Abstract
Ultraviolet (UV)-induced DNA damage causes an efficient block of elongating replication forks. The checkpoint kinase, CHK1 has been shown to stabilize replication forks following hydroxyurea treatment. Therefore, we wanted to test if the increased UV sensitivity caused by the unspecific kinase inhibitor caffeine—inhibiting ATM and ATR amongst other kinases—is explained by inability to activate the CHK1 kinase to stabilize replicative structures. For this, we used cells deficient in polymerase η (Polη), a translesion synthesis polymerase capable of properly bypassing the UV-induced cis–syn TT pyrimidine dimer, which blocks replication. These cells accumulate gaps behind progressing replication forks after UV exposure. We demonstrate that both caffeine and CHK1 inhibition, equally retards continuous replication fork elongation after UV treatment. Interestingly, we found more pronounced UV-sensitization by caffeine than with the CHK1 inhibitor in clonogenic survival experiments. Furthermore, we demonstrate an increased collapse of replicative structures after caffeine treatment, but not after CHK1 inhibition, in UV-irradiated cells. This demonstrates that CHK1 activity is not required for stabilization of gaps induced during replication of UV-damaged DNA. These data suggest that elongation and stabilization of replicative structures at UV-induced DNA damage are distinct mechanisms, and that CHK1 is only involved in replication elongation.
INTRODUCTION
The PI-3 kinase-related kinases ATM, ATR and DNA-PKcs are activated following DNA damage and constitute major components to orchestrate the DNA damage response. While ATM and DNA-PK are activated at DNA double-strand breaks (DSBs), the ATR kinase is activated at single-stranded DNA (ssDNA) generated upon replication stress through interaction with ATRIP, which interacts with the ssDNA binding protein RPA (1). In turn, the ATR kinase phosphorylates the CHK1 kinase, which is a key component for mediating cell cycle arrest in the S and G2/M phases of the cell cycle (2). In S phase, CHK1 suppresses origin firing after DNA damage through phosphorylation of CDC25A (3). In addition, the CHK1 protein has many other functions during S phase, for instance in directly promoting homologous recombination at stalled forks by assisting the exchange of RPA with RAD51 and phosphorylation of RAD51 directly (4,5). ATR and CHK1 but not ATM or CHK2 are critical for maintenance of fragile sites (6,7). Also, CHK1 has a very important role to protect hydroxyurea stalled replication forks from collapsing (8), which seems to be a conserved role from Saccharomyces cerevisiae, where CHK1/CHK2 ortholog rad53 mutants fails to fully protect stalled forks from collapse (9), consistent with the role of Cds1 in budding yeast (10). However, replication collapse in methyl methanosulphonate-treated rad53 mutants is dependent on EXO1 and it is clear that the minor S. cerevisiae checkpoint kinase CHK1 also has RAD53-independent roles for stabilizing stalled replication forks (11). This demonstrates that the process of preventing stalled forks from collapse is a more complex pathway in S. cerevisiae than previously anticipated.
In mammalian cells the CHK1 kinase has an important role promoting replication elongation (12), which has generally been thought to be linked with its role in maintaining fork stability. However, more recent data demonstrate that the role of CHK1 in promoting replication elongation is through its role in suppressing origin firing (13).
The ATR–CHK1 pathway is activated upon ultraviolet (UV)-induced replication block (14,15), which is important to mediate the intra--S phase arrest to prevent replication initiation after UV damage (16). ATR also signals through Claspin (17), the TIM/TIPIN complex (18,19) and CHK1 (14,16,20) to slow down replication forks after UV treatment. UV-induced fork slowing also requires the RAD51 and XRCC3 proteins (21,22).
Bypass of physical DNA damage including UV-induced lesions has been shown to occur behind the replication fork (23,24). During replication of UV-damaged DNA, replication forks continue past the lesion, leaving behind ssDNA gaps in the newly replicated DNA (25–27). These ssDNA regions are likely to trigger monoubiquitination of proliferating cell nuclear antigen (PCNA) via the RAD6–RAD18 pathway (28,29), increasing the affinity of PCNA for translesion synthesis (TLS) polymerases, a process recently reviewed in (30). The ssDNA also serves as a signal for ATR-mediated CHK1 phosphorylation (31). In addition to gap filling by TLS, the cell may employ a recombinational pathway for DNA damage bypass (21,22).
Caffeine is an unspecific kinase inhibitor that blocks checkpoint signalling and other important cellular functions, and has been shown to inhibit both ATM and ATR in a dose-dependent manner (32). Caffeine treatment after UV irradiation causes premature chromatin condensation, a hallmark of cells entering mitosis before completion of DNA replication (33). This occurs via inhibition of CHK1 through ATR inhibition, and is not induced by ATM inhibition (33). In addition, caffeine treatment but not ATM inhibitor KU55933 abrogates the G2 checkpoint induced by unfilled ssDNA gaps following UV irradiation (25). In Xenopus egg extracts, extended stretches of ssDNA coated with RPA stops DNA replication in an ATR but not ATM-dependent manner, a process that is inhibited by caffeine (34). Caffeine has been extensively used when studying xeroderma pigmentosum variant (XP-V) cells as it sensitises these cells to UV exposure (35). These cells, derived from patients with the sunlight-induced syndrome XP-V, are deficient in the TLS polymerase η (Polη) (36–38), which is capable of correctly bypassing the most common UV-induced DNA lesion, cis–syn thymine–thymine cyclobutane pyrimidine dimer (CPD) (39–41). More recently, it was reported that resumption of DNA replication and survival in Polη-deficient cells is dependent on the ATR–CHK1 pathway (31).
