Abstract
The crystal structure of a Wnt morphogen bound to its Frizzled receptor ectodomain provides insights into the evolutionary provenance of this complex fold, and offers an explanation for why Wnts utilize both lipid- and protein-mediated contacts to engage Frizzleds.
The core repertoire of cell signaling pathways that guide the development of metazoan organisms notably include two systems, Wnt and Hedgehog, that appear to have an enigmatically intertwined evolutionary history (Kalderon, 2002; Nusse, 2003; Buechling and Boutros, 2011). The molecular parallels center on their respective signaling receptor types, Frizzled and Smoothened (Smo), that are related heptahelical transmembrane molecules capped by N-terminal cysteine-rich Frizzled (Fz) ectodomains (Kalderon, 2002; Nusse, 2003; Krishnan et al., 2012). These latter Fz modules were conjectured to have an ancient sterol or lipid-binding function by their distant structural homology to the cholesterol-carrying domain of the Niemann-Pick type C (NPC) transporter NPC1, and the riboflavin-bearing module of a folate receptor-like protein (Kwon et al., 2009; Bazan and de Sauvage, 2009). While Wnt and Hedgehog morphogens are unrelated proteins, similar enzymatic machinery perform critical lipid modifications that dictate their engagement to the Fz domains of Frizzled receptors—in the case of Wnts—or indirectly activate Smo, perhaps through its binding of a sterol-like molecule, by Hedgehog interaction with twelve-transmembrane Patched transporters (Hausmann et al., 2009; Nachtergaele et al., 2012).
While the structure of unlipidated Hedgehog has been captured both free and in complex with several cell surface molecules (Beachy et al., 2011), and partly discloses the nature of its affinity for Patched (Bosanac et al., 2009), Wnts have steadfastly eluded crystallographic analysis because of the inherent difficulties in producing recombinant versions of the lipidated and glycosylated, cysteine-rich 350–400 amino acid chains. By coexpressing the Xenopus Wnt8 protein with the receptive Fz domain of the human Fz8 receptor, Janda et al. (2012) succeeded in capturing a stable complex in solution, and reveal for the first time the X-ray crystal structure of a Fz domain in the embrace of a Wnt morphogen. The overall structure of the Wnt is remarkable, having the appearance of a grasping right hand with thumb and forefinger jutting out from a thicker palm and knuckles base, pinching the globular Fz8 ectodomain (Figure 1A). Both thumb and finger extensions in Wnt are long β-strand hairpin loops crossbraced by disulfide bridges; from Ser187 at the tip of the thumb, a palmitoleic acid (PAM) lipid group projects into a hydrophobic groove in the captive Fz domain, a binding Site 1 coincident with the predicted lipid or sterol pocket in Fz folds (Bazan and de Sauvage, 2009). In contrast, the Wnt finger β-hairpin makes a protein-protein Site 2 contact with a shallow pocket on the reverse face of the Fz domain, utilizing a rare vicinal disulfide link (Carugo et al., 2003) between Cys320-Cys321 as part of the recognition probe (Janda et al., 2012).
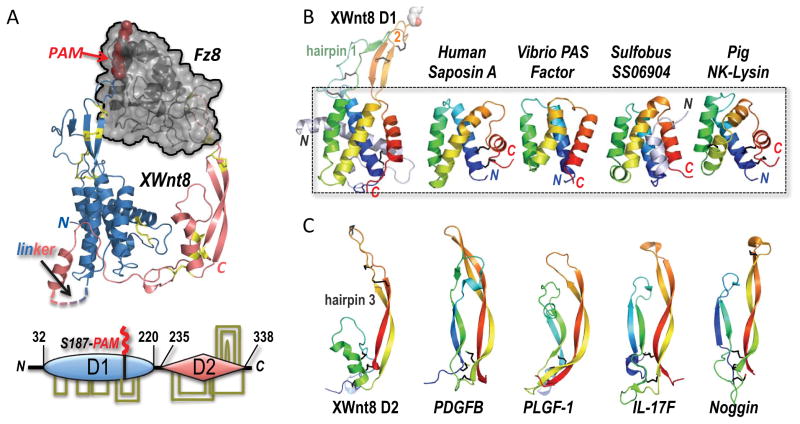
Figure 1. Modular architecture of Wnts suggests an evolutionary fusion of membrane-seeking and receptor-binding domains.
