Abstract
One of the most important and overlooked function of the gastrointestinal tract is to provide a dynamic barrier to tightly control antigen trafficking both through the transcellular and paracellular pathways. Intercellular tight junctions (TJ) are the key structures regulating paracellular trafficking of macromolecules. While steady progress has been made in understanding TJ ultrastructure, relatively little is known about their pathophysiological regulation. Our discovery of zonulin, the only known physiologic modulator of intercellular TJ described so far, increased understanding of the intricate mechanisms that regulate gut permeability and led us appreciate that its up-regulation in genetically susceptible individuals may lead to immune-mediated diseases. This information has translational implications, since the zonulin pathway is currently exploited to develop both diagnostic and therapeutic applications pertinent to a variety of immune-mediated diseases.
1. Technological Primer
Recent studies indicate that beside water and salt homeostasis and digestion and absorption of nutrients, another key function of the intestine is to regulate the trafficking of environmental antigens across the host mucosal barrier (1). Intestinal tight junctions (TJ) are responsible for the paracellular trafficking of macromolecules and, therefore, they contribute to the balance between tolerance and immune response to non-self antigens (1). While considerable knowledge exists about TJ ultrastructure, relatively little is known about their patho-physiological regulation leading to local and/or systemic inflammation. Technologies capable to restore intestinal barrier function and, therefore, proper antigen trafficking, may represent an innovative approach to prevent and/or treat immune-mediated diseases in which increased intestinal permeability seems to be integral part of their pathogenesis.
2. What are the findings
Regulation of intestinal permeability: The zonulin pathway
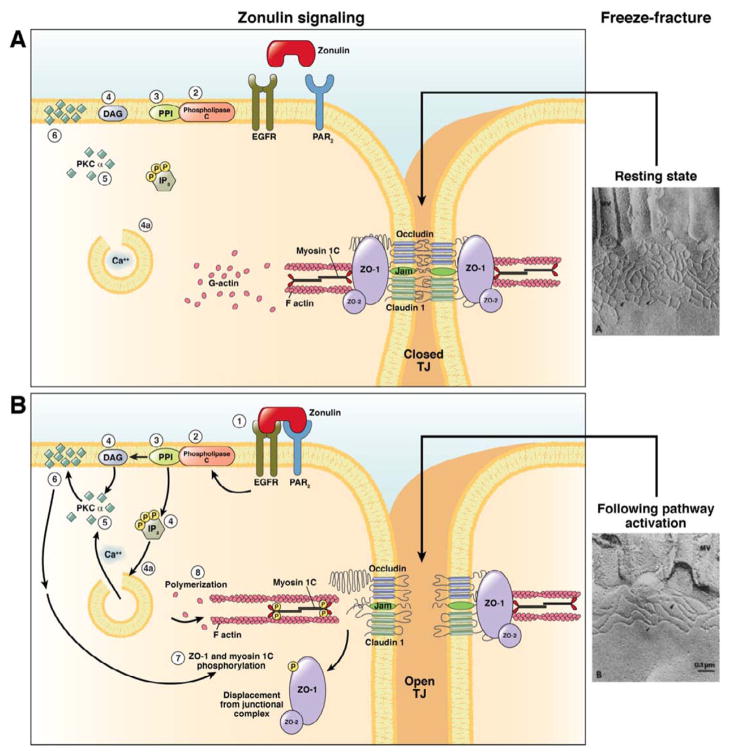
In the past decade we have focused our research effort on the discovery of physiologic modulators of intestinal TJ. Our studies led to the discovery and characterization of zonulin as the only human protein discovered to date that is known to reversibly regulate intestinal permeability by modulating intercellular TJs (2) (Figure 1). Through proteomic analysis of human sera, we have recently identified zonulin as pre-haptoglobin (HP)2 (3)), a molecule that, to date, has only been regarded as the inactive precursor for HP2, one of the two genetic variants (together with HP1) of human HPs. Our data suggest that pre-HP2, a multifunctional protein that, in its intact single chain form (i.e.; zonulin), regulates intestinal permeability caused by EGFR transactivation through proteinase activate receptor 2 (PAR2) (3), while in its cleaved two-chain form acts as a Hb scavenger.
Figure 1.