Here, we wanted to study the stability of replication structures on UV-damaged DNA after inhibition of the CHK1 kinase pathway using the selective CHK1 kinase inhibitor CEP-3891. As a comparison, we also investigated how elongation and stabilization of replication forks is affected by caffeine during replication on UV-damaged DNA. We report that caffeine and the CHK1 inhibitor CEP-3891 similarly affects continuous replication elongation. Surprisingly, we find that CHK1 activity is not required for stabilization of UV-induced gaps behind the replication fork, which separates the role for CHK1 in replication fork elongation and stabilization of UV-induced replicative gaps. Furthermore, we report that in Polη-deficient cells, the collapse of replicative structures after UV irradiation is induced by the presence of caffeine but independent of CHK1 activity. This suggests that stabilization of replicative structures after UV irradiation, in analogy with S. cerevisiae, are maintained in a complex kinase network.
MATERIALS AND METHODS
Cell culture
The SV40-transformed, Polη-deficient fibroblast cell line XP30RO originally obtained from a patient and containing an empty vector, and the restored cell line XP30RO+Polη stably expressing Polη from a vector (42), were a kind gift from Dr Alan Lehmann, University of Sussex, UK. Cells were grown in Dulbecco’s Eagle’s minimum essential medium (DMEM) with 10% foetal calf serum and 1% streptomycin–penicillin, restored cells in the presence of 100 µg/ml zeocin. Cells were cultured and treated in an incubator at 37°C with 5% CO2. The XP30RO cells have a 13 bp deletion leading to a frame shift, yielding a 42 amino acids protein (38).
UVC irradiation
Cells were washed with cold Hank’s balanced salt solution (HBSS) before irradiation at room temperature under a 254 nm UVC low-pressure mercury lamp (Phillips TUV 15 W) at indicated doses. Dose rates used were 0.18 and 0.10 J/m2s. Exposure times were controlled using a fast magnetic shutter mounted within the apparatus and controlled by a timer.
Inhibitors
Caffeine (Sigma) was dissolved in DMEM to a concentration of 50 mM half an hour before use, and diluted to a working concentration of 1 mM. CEP-3891 (obtained from Stephen Trusko, Cephalon Inc.) was dissolved in DMSO (0.5 mM) and diluted to 0.5 µM. Gö6976 (Calbiochem) was dissolved in DMSO (1.32 mM) and diluted to 1 µM. SB-218078 (Calbiochem) was dissolved in DMSO (2 mM) and diluted to 2 µM. All inhibitors were diluted to their final concentration in pre-warmed DMEM 15 min before use.
Clonogenic survival
In 10 cm tissue culture dishes, 5 × 105 cells were seeded and allowed to grow for 24 h before irradiation with different doses of UVC on ice. After 24 h recovery, in presence or absence of inhibitors added directly after treatment, a sufficient number of cells were re-seeded in triplicates onto 10 cm tissue culture dishes and grown in DMEM. After 10 days, colonies were fixed and stained with methylene blue dissolved in methanol (4 g/l).
Immunofluorescence
Cells were seeded on cover slips and allowed to grow for at least 18 h before being pulse labelled with EdU (10 µM) for 10 min directly followed by UVC irradiation (2.5 J/m2). The cells were then allowed to repair for in the presence of absence of inhibitors for 6 h before fixation with 1% paraformaldehyde for 20 min, permeabilized for 10 min with 0.3% Triton X-100 in phosphate buffered saline (PBS), blocked with 3% bovine serum albumin (BSA) in PBS for 40 min, incubated with primary antibodies against γH2AX (Upstate, mouse, clone JBW301) for 2 h at room temperature, washed and permeabilized followed by EdU staining using Click-iT Alexa Fluor 488 (Invitrogen) following the manufacturer’s instructions. The slides were then washed and permeabilized again before incubation with fluorophore-conjugated secondary antibody (Alexa 633 goat anti mouse, Molecular Probes) for 1 h at room temperature, washed and mounted in ProLong Gold (Invitrogen). Antibodies were diluted 1:1000 in PBS with 3% BSA. Fluorescence images were captured using a Zeiss LSM 510 inverted confocal microscope using planapochromat 63×/NA 1.4 oil immersion objective, excitation wavelengths of 488 and 630 nm and processed using the LSM software. Only cells with distinct EdU foci were analysed for γH2AX foci number, size and intensity. Analysis was performed using ImageJ software (43). In total 300–500 foci from coded samples in two separate experiments were analysed.
Replication fork elongation using the alkaline DNA unwinding technique
Continuous replication fork elongation was measured as described previously (22). Shortly, 3H-thymidine is incorporated into ongoing forks during 30 min, and the forks are allowed to progress from the labelled area for 4–8 h. By addition of alkaline solution, DNA unwinding is initiated from free DNA ends. When unwinding is initiated from the free DNA ends at a replication fork, the fraction of labelled ssDNA is a measurement of fork progression from the labelled area, decreasing as the fork progresses. Cells plated in 24-well plates (70 000 cells/well) were allowed to grow for 18 h before pulse labelling (0.5 h) with 37 kBq/ml 3H-thymidine (GE Healthcare) in DMEM, directly followed by UVC irradiation with indicated doses and incubated in pre-warmed DMEM with or without inhibitors for indicated times. Replication was terminated by the addition of 0.5 ml of ice-cold 0.15 M NaCl and 0.03 M NaOH unwinding solution, and 30 min incubation on ice in darkness. Unwinding was stopped by the addition of 1 ml of 0.02 M NaH2PO4. DNA was fragmented to ∼3 kb by sonication (B-12 sonifier with micro-tip; Branson) for 15 s, and sodium dodecyl sulphate (SDS) was added to a final concentration of 0.25%. After overnight storage at −20°C, single-and double-stranded DNA was separated on hydroxy apatite columns at 60°C, and radioactive decay was determined.