(A) The structure of the binary complex between Xwnt8 and the Fz8 CRD (PDB ID 4F0A) shows that the Wnt utilizes an unusual pair of extended β-hairpin fingers to grasp opposite sides of the (black) Fz domain. The blue N-terminal, mostly helical Wnt domain is lipid-modified (red PAM moiety) at Ser187 at the tip of β-hairpin 2; a hydrophobic groove in the Fz domain sequesters the PAM moiety. The red, mostly β-stranded C-terminal Wnt domain utilizes the tip of another β-hairpin finger to dock a distinct pocket in Fz8. Eleven conserved disulfide bridges are shown in stick format and colored yellow; these links are also drawn in the bottom schematic of the two-domain (D1 and D2) Wnt structure, and fully detailed in Figure 2A.
(B) A saposin-like four-α-helix bundle fold forms the core of the D1 domain, and superposes in the 2.85 to 3.9 Å RMSD range to the structures of human Saposin A (PDB ID 2DOB) and Pig NK-Lysin (1NKL)—both conventional saposins with an identifying trio of disulfide bridges, and Vibrio vulnificus PAS factor (PDB ID 2B8I) and Sulfobus solfataricus SS06904 protein (2KJG), examples of atypical, disulfide-free saposins. Hairpins 1 and 2 represent insertions into the first and third interhelical loops, respectively, in the XWnt8 D1 core domain.
(C) The structure of the Wnt D2 domain reveals a distinct resemblance to the monomer folds of cystine-knot growth factors, here represented by PDGFB (PDB ID 3MJG), PLGF-1 (PDB ID 1FZV), IL-17F (PDB ID 1JPY) and Noggin (PDB ID 1M4U) (Shim et al., 2010; Iyer et al., 2001; Hymowitz et al., 2001; Groppe et al., 2002). These structures superpose with the XWnt8 D2 domain in the 3 to 3.7 Å RMSD range over 41–46 Cα positions (Krissinel and Henrick, 2004).
A Wnt membrane-targeting domain resembles saposins
The evolutionary origin of these unusual, dual Fz recognition heads in Wnt was not immediately obvious. However upon deeper inspection, we find that it can best be read from their supporting scaffolds in the palm and knuckle regions of the structure that form two distinct protein domains (D1 and D2) separated naturally in the protein chains of Wnts by a length and sequence-variable linker (Figure 1A and S1A–C). N-terminal domain D1 is largely a helical bundle (the ‘palm’) with two long β-hairpin loop excursions (and their respective disulfide link rungs) packed alongside each other, the second or β-hairpin 2 bearing the lipidated Ser187, while β-hairpin 1 is less well structured, partly collapsed and more degenerate in sequence. The four helices supporting these β-hairpins form a core αC-αF antiparallel bundle with a faint internal repeat of a foundational helix-helix unit (Figure 2 and S1A). Using the PDBeFold server (Krissinel and Henrick, 2004), the four-helical core structure of D1 is significantly superposed with saposin-like proteins, an ancient class of multi-purpose lipid-interacting and carrier helical folds (Bruhn, 2005) (Figure 1B and S1B); lacking the interhelical disulfide bridges common to mammalian saposins, the Wnt D1 helical module is more closely set in the mold of atypical saposin-like proteins found in bacteria (Lee et al., 2006). Instructively, saposins can serve as the loading machinery for the lipid cargo presented by the non-classical Major Histocompatibility Complex (MHC) molecule CD1 (Darmoise et al., 2010; León et al, 2012). Thus, a plausible evolutionary path for the Wnt D1 domain involves the covalent acquisition of a lipid that was previously engaged non-covalently. In this way, Wnts could have exploited the inherent lipid affinity of the saposin-like fold to fulfill a functional requirement to associate with the plasma membrane, and act as a locally acting morphogen for a primitive, lipid-interacting Fz receptor.
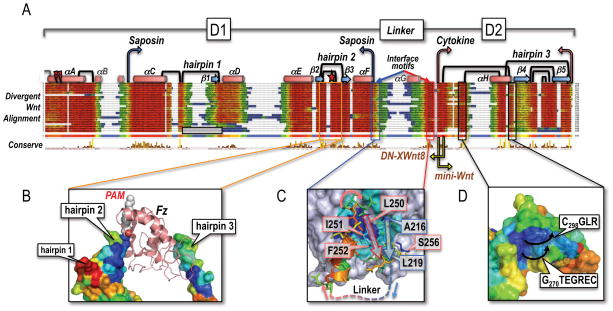
Figure 2. The composite Wnt fold and structural diversity of primitive Wnts.
