Abstract
Diffuse large B cell lymphomas (DLBCL) are aggressive B-cell lymphomas that are clinically, pathologically and genetically diverse, in part reflecting the functional diversity of the B-cell system. The focus in recent years has been towards incorporation of clinical features, morphology, immunohistochemistry and ever evolving genetic data into the classification scheme. The 2008 WHO classification reflects this complexity with the addition of several new entities and variants. The discovery of distinct subtypes by gene expression profiling (GEP) heralded a new era with a focus on pathways of transformation as well as a promise of more targeted therapies, directed at specific pathways. Some DLBCLs exhibit unique clinical characteristics with a predilection for specific anatomic sites; the anatomic site often reflects underlying biological distinctions. Recently, the spectrum of EBV-driven B-cell proliferations in patients without iatrogenic or congenital immunosuppression has been better characterized; most of these occur in patients of advanced age, and include EBV-positive large B-cell lymphoma of the elderly. HHV-8 is involved in the pathogenesis of primary effusion lymphoma, which can present as a “solid variant.” Two borderline categories were created; one deals with tumors at the interface between classical Hodgkin lymphoma (cHL) and DLBCL. The second confronts the interface between Burkitt Lymphoma (BL) and DLBCL, so called “B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma” in the 2008 classification. Most cases harbor both MYC and BCL2 translocations, and are highly aggressive. Another interesting entity is ALK+ DLBCL, which renders itself potentially targetable by ALK inhibitors. Ongoing investigations at the genomic level, with both exome and whole genome sequencing, are sure to reveal new pathways of transformation in the future.
Keywords: Diffuse large B-cell lymphoma, plasmablastic lymphoma, Burkitt lymphoma, double hit lymphoma, grey zone lymphoma, Hodgkin’s lymphoma, cutaneous lymphoma, central nervous system, immunophenotyping
Introduction
Diffuse large B-cell lymphomas (DLBCL) are aggressive lymphomas with tremendous heterogeneity in terms of clinicopathologic and molecular genetic features. From a morphological standpoint, the current 2008 WHO classification defines DLBCL as a diffuse growth of neoplastic large B lymphoid cells with a nuclear size equal to or exceeding normal macrophage nuclei. 1 DLBCL is the most common lymphoma subtype and accounts for 30–40% of adult non-Hodgkin lymphoma. The diversity of the DLBCL group is being slowly unraveled and the 2008 WHO classification lists a large number of DLBCL sub-groups primarily based on distinct morphologic, biologic, immunophenotypic or clinical parameters.1 A significant proportion of DLBCL remains biologically heterogeneous and does not fit into any specific disease sub-groups; these are defined as diffuse large B-cell lymphoma, not otherwise specified (DLBCL-NOS). While substantial progress has been made towards molecular sub-classification of this entity, the translation to effective treatment strategies remains a challenge.
Historically, lymphoma classification has been based on the presumed normal counterpart. Thus, some of the subtypes of diffuse large B-cell lymphomas reflect the putative cell of origin, e.g. mature B-cell vs. more differentiated plasma cells. However, adopting this methodology as the sole means of classification is perhaps inadequate or incomplete in defining certain subtypes of lymphoma, e.g. intravascular large B-cell lymphoma. Additionally, the focus has shifted towards pathogenetic pathways that might be the subject of molecularly targeted therapy. As such, a number of aggressive B-cell lymphomas are defined primarily on the basis of either specific genetic aberrations, such as ALK-positive large B-cell lymphoma, or viruses involved in B-cell transformation, e.g., primary effusion lymphoma. This review will emphasize some of the implications of gene expression studies in DLBCL-NOS, entities that have been recognized more recently, as well as areas of diagnostic challenge, such as the interface between DLBCL and Burkitt lymphoma.
DIFFUSE LARGE B-CELL LYMPHOMA, NOT OTHERWISE SPECIFIED (DLBCL, NOS)
DLBCL, NOS is the most common type of lymphoma on a worldwide basis accounting for 25–30% of non-Hodgkin lymphomas. Although most cases of DLBCL, NOS are de novo, this diagnosis also includes histologically transformed low grade B-cell malignancy, such as follicular lymphoma or chronic lymphocytic leukemia (CLL), so-called Richter’s transformation. DLBCL, NOS is more common in the elderly but occurs in all age groups. Males are more commonly affected and sites of involvement include lymph nodes or extranodal sites (bone, skin, thyroid, gastrointestinal tract and lung). The most common genetic aberrations in DLBCL, NOS involve the BCL6 gene, seen in 30% of cases. 2 Translocation involving MYC (up to 10% of cases) 3, 4and BCL2 5 are frequent in lymphomas of the germinal center B-cell subgroup (see below).
DLBCL, NOS is a diagnosis of exclusion, not corresponding to one of the specific subtype described below. The focus in recent years has been towards identification of molecular alterations and identification of specific pathways leading to transformation. 6–8 Gene expression profiling (GEP) of diffuse large B-cell lymphomas has identified molecular subtypes, which correlate with not only prognosis, may have relevance for treatment based on signaling pathways. Staudt and colleagues showed that at least two major types of DLBCL could be identified by GEP, resembling either germinal center B-cells (GCB) or activated B-cells (ABC), establishing a putative “cell of origin”. 9–11 The original gene expression profiling studies by Alizadeh et al. employed samples from patients treated with CHOP and related regimens prior to the Rituximab era and demonstrated a significantly worse prognosis for the ABC subtype, independent of other clinical factors. 10 While the introduction of Rituximab to traditional CHOP therapy has improved outcomes in the ABC subtype, this subtype still remains less responsive to therapy than the GCB subtype. 12 Prognostic models based on limited sets of genes have been proposed. 13 Other studies used GEP to examine aspects of cell metabolism or tumor microenvironment. 14 Immune escape of the tumor cells from T-cell surveillance was the subject of still other studies. 15–17 A detailed review is beyond the scope of this article.
The ABC and GCB DLBCL subtypes, originally formulated based on a cell of origin model, have more recently been shown to harbor different pathways of cellular transformation and oncogenesis. 18, 19 Regarding the ABC subtype, the major signaling alteration appears to be the constitutive activation of the NFκB pathway through chronic stimulation of the B-cell receptor (BCR) pathway. Ngo et al. have demonstrated the role of the CBM complex, CARD11, BCL10 and MAL1 downstream of BCR in NFκB activation. 20 Mutations in CARD11 are observed in approximately 10% of ABC DLBCLs. A majority of other ABC DLBCLs have been shown to have chronic activation of the B-cell receptor pathway through various other mechanisms including activating mutations of CD79A and CD79B and recruitment of Bruton’s tyrosine kinase, which is required for CARD11 signaling. 6 At present, GEP data does not dictate therapy by current standards of care. However, recent data have shown the efficacy of Bortezomib, a proteasome inhibitor that blocks NFκB signaling, in improving response rate and median survival for the ABC subtype of DLBCL but predictably not for the GCB subtype in the relapsed setting. 21 Several studies have also addressed the potential of BCR pathway down regulation by blocking various downstream signaling molecules including Btk, Phosphatidylinositol 3 kinases, Spleen tyrosine kinase, mammalian target of rapamycin and SRC family kinases. 22 The efficacy of small molecule inhibitors against several of these targets has been described via either in vitro experiments or clinical trials.
Because of the technical difficulties in performing GEP on every case, various immunohistochemical profiles have been proposed as surrogates of the GEP. While the correspondence is not exact, similar prognostic correlations can be drawn with immunohistochemically defined groups. 23–25 Several genes characteristic of germinal center derivation have been demonstrated to be more highly expressed in the GCB subtype, e.g. BCL6, CD10, LMO2, BCL7A etc. 10 The classic Hans algorithm utilized protein expression of BCL6, CD10 and MUM-1/IRF4, but the panel has been expanded in newer iterations known as “Choi” and “Tally” algorithms, with greater predictability of outcome (Figure 1). 25, 26 However, the concordance rate between the immunohistochemically defined subtype, ABC vs. GCB, and GEP has been variable. 25, 27 A recent study showed the continued relevance of the GEP in a clinical trial utilizing rituximab plus chemotherapy, but none of the immunohistochemical algorithms employed could reproduce this result. 28 As highlighted by several studies examining reproducibility among different laboratories, this lack of concordance may be in part due to variability in performing and scoring the immunohistochemical studies. 29, 30 The other consistent issue is the existence of a small percentage of “unclassifiable cases” by immunohistochemistry. Recently, a report from the International DLBCL Rituximab-CHOP consortium introduced a new algorithm “Visco-Young” based on expression of CD10, FOXP1 and BCL6 which demonstrated a 92.6% concordance with GEP and ability to independently predict progression-free and overall survival (Figure 1C). 31
Figure 1.