Schematic representation of the zonulin mechanism of action. A. Resting state: During the resting state TJ proteins are angeged in both homophilic and heterophilic protein-protein interactions that keep TJ in a competent state (TJ) closed as reflected by the complexity of TJ meshwork shown in the freeze fracture electron microscopy photograph. B. Following zonulin pathway activation: Zonulin transactivates EGFR through PAR2 (1). The protein then activates phospholipase C (2) that hydrolyzes phosphatidyl inositol (PPI) (3) to release inositol 1,4,5-tris hosphate (IP-3) and diacylglycerol (DAG) (4). PKCα is then activated (5), either directly (via DAG) (4) or through the release of intracellular Ca2+ (via IP-3) (4a). Membrane-associated, activated PKCα (6) catalyzes the phosphorylation of target protein(s), including ZO1 and myosin 1C, as well as polymerization of soluble G-actin in F-actin (7). The combination of tight junction protein phosphorylation and actin polymerization causes the rearrangement of the filaments of actin and the subsequent displacement of proteins (including ZO-1) from the junctional complex (8). As a result, intestinal TJ become looser (see freeze fracture electron microscopy). Once the zonulin signaling is over, the TJ resume their baseline steady state.
Environmental stimuli causing intestinal zonulin release
Among the several potential intestinal stimuli that can trigger zonulin release, small intestinal exposure to bacteria and gluten are the two triggers that have been identified so far (2). Enteric infections have been implicated in the pathogenesis of several pathological conditions, including allergic, autoimmune, and inflammatory diseases, by causing impairment of the intestinal barrier. We have generated evidence that small intestines exposed to enteric bacteria secreted zonulin (2). This secretion was independent of the virulence of the microorganisms tested, occurred only on the luminal aspect of the bacteria-exposed small intestinal mucosa, and was followed by an increase in intestinal permeability coincident with the disengagement of the protein zonula occludens (ZO)-1 from the tight junctional complex (4). This zonulin-driven opening of the paracellular pathway may represent a defensive mechanism, which flushes out microorganisms so contributing to the innate immune response of the host against bacterial colonization of the small intestine.
Beside bacterial exposure, we have shown that gliadin, the main staple protein in wheat also affects the intestinal barrier function by releasing zonulin by engaging the chemokine receptor CXCR3 (5). Our data demonstrate that in the intestinal epithelium, CXCR3 is expressed at the luminal level, is over-expressed in celiac disease (CD) patients, co-localizes with specific gliadin peptides, and that this interaction coincides with recruitment of the adapter protein, MyD88, to the receptor (5).
3. Why is this important?
Shift of paradigm in the pathogenesis of autoimmune diseases
It is generally accepted that it is the interplay between environmental factors and specific susceptibility genes that underlies the aberrant immune response responsible for the onset of these diseases. Less than 10% of those with increased genetic susceptibility progress to clinical disease, suggesting a strong environmental trigger in the pre-disease state (1). Environmental factors are also likely affecting the outcome of the process and the rate of progression to disease in those who develop pathologic outcomes. One theory is that antigens absorbed through the gut may be involved (1).
A common denominator of autoimmune diseases is the presence of several preexisting conditions leading to an autoimmune process. The first is a genetic susceptibility for the host immune system to recognize, and potentially misinterpret, an environmental antigen presented within the GI tract. Second, the host must be exposed to the antigen. Finally, the antigen must be presented to the GI mucosal immune system following its paracellular passage (normally prevented by the TJ competency) from the intestinal lumen to the gut submucosa. In many cases, increased permeability appears to precede disease and causes an abnormality in antigen delivery that triggers the multiorgan process leading to the autoimmune response.
Therefore, the following novel hypothesis can be formulated to explain the pathogenesis of autoimmune diseases that encompasses the following three key points:
Autoimmune diseases involve a miscommunication between innate and adaptive immunity.
The classical autoimmune theories, molecular mimicry or bystander effect alone may not explain entirely the complex events involved in the pathogenesis of autoimmune diseases. Rather, the continuous stimulation by non-self antigens (environmental triggers) appears necessary to perpetuate the process. This concept implies that the autoimmune response can be theoretically stopped and perhaps reversed if the interplay between autoimmune predisposing genes and trigger(s) is prevented or eliminated.
In addition to genetic predisposition and the exposure to the triggering non-self antigen, the third key element necessary to develop autoimmunity is the loss of the protective function of mucosal barriers that interface with the environment (mainly the GI mucosa).
Role of zonulin in autoimmune diseases
Celiac disease
celiac disease (CD) represents the best testimonial of this theory. CD is a unique model of autoimmunity in which, in contrast to most other autoimmune diseases, a close genetic association with HLA genes, a highly specific humoral autoimmune response against tissue transglutaminase, and, most importantly, the triggering environmental factor (gliadin), are all known (6). Early in the disease, TJ are opened secondary to zonulin up-regulation directly induced by the exposure to the disease’s antigenic trigger gliadin (see above), causing an increased paracellular passage of antigens, including gliadin, in the gut submucosa (6). In genetically predisposed individuals this, in turn, trigger the gluten-specific adaptive immune response causing the autoimmune insult of the intestinal mucosa seen in patients with CD (6). Once gluten is removed from the diet, serum zonulin levels decrease, the intestine resumes its baseline barrier function, the autoantibody titers are normalized, the autoimmune process shuts off and, consequently, the intestinal damage (that represents the biological outcome of the autoimmune process) heals completely.