(A) Structure-guided multiple sequence alignment of divergent Wnts to the template structure of XWnt8, and the human Wnt3a chain (Janda et al., 2012). Rather than a uniform set of vertebrate Wnts, we collected very divergent, primitive Wnts from the opposite end of the metazoan spectrum (Pang et al., 2010; Riddiford and Olson, 2011) that would better illustrate the plasticity of the Wnt fold. Sequences were harvested by iterative PsiBLAST searches (Altschul et al., 1997) from Genbank, using as bait the unclassified Wnt subtype chains of available Planaria (Schmidtea mediterranea), Tunicate (Oikopleura dioica), Cnidaria (Nematostella vectensis), Placozoa (Trichoplax adherens), Porifera (Oscarella lobularis and Amphimedon queenslandica) and Ctenophore (Mnemiopsis leidy) genomes; see Supplemental Data for Methods and References. Also included in the screen were the outlier C. elegans Wnts lin-44 and mom-2, and D. melanogaster WntD (Ching et al., 2008). Alignments were captured in Jalview with the ClustalX color scheme (Waterhouse et al., 2009); the consensus sequence logo is displayed in pink. Helices as drawn as red cylinders and labeled αA-αH (similar to the nomenclature of Janda et al., 2012), while β-strands are depicted as blue arrows and called β1-β5 (different from Janda et al., 2012). The boundaries of the saposin-like D1 and cytokine-like D2 domains are noted with blue and pink arrows, respectively; yellow arrows mark the N-terminal end of the Janda et al. (2012) mini-Wnt construct, and the C-terminal boundary of the DN-XWnt8 truncation (Hoppler et al., 1996). The Tiki1 processing sites in the XWnt8 helix αA are indicated by red lightning bolts (Zhang et al., 2012). The eleven X-ray-defined disulfide bridges (Figure 1A) are drawn as black links; an N-terminal grey half-link notes a likely, additional disulfide bridge in some Wnts, but absent in XWnt8. The three hairpin structures are also numbered, with the lipidated Ser187 (marked with a red star) located in hairpin 2, and the vicinal disulfide bridge in hairpin 3. Hairpin 1 is notably absent in the Oikopleura Wnts, as noted by a grey, empty box. The length and sequence-variable linker region between D1 and D2 domains is bordered by well conserved interface motifs that closely associate in a near parallel β-strand fashion at the base of the saposin-like helical bundle; the intervening sequence is largely unstructured (and not visible in the XWnt8 structure, save for a short helix αG).
(B) The multiple alignment of deeply divergent Wnts was used to color the molecular surface of the XWnt8 structure using the ConSurf program (Ashkenazy et al., 2010), showing nearly invariant (blue) to completely unconserved (red) sequence patches. The present view is of the the captured Fz domain, with a highly divergent (and collapsed) hairpin 1, a very well conserved hairpin 2 (lipidated with the PAM group bound by Fz; orange boxed region), and only a moderately conserved hairpin 3, that is involved in protein-protein contacts with the second Fz binding site.
(C) Close-up view of the two conserved interface motifs in blue (D1 domain) and red (D2 domain) that associate in a parallel fashion; blue and red arrows illustrate the path of the underlying chain. The missing linker region is noted by a dotted line. Key conserved residues in XWnt8 are boxed and numbered.
(D) A conserved, convex epitope at the base of the D2 domain in Wnts that may indicate the interaction site with LRP5/6, by analogy with the sclerostin contact site (Bourhis et al., 2011; Bao et al., 2012). The black arrows indicate the direction of the two loops in XWnt8 that respectively follow Gly270 and Cys298.
As saposins have been captured in two different configurations, it is important to note that the Wnt D1 saposin-like domain resembles the closed or apo helical form (with an innate membrane-targeting ability), in contrast to the open or lipid-bound state that features V-shaped monomers clasped as dimers, or forming larger lipoprotein aggregates (Ahn et al., 2003; Rossmann et al., 2008; Popovic et al., 2012). All together, the Wnt D1 domain retains an ancestral saposin-like fold but is no longer capable of sequestering the covalently linked lipid due to the packing of additional N-terminal helices, inserted β-hairpins, and fused D2 domain.