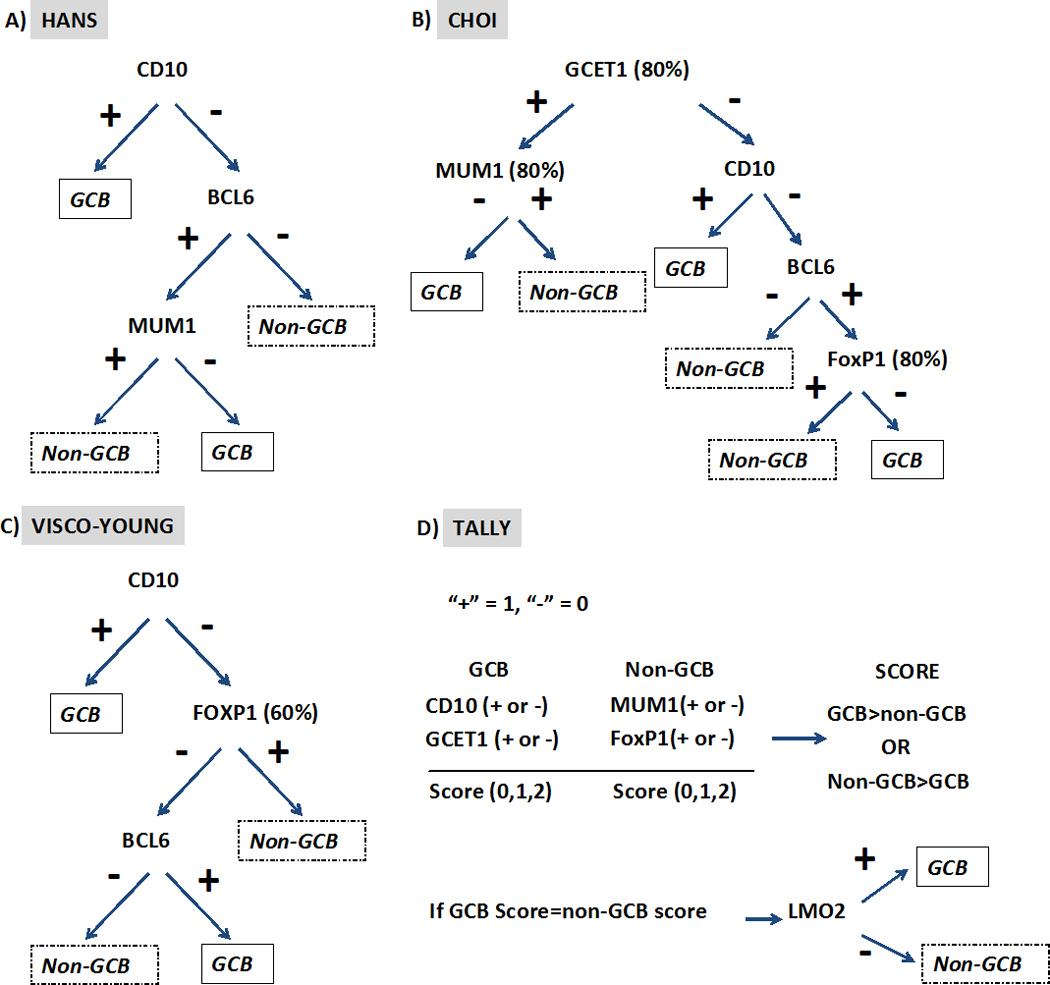
Immunohistochemistry algorithms for determining molecular subtype. All algorithms use a positivity cut-off in tumor cells of ≥ 30% for immunohistochemical markers unless otherwise indicated in the figure. (GCB-Germinal center B-cells)
One might question if morphological features still have relevance for the subclassification of DLBCL, such as the recognition of centroblastic, immunoblastic and anaplastic subtypes. Historical studies suggested that tumors composed predominantly of centroblasts had a better prognosis than those composed of immunoblasts. 32 This is likely due to a partial correlation with GEP as the immunoblastic subtype is enriched for cases with an ABC profile, while purely centroblastic neoplasms are more often GCB. 33 However, reproducibility has been less than satisfactory when applied to a broad spectrum of tumors, probably reflecting inter-observer variability and different criteria for designating lymphomas as the “immunoblastic” subtype. The use of cytological criteria was recently resurrected by Ott et al., 34 who found that immunoblastic morphology was highly significant in predicting an adverse outcome. However, in a trial of 949 patients only 7.4% of the cases were classified as immunoblastic, which is significantly less that what would be expected for the ABC subtype based on GEP. While authors were able to apply very stringent criteria to identify a prognostically relevant subset, because of the rarity of these lesions the utility of this approach in general practice is limited.
DIFFUSE LARGE B-CELL LYMPHOMA SUBTYPES IN SPECIFIC SITES
Several variants or subtypes of DLBCL have been segregated out in the WHO classification because of a propensity to affect distinct sites. These include primary DLBCL of the central nervous system (CNS), primary cutaneous DLBCL, leg type, and intravascular large B-cell lymphoma.
Primary CNS DLBCL includes those cases presenting with intracerebral or intraocular disease. The majority of patients with intraocular lesions develop contralateral tumors and cerebral lesions. The median age of presentation is 60 years and there appears to be a male preponderance. GEP studies have demonstrated some unique features in primary CNS tumors. 35–37 However, a consistent pattern as ABC or GCB has not emerged. It is interesting that there appears to be a link between primary CNS DLBCL, and DLBCL presenting in the testis, perhaps because both are immune privileged sites and tend to show decreased or absent expression of HLA class I and II proteins allowing further dodging of the immune system. 38, 39 Novel chemotherapy protocols including high-dose methotrexate have remarkably improved the previously poor prognosis. 40, 41
Primary cutaneous DLBCL, leg-type, is clinically more aggressive than other cutaneous B-cell lymphomas composed of large B-cells. 42 By GEP and immunophenotype, it resembles the activated B-cell type of nodal DLBCL. 43 As with nodal DLBCL, strong BCL-2 expression is an adverse prognostic factor and correlates with the ABC GEP. 44 Primary cutaneous follicle center lymphomas may be composed predominantly of large B-cells in some cases, but nevertheless have an indolent clinical course and generally do not require chemotherapy for clinical management unlike DLBCL, leg type.
Intravascular large B-cell lymphoma is a rare form of DLBCL characterized by the presence of large B-cells only in the lumens of small vessels, particularly capillaries of various organs; lymph node involvement is rare. 45, 46 There are two distinct patterns of clinical presentation, a Western form with neurological and cutaneous manifestations and an Asian form with pancytopenia, hepatosplenomegaly, multiorgan failure and hemophagocytic syndrome.40, 47 The disease is often not diagnosed until autopsy, because of the lack of definitive radiological or clinical evidence of disease, and diverse symptomatology. Patients presenting with cutaneous disease appear to have a somewhat better prognosis, probably because of early detection. Using immunophenotyping as a surrogate for the GEP, the majority of intravascular DLBCL has an ABC phenotype 47 and generally also expresses CD5.