Type 1 diabetes
recent studies have shown that altered intestinal permeability occurs in both type 1 and type 2 diabetes prior to the onset of complications (7). This has led to the suggestion that an increased intestinal permeability due to alteration in intestinal TJ is responsible for the onset of diabetes. This hypothesis is supported by studies performed in an animal model that develops type 1 diabetes (T1D) spontaneously that showed an increased permeability of the small intestine (but not of the colon) of the BioBreeding diabetic prone (BBDP) rats that preceded the onset of diabetes by at least a month (8). Further, histological evidence of pancreatic islet destruction was absent at the time of increased permeability but clearly present at a later time (2,8). Therefore, these studies provided evidence that increased permeability occurred before either histological or overt manifestation of diabetes in this animal model. We confirmed these data by reporting in the same rat model that zonulin-dependent increase in intestinal permeability precedes the onset of T1D by 2–3 weeks (2). This goal was achieved by using the zonulin inhibitor, AT1001 (subsequently used for clinical trial with the name Larazotide acetate, see below), an ectapeptide that prevents both Zot- and zonulin- and Zonula occludens toxin (Zot), zonulin prokaryotic counterpart elaborated by Vibrio cholerae)-induced TJ disassembly (9). Oral administration of AT1001 to BBDP rats blocked autoantibody formation and zonulin-induced increases in intestinal permeability, so reducing the incidence of diabetes by 70% (10). These studies suggest that the zonulin-dependent loss of intestinal barrier function is one of the initial steps in the pathogenesis of T1D in the BBDP animal model of the disease (10). The involvement of zonulin in T1D pathogenesis was corroborated by our studies in humans showing that ~50% of T1D patients has elevated serum zonulin levels that correlated with increased intestinal permeability (11). We also provided preliminary evidence suggesting that, as in the BBDP rat model of the disease, zonulin up-regulation precedes the onset of diabetes in T1D patients (11). Recently, we have also reported a direct link between antibodies to Glo-3a (a wheat-related protein), zonulin upregulation, and islet autoimmunity in children at increased risk for T1D (12). Similarly to CD, these data link exposure to gluten and subsequent zonulin upregulation to impairment gut barrier function and subsequent onset of T1D in genetically predisposed individuals.
Zonulin, gut inflammation, obesity, and insulin resistance
Recent evidence suggests a possible role of gut intestinal permeability in obesity (7). In obese patients, intestinal permeability parameters are correlated to metabolic syndrome risk factors, obesity-induced inflammation, and non-alcoholic fatty liver disease (7). More recently it has been reported that zonulin is associated with obesity-associated insulin resistance (13). Interestingly, circulating zonulin increased with body mass index, waist to hip ratio, fasting insulin, fasting triglycerides, uric acid and IL-6. This last observation is of particular interest, since it suggests that the relationship between insulin sensitivity and circulating zonulin might be mediated through the obesity-related circulating IL-6 increase, a cytokine that regulates zonulin expression by interacting with its gene promoters through STAT 3 activation (13).
Role of zonulin in other immune-mediated diseases
Since the discovery of zonulin as the precursor of HP2, this protein has been used as a biomarker of several immune-mediated diseases. Table 1 summarized the three major categories of immune-mediated conditions, namely autoimmune diseases, tumoral diseases, and neuro-inflammatory diseases, in which zonulin is up-regulated.
Table 1.
Major diseases associated to zonulin (pre-HP2) biomarker
1. Autoimmune diseases
|
2. Cancers
|
3. Disease of the nervous system
|
4. How can this be translated in routine clinical diagnosis/treatment?
Zonulin as a diagnostic tool
With the appreciation that zonulin is associated to a series of immune-mediated diseases, a quantitative sandwich enzyme linked immunosorbent assay (ELISA) has been developed to use its serum levels as a biomarker of intestinal barrier integrity in several inflammatory diseases (3). Further, it has been reported that carrying the HP2 allele (alias, zonulin gene) correlates with higher risk of developing inflammatory diseases and HP2 homozygosis (two copied of the zonulin gene) is associated with increased morbidity. We have recently developed a single-step RT-PCR protocol using specific primers to amplify both HP1 and HP2 genes, to perform zonulin genotyping to be correlated with the risk and severity of immune-mediated diseases.