A dominant-negative variant of XWnt8 (DN-XWnt8) that was earlier characterized by Hoppler et al. (1996) acts as a receptor inhibitor, and its deduced chain boundaries suggests that a free Wnt D1 domain retains Site 1 Fz-binding activity. The DN-XWnt8 is effectively truncated at the N-terminal boundary of the D2 domain, critically preserving the second interface motif strand that docks against the D1 saposin-like helical bundle (Fig. 2A, C); the absence of this motif (in a 30 residue-shorter truncation) destabilizes the D1 fold and results in a much weaker Fz inhibitor (Hoppler et al., 1996). This DN form of Wnt D1, analogously to the mini-Wnt construct of Janda et al. (2012) that captures a free D2 domain, could be used to probe the looser protein-protein specificity determinants of Site 1, which is dominated by the lipid contacts. Another alteration of the Wnt D1 chain that results in a loss of Ser187 lipidation and Fz binding activity is the cleavage of an N-terminal helix (the packs against the saposin-like helical bundle; Fig. 1A) by the Tiki1 metalloprotease (Zhang et al., 2012). This N-terminal Wnt processing may disturb the folding of the D1 domain, and/or affect a particular Wnt surface epitope necessary for Porc membrane-bound O-acyltransferase (MBOAT) recognition and activity, resulting in the secretion of a non-lipidated, nonfunctional Wnt. Yet, the Ser187 position, while highly conserved, is not invariant in Wnts (Figure 2); for instance, a Gln residue at this position enables the Drosophila WntD to be secreted in a lipid-independent manner (Ching et al., 2008) and it retains a biological role in primordial germ cell migration through engagement of the Fz4 receptor (McElwain et al., 2011)—we speculate that without a lipid anchor, an enlarged β-hairpin 2 loop in WntD may drive binding to the Fz4 hydrophobic groove with sufficient affinity to cement an altered Site 1 contact (Figure 2).
The β-hairpin extrusions of the Wnt D1 domain independently exhibit a structural resemblance (not shown) to an ancient class of antimicrobial peptides that share a common β-hairpin fold, frequently stiched together by multiple disulfide bridges; these β-hairpin molecules are typically amphipathic, membrane-active effector proteins that function in innate host defense (Yeaman and Yount, 2007). Speculatively, the ancestral, duplicated β-hairpin/helical hairpin motif of Wnt D1 (Figure S1A) is doubly endowed with protein motifs that are known to engage membrane lipids. These embedded β-hairpins may indicate a deep link to an ancient host defense role for the progenitor Wnt D1 molecule. Intriguingly, immune defense functions are catalogued for saposin-like proteins, from humans to primitive eukaryotes (Darmoise et al., 2010), with direct membrane-permeabilizing and pore-forming activities observed against pathogenic bacteria (Hoeckendorf et al., 2012).
A cytokine-like domain in Wnt
The most striking feature of the Wnt D2 domain is its exposed finger fold formed by a long and curled, antiparallel β-strand column crowned by a β-hairpin 3 structure pinned together by two disulfide bridges, and presenting a third, vicinal disulfide link at the tip (Figure 1C). The Wnt D2 hairpin and its ‘knuckle’ base intriguingly superpose with a diverse class of cystine-knot cytokines like platelet-derived growth factor (PDGF) and interleukin-17 (IL-17) that typically form side-by-side homo- or heterodimers (Hymowitz et al., 2001) (Figure S1C–E); nonetheless, the most primitive examples of the cystine-knot architecture—captured in the folds of horseshoe crab coagulogen and the mammalian Wnt signaling regulator sclerostin (Bergner et al., 1996; Veverka et al., 2009)—are monomers in solution, while the BMP inhibitor Noggin forms an unusual back-to-back structure (Groppe et al., 2002). The alignment of the long β-hairpins of representative cystine knot factors with the equivalent Wnt D2 structure also brings into proximity the eponymous, compact disulfide network of cystine knots with the constellation formed by Cys260-Cys298, Cys276-Cys291 and Cys295-Cys337 in Wnt D2 (Figure S1C). The structure of IL-17F showed that only two of the three disulfide bridges of cystine knot factors are conserved in the superfamily (Hymowitz et al., 2001), and one link of this pair is a topological match with the Cys295-Cys337 disulfide bridge in Wnt D2 (Figure S1C).
Overall, we suggest that the Wnt D2 domain is a degenerate cystine knot cytokine, with the more variable, N-terminal β-hairpin of cystine knot structures replaced by the underlying loop and short helix architecture of the Wnt D2 ‘knuckle’; the conserved β-strand-like interface motif that docks to the saposin-like domain could also have been repurposed from the first β-strand of the unraveled cystine-knot (Figure 1C, 2A). The manner in which the long β-hairpin finger tip of the D2 domain makes a discriminating contact with a slight pocket on the surface of the bound Fz domain is reminiscent of cytokine-receptor interfaces seen in the artemin-GFRα3 complex (Wang et al., 2006) and a TGFβ-TGFβR2 assembly (Hart et al., 2002) (Figure S1D). The architectural resemblance of the non-lipidic Fz recognition fragment of Wnt to a receptor-binding cytokine fold is suggestive of an ancient relationship to a monomeric cystine-knot factor, and the ability to express this compact D2 domain as a selective ‘mini-Wnt’ Fz binder by Janda et al. (2012) further supports this notion (Figure 2A). Intriguingly, Norrin, a cystine knot factor of the sclerostin family, has been experimentally defined as an agonist ligand of the Fz4 receptor (Xu et al., 2004; Smallwood et al., 2007), and the present analysis posits that it may mimic the Wnt D2 structure and mode of Fz engagement. In particular, the mutagenic screen of the Norrin chain paints a potential Fz4 interaction patch in the adjacent β-hairpins of a cystine-knot fold (Smallwood et al., 2007; our modeling, not shown), similar to the conserved Wnt hairpin 3 surfaces (Fig. S1B).