T-CELL/HISTIOCYTE RICH LARGE B-CELL LYMPHOMA (T/HRLBCL)
T-cell/histiocyte-rich large B-cell lymphoma is a morphological variant of DLBCL, but one with many distinctive clinical features. 48, 49 T/HRLBCL tends to present in a relatively younger age group with a median age in the fourth decade and demonstrates male predominance. It has an aggressive clinical course, and often presents with advanced stage and spleen, liver and bone marrow (1/3rd of cases) involvement. 48–50 Recent studies have focused on the mechanisms of recruitment of inflammatory cells, and the relationship to the microenvironment. 51 Some cases appear related to nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), with the neoplastic cells being epithelial membrane antigen (EMA)-positive and having an LP-like morphology. 52 Additionally, NLPHL may progress to a process indistinguishable from de novo T/HRLBCL. The relationship of primary and secondary T/HRLBCL is not fully resolved, and in some instances the distinction between NLPHL and T/HRLBCL can be problematic in primary biopsy specimens. Also, the diagnosis of T/HRLBCL is best rendered on excision biopsies. Needle core biopsies should be avoided for primary diagnosis and classification for this and other lymphoma subtypes.
There are several key histological features that help distinguish T/HRLBCL from NLPHL; 1) absence of small B-cells 2) lack of a follicular structure as demonstrated by absence of follicular dendritic cells 3) Absence of T-cell rosettes around atypical B-cells despite a rich T-cell background. In addition, unlike NLPHL, T/HRLBCL very rarely presents with localized Stage I disease. Other cases with an overlapping morphological appearance may be composed of EBV-positive cells resembling Hodgkin/ Reed-Sternberg (HRS)-like cells in a background rich in T-cells. 53 Once considered a subtype of T/HRLBCL, EBV-positive cases are now included within EBV-positive large B-cell lymphoma. T/HRLBCL patients are generally treated similarly to their DLBCL, NOS counterparts with anthracycline containing chemotherapy, prednisone and Rituximab. 50 Interestingly, T/HRLBCL is rare in pediatric patients, but when it does occur is most often a progression of NLPHL. 54
EBV-POSITIVE DIFFUSE LARGE B-CELL LYMPHOMA
This entity is included as a provisional category in the WHO classification as EBV positive diffuse large B-cell lymphoma of the elderly. Oyama et al. first noted that EBV positive B-cell lymphoproliferative disorders were more likely to occur at advanced age, and had a clinically aggressive course. 55 Currently, it is defined as a histologically malignant polymorphic or monomorphic EBV-positive B-cell lymphoproliferation in patients who are generally over the age of 50 without any known immunodeficiency, transplantation or prior lymphoma. These patients have frequent extranodal presentation (e.g stomach, lung, tonsils and skin) and overall poor prognosis. Some cases show morphological overlap with classical Hodgkin lymphoma (cHL), also encountered in the elderly, but said to have a better prognosis. 56 The lesion is thought to be related to defective immune surveillance of EBV secondary to immunosenescence, the natural decay of the immune system as a consequence of aging. 55 Dojcinov et al. report a large series of patients from the Western world with overall similar clinical and pathological features; median age at presentation of 75 and an aggressive clinical course. 57 However, identical lesions can also be seen more rarely in younger patients, sometimes occurring close to the time of primary EBV-infection. 58 Dojcinov et al. also described EBV-driven proliferations presenting in cutaneous or mucosal sites, termed mucocutaneous ulcer, that are histologically alarming, but have a much more indolent and often self-limited clinical course. 57, 59 The cells in mucocutaneous ulcer often have an immunophenotype resembling cHL, with expression of CD30, CD15 and variable CD20.
The differential diagnoses include various EBV related benign lymphoproliferations, EBV reactivation in an older age group as well as other lymphomas like lymphomatoid granulomatosis, plasmablastic lymphoma and Hodgkin lymphoma. In some cases, distinction from Hodgkin lymphoma may be particularly problematic. Historically, cells resembling Reed-Sternberg cells have been described in many EBV-associated conditions ranging from infectious mononucleosis 60 to methotrexate-associated lymphoproliferative disease. 61 The correct diagnosis requires integration of clinical and pathological features, with correct interpretation of the immunophenotype in the clinical and pathological context.
Another distinctive form of EBV-positive DLBCL is DLBCL associated with chronic inflammation. This lesion was first recognized in patients with long standing chronic pyothorax. 62 However, an identical process may be seen in areas of chronic inflammation affecting body cavities or restricted spaces, such as joints. 63 This subtype manifests in the median age group of 65–70 years with a striking male predominance. The typical sites of involvement include pleural cavity, bone (especially femur), joints and periarticular soft tissue. In general, the tumor cells exhibit type III EBV latency with positive EBER and LMP1 expression. They exhibit aggressive clinical behavior and 5 year survival ranges from 20–35 %. 64, 65
MEDIASTINAL (THYMIC) LARGE B-CELL LYMPHOMA (PMBL)
PMBL has been recognized for a number of years as a distinct entity, based on its unique clinical and molecular features, 66–69 although cytologically, it resembles many other DLBCL. It occurs most commonly in female adolescents and young adults (median age at presentation in the fourth decade), but can also affect the pediatric age group. 70 It presents most often as early stage, bulky disease in the mediastinum with local extension to the lung, chest wall, pleura, pericardium with pleural and pericardial effusions. Distant extranodal disease at presentation is uncommon; however, relapse most often occurs in extranodal sites including kidneys, adrenals, liver and central nervous system. Bone marrow involvement is rare. The tumor is thought to be derived from medullary B cells within the thymus gland. The cells express CD20, CD79a, OCT-2 and BOB-1 but do not express surface immunoglobulin or CD10. CD23 is often positive. Pivotal studies showed a GEP differing from other DLBCL and resembling cHL, with upregulation of the NFκB pathway. 71, 72 TRAF-1 and c-REL expression was also observed, reflecting activation of NFκB pathway. 73 While there are many similarities between cHL and PMBL including young age of onset, presence of mediastinal mass, lack of surface immunoglobulin and shared pathways of activation, generally, histologic and immunophenotypic distinction is possible. However, CD30 is often positive (albeit weak and heterogeneous), and in some cases the distinction from nodular sclerosis cHL can be difficult, so-called “grey zone lymphomas”. 74 Regarding prognosis, a PMBL molecular signature was shown to be associated with better survival as compared to ABC and GCB DLBCL. 71 Traditional studies have suggested a need for radiation therapy in addition to CHOP-based chemotherapy for sustained complete remission in PMBL. The application of more intensive regimens, such as dose adjusted EPOCH (DA-EPOCH-R), with the addition of rituximab appear to obviate the need for radiation, reducing long term complication. 75–77
DIFFUSE LARGE B-CELL LYMPHOMAS WITH PLASMA CELL IMMUNOPHENOTYPE
Several types of diffuse large B-cell lymphoma exhibit a plasmablastic phenotype i.e. acquisition of plasma cell markers such as CD38/CD138 with loss of or weak B-cell markers and MUM-1 positivity. These include ALK-positive large B-cell lymphoma, plasmablastic lymphoma and primary effusion lymphoma.
ALK-positive large B-cell lymphoma is a rare subtype (<1% of DLBCL) in which the neoplastic cells resemble transformed cells with a terminally differentiated B-cell/plasma cell phenotype. 78 It occurs in all age groups (9–70 yrs) with a male predominance and mainly involves lymph nodes sometimes with mediastinal involvement. 79, 80 Several translocations involving leading to growth-factor independent activation of ALK have been demonstrated; the t(2;17) involving ALK and clathrin is the most common of these, but the classical t(2;5) of anaplastic large cell lymphoma can also be seen. Recently, cytogenetically complex SEC31A-ALK fusion was shown to be recurrent. 81 Most cases express cytoplasmic IgA, and there is often a downregulation of mature B-cell associated antigens such as CD20 and PAX-5. In contrast to ALK-positive anaplastic large cell lymphoma, CD30 is negative, CD138 is positive and prognosis is dismal with poor response to conventional CHOP based chemotherapy. 82 Small molecule inhibitors of ALK carry a promising future, especially with the recent approval of crizotinib (Xalkori Capsules, Pfizer, Inc.), the small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases for treatment of non-small cell lung carcinoma. 83 There is at least one clinical trial assessing the use of Crizotinib in ALK positive tumors in a non-lung cancer context (NCT01121588).