Pulsed-field gel electrophoresis
Directly after 0.5 h labelling with 14C-TdR (4.39 mM, 9.25 kBq/ml), cells were UVC irradiated (10 J/m2), and allowed to repair in pre-warmed DMEM with or without inhibitors for indicated times. The cells were then harvested and 106 cells melted into 10 mg/ml InCert agarose (Cambrex) plugs and incubated for 48 h in 0.5 M EDTA, 1% N-laurylsarcosyl, and 2 mg/ml proteinase K at 20°C in darkness. After four washings in Tris–ethylenediaminetetraacetic acid (EDTA) buffer, separation was performed on agarose gel (1% certified megabase agarose, Bio-Rad) on a CHEF DR III apparatus for 20 h (Bio-Rad, 120° angle, 4 V/cm, switch time 60/240 s, 14°C). The gel was stained with ethidium bromide, and DNA was transferred to a nylon membrane (Hybond-N, Amersham Pharmacia Biotech) according to the manufacturer’s protocol. The membrane was dried at 50°C over night, and exposed onto a phosphoimager plate (FujiFilm) for quantification employing ImageGauge software (FLA-3000, FujiFilm).
SDS–PAGE and western blotting
Harvested cells were lysed on ice in lysis buffer (100 mM Tris, 150 mM NaCl, 1% NP-40) containing 1× Complete protease inhibitor cocktail (Roche) and 1× phosphatase inhibitor mix (Thermo Scientific). Cell lysates equivalent to 50 µg protein, were separated by SDS-polyacrylamide 4–12% Bis–Tris gels (BioRad) and transferred to nitrocellulose membranes (GE Healthcare). Antibodies and concentrations used were phosphorylated ATM (mouse monoclonal, Santa Cruz, 10H11.E12, 1:500), ATM (mouse monoclonal, Abcam, [2C1(1A1)], 1:1500), γH2AX (mouse monoclonal, Abcam, [3F2], 1:2000), CHK1 and phosphorylated CHK1 (S345) (rabbit polyclonal, Cell Signaling Technology, 1:300).
RESULTS
CHK1 inhibitor and caffeine delays replication fork elongation in Polη-deficient cells
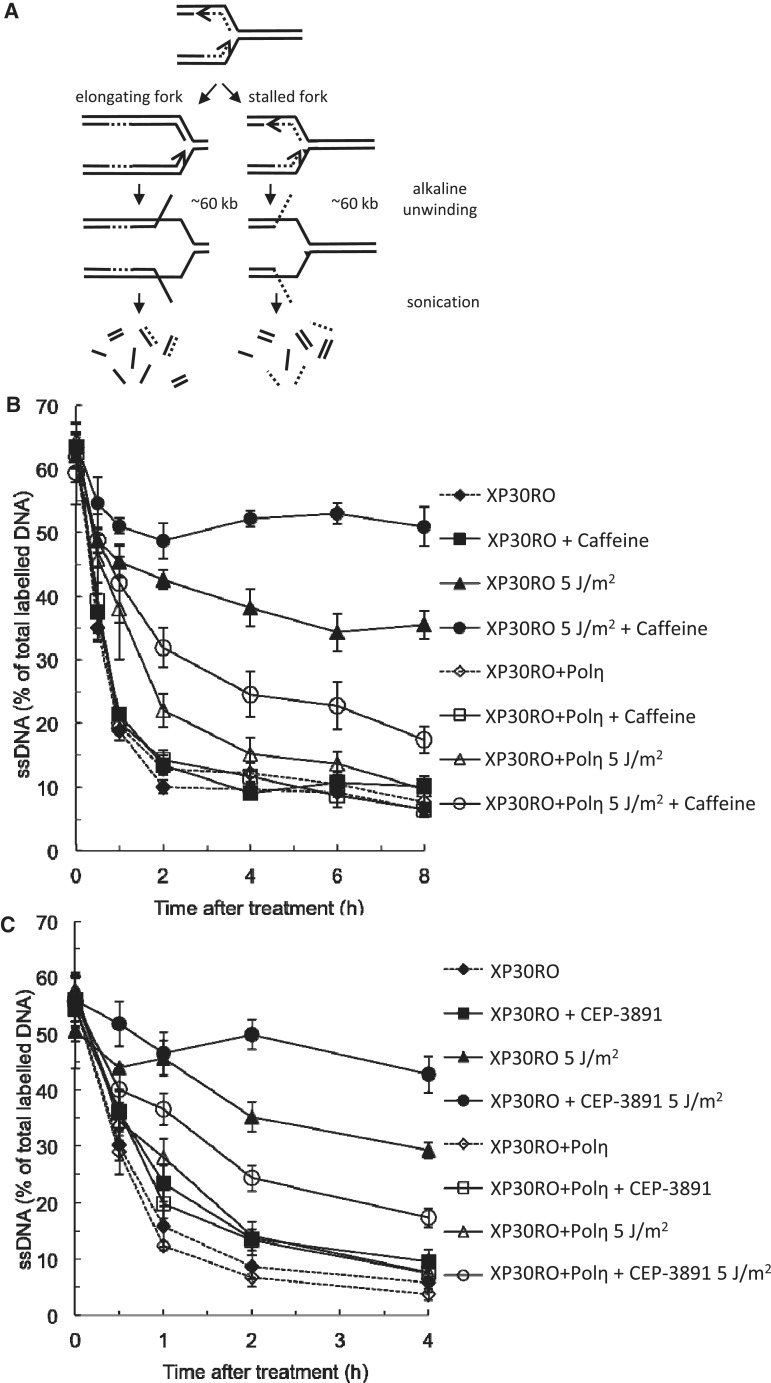
To monitor the effects on replication fork elongation after exposure to short-wave UV, we used the alkaline DNA unwinding method. For this, Polη-deficient XP30RO cells and cells with rescue expression of Polη (XP30RO+Polη) were pulse labelled directly before UV irradiation and allowed to progress from the labelled area in the presence or absence of CHK1 inhibitor or caffeine. At different time points, replication was stopped and unwinding of DNA from open ends was started (Figure 1A). There is no difference in the progression on undamaged DNA (Figure 1B and C), as reported previously (44). UV irradiation delays the continuous replication fork elongation slightly in restored cells, but induces a considerably stronger block in Polη-deficient XP30RO cells (Figure 1B and C). We find that caffeine treatment further delays the continuous replication fork elongation in UV-irradiated XP30RO cells (Figure 1B). We show that replication fork progression is also reduced in UV-irradiated XP30RO cells with Polη rescue expression after caffeine treatment. Interestingly, addition of CHK1 inhibitor CEP-3891 decreases the continuous replication fork elongation after exposure to UV (Figure 1C), to levels similar to the effect of caffeine in both cell lines. CHK1 inhibition also slightly decreases continuous replication fork elongation in unirradiated cells. This is in agreement with an overall reduction of replication elongation by inhibition or siRNA depletion of CHK1 (12,45) and may be explained by an increase in replication initiation following CHK1 inhibition (13). The effect of CHK1 inhibition on irradiated samples is considerably greater compared to unirradiated samples. As for caffeine, the replication elongation block induced by CHK1 inhibitor CEP-3891 is more severe in Polη-deficient cells compared to restored cells, in agreement with a previous study using a different CHK1 inhibitor, UCN-01 (31).