Assembly of Wnt ternary complexes
The binary complex of a Wnt bound to a Fz domain is the organizing element of the three main signaling modes catalogued for Wnts (the canonical or β-catenin pathway, the noncanonical or planar cell polarity/PCP pathway, and the G-protein-coupled receptor/GPCR or Ca2+ pathway) that diverge at the receptor level by the nature of the accessory proteins (Buechling and Boutros, 2010). The best understood Wnt signaling complex (that drives β-catenin stabilization in the cytoplasm) utilizes LDL-receptor-related proteins LRP5 or 6 to complete a ternary assembly (Bourhis et al., 2011; Bao et al., 2012). A conservation analysis of the present structure of XWnt8 suggests two potential LRP5/6 interaction sites that lie adjacent to each other on the top and rear faces of the Wnt D2 ‘knuckle’ domain, one concave pocket (described by Janda et al., 2012) and another convex feature formed by two stacked loops at the base of the cystine knot-like β-hairpin structure (Figure 2D). The available structures of Wnt antagonist fragments bound to ectodomain repeats of LRP5/6 point to a preferred Asn-X-Ile loop peptide as a docking motif for central toroidal pockets in LRP5/6 β-propellers (and is also employed in Laminin-Nidogen and Agrin-LRP4 complexes) (Ahn et al., 2011; Bourhis et al., 2011; Chen et al., 2011; Cheng et al., 2011; Bao et al., 2012). The cystine-knot factor sclerostin notably bears this distinctive Asn-X-Ile LRP5/6-binding motif in a long loop at the base of the characteristic β-hairpin ‘finger’ of the fold (Bourhis et al., 2001; Veverka et al., 2009), which is coincident with the proposed approach of the LRP chain to the conserved surface patches of the present Wnt D2 domain in the binary Fz complex (Figure 2A, 3). However, the Asn-X-Ile motif is wholly absent from the Wnt D2 conserved patches (or any other preserved sequence stretches in Wnts; Figure 2A), and instead suggests that the Wnt-LRP5/6 interaction will employ a very distinct recognition mode, akin to the alternative interface captured for the C-terminal globular domain of the Dickkopf inhibitor DKK1 by the E3 β-propeller of LRP6, versus the linchpin Asn-X-Ile interaction from an unstructured N-terminal segment of DKK1 (Cheng et al., 2011; Bao et al., 2012).
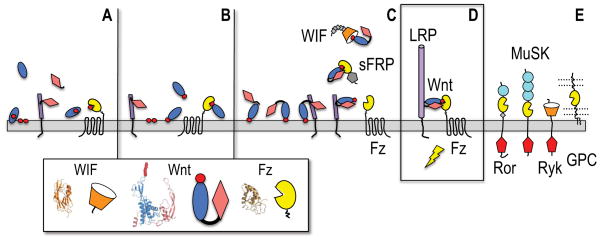
Figure 3. Evolutionary model for the emergence of Wnts as Fz receptor-binding morphogens.
(A) A membrane-interacting saposin-like domain (blue oval) has a (red circle) lipid-loading role for a Fz-like GPCR. A secreted cystine knot cytokine (pink diamond) binds a cell surface LRP-like progenitor (purple cylinder).
(B) The saposin-like domain gains a Ser-acylation site in its membrane-interacting loops and can then directly bind the Fz domain of the GPCR.
(C) A hydrophobic motif in the cytokine N-terminal extension can dock to the saposin helical domain (Figure S1), and a fortuitous gene fusion of these interacting proteins creates a two domain Wnt progenitor that retains cytokine-mediated binding to a primitive LRP, and can be presented together to the Fz GPCR principally by lipid docking. They cytokine portion of the Wnt has an evolved affinity for the interacting Fz and WIF domains, on the receptors or soluble binding proteins (sFRPs, WIF). Small grey diamonds mark the EGF repeat tail in WIF, while a grey pentagon in sFRP illustrates a C-terminal netrin module.
(D) The Wnt remains the critical bridging element in the active ternary receptor complex with LRP5/6 and a Fz GPCR. The purple cylinder in the LRP chain (or its shorter progenitor LRP) denotes the four-fold-repeat of β-propeller and EGF domains.