Plasmablastic lymphoma (PBL) was initially described in the oral cavity in the setting of HIV-infection. 84 However, PBL can occur in other settings of decreased immune surveillance, such as advanced age and congenital or acquired iatrogenic immunosuppression.85 The median age of presentation is 50 years with a male predominance; most patients are at an advanced stage (stage III or IV). The majority of cases is EBV-positive with a latency I phenotype. They typically present in extranodal sites, often with a mucosal or cutaneous localization. The morphology of neoplastic cells ranges from plasmablastic to immunoblastic. Some cases demonstrate more mature appearing plasma cells, and the immunophenotypic profile overlaps with that of plasma cell myeloma. 85, 86 Recent studies have shown a high incidence of MYC translocations in PBL, 87, 88 and similar genetic anomalies in plasma cell myeloma undergoing blastic transformation provide evidence for a link between myeloma and PBL. 89 PBL demonstrates an early response to therapy albeit with high relapse rates and overall poor prognosis. 90 Recent studies show that prognosis remains poor in HIV positive patients despite HAART therapy. 91
Primary effusion lymphoma (PEL) is a lymphoproliferative disorder associated with human herpesvirus 8 (HHV-8) infection. 92 It occurs most commonly in young or middle-aged males with HIV infection. Usually, there is co-infection with EBV. In the absence of HIV infection, it is seen predominantly in the elderly, especially in individuals of Mediterranean origin, which has high HHV-8 infection prevalence. 93 Some affected patients also have a history of Kaposi sarcoma, and less commonly multicentric Castleman’s disease. 94 The most common sites of involvement are the pleural, pericardial and peritoneal cavities. Some cases may present with tumor masses involving the gastrointestinal tract, soft tissue and other extranodal sites, termed extracavitary PEL. 95
The morphology of the tumor cells varies from plasmablastic, immunoblastic to frankly anaplastic. The differential diagnosis includes pyothorax-associated DLBCL, which presents with a pleura based lesion and is usually EBV-positive, but HHV-8 negative. Both tumor types can have aberrant cytoplasmic CD3 expression, which is a diagnostic pitfall as B-cell markers can be lost. 96, 97 Immunohistochemistry for the HHV8/KSHV-associated latent protein (LANA) is positive. Generally, EBV encoded RNA-ISH (EBER-ISH) is positive and LMP-1 is negative. Interestingly, stabilization of MYC also has been implicated in pathogenesis of PEL through unknown mechanisms. 98, 99 The prognosis is extremely poor with a median survival reported to be less than 6 months.
A related disorder is large B-cell lymphoma arising in HHV-8 associated multicentric Castleman disease (MCD). 100 The patient group is similar to that described for PEL with HIV infection, frequent Kaposi sarcoma and enlarged lymph nodes and spleen. Activation of the IL-6 signaling pathway mediates many of the clinical stigmata in MCD and in these patients. 101 This process represents an expansion of plasmablastic cells within a lymph node in the setting of MCD. The cells express IgM lambda, but monoclonality has been absent at the genetic level. Generally EBV co-infection is not observed; however, variations on this theme with unusual lymphoid proliferations co-infected with both EBV and HHV-8 have been described more recently. 102
B-CELL LYMPHOMA, UNCLASSIFIABLE, WITH FEATURES INTERMEDIATE BETWEEN DIFFUSE LARGE B-CELL LYMPHOMA AND BURKITT LYMPHOMA (B-UNC/BL/DLBCL)
This category was introduced in the 2008 WHO classification to address cases that have features intermediate between DLBCL and BL in terms of morphology, immunophenotype or cytogenetics and generally behave clinically in a more aggressive fashion. 103 This is a relatively rare entity that is seen mostly in adults with widespread nodal and extranodal disease along with leukemic manifestations in some patients. The distinction of BL from morphologically similar aggressive B-cell lymphomas has been problematic for pathologists and clinicians. For years a variety of terms have been used to describe lymphomas with morphological features resembling BL, but of uncertain biological linkage: small non-cleaved, non-Burkitt, atypical BL, Burkitt-like lymphoma. The use of these terminologies is discouraged in the context of the current WHO 2008 lymphoma lexicon.
GEP studies have shed some light on the spectrum of aggressive B-cell lymphomas bordering on BL. BL has a characteristic GEP, but there are cases morphologically within the spectrum of DLBCL with a similar GEP. 104, 105 Some harbor MYC translocations as a sole abnormality (MYC simple karyotype) while others have more complex abnormalities in association with MYC translocation (MYC complex karyotype). Sometimes, MYC translocations are detected along with either BCL2 (more frequent) or BCL6 translocations, so called “double-hit lymphomas”. Rarely, translocations involving MYC, BCL2 and BCL6 are seen together, hence the terminology “triple-hit lymphomas”. Double hit lymphomas are generally refractory to standard chemotherapy regimens, and have a poor prognosis. 106–108 The poor prognosis of this subgroup is most likely due to the additive effect of having MYC and BCL2 dysregulation leading to simultaneous increased proliferation and decreased apoptosis respectively, in combination with the genomic complexity seen in these patients. 103 A recent study has suggested that high expression of MYC and BCL2 proteins carries the same prognostic significance; even if genetic studies are negative for translocation. 109 These double hit and triple hit lymphomas comprise the largest cohort within the category of B-UNC/BL/DLBCL. Other scenarios that might qualify for an entry into this diagnostic entity include 1) cases with morphological features intermediate between DLBCL and BL, with a proliferation index approaching 100%, starry sky appearance and immunophenotype typical of BL or 2) a morphologically typical BL with an aberrant immunophenotype (especially strong to moderate BCL2 expression, or Ki-67 proliferative index <95%).
Importantly, the absence of MYC translocation in an otherwise typical BL (morphologically and immunophenotypically) does not exclude the diagnosis of BL. Up to 10% of otherwise typical BL have been shown to be negative for MYC translocation by FISH. 40, 105, 110 While technical issues for FISH assays might be a consideration in some cases, other pathways leading to MYC activation (e.g. miRNA deregulation) 111 have been demonstrated. Immunohistochemistry for MYC protein may prove to be informative in these cases. Conversely, the mere presence of MYC translocation or high proliferation index (100%) by itself in an otherwise typical DLBCL would also not qualify for an entry into this category. The clinical significance of a MYC translocation in DLBCL is not yet resolved, and may in part depend on the nature of the therapy employed. Recent studies have suggested that MYC-positive DLBCLs treated with R-CHOP have a poor prognosis, 4, 112 but in preliminary data an adverse effect was not observed in patients treated with DA-EPOCH-R at the NCI. 3 Additionally, many of the MYC-positive DLBCL in the published series did not have MYC as a sole genetic abnormality.
Certain problem areas still remain in terms of classification. One example is DLBCL with a very high proliferation rate approaching 100%, and an immunophenotype typical for BL: CD20+, CD10+, BCL-6+, BCL-2-, but lacking a demonstrable MYC translocation. At present our view is to retain these cases within DLBCL and not B-UNC/BL/DLBCL. However, as discussed above, possibility of MYC deregulation might still be pursued via immunohistochemistry. Another ambiguous subset is composed of cases that otherwise would be classified as BL, but exhibit minor morphologic variability or weak BCL-2 expression. In the presence of a classical cytogenetic profile (i.e. isolated MYC translocation), such cases can be retained as BL, and previously were referred to as atypical BL in the 3rd Edition of the WHO classification.113 In this setting cytogenetic studies for BCL2 should be performed to rule out an associated BCL2 translocation.