Figure 1.
Caffeine and CHK1 inhibition both retards replication fork progression in Polη-deficient cells. (A) Outline of the alkaline DNA unwinding assay to monitor replication fork elongation. Unwinding starts from DNA ends such as those present at a replication fork, allowing the monitoring of the fraction of labelled DNA that ends up in the single-stranded and double-stranded fractions, respectively. (B) Replication fork progression after UVC irradiation (5 J/m2) and the presence of 1 mM caffeine in Polη-deficient XP30RO and restored cells. (C) Replication fork progression after UVC irradiation (5 J/m2) and the presence of 0.5 µM CEP-3891 in Polη-deficient XP30RO and restored cells.
CHK1 inhibition and caffeine affects checkpoint signalling and reduces survival in UV-irradiated cells
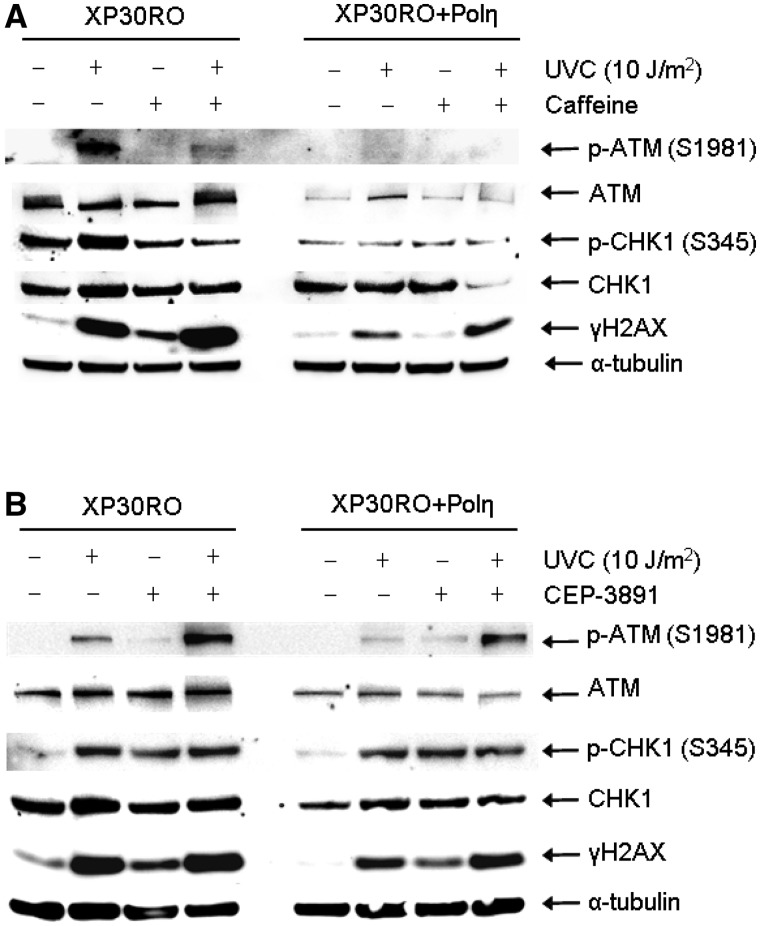
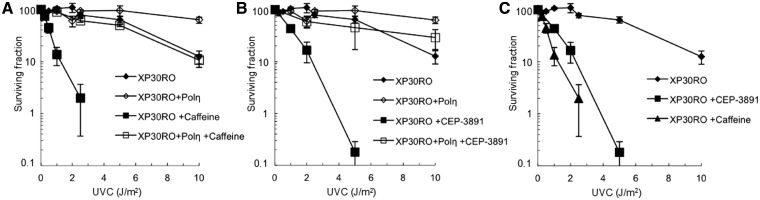
After UV damage, the ATR kinase is activated by ssDNA regions and in turn activates ATM (46) and CHK1 (14) via phosphorylation. Here, we investigated the checkpoint signalling pattern after treatment with caffeine or CEP-3891. We found that addition of caffeine reduced the ATR dependent UV-induced phosphorylation of ATM on S1981, as well as of CHK1 on S345 (Figure 2A). Also, γH2AX levels are increased by addition of caffeine, as expected (Figure 2A). It is well established that inhibition or depletion of CHK1 causes hyperactivation of the upstream ATR kinase (8). In agreement with this, we found that ATR-dependent phosphorylation of both ATM and CHK1 are increased following CHK1 inhibition (Figure 2B). Although CHK1 is hyperphosphorylated, it will remain inactive due to the presence of the inhibitor. Also, an increased level of γH2AX signal is present in CHK1 inhibited cells. Taken together, caffeine treatment and CHK1 inhibition after UV exposure have very similar effect on continuous replication fork progression and checkpoint signalling in these cell lines. These results indicate that caffeine may be inhibiting the ATR pathway and the CHK1 kinase at the conditions used in these experiments. To further investigate this, we compared survival rates of XP30RO and XP30RO+Polη cells after treatment with caffeine or CEP-3891. Caffeine has been shown to decrease survival in UV-irradiated XP30RO cells following UV irradiation (35). Here, we confirmed the sensitization of XP30RO cells by caffeine, and also demonstrate that the CHK1 inhibitor CEP-3891 causes a similar but weaker sensitization of XP30RO cells after UV exposure (Figure 3A and B). Interestingly, the effect on UV-irradiated XP30RO cells by caffeine at the doses used here is stronger than the impairing of survival after CHK1 inhibition (clarified in Figure 3C). Since CEP-3891 and caffeine similarly reduced replication elongation on a UV-damaged template, this may imply that caffeine affects another pathway required for survival in XP30RO cells following UV exposure.