(E) Additional signaling receptors (Ror, MuSK and Ryk) and presentation molecules (glypican or GPC) can potentially engage lipidated Wnts. Light blue circles in Ror and MuSK ectodomains mark Ig modules, while intracellular tyrosine kinases are noted by red pentagons.
The architectural division of the Wnt into fused saposin- and cytokine-like halves advances an evolutionary schema for the structural and functional provenance of this complex fold and its dual mechanism for Fz recognition. In the present configuration, the lipidated ‘thumb’ of the saposin-like D1 domain provides a generic targeting function for the specificity-determining cytokine-like D2 domain of Wnts, allowing it to potentially couple to a range of Fz receptors, accessory receptors (like Ror and MuSK), presentation proteins (glypicans) and carrier proteins (soluble Fz-related proteins or sFRPs) that carry embedded, lipid-binding Fz domains (Figure 3) (Bazan and de Sauvage, 2009). This lipidated-Wnt interaction role is recapitulated by the fold of the Wnt inhibitory factor (WIF) that, distinctly from the helical Fz fold (Janda et al., 2012), uses the hydrophobic interior of an immunoglobulin domain to sequester phospholipid acyl chains (Malinauskas et al., 2011). However, while the C-terminal EGF repeats of WIF1 aid in fully capturing Wnts, the spartan WIF ectodomain of the Ryk tyrosine kinase may bind the lipidated thumb of Wnts in a similar manner to WIF1 (Malinauskas et al., 2011) without perhaps fully engaging the cytokine-like D2 portion of Wnt. For noncanonical Wnt receptors Ryk and Ror1/2, and likely MuSK, there is evidence to suggest that their WIF and Fz domains—dedicated binders of the Wnt lipid moiety—allow them to form both Fz-independent as well as inclusive Wnt signaling complexes (Buechling and Boutros, 2011; Clark et al., 2012).
As mentioned, saposins not only interact with lipid membranes, but also serve as lipid presentation or loading molecules for receptors (Leon et al., 2012), or drive the membrane association of bound enzymes (Atrian et al., 2007), and we suggest that a critical event in the early evolution of Wnts was the functionally advantageous gain of a Ser-acylation motif in the exposed hairpin 2 of a D1 domain progenitor that was a saposin-like lipid carrier protein for an ancestral Fz receptor (Figure 3). The closer homology of Porc and Hhat within the MBOAT family, the two enzymes that are respectively charged with modifying unrelated Wnt and Hedgehog morphogens, is one of the intriguing mechanistic parallels between the two signaling pathways (Nusse, 2003; Buglino and Resh, 2010; Chang et al., 2011). However, the Ser acylation motif of Wnt in a well ordered loop of the folded D1 domain, is structurally and evolutionarily distinct from the N-terminal Cys acylation site of Hedgehog, in disordered chain tethered to the globular protein (Nusse, 2003; Buglino and Resh, 2010).
Functional imperatives in the evolution of Wnt structure
As an example of a preexisting functional linkage like that proposed between primitive saposin-like proteins and Fz-like receptors, the NPC1 Fz domain utilizes a dedicated, secreted carrier protein called NPC2 (that has a WIF-like immunoglobulin fold) to transiently couple to the Fz domain and transfer loaded cholesterol molecules to its hydrophobic groove (Figure S2) (Kwon et al., 2009, 2011). The fortuitous fusion of this lipidated saposin-like protein with a primitive cystine-knot cytokine (or D2 domain progenitor) may have shackled the Fz-targeting function of a lipidated D1 domain to a primitive D2 cytokine with a binding allegiance to a different transmembrane receptor, prospectively an LRP-like molecule, through a sclerostin-like rear-mounted contact site (Figure 3). The evolution of this ancestral, full-length Wnt factor into the present form would then require the selective honing of a binding affinity of the exposed D2 hairpin 3 ‘forefinger’ for lipid-coupled Fz domains; this opportunistic and late development of a Fz-binding ability is mirrored in the limited footprint and micromolar affinity of the Wnt D2 cytokine domain with Fz (Janda et al., 2012). The retention of LRP-binding ability by the primitive D2 domain would have granted the ancestral Wnt the ability to bridge an LRP chain with the Fz GPCR, and form an active ternary complex coupled to a pre-existing β-catenin pathway (Valenta et al., 2012) (Figure 2). An analogous, piecemeal evolution of the composite Hedgehog morphogen precursor chain (a fusion of ancestral zinc protease and intein domains) and its activating interjection into a primitive Patched-Smo pathway has been likewise proposed (Hausmann et al., 2009; Ingham et al., 2011).