B-CELL LYMPHOMA, UNCLASSIFIABLE, WITH FEATURES INTERMEDIATE BETWEEN DIFFUSE LARGE B-CELL LYMPHOMA AND HODGKIN LYMPHOMA
This category of large B-cell lymphomas (also referred to as grey zone lymphoma, GZL) is reserved for those cases that demonstrate clinical, morphologic and immunophenotypic overlap between cHL and DLBCL, especially the PMBL type. These demonstrate propensity for mediastinal involvement in young male adults unlike cHL and PMBL, which occur more commonly in young females.114 This category is felt to overlap with what had been termed Hodgkin-like anaplastic large cell lymphoma in the older literature.115
GZL are composed of large pleomorphic tumor cells that sheet-out, often in a diffusely fibrotic stroma. The majority of the pleomorphic cells resemble Hodgkin cells, while in some areas they are more reminiscent of PMBL. A characteristic feature is the broad spectrum of cytologic appearance. The inflammatory infiltrate is usually sparse, but scattered eosinophils, lymphocytes, and histiocytes may be present. The immunophenotypic features of GZL are also intermediate, with frequent asynchrony between the morphology and immunophenotype.74 These include cases that resemble PMBL morphologically but demonstrate CD15 positivity or loss of CD20. On the other hand, cases that cytologically appear closer to cHL might express strong CD20 expression with retention of B-cell program in addition to CD30 and CD15 positivity. While a close relationship between cHL and PMBL has been demonstrated via GEP, gene profiling studies of grey zone lymphomas have not yet been undertaken. A recent large-scale methylation analysis demonstrated a close epigenetic relationship between GZL, cHL and PMBL, but quite different from that of DLBCL. In addition, principle component analysis indicated that GZL did not cluster with either cHL or PMBL, but demonstrated a unique epigenetic signature.116
The optimal treatment of GZL is not established. Despite the many similarities between cHL, PMBL and GZL including cytogenetic aberrations,76, 114, 115 GZL is considerably more aggressive and patients have had a relatively poor outcome with both cHL and NHL regimens.87, 90–91 Prospective studies from NCI have shown that GZL patients with clinical features similar to the PMBL cohort treated with DA-EPOCH-R had significantly inferior event free survival and often required consolidation mediastinal radiation.76 Currently for CD20-positive GZL, immunochemotherapy with rituximab, followed by radiation treatment if there is persistent PET-positive disease is the preferred approach. 76
Conclusion
The heterogeneity of the diffuse large B-cell lymphomas reflects the heterogeneity of B-cell neoplasms in general, and this complexity had led to an expanding number of entities in the WHO classification. GEP has delineated at least two major subsets, based on a cell of origin model, GCB and ABC. Newer technologies and platforms may make such testing routinely available in the clinical practice setting. 110,111 Along the same lines, better markers and improved algorithms for immunohistochemistry as a surrogate for GEP may improve categorization short of GEP based on RNA expression data.
The use of high throughput genomic techniques will continue to add complexity to this picture. While several criteria including clinical manifestation, morphology, immunophenotype and certain cytogenetic features determine the final subtype, the treatment of DLBCL has not varied in recent years, with R-CHOP being the standard in many clinical practices. The addition of rituximab to CHOP improved survival for most patients, but outcome was still inferior for the ABC subset in comparison with GCB. Recent data suggest that DA-EPOCH-R provides durable remissions in diffuse large B-cell lymphoma and is effective in both germinal center and non-germinal center B-cell subtypes. 117 Further studies are needed to determine the optimal treatment approach for other high risk categories, such as B-UNC/BL/DLBCL, with complex karyotypes. High throughput genomic studies have paved the way for better understanding of DLBCL and have led to new drug development. For example, the identification of mutations affecting B-cell signaling have led to trials using small molecule BTK inhibitors targeting the B-cell receptor pathway .6,17,109 In addition, targeted therapy of tumors with upregulation of ALK may be effective, similar to the approach utilized for ALK-positive lung cancer.
Figure 2.
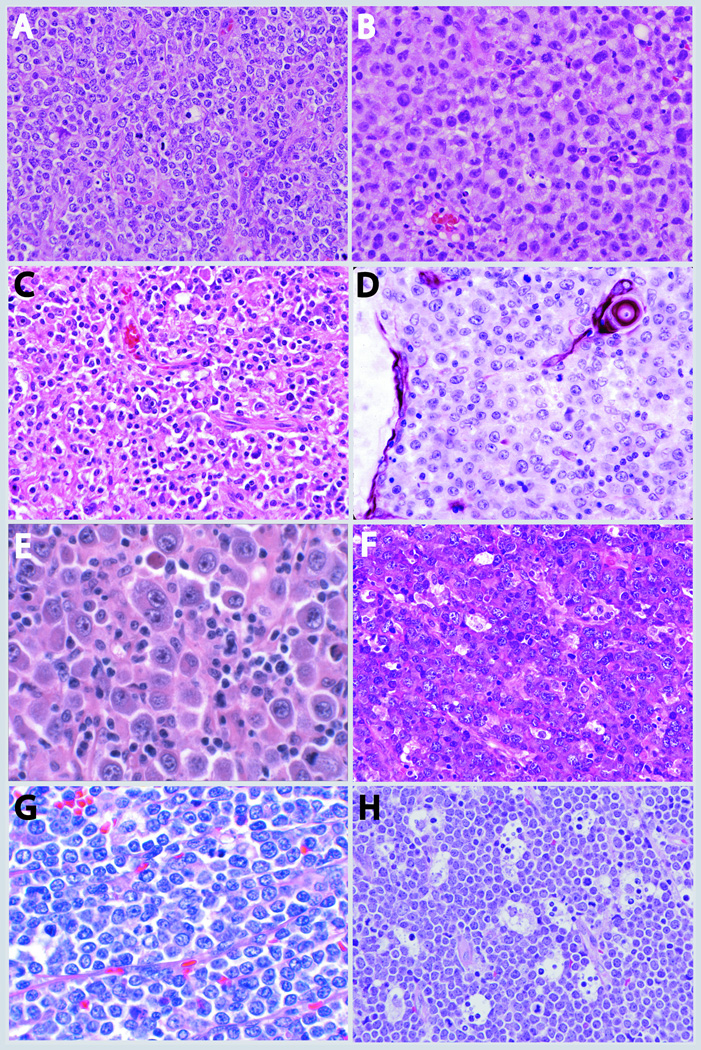
DLBCL subtypes. A) DLBCL GCB type which is enriched in centroblasts B) DLBCL ABC type with an immunoblastic morphology C) EBV positive DLBCL of elderly with a polymorphous background and presence of Reed-Sternberg like cells D) PMBL with cytokeratin immunostain highlighting thymic Hassall’s corpuscle E) ALK positive DLBCL demonstrating immunoblastic and plasmablastic features F) Plasmablastic lymphoma in a HIV positive patient demonstrating diffuse sheets of plasmablastic and immunoblastic cells with frequent mitoses, tingible body macrophages and apoptosis G) Double Hit DLBCL containing sheets of monomorphic medium to large cells without a starry sky pattern H) Burkitt lymphoma with sheets of medium sized cells with frequent mitoses and tingible-body macrophages imparting a starry sky pattern.
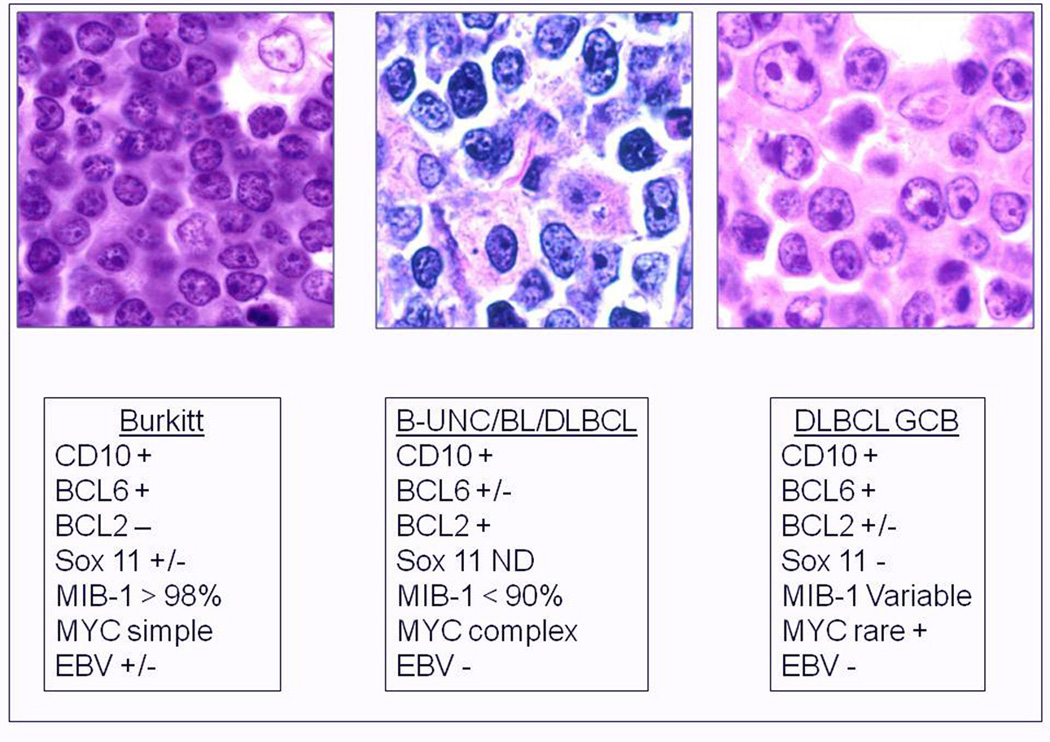
Figure 3.