Figure 2.
Influence of caffeine and CHK1 inhibition on checkpoint signalling. (A) Levels of phospho-ATM (S1981), ATM, phospho-CHK1 (S345), CHK1, γH2AX and α-tubulin in Polη-deficient XP30RO and restored cells after 6 h repair in the absence or presence of 1 mM caffeine, following UVC irradiation (10 J/m2) as determined by western blotting. (B) Levels of phospho-ATM (S1981), ATM, phospho-CHK1 (S345), CHK1, γH2AX and α-tubulin in Polη-deficient XP30RO and restored cells, UVC irradiated (10 J/m2) and allowed to repair for 6 h in the absence or presence of 0.5 µM CHK1 inhibitor CEP-3891, as visualized by western blotting.
Figure 3.
UVC-irradiated cells display impaired survival after caffeine or CHK1 inhibition. (A) Survival after UVC irradiation in Polη-deficient XP30RO and restored cells in the absence, and presence of 1 mM caffeine. Error bars indicate SEM of at least three independent experiments. (B) Survival after UVC irradiation in XP30RO and restored XP30RO+Polη cells in the absence and presence of 0.5 µM CHK1 inhibitor CEP-3891. Error bars indicate SEM of at least three independent experiments. (C) The effect of caffeine and CEP-3891 on survival after UVC irradiation of XP30RO cells only, juxtaposed in a separate graph.
Caffeine but not CHK1 inhibition induces replication-associated DSBs after UV irradiation
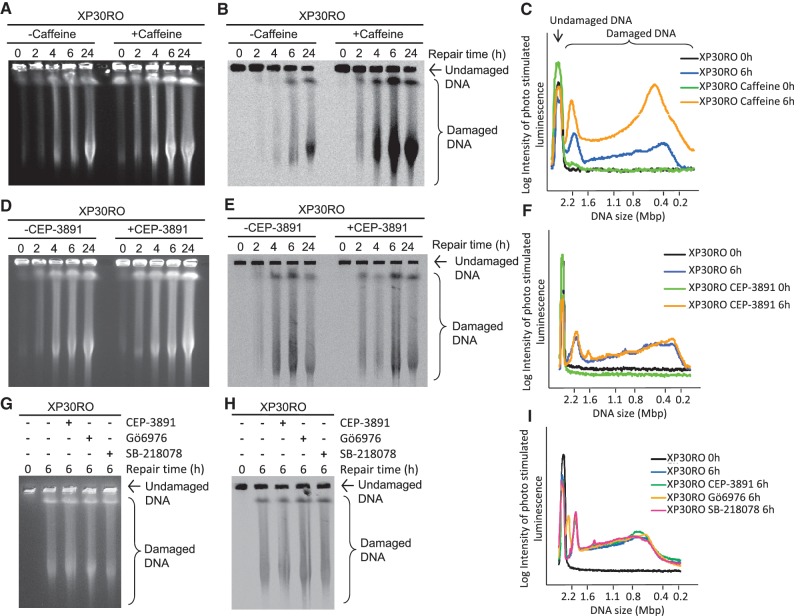
Treatment with either caffeine or CEP-3891 induces a γH2AX response (Figure 2A and B), a well-known marker of DNA DSBs but which has also been shown to occur after treatments causing replication elongation problems also in absence of DSBs (47,48). We decided to specifically test whether caffeine treatment and CHK1 inhibition even increases the collapse of replication forks into DSBs after UV exposure. We pre-labelled cells for 0.5 h with 14C-thymidine to specifically study UV-induced DSBs that are associated with replication. We performed this study in XP30RO cells as they display an enhanced UV sensitivity in the presence of caffeine or CHK1 inhibitor. Directly after labelling, cells were exposed to UV irradiation and allowed to repair in the presence or absence of caffeine. Cells were collected at different time points (0, 2, 4, 6, 24 h) to allow DNA separation by pulsed-field gel electrophoresis. This allows visualisation of all induced DSBs after staining with ethidium bromide (Figure 4A) and detection of replication-associated DSB visualized by autoradiography (Figure 4B). We found that caffeine treatment increased the amount of UV-induced replication-associated DSBs (Figure 4C). This is in agreement with the increase of γH2AX signal after caffeine treatment. In sharp contrast, when we repeated the experiment using CHK1 inhibitor CEP-3891, we find very little increase in UV-induced replication-associated DSBs after inhibition of CHK1 (Figure 4D–F). In line with this, we also see that caffeine, but not CEP-3891, treatment causes an increase in both foci intensity and foci area (P < 0.05) in cells replicating when irradiated, although the number of foci per cell does not significantly differ between the treatment groups (Supplementary Figure S1). To ensure that the absence of active CHK1 does not affect the formation of replication-associated DNA DSBs after UV irradiation, we repeated the experiment with two additional CHK1 inhibitors (Gö6976 and SB-218078). Consistent with the effect of CEP-3891, replication-associated DNA DSBs are induced after UV exposure, but the presence of either of the two additional CHK1 inhibitors does not affect this (Figure 4G–I). These two additional CHK1 inhibitors have the same effect on continuous replication fork elongation, measured in the same way as in Figure 1 (Supplementary Figure S2A and B). It is somewhat unexpected that CHK1 activity does not affect the stability of replication structures formed by forks stalled by UV irradiation, as it has previously been reported that CHK1 is important for stabilization of hydroxyurea stalled replication forks, however at late time points (8). Consistent with this, the presence of a CHK1 inhibitor does induce a small increase in DSB levels 24 h after UVC irradiation (Figure 4D and E). To confirm the activity of our CHK1 inhibitor, we measured the induction of DNA DBSs by this inhibitor alone, seeing an induction of DSBs by the inhibitor alone confirming previous results (8) that are prevented if replication is inhibited by hydroxyurea (Supplementary Figure S3).
Figure 4.