The question that has bedeviled genomic prospectors in the Wnt field concerns the evolutionary origins of Wnt morphogens in the metazoan lineage. With remarkable consistency, Wnt genes can be classified into 13 subfamilies (12 of which encompass the 19 Wnts of the human repertoire) that persist from some of the earliest branches of the metazoan tree (Pang et al., 2010; Riddiford and Olson, 2011). For instance, Cnidaria, represented by the genomes of the sea anemone Nematostella vectensis, and the freshwater Hydra magnapapillata, surprisingly yield an almost complete deck of Wnts. The most primitive Wnts are the handful of unclassified molecules unearthed in Placozoa (Tricoplax adherens), Porifera (the sponges Oscarella lobularis and Amphimedon queenslandica) and Ctenophores (the comb jelly Mnemiopsis leidyi), and while these show a much greater divergence of sequence and frequent loss of disulfide bridges from bilaterian Wnts, they are still full-length, lipidated D1–D2 domain Wnt proteins (Figure 2A). A critical difference of these primitive Wnts with bilaterian Wnts is the lack of sequence and length conservation of the hairpin 3 extension that forms the present Fz contact site (Janda et al., 2012), arguing that this particular, molecular attachment for D2 was the last to evolve. The presence of Fz- and Smo-like GPCRs in the genome of fungi and social amoeba Dictyostelium discoideum, without identifiable Wnt ligands, suggests that these lipid- and sterol-sensing receptors predate the emergence of Wnts (Harwood, 2008; Krishnan et al., 2012). The compelling structure of a Wnt clasping a Fz receptor domain (Janda et al., 2012) allows us to draw a plausible molecular path for the evolution of this critical morphogen-receptor pairing.
Supplementary Material
Abbreviations
- Fz
frizzled
- Smo
Smoothened
- Wnt
Wingless-Int-1
- PAM
palmitoleic acid
Footnotes
Supplemental Data includes two figures and experimental procedures, and can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn VE, Chu ML, Choi HJ, Tran D, Abo A, Weis WI. Dev Cell. 2011;21:862–873. doi: 10.1016/j.devcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. Nucl Acids Res. 2010;38(Web Server Issue):W529–W533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Zheng JJ, Wu D. Science Signal. 2012;5:pe22. doi: 10.1126/scisignal.2003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF, de Sauvage FJ. Cell. 2009;138:1055–1056. doi: 10.1016/j.cell.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Genes Dev. 2010;24:2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosanac I, Maun HR, Scales SJ, Wen X, Lingel A, Bazan JF, de Sauvage FJ, Hymowitz SG, Lazarus RA. Nature Struct Molec Biol. 2009;16:691–697. doi: 10.1038/nsmb.1632. [DOI] [PubMed] [Google Scholar]
- Bourhis E, Wang W, Tam C, Hwang J, Zhang Y, Spittler D, Huang OW, Gong Y, Estevez A, Zilberleyb I, Rouge L, Chiu C, Wu Y, Costa M, Hannoush RN, Franke Y, Cochran AG. Structure. 2011;19:1433–1442. doi: 10.1016/j.str.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Bruhn H. Biochem J. 2005;389:249–257. doi: 10.1042/BJ20050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglino JA, Resh MD. PLoS ONE. 2010;5:e11195. doi: 10.1371/journal.pone.0011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechling T, Boutros M. Curr Topics Dev Biol. 2011;97:21–53. doi: 10.1016/B978-0-12-385975-4.00008-5. [DOI] [PubMed] [Google Scholar]
- Carugo O, Cemazar M, Zahariev S, Hudáky I, Gáspári Z, Perczel A, Pongor S. Protein Engin. 2003;16:637–639. doi: 10.1093/protein/gzg088. [DOI] [PubMed] [Google Scholar]
- Chang CCY, Sun J, Chang TY. Front Biol. 2011;6:177–182. [Google Scholar]
- Chen S, Bubeck D, MacDonald BT, Liang WX, Mao JH, Malinauskas T, Llorca O, Aricescu AR, Siebold C, He X, Jones EY. Dev Cell. 2011;21:848–861. doi: 10.1016/j.devcel.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Biechele T, Wei Z, Morrone S, Moon RT, Wang L, Xu W. Nature Struct Molec Biol. 2011;18:1204–1210. doi: 10.1038/nsmb.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Hang HC, Nusse R. J Biol Chem. 2008;283:17902–17908. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CE, Nourse CC, Cooper HM. Neurosignals. 2012;20:202–220. doi: 10.1159/000332153. [DOI] [PubMed] [Google Scholar]