Zonulin inhibitor Larazotide for the treatment of autoimmune diseases
CD and type 1 diabetes autoimmune models suggest that, when the finely tuned trafficking of macromolecules is deregulated due to a leaky gut, autoimmune disorders can occur in genetically susceptible individuals (1,2). This theory implies that removing any of the three key elements function) should block the autoimmune process. To challenge this hypothesis, zonulin inhibitor Larazotide acetate was used with encouraging results in the BBDP rat model of autoimmunity (2). Besides preventing the loss of intestinal barrier function, the appearance of autoantibodies, and the onset of disease, pretreatment with Larazotide acetate protected against the insult of pancreatic islets and, therefore, of the insulitis responsible for the onset of type 1 diabetes (2). This proof-of-concept in an animal model of autoimmunity provided the rationale to design human clinical trials in which Larazotide acetate was initially tested in an inpatient, double-blind, randomized placebo controlled trial to determine its safety, tolerability, and preliminary efficacy (14). No increase in adverse events was recorded among patients exposed to Larazotide as compared to placebo. Following acute gluten exposure, a 70% increase in intestinal permeability was detected in the placebo group, while no changes were seen in the Larazotide acetate group (14). Gastrointestinal symptoms were significantly more frequent among patients of the placebo group as compared to the Larazotide acetate group (14). Larazotide acetate has now been tested in approximately 500 subjects with excellent safety profile and promising efficacy as concern protection against symptoms caused by gluten exposure in CD patients (15).
5. What are the roadblocks and/or limitations?
The role of intestinal permeability in the pathogenesis of immune-mediated diseases is a relatively new field of translational science that only recently has received proper attention. While the zonulin pathway is the only physiologic mechanism described so far, it is likely that other pathways are involved in physiologic TJ modulation. The prophylactic efficacy of zonulin inhibitors in preventing disease status has been proved both in animal models and humans. However, its efficacy in treating already established diseases or in slowing down progression of disease states remains to be established. Translating basic observations to clinical diagnostic and therapeutic applicability requires a strong academia-industry partnership in order to secure proper know-how, expertise, and economic resources. In a volatile global economy and with NIH funding suffering possible cuts, the major roadblock to bring technologies the zonulin technology to clinical applicability remains the availability of funds to support proper clinical trials.
6. Conclusions
The discovery of zonulin has increased understanding of the intricate mechanisms that regulate the intestinal epithelial paracellular pathway and the role of intestinal permeability in health and disease. Zonulin can be used as a biomarker of impaired gut barrier function for several autoimmune, neurodegenerative, and tumoral diseases and can be a potential therapeutic target for the treatment of these devastating conditions.
Acknowledgments
Funding: Work presented in this review was supported in parts by grants from the National Institutes of Health Grants DK-48373 and DK-078699 to AF.
Footnotes
Conflicts of interest: The author is a share-holder of Alba Therapeutics
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:416–422. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106:16799–804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-Dependent Activation of the Zonulin System is Involved in the Impairment of the Gut Barrier Function Following Bacterial Colonization. Gastroenterol. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 5.Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasano A. Surprises from celiac disease. Sci Am. 2009;301:54–61. doi: 10.1038/scientificamerican0809-54. [DOI] [PubMed] [Google Scholar]
- 7.de Kort S, Keszthelyi D, Masclee AA. Leaky gut and diabetes mellitus: what is the link? Obes Rev. 2011;12:449–58. doi: 10.1111/j.1467-789X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 8.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999;276:G951–7. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- 9.Di Pierro M, Lu R, Uzzau S, Wang W, Margaretten K, Pazzani C, Maimone F, Fasano A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J Biol Chem. 2001;276:19160–5. doi: 10.1074/jbc.M009674200. [DOI] [PubMed] [Google Scholar]
- 10.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A. 2005;102:2916–21. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–9. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 12.Simpson M, Mojibian M, Barriga K, Scott FW, Fasano A, Rewers M, Norris JM. An exploration of Glo-3A antibody levels in children at increased risk for type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:563–72. doi: 10.1111/j.1399-5448.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7(5):e37160. doi: 10.1371/journal.pone.0037160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–66. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 15.Leffler DA, Kelly CP, Abdallah HZ, Colatrella AM, Harris LA, Leon F, Arterburn LA, Paterson BM, Lan ZH, Murray JA. A Randomized, Double-Blind Study of Larazotide Acetate to Prevent the Activation of Celiac Disease During Gluten Challenge. Am J Gastroenterol. doi: 10.1038/ajg.2012.211. [epub ahed of print] [DOI] [PMC free article] [PubMed] [Google Scholar]