Caffeine treatment but not CHK1 inhibition induces replication-associated DSBs after UV irradiation. UVC-induced (10 J/m2) DSBs in Polη-deficient XP30RO cells visualized by pulsed-field gel electrophoresis. All cells were pulse labelled (0.5 h) with 14C-TdR prior to UVC irradiation (10 J/m2) and allowed to repair in the presence of 1 mM caffeine, 0.5 µM CEP-3891 or mock treatment for indicated times. (A) Ethidium bromide staining of a representative gel showing caffeine-treated samples before (B) autoradiography analysis to determine replication-associated DSBs. (C) Quantification of the intensity of radioactively labelled DNA fragments released in pulse-labelled XP30RO cells repairing in the absence or presence of 1 mM caffeine, 0 h (black and green lines, respectively) and 6 h (blue and yellow lines, respectively) after UVC irradiation. (D) Ethidium bromide staining before (E) autoradiography analysis of a representative gel showing replication-associated DSBs induced by CEP-3891 treatment after UV irradiation. (F) Quantification of the intensity of radioactively labelled DNA fragments released in pulse-labelled XP30RO cells repairing in the absence or presence of 0.5 µM CEP-3891, 0 h (black and green lines, respectively) and 6 h (blue and yellow lines, respectively) after UVC irradiation. (G) Ethidium bromide staining before (H) autoradiography analysis of a representative gel showing replication-associated DSBs formed after UV irradiation, in the presence of CHK1 inhibitors CEP-3891, Gö6976 or SB-218078. (F) Quantification of the intensity of radioactively labelled DNA fragments released in pulse-labelled XP30RO cells at 0 h (black line) or 6 h after UV exposure. Cells were allowed to repair in the absence (blue line) or presence of 0.5 µM CEP-3891 (green line), 1 µM Gö6976 (yellow line) or 2 µM SB-218078 (pink line). Representative gels of at least three independent experiments are shown.
DISCUSSION
The CHK1 kinase is a key mediator in maintaining replication fork stability (5,8), due to its role in controlling replication origin firing (13). Interestingly, we show that CHK1 inhibition does not affect the collapse of replication structures after UV irradiation, in sharp contrast to its role after hydroxyurea treatment. This could partially be explained by hydroxyurea studies (5,8) generally employing longer treatments than we did here. However, the difference may be largely explained by the difference in structures formed depending on the type of fork-interfering agent. While hydroxyurea slows down replication and causes replication forks to halt due to nucleotide depletion, physical damage such as UV-induced pyrimidine dimers or methylated bases causes an initial total block to replication fork elongation. However, despite such DNA damage, replication is believed to continue leaving unreplicated gaps behind the replication fork (27,44). It was recently shown that budding yeast replisome complexes remain intact at early time points after UV exposure despite formation of ssDNA gaps (49). Interestingly, replication fork integrity was independent of the main yeast S phase checkpoint kinase Rad53 (49). The direct stabilization of replication structures on UV-damaged DNA appears to be controlled by a complex network of proteins, however the induction of replication-associated DSBs after UV exposure occurs independently of CHK1, but is enhanced by caffeine treatment. From our experiments, it is obvious that DNA structures formed after replication of UV-damaged DNA are stabilized by caffeine-sensitive kinase(s) but the stabilization is unrelated to CHK1. Interestingly, a recent report shows that the physical presence of CHK1 protein may affect replication fork progression after UV exposure as ATR knockdown and CHK1 inhibition equally retards fork progression, but CHK1 knockdown induces a further slowing of replication fork progression (50).
CHK1 has been shown to promote ubiquitination of PCNA in a kinase-independent manner (51). Although this modification enhances TLS activity (52–54), we see a strikingly more pronounced block on replication elongation on UV-damaged DNA from removal of Polη expression compared to CHK1 inhibition. This suggests that there are other factors than CHK1 which are largely contributing to TLS activity after UV exposure. It is possible that the effect of CHK1 on replication fork elongation after UV in Polη-proficient cells is solely explained by decreased PCNA ubiquitination. This suggests that the effect of CHK1 inhibition on continuous replication fork elongation after UV exposure in Polη-deficient cells is explained by decreased activity of other TLS polymerases.