- Croce JC, McClay DR. Meth Molec Biol. 2008;469:3–18. doi: 10.1007/978-1-60327-469-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, Affolter M, Vale WW, Belmonte JC, Choe S. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP. Nature Struct Biol. 2002;9:203–208. doi: 10.1038/nsb766. [DOI] [PubMed] [Google Scholar]
- Hausmann G, von Mering C, Basler K. PLoS Biol. 2009;7:e1000146. doi: 10.1371/journal.pbio.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, Gurney AL, de Vos AM, Starovasnik MA. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, Nakano Y, Seger C. Nature Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- Iyer S, Leonidas DD, Swaminathan GJ, Maglione D, Battisti M, Tucci M, Persico MG, Acharya KR. J Biol Chem. 2001;276:12153–12161. doi: 10.1074/jbc.M008055200. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood AJ. Meth Molec Biol. 2008;469:21–32. doi: 10.1007/978-1-60327-469-2_2. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Brown JD, Moon RT. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- Kalderon D. Trends Cell Biol. 2002;12:523–531. doi: 10.1016/s0962-8924(02)02388-7. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Almén MS, Fredriksson R, Schiöth HB. PLoS ONE. 2012;7:e29817. doi: 10.1371/journal.pone.0029817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Acta Cryst. 2004;D60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Abi-Mosieh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Palnitkar M, Deisenhofer J. PLoS One. 2011;6:e18722. doi: 10.1371/journal.pone.0018722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yang ST, Rho SH, Im YJ, Kim SY, Kim YR, Kim MK, Kang GB, Kim JI, Rhee JH, Eom SH. J Molec Biol. 2006;355:491–500. doi: 10.1016/j.jmb.2005.10.074. [DOI] [PubMed] [Google Scholar]
- León L, Tatituri RV, Grenha R, Sun Y, Barral DC, Minnaard AJ, Bhowruth V, Veerapen N, Besra GS, Kasmar A, Peng W, Moody DB, Grabowski GA, Brenner MB. Proc Natl Acad Sci USA. 2012;109:4357–4364. doi: 10.1073/pnas.1200764109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinauskas T, Aricescu AR, Lu W, Siebold C, Jones EY. Nature Struct Molec Biol. 2011;18:886–893. doi: 10.1038/nsmb.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwain MA, Ko DC, Gordon MD, Fyrst H, Saba JD, Nusse R. PLoS ONE. 2011;6:e26993. doi: 10.1371/journal.pone.0026993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Nature Chem Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Development. 2003;130:5297–5305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- Pang K, Ryan JF, Mullikin JC, Baxevanis AD, Martindale MQ NISC Comp. Seq. Prog. EvoDevo. 2010;1:10. doi: 10.1186/2041-9139-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic K, Holyoake J, Pomes R, Privé G. Proc Natl Acad Sci USA. 2012;109:2908–2912. doi: 10.1073/pnas.1115743109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey JP, Ellies DL. Dev Dyn. 2010;23:102–114. doi: 10.1002/dvdy.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford N, Olson PD. Dev Genes Evol. 2011;221:187–197. doi: 10.1007/s00427-011-0370-8. [DOI] [PubMed] [Google Scholar]
- Rossmann M, Schultz-Heienbrok R, Behlke J, Remmel N, Ailings C, Sandhoff K, Saenger W, Maier T. Structure. 2008;16:809–817. doi: 10.1016/j.str.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. J Biol Chem. 2007;282:4057–4068. doi: 10.1074/jbc.M609618200. [DOI] [PubMed] [Google Scholar]
- Shim AH, Liu H, Focia PJ, Chen X, Lin PC, He X. Proc Natl Acad Sci USA. 2010;107:11307–11312. doi: 10.1073/pnas.1000806107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veverka V, Henry AJ, Slocombe PM, Ventom A, Mulloy B, Muskett FW, Muzylak M, Greenslade K, Moore A, Zhang L, Gong J, Qian X, Paszty C, Taylor RJ, Robinson MK, Carr MD. J Biol Chem. 2009;284:10890–10900. doi: 10.1074/jbc.M807994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitt UA, Hsu SY, Hsueh AJW. Molec Endo. 2001;15:681–694. doi: 10.1210/mend.15.5.0639. [DOI] [PubMed] [Google Scholar]
- Wang X, Baloh RH, Milbrandt J, Garcia KC. Structure. 2006;14:1083–1092. doi: 10.1016/j.str.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Zhang X, Abreu JG, MacDonald BT, Singh S, Coburn KL, Cheong SM, Zhang MM, Ye QZ, Hang HC, He X. Cell. 2012;149:1565–1577. doi: 10.1016/j.cell.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.