(Adapted from Jaffe et al. 118) Burkitt lymphoma, B-UNC/BL/DLBCL, and DLBCL of the GCB type. B-UNC/BL/DLBCL are generally associated with a complex karyotype with both MYC and BCL2 translocations. BCL2 translocations are found in approximately 30% of the GCB type of DLBCL, but MYC translocation should be absent, and if a dual translocation is found, the case should be classified as B-UNC/BL/DLBCL. Morphologically BL is composed of medium sized cells, with minimal nuclear variation. Cell size is largest in DLBCL, and B-UNC/BL/DLBCL is generally composed of cells of intermediate to large size, with greater variability than Burkitt lymphoma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. May 12;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito M, Gao J, Basso K, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007 Sep;12(3):280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Dunleavy K, Pittaluga S, Wayne As, et al. MYC + aggressive-B-cell lymphomas: novel therapy of untreated Burkitt lymphoma (BL) AND MYC + Diffuse large B-cell lymphoma (DLBCL) WITH DA-EPOCH-R. Ann Oncol. 2011;22(suppl 4):106. [Google Scholar]
- 4.Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009 Oct 22;114(17):3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal J, Sanger WG, Horsman DE, et al. BCL2 Translocation Defines a Unique Tumor Subset within the Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. Am J Pathol. 2004 Jul;165(1):159–166. doi: 10.1016/s0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. Jan 7;463(7277):88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. Feb 3;470(7332):115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. Sep;43(9):830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 10.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 11.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expressionbased method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008 Nov 27;359(22):2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-Bcell lymphoma based on the expression of six genes. N Engl J Med. 2004 Apr 29;350(18):1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 14.Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8(1):68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 15.Rimsza LM, Roberts RA, Miller TP, et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood. 2004 Jun 1;103(11):4251–4258. doi: 10.1182/blood-2003-07-2365. [DOI] [PubMed] [Google Scholar]
- 16.Rimsza LM, Roberts RA, Campo E, et al. Loss of major histocompatibility class II expression in non-immune-privileged site diffuse large B-cell lymphoma is highly coordinated and not due to chromosomal deletions. Blood. 2006 Feb 1;107(3):1101–1107. doi: 10.1182/blood-2005-04-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of beta2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. Dec 13;20(6):728–740. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008 Mar 21;319(5870):1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 19.Milhollen MA, Traore T, Adams-Duffy J, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. Sep 2;116(9):1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 20.Ngo VN, Davis RE, Lamy L, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006 May 4;441(7089):106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 21.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009 Jun 11;113(24):6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweetenham JW. Molecular signatures in the diagnosis and management of diffuse large B-cell lymphoma. Curr Opin Hematol. Jul;18(4):288–292. doi: 10.1097/MOH.0b013e32834706ee. [DOI] [PubMed] [Google Scholar]
- 23.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004 Jan 1;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 24.Salles G, de Jong D, Xie W, et al. Prognostic significance of immunohistochemical biomarkers in diffuse large B-cell lymphoma: a study from the Lunenburg Lymphoma Biomarker Consortium. Blood. Jun 30;117(26):7070–7078. doi: 10.1182/blood-2011-04-345256. [DOI] [PubMed] [Google Scholar]
- 25.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. Jan 10;29(2):200–207. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009 Sep 1;15(17):5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, Banham AH, Leppa S. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol. 2009 Aug;22(8):1094–1101. doi: 10.1038/modpathol.2009.73. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. May 5;117(18):4836–4843. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]
- 29.Zu Y, Steinberg SM, Campo E, et al. Validation of tissue microarray immunohistochemistry staining and interpretation in diffuse large B-cell lymphoma. Leuk Lymphoma. 2005 May;46(5):693–701. doi: 10.1080/10428190500051844. [DOI] [PubMed] [Google Scholar]
- 30.de Jong D, Xie W, Rosenwald A, et al. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications (a study from the Lunenburg Lymphoma Biomarker Consortium) J Clin Pathol. 2009 Feb;62(2):128–138. doi: 10.1136/jcp.2008.057257. [DOI] [PubMed] [Google Scholar]
- 31.Visco C, Li Y, Xu-Monette ZY, et al. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: A report from the International DLBCL Rituximab-CHOP consortium program study. Leukemia. Mar 22; doi: 10.1038/leu.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelhard M, Brittinger G, Huhn D, et al. Subclassification of diffuse large B-cell lymphomas according to the Kiel classification: distinction of centroblastic and immunoblastic lymphomas is a significant prognostic risk factor. Blood. 1997;89(7):2291–2297. [PubMed] [Google Scholar]
- 33.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002 Jun 20;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 34.Ott G, Ziepert M, Klapper W, et al. Immunoblastic morphology but not the immunohistochemical GCB/nonGCB classifier predicts outcome in diffuse large B-cell lymphoma in the RICOVER-60 trial of the DSHNHL. Blood. Dec 2;116(23):4916–4925. doi: 10.1182/blood-2010-03-276766. [DOI] [PubMed] [Google Scholar]
- 35.Tun HW, Personett D, Baskerville KA, et al. Pathway analysis of primary central nervous system lymphoma. Blood. 2008 Mar 15;111(6):3200–3210. doi: 10.1182/blood-2007-10-119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung CO, Kim SC, Karnan S, et al. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood. Jan 27;117(4):1291–1300. doi: 10.1182/blood-2010-07-297861. [DOI] [PubMed] [Google Scholar]
- 37.Rubenstein JL, Shen A, Batchelor TT, Kadoch C, Treseler P, Shuman MA. Differential gene expression in central nervous system lymphoma. Blood. 2009 Jan 1;113(1):266–267. doi: 10.1182/blood-2008-04-152835. author reply 267–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Abbadi MA, Hattab EM, Tarawneh MS, Amr SS, Orazi A, Ulbright TM. Primary testicular diffuse large B-cell lymphoma belongs to the nongerminal center B-cell-like subgroup: A study of 18 cases. Mod Pathol. 2006 Dec;19(12):1521–1527. doi: 10.1038/modpathol.3800691. [DOI] [PubMed] [Google Scholar]
- 39.Booman M, Szuhai K, Rosenwald A, et al. Genomic alterations and gene expression in primary diffuse large B-cell lymphomas of immune-privileged sites: the importance of apoptosis and immunomodulatory pathways. J Pathol. 2008 Oct;216(2):209–217. doi: 10.1002/path.2399. [DOI] [PubMed] [Google Scholar]
- 40.Swerdlow SHCE, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 41.Ferreri AJ. How I treat primary CNS lymphoma. Blood. Jul 21;118(3):510–522. doi: 10.1182/blood-2011-03-321349. [DOI] [PubMed] [Google Scholar]
- 42.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005 May 15;105(10):3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 43.Hoefnagel JJ, Dijkman R, Basso K, et al. Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood. 2005 May 1;105(9):3671–3678. doi: 10.1182/blood-2004-04-1594. [DOI] [PubMed] [Google Scholar]