In conclusion, we show that CHK1 is important for replication elongation on UV-damaged DNA, similar to the role of CHK1 after replication fork slowing by hydroxyurea treatment. However, we show that there is at least one pathway needed to protect and stabilize replication structures after UV irradiation, which is independent of CHK1 but inhibited by caffeine.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Figures 1–3.
FUNDING
Swedish Cancer Society; Swedish Children's Cancer Foundation; Swedish Research Council; Swedish Pain Relief Foundation; Torsten and Ragnar Söderberg Foundation. Funding for open access charge: Swedish Research Council.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Alan Lehmann, University of Sussex, for helpful discussions and the XP30RO and XP30RO+Polη cell lines, and Dr Stephen Trusko for materials.
REFERENCES
- 1.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPAssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 2.Zachos G, Rainey MD, Gillespie DA. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 2003;22:713–723. doi: 10.1093/emboj/cdg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, Zhou BB, Bartek J, Lukas J. Chk1 regulates the S-phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 4.Sleeth KM, Sorensen CS, Issaeva N, Dziegielewski J, Bartek J, Helleday T. RPA mediates recombination repair during replication stress and is displaced from DNA by checkpoint signalling in human cells. J. Mol. Biol. 2007;373:38–47. doi: 10.1016/j.jmb.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat. Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 6.Casper AM, Nghiem P, Arlt MF, Glover TW. ATR regulates fragile site stability. Cell. 2002;111:779–789. doi: 10.1016/s0092-8674(02)01113-3. [DOI] [PubMed] [Google Scholar]
- 7.Durkin SG, Arlt MF, Howlett NG, Glover TW. Depletion of CHK1, but not CHK2, induces chromosomal instability and breaks at common fragile sites. Oncogene. 2006;25:4381–4388. doi: 10.1038/sj.onc.1209466. [DOI] [PubMed] [Google Scholar]
- 8.Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol. Cell. Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M. The DNA replication checkpoint response stabilizes stalled replication forks. Nature. 2001;412:557–561. doi: 10.1038/35087613. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay HD, Griffiths DJ, Edwards RJ, Christensen PU, Murray JM, Osman F, Walworth N, Carr AM. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segurado M, Diffley JF. Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks. Genes Dev. 2008;22:1816–1827. doi: 10.1101/gad.477208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petermann E, Maya-Mendoza A, Zachos G, Gillespie DA, Jackson DA, Caldecott KW. Chk1 requirement for high global rates of replication fork progression during normal vertebrate S-phase. Mol. Cell. Biol. 2006;26:3319–3326. doi: 10.1128/MCB.26.8.3319-3326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petermann E, Woodcock M, Helleday T. Chk1 promotes replication fork progression by controlling replication initiation. Proc. Natl Acad. Sci. USA. 2010;107:16090–16095. doi: 10.1073/pnas.1005031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Z, Kumagai A, Wang SX, Dunphy WG. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 2000;14:2745–2756. doi: 10.1101/gad.842500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward IM, Minn K, Chen J. UV-induced ataxia-telangiectasia-mutated and Rad3-related (ATR) activation requires replication stress. J. Biol. Chem. 2004;279:9677–9680. doi: 10.1074/jbc.C300554200. [DOI] [PubMed] [Google Scholar]
- 16.Heffernan TP, Simpson DA, Frank AR, Heinloth AN, Paules RS, Cordeiro-Stone M, Kaufmann WK. An ATR-and Chk1-dependent S checkpoint inhibits replicon initiation following UVC-induced DNA damage. Mol. Cell. Biol. 2002;22:8552–8561. doi: 10.1128/MCB.22.24.8552-8561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chini CC, Chen J. Human claspin is required for replication checkpoint control. J. Biol. Chem. 2003;278:30057–30062. doi: 10.1074/jbc.M301136200. [DOI] [PubMed] [Google Scholar]
- 18.Unsal-Kacmaz K, Mullen TE, Kaufmann WK, Sancar A. Coupling of human circadian and cell cycles by the timeless protein. Mol. Cell Biol. 2005;25:3109–3116. doi: 10.1128/MCB.25.8.3109-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unsal-Kacmaz K, Chastain PD, Qu PP, Minoo P, Cordeiro-Stone M, Sancar A, Kaufmann WK. The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol. Cell Biol. 2007;27:3131–3142. doi: 10.1128/MCB.02190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 21.Henry-Mowatt J, Jackson D, Masson JY, Johnson PA, Clements PM, Benson FE, Thompson LH, Takeda S, West SC, Caldecott KW. XRCC3 and Rad51 modulate replication fork progression on damaged vertebrate chromosomes. Mol. Cell. 2003;11:1109–1117. doi: 10.1016/s1097-2765(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 22.Johansson F, Lagerqvist A, Erixon K, Jenssen D. A method to monitor replication fork progression in mammalian cells: nucleotide excision repair enhances and homologous recombination delays elongation along damaged DNA. Nucleic Acids Res. 2004;32:e157. doi: 10.1093/nar/gnh154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daigaku Y, Davies AA, Ulrich HD. Ubiquitin-dependent DNA damage bypass is separable from genome replication. Nature. 2010;465:951–955. doi: 10.1038/nature09097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karras GI, Jentsch S. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S-phase. Cell. 2010;141:255–267. doi: 10.1016/j.cell.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Wigan M, Pinder A, Giles N, Pavey S, Burgess A, Wong S, Sturm RA, Gabrielli B. A UVR-induced G2-phase checkpoint response to ssDNA gaps produced by replication fork bypass of unrepaired lesions is defective in melanoma. J. Invest. Dermatol. 2012;132:1681–1688. doi: 10.1038/jid.2012.41. [DOI] [PubMed] [Google Scholar]
