenaline-stimulated adenylate cyclase in rat adipose tissue. FEBS Lett. 1979 Sep 1;105(1):19–22. doi: 10.1016/0014-5793(79)80878-9. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Butcher F. R., Krebs E. G. Early effect of progesterone on levels of cyclic adenosine 3':5'-monophosphate in Xenopus oocytes. J Biol Chem. 1979 Feb 10;254(3):579–582. [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Mar 10;252(5):1712–1718. [PubMed] [Google Scholar]
- Masui Y., Clarke H. J. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Mavier P., Hanoune J. Adenylate cyclase from rat-liver plasma membrane: inhibition by mersalyl and other mercurial derivatives. Eur J Biochem. 1975 Nov 15;59(2):593–599. doi: 10.1111/j.1432-1033.1975.tb02487.x. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Moreau M., Vilain J. P., Guerrier P. Free calcium changes associated with hormone action in amphibian oocytes. Dev Biol. 1980 Jul;78(1):201–214. doi: 10.1016/0012-1606(80)90329-2. [DOI] [PubMed] [Google Scholar]
- Morrill G. A., Schatz F., Kostellow A. B., Poupko J. M. Changes in cyclic AMP levels in the amphibian ovarian follicle following progesterone induction of meiotic maturation. Effect of phosphodiesterase inhibitors and exogenous calcium on germinal vesicle breakdown. Differentiation. 1977 Aug 11;8(2):97–104. doi: 10.1111/j.1432-0436.1977.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Mulner O., Cartaud A., Ozon R. Cyclic AMP phosphodiesterase activities in Xenopus laevis oocytes. Differentiation. 1980 Feb;16(1):31–39. doi: 10.1111/j.1432-0436.1980.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Mulner O., Huchon D., Thibier C., Ozon R. Cyclic AMP synthesis in Xenopus laevis oocytes: inhibition by progesterone. Biochim Biophys Acta. 1979 Jan 4;582(1):179–184. doi: 10.1016/0304-4165(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Freedman R., Hoffer B. J. Lanthanum inhibits brain adenylate cyclase and blocks noradrenergic depression of Purkinje cell discharge independent of calcium. Nature. 1976 May 27;261(5558):330–332. doi: 10.1038/261330a0. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Smith L. D. Inhibition of oocyte maturation by theophylline: possible mechanism of action. Dev Biol. 1976 Sep;52(2):318–322. doi: 10.1016/0012-1606(76)90249-9. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Reynhout J. K., Smith L. D. Studies on the appearance and nature of a maturation-inducing factor in the cytoplasm of amphibian oocytes exposed to progesterone. Dev Biol. 1974 Jun;38(2):394–400. doi: 10.1016/0012-1606(74)90016-5. [DOI] [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem. 1981 Jun 25;256(12):6368–6373. [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Drury K. C. Progesterone induced maturation in oocytes of Xenopus laevis. Appearance of a 'maturation promoting factor' in enucleated oocytes. Cell Differ. 1973 Oct;2(4):247–254. doi: 10.1016/0045-6039(73)90013-4. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Finidori-Lepicard J., Hanoune J., Baulieu E. E. Effect of a methyl-transferase inhibitor, 5'-deoxy'5'-S-isobutyl-thioadenosine (SIBA) on cAMP level and progesterone induced meiosis reinitiation in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1981 May 29;100(2):544–550. doi: 10.1016/s0006-291x(81)80211-2. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. E. Inhibition of the progesterone-dependent induction of meiosis by gammexane in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1977 Jul 25;77(2):496–502. doi: 10.1016/s0006-291x(77)80007-7. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. E. Progesterone-induced meiotic reinitation in vitro in Xenopus laevis oocytes: a role for the displacement of membrane-bound calcium. Differentiation. 1977 Oct 20;9(1-2):67–76. doi: 10.1111/j.1432-0436.1977.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Boquet P., Godeau F., Baulieu E. E. Progesterone-induced meiosis in Xenopus laevis oocytes: a role for cAMP at the "maturation-promoting factor" level. Cell. 1978 Dec;15(4):1269–1275. doi: 10.1016/0092-8674(78)90052-1. [DOI] [PubMed] [Google Scholar]
- Schutter A. P., Kram R., Hubert E., Brachet J. Cyclic nucleotides and amphibian development. Exp Cell Res. 1975 Nov;96(1):7–14. doi: 10.1016/s0014-4827(75)80030-9. [DOI] [PubMed] [Google Scholar]
- Speaker M. G., Butcher F. R. Cyclic nucleotide fluctuations during steroid induced meiotic maturation of frog oocytes. Nature. 1977 Jun 30;267(5614):848–850. doi: 10.1038/267848a0. [DOI] [PubMed] [Google Scholar]
- Sunkara P. S., Wright D. A., Rao P. N. Mitotic factors from mammalian cells induce germinal vesicle breakdown and chromosome condensation in amphibian oocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2799–2802. doi: 10.1073/pnas.76.6.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. B., Houslay M. D., Metcalfe J. C., Birdsall N. J. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature. 1975 Jun 26;255(5511):684–687. doi: 10.1038/255684a0. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Pinto L. H., O'Connor C. M., Smith L. D. Progesterone induces a rapid increase in [Ca2+]in of Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1534–1536. doi: 10.1073/pnas.77.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]