- 44.Grange F, Petrella T, Beylot-Barry M, et al. Bcl-2 protein expression is the strongest independent prognostic factor of survival in primary cutaneous large B-cell lymphomas. Blood. 2004 May 15;103(10):3662–3668. doi: 10.1182/blood-2003-08-2726. [DOI] [PubMed] [Google Scholar]
- 45.Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007 Jul 20;25(21):3168–3173. doi: 10.1200/JCO.2006.08.2313. [DOI] [PubMed] [Google Scholar]
- 46.Burg G, Kempf W, Cozzio A, et al. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J Cutan Pathol. 2005 Nov;32(10):647–674. doi: 10.1111/j.0303-6987.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 47.Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007 Jan 15;109(2):478–485. doi: 10.1182/blood-2006-01-021253. [DOI] [PubMed] [Google Scholar]
- 48.Achten R, Verhoef G, Vanuytsel L, De Wolf-Peeters C. Histiocyte-rich, T-cell-rich Bcell lymphoma: a distinct diffuse large B-cell lymphoma subtype showing characteristic morphologic and immunophenotypic features. Histopathology. 2002;40(1):31–45. doi: 10.1046/j.1365-2559.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- 49.Achten R, Verhoef G, Vanuytsel L, De Wolf-Peeters C. T-cell/histiocyte--rich large Bcell lymphoma: a distinct clinicopathologic entity. J Clin Oncol. 2002;20(5):1269–1277. doi: 10.1200/JCO.2002.20.5.1269. [DOI] [PubMed] [Google Scholar]
- 50.Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006 Apr;11(4):384–392. doi: 10.1634/theoncologist.11-4-384. [DOI] [PubMed] [Google Scholar]
- 51.Van Loo P, Tousseyn T, Vanhentenrijk V, et al. T-cell/histiocyte-rich large B-cell lymphoma shows transcriptional features suggestive of a tolerogenic host immune response. Haematologica. Mar;95(3):440–448. doi: 10.3324/haematol.2009.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pittaluga S, Jaffe ES. T-cell/histiocyte-rich large B-cell lymphoma. Haematologica. Mar;95(3):352–356. doi: 10.3324/haematol.2009.016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim MS, Beaty M, Sorbara L, et al. T-cell/histiocyte-rich large B-cell lymphoma: a heterogeneous entity with derivation from germinal center B cells. Am J Surg Pathol. 2002;26(11):1458–1466. doi: 10.1097/00000478-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Tiemann M, Riener MO, Claviez A, et al. Proliferation rate and outcome in children with T-cell rich B-cell lymphoma: a clinicopathologic study from the NHL-BFM-study group. Leuk Lymphoma. 2005 Sep;46(9):1295–1300. doi: 10.1080/10428190500083326. [DOI] [PubMed] [Google Scholar]
- 55.Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007 Sep 1;13(17):5124–5132. doi: 10.1158/1078-0432.CCR-06-2823. [DOI] [PubMed] [Google Scholar]
- 56.Asano N, Yamamoto K, Tamaru J, et al. Age-related Epstein-Barr virus (EBV)-associated B-cell lymphoproliferative disorders: comparison with EBV-positive classic Hodgkin lymphoma in elderly patients. Blood. 2009 Mar 19;113(12):2629–2636. doi: 10.1182/blood-2008-06-164806. [DOI] [PubMed] [Google Scholar]
- 57.Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. May 5;117(18):4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beltran BE, Morales D, Quinones P, Medeiros LJ, Miranda RN, Castillo JJ. EBVpositive diffuse large b-cell lymphoma in young immunocompetent individuals. Clin Lymphoma Myeloma Leuk. Dec;11(6):512–516. doi: 10.1016/j.clml.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. Mar;34(3):405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abbondanzo SL, Sato N, Straus SE, Jaffe ES. Acute infectious mononucleosis. CD30 (Ki-1) antigen expression and histologic correlations. Am J Clin Pathol. 1990;93(5):698–702. doi: 10.1093/ajcp/93.5.698. [DOI] [PubMed] [Google Scholar]
- 61.Kamel OW, Weiss LM, van de Rijn M, Colby TV, Kingma DW, Jaffe ES. Hodgkin’s disease and lymphoproliferations resembling Hodgkin’s disease in patients receiving long-term low-dose methotrexate therapy. Am J Surg Pathol. 1996;20(10):1279–1287. doi: 10.1097/00000478-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Aozasa K, Takakuwa T, Nakatsuka S. Pyothorax-associated lymphoma: a lymphoma developing in chronic inflammation. Adv Anat Pathol. 2005 Nov;12(6):324–331. doi: 10.1097/01.pap.0000194627.50878.02. [DOI] [PubMed] [Google Scholar]
- 63.Loong F, Chan AC, Ho BC, et al. Diffuse large B-cell lymphoma associated with chronic inflammation as an incidental finding and new clinical scenarios. Mod Pathol. Apr;23(4):493–501. doi: 10.1038/modpathol.2009.168. [DOI] [PubMed] [Google Scholar]
- 64.Narimatsu H, Ota Y, Kami M, et al. Clinicopathological features of pyothorax-associated lymphoma; a retrospective survey involving 98 patients. Ann Oncol. 2007 Jan;18(1):122–128. doi: 10.1093/annonc/mdl349. [DOI] [PubMed] [Google Scholar]
- 65.Nakatsuka S, Yao M, Hoshida Y, Yamamoto S, Iuchi K, Aozasa K. Pyothorax-associated lymphoma: a review of 106 cases. J Clin Oncol. 2002 Oct 15;20(20):4255–4260. doi: 10.1200/JCO.2002.09.021. [DOI] [PubMed] [Google Scholar]
- 66.Barth TF, Leithauser F, Joos S, Bentz M, Moller P. Mediastinal (thymic) large B-cell lymphoma: where do we stand? Lancet Oncol. 2002;3(4):229–234. doi: 10.1016/s1470-2045(02)00714-3. [DOI] [PubMed] [Google Scholar]
- 67.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. Oct 28;116(17):3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feuerhake F, Kutok JL, Monti S, et al. NFkappaB activity, function, and target-gene signatures in primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma subtypes. Blood. 2005 Aug 15;106(4):1392–1399. doi: 10.1182/blood-2004-12-4901. [DOI] [PubMed] [Google Scholar]
- 69.Rui L, Emre NC, Kruhlak MJ, et al. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell. Dec 14;18(6):590–605. doi: 10.1016/j.ccr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oschlies I, Burkhardt B, Salaverria I, et al. Clinical, pathological and genetic features of primary mediastinal large B-cell lymphomas and mediastinal gray zone lymphomas in children. Haematologica. Feb;96(2):262–268. doi: 10.3324/haematol.2010.030809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003 Sep 15;198(6):851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003 Dec 1;102(12):3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 73.Rodig SJ, Savage KJ, LaCasce AS, et al. Expression of TRAF1 and nuclear c-Rel distinguishes primary mediastinal large cell lymphoma from other types of diffuse large B-cell lymphoma. Am J Surg Pathol. 2007 Jan;31(1):106–112. doi: 10.1097/01.pas.0000213334.40358.0e. [DOI] [PubMed] [Google Scholar]
- 74.Traverse-Glehen A, Pittaluga S, Gaulard P, et al. Mediastinal Gray Zone Lymphoma: The Missing Link Between Classic Hodgkin’s Lymphoma and Mediastinal Large B-Cell Lymphoma. Am J Surg Pathol. 2005 Nov;29(11):1411–1421. doi: 10.1097/01.pas.0000180856.74572.73. [DOI] [PubMed] [Google Scholar]
- 75.Grant C, Dunleavy K, Eberle FC, Pittaluga S, Wilson WH, Jaffe ES. Primary mediastinal large B-cell lymphoma, classic Hodgkin lymphoma presenting in the mediastinum, and mediastinal gray zone lymphoma: what is the oncologist to do? Curr Hematol Malig Rep. Sep;6(3):157–163. doi: 10.1007/s11899-011-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dunleavy KPS, Shovlin M, et al. Untreated primary mediastinal B-cell (PMBL) and mediastinal grey zone (MGZL) lymphomas: comparison of biological features and clinical outcome following DA-EPOCH-R without radiation. Ann Oncol. 2011;22(suppl 4):134. [Google Scholar]
- 77.Zinzani PL, Piccaluga PP. Primary mediastinal DLBCL: evolving biologic understanding and therapeutic strategies. Curr Oncol Rep. Oct;13(5):407–415. doi: 10.1007/s11912-011-0189-5. [DOI] [PubMed] [Google Scholar]
- 78.Delsol G, Lamant L, Mariame B, et al. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2; 5 translocation. Blood. 1997;89(5):1483–1490. [PubMed] [Google Scholar]
- 79.Gesk S, Gascoyne RD, Schnitzer B, et al. ALK-positive diffuse large B-cell lymphoma with ALK-Clathrin fusion belongs to the spectrum of pediatric lymphomas. Leukemia. 2005 Oct;19(10):1839–1840. doi: 10.1038/sj.leu.2403921. [DOI] [PubMed] [Google Scholar]