- 26.Elvers I, Johansson F, Groth P, Erixon K, Helleday T. UV stalled replication forks restart by re-priming in human fibroblasts. Nucleic Acids Res. 2011;39:7049–7057. doi: 10.1093/nar/gkr420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes M, Foiani M, Sogo JM. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell. 2006;21:15–27. doi: 10.1016/j.molcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji Y, Watanabe K, Araki K, Shinohara M, Yamagata Y, Tsurimoto T, Hanaoka F, Yamamura K, Yamaizumi M, Tateishi S. Recognition of forked and single-stranded DNA structures by human RAD18 complexed with RAD6B protein triggers its recruitment to stalled replication forks. Genes Cells. 2008;13:343–354. doi: 10.1111/j.1365-2443.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 30.Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat. Rev. Mol. Cell. Biol. 2012;13:141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Despras E, Daboussi F, Hyrien O, Marheineke K, Kannouche PL. ATR/Chk1 pathway is essential for resumption of DNA synthesis and cell survival in UV-irradiated XP variant cells. Hum. Mol. Genet. 2010;19:1690–1701. doi: 10.1093/hmg/ddq046. [DOI] [PubMed] [Google Scholar]
- 32.Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 33.Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc. Natl Acad. Sci. USA. 2001;98:9092–9097. doi: 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costanzo V, Shechter D, Lupardus PJ, Cimprich KA, Gottesman M, Gautier J. An ATR-and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol. Cell. 2003;11:203–213. doi: 10.1016/s1097-2765(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 35.Arlett CF, Harcourt SA, Broughton BC. The influence of caffeine on cell survival in excision-proficient and excision-deficient xeroderma pigmentosum and normal human cell strains following ultraviolet-light irradiation. Mutat. Res. 1975;33:341–346. doi: 10.1016/0027-5107(75)90209-2. [DOI] [PubMed] [Google Scholar]
- 36.Johnson RE, Kondratick CM, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 37.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 39.Masutani C, Kusumoto R, Iwai S, Hanaoka F. Mechanisms of accurate translesion synthesis by human DNA polymerase eta. EMBO J. 2000;19:3100–3109. doi: 10.1093/emboj/19.12.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCulloch SD, Kokoska RJ, Masutani C, Iwai S, Hanaoka F, Kunkel TA. Preferential cis-syn thymine dimer bypass by DNA polymerase eta occurs with biased fidelity. Nature. 2004;428:97–100. doi: 10.1038/nature02352. [DOI] [PubMed] [Google Scholar]
- 41.Johnson RE, Prakash S, Prakash L. Efficient bypass of a thymine–thymine dimer by yeast DNA polymerase, Poleta. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 42.Stary A, Kannouche P, Lehmann AR, Sarasin A. Role of DNA polymerase eta in the UV mutation spectrum in human cells. J. Biol. Chem. 2003;278:18767–18775. doi: 10.1074/jbc.M211838200. [DOI] [PubMed] [Google Scholar]
- 43.Rasband WS. Maryland, USA: Bethesda; 1997–2011. U.S. National Institutes of Health. http://imagej.nih.gov/ij/ [Google Scholar]
- 44.Lehmann AR, Kirk-Bell S, Arlett CF, Paterson MC, Lohman PH, de Weerd-Kastelein EA, Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc. Natl Acad. Sci. USA. 1975;72:219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petermann E, Helleday T, Caldecott KW. Claspin promotes normal replication fork rates in human cells. Mol. Biol. Cell. 2008;19:2373–2378. doi: 10.1091/mbc.E07-10-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stiff T, Walker SA, Cerosaletti K, Goodarzi AA, Petermann E, Concannon P, O'Driscoll M, Jeggo PA. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 2006;25:5775–5782. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundin C, North M, Erixon K, Walters K, Jenssen D, Goldman AS, Helleday T. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 2005;33:3799–3811. doi: 10.1093/nar/gki681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyurea–stalled replication forks become progressively inactivated and require two different RAD51–mediated pathways for restart and repair. Mol. Cell. 2010;37:492–502. doi: 10.1016/j.molcel.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Piccoli G, Katou Y, Itoh T, Nakato R, Shirahige K, Labib K. Replisome stability at defective DNA replication forks is independent of S phase checkpoint kinases. Mol. Cell. 2012;45:696–704. doi: 10.1016/j.molcel.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Speroni J, Federico MB, Mansilla SF, Soria G, Gottifredi V. Kinase-independent function of checkpoint kinase 1 (Chk1) in the replication of damaged DNA. Proc. Natl Acad. Sci. USA. 2012;109:7344–7349. doi: 10.1073/pnas.1116345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang XH, Shiotani B, Classon M, Zou L. Chk1 and Claspin potentiate PCNA ubiquitination. Genes Dev. 2008;22:1147–1152. doi: 10.1101/gad.1632808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell. 2004;14:491–500. doi: 10.1016/s1097-2765(04)00259-x. [DOI] [PubMed] [Google Scholar]
- 53.Guo C, Sonoda E, Tang TS, Parker JL, Bielen AB, Takeda S, Ulrich HD, Friedberg EC. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell. 2006;23:265–271. doi: 10.1016/j.molcel.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 54.Plosky BS, Vidal AE, Fernandez de Henestrosa AR, McLenigan MP, McDonald JP, Mead S, Woodgate R. Controlling the subcellular localization of DNA polymerases iota and eta via interactions with ubiquitin. EMBO J. 2006;25:2847–2855. doi: 10.1038/sj.emboj.7601178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.