- 80.Laurent C, Do C, Gascoyne RD, et al. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma: a rare clinicopathologic entity with poor prognosis. J Clin Oncol. 2009 Sep 1;27(25):4211–4216. doi: 10.1200/JCO.2008.21.5020. [DOI] [PubMed] [Google Scholar]
- 81.Bedwell C, Rowe D, Moulton D, Jones G, Bown N, Bacon CM. Cytogenetically complex SEC31A-ALK fusions are recurrent in ALK-positive large B-cell lymphomas. Haematologica. Feb;96(2):343–346. doi: 10.3324/haematol.2010.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morgan EA, Nascimento AF. Anaplastic lymphoma kinase-positive large B-cell lymphoma: an underrecognized aggressive lymphoma. Adv Hematol. 2012:529572. doi: 10.1155/2012/529572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chabner BA. Early accelerated approval for highly targeted cancer drugs. N Engl J Med. Mar 24;364(12):1087–1089. doi: 10.1056/NEJMp1100548. [DOI] [PubMed] [Google Scholar]
- 84.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89(4):1413–1420. [PubMed] [Google Scholar]
- 85.Colomo L, Loong F, Rives S, et al. Diffuse Large B-cell Lymphomas With Plasmablastic Differentiation Represent a Heterogeneous Group of Disease Entities. Am J Surg Pathol. 2004 Jun;28(6):736–747. doi: 10.1097/01.pas.0000126781.87158.e3. [DOI] [PubMed] [Google Scholar]
- 86.Vega F, Chang CC, Medeiros LJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol. 2005 Jun;18(6):806–815. doi: 10.1038/modpathol.3800355. [DOI] [PubMed] [Google Scholar]
- 87.Taddesse-Heath L, Meloni-Ehrig A, Scheerle J, Kelly JC, Jaffe ES. Plasmablastic lymphoma with MYC translocation: evidence for a common pathway in the generation of plasmablastic features. Mod Pathol. Jul;23(7):991–999. doi: 10.1038/modpathol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valera A, Balague O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol. Nov;34(11):1686–1694. doi: 10.1097/PAS.0b013e3181f3e29f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gabrea A, Martelli ML, Qi Y, et al. Secondary genomic rearrangements involving immunoglobulin or MYC loci show similar prevalences in hyperdiploid and nonhyperdiploid myeloma tumors. Genes Chromosomes Cancer. 2008 Jul;47(7):573–590. doi: 10.1002/gcc.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castillo JJ. Plasmablastic lymphoma: are more intensive regimens needed? Leuk Res. Dec;35(12):1547–1548. doi: 10.1016/j.leukres.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 91.Castillo JJ, Furman M, Beltran BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: Poor prognosis in the era of highly active antiretroviral therapy. Cancer. Apr 17; doi: 10.1002/cncr.27551. [DOI] [PubMed] [Google Scholar]
- 92.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995 May 4;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 93.Cobo F, Hernandez S, Hernandez L, et al. Expression of potentially oncogenic HHV-8 genes in an EBV-negative primary effusion lymphoma occurring in an HIV-seronegative patient. J Pathol. 1999;189(2):288–293. doi: 10.1002/(SICI)1096-9896(199910)189:2<288::AID-PATH419>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 94.Teruya-Feldstein J, Zauber P, Setsuda JE, et al. Expression of human herpesvirus-8 oncogene and cytokine homologues in an HIV-seronegative patient with multicentric Castleman’s disease and primary effusion lymphoma. Lab Invest. 1998 Dec;78(12):1637–1642. [PubMed] [Google Scholar]
- 95.Chadburn A, Hyjek E, Mathew S, Cesarman E, Said J, Knowles DM. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol. 2004 Nov;28(11):1401–1416. doi: 10.1097/01.pas.0000138177.10829.5c. [DOI] [PubMed] [Google Scholar]
- 96.Petitjean B, Jardin F, Joly B, et al. Pyothorax-associated lymphoma: a peculiar clinicopathologic entity derived from B cells at late stage of differentiation and with occasional aberrant dual B-and T-cell phenotype. Am J Surg Pathol. 2002 Jun;26(6):724–732. doi: 10.1097/00000478-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Beaty MW, Kumar S, Sorbara L, Miller K, Raffeld M, Jaffe ES. A biophenotypic human herpesvirus 8--associated primary bowel lymphoma. Am J Surg Pathol. 1999;23(8):992–994. doi: 10.1097/00000478-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 98.Ahmad A, Groshong JS, Matta H, et al. Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 cooperates with Myc to promote lymphoma in mice. Cancer Biol Ther. Nov;10(10):1033–1040. doi: 10.4161/cbt.10.10.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bubman D, Guasparri I, Cesarman E. Deregulation of c-Myc in primary effusion lymphoma by Kaposi’s sarcoma herpesvirus latency-associated nuclear antigen. Oncogene. 2007 Jul 26;26(34):4979–4986. doi: 10.1038/sj.onc.1210299. [DOI] [PubMed] [Google Scholar]
- 100.Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406–1412. [PubMed] [Google Scholar]
- 101.van Rhee F, Stone K, Szmania S, Barlogie B, Singh Z. Castleman disease in the 21st century: an update on diagnosis, assessment, and therapy. Clin Adv Hematol Oncol. Jul;8(7):486–498. [PubMed] [Google Scholar]
- 102.Seliem RM, Griffith RC, Harris NL, et al. HHV-8+, EBV+ multicentric plasmablastic microlymphoma in an HIV+ Man: the spectrum of HHV-8+ lymphoproliferative disorders expands. Am J Surg Pathol. 2007 Sep;31(9):1439–1445. doi: 10.1097/PAS.0b013e31804d43d8. [DOI] [PubMed] [Google Scholar]
- 103.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. Feb 24;117(8):2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 104.Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006 Jun 8;354(23):2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 105.Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006 Jun 8;354(23):2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 106.Tomita N, Tokunaka M, Nakamura N, et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica. 2009 Jul;94(7):935–943. doi: 10.3324/haematol.2008.005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. Mar;34(3):327–340. doi: 10.1097/PAS.0b013e3181cd3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009 Sep 10;114(11):2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in R-CHOP treated diffuse large B cell lymphoma. Journal of Clinical Oncology. 2012 doi: 10.1200/JCO.2011.41.0985. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haralambieva E, Boerma EJ, van Imhoff GW, et al. Clinical, immunophenotypic, and genetic analysis of adult lymphomas with morphologic features of Burkitt lymphoma. Am J Surg Pathol. 2005 Aug;29(8):1086–1094. [PubMed] [Google Scholar]
- 111.Onnis A, De Falco G, Antonicelli G, et al. Alteration of microRNAs regulated by c-Myc in Burkitt lymphoma. PLoS One. 5(9) doi: 10.1371/journal.pone.0012960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. Jul 10;28(20):3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- 113.Jaffe ES, Harris NL, Stein H, Vardiman J. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 114.Eberle FC, Salaverria I, Steidl C, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol. Dec;24(12):1586–1597. doi: 10.1038/modpathol.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zinzani PL, Bendandi M, Martelli M, et al. Anaplastic large-cell lymphoma: clinical and prognostic evaluation of 90 adult patients. J Clin Oncol. 1996 Mar;14(3):955–962. doi: 10.1200/JCO.1996.14.3.955. [DOI] [PubMed] [Google Scholar]
- 116.Eberle FC, Rodriguez-Canales J, Wei L, et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma. Haematologica. Apr;96(4):558–566. doi: 10.3324/haematol.2010.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilson WH, Jung SH, Porcu P, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. May;97(5):758–765. doi: 10.3324/haematol.2011.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jaffe ES, Pittaluga S. Aggressive B-cell lymphomas: a review of new and old entities in the WHO classification. Hematology Am Soc Hematol Educ Program. 2011:506–514. doi: 10.1182/asheducation-2011.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]