Abstract
Aim
The Kir 6.1 Katp channel is believed to play an important role in ventricular repolarization as determined from both functional and genetic studies of the potassium inwardly-rectifying channel, subfamily J, member 8 (KCNJ8)-S422L missense mutation in patients with J-wave syndromes. Although Kir6.1 is also present in atrial tissue, it is unknown whether this channel modulates atrial repolarization and hence whether the S422L mutation portends a greater risk of atrial arrhythmias. This study sought to examine whether there was an increased frequency of the KCNJ8-S422L mutation among patients with atrial fibrillation (AF) and early repolarization (ER) as a possible novel susceptibility gene for AF.
Methods and results
A total of 325 lone AF probands were identified from the Vanderbilt AF Registry, a collection of clinical data and DNA from consented, consecutively enrolled participants. The coding regions of KCNJ8 were sequenced, and the patient's presenting electrocardiogram (ECG) was reviewed by two independent physicians for ER abnormalities. The KCNJ8-S422L mutation was identified in two AF probands while no other candidate gene variants were identified in these cases. Twenty-two (7%) patients were found to have ER on the ECG, including the two probands carrying the S422L variant. In one small AF kindred, the S422L variant co-segregated with AF and ER.
Conclusions
The KCNJ8-S422L variant is associated with both increased AF susceptibility and ER indicating a role for Kir 6.1 Katp channel in both ventricular and atrial repolarization.
Keywords: Atrial fibrillation, Early repolarization, KCNJ8, Kir6.1, Mutation
Introduction
The electrocardiographic (electrocardiogram, ECG) finding of early repolarization (ER) is common in the general population with a prevalence of 1–2%, and was previously thought to be benign.1 However, ER has recently been associated with an increased risk of ventricular fibrillation and sudden cardiac death,2 and it has been suggested that it represents one facet of a larger group of ‘J-wave syndromes’, a spectrum of ER-associated condition, including Brugada syndrome.3
From previous reports, S422L a missense mutation in the gene encoding the cardiac Katp channel Kir6.1 potassium inwardly-rectifying channel, subfamily J, member 8 (KCNJ8), has been identified as a candidate variant for J-wave syndromes.4,5 In the first case report, a 14-year-old female presented with multiple occurrences of VF and the isolated clinical finding of ER on her ECG.4 In a subsequent case report, two individuals from a J-wave cohort were identified to carry the S422L variants after screening, one with ER on the ECG and the second with spontaneous Brugada Type 1 ECG pattern.5 Screening among a second cohort of Brugada and ER probands and their family members identified five additional individuals carrying the S442L variant, further supporting this variant as a disease-causing variant.6
The Kir6.1 channel facilitates a non-voltage-gated inwardly rectifying potassium current, leading to a shortening of the action potential (AP) duration under conditions of metabolic stress.7 The S422L variant leads to a gain-of-function of the Kir61.1 channel, increasing IKATP in heterologous expression with Simian fibroblast cells (COS-1) and human embryonal kidney cells (TSA201).5,6 Shortening of the AP duration is an important component of atrial fibrillation (AF) initiation and mutations in ion channels leading to a shortened AP have been previously described in lone AF.8–10 Kir6.1 is present in both atrial and ventricular myocytes, and although a ventricular phenotype has been described with J-wave syndromes, the effect of this variant in atrial myocytes is unknown. Of note, Barajas-Martinez et al. reported symptomatic AF among three of the five carriers of S422L, hinting at an atrial phenotype. In this study we sought to determine whether there was an increased frequency of the KCNJ8-S422L mutation among patients with AF and ER and determine if KCNJ8 may be a novel susceptibility gene for AF.
Methods
Study population
Over an 8 year period, 2095 patients were enrolled in the Vanderbilt AF Registry, and were recruited from Vanderbilt Arrhythmia Clinics, the emergency room and inpatient services.11 Individuals ≥18 years of age and with documented AF on ECG, rhythm strips or Holter monitor and who were diagnosed during routine physical examination, were included in the study. At enrollment a detailed medical history, drug and family history was obtained on all patients. Whenever appropriate, first- and second-degree relatives were contacted directly to obtain medical records and documentation of AF. The study protocol and written informed consents were approved by the Vanderbilt University Institutional Review Board.
Control population
Controls were selected from the Vanderbilt Cardiac Surgery Registry, a registry of consecutive patients undergoing cardiac surgery.12 Controls were selected among those who did not develop AF in the post-operative period from cardiac surgery. The study protocol and written informed consents were approved by the Vanderbilt University Institutional Review Board.
Definition
In order to better characterize the course of AF, recruited subjects were clinically classified using a consistently applied set of definitions. AF was defined as presence of irregular fibrillatory waves and the presence of an irregular ventricular response in patients with intact atrioventricular conduction. Lone AF was defined as AF occurring in individuals' ≤65 years of age in the absence of cardiovascular disease, diabetes mellitus, hypertension, morbid obesity, obstructive sleep apnea, or thyroid dysfunction, which was determined by clinical examination, ECG, echocardiography, body-mass index measurements, and thyroid function tests.
Paroxysmal AF was defined as recurrent AF (≥2 episodes) that terminates spontaneously within 7 days. Persistent AF was defined as AF that was present for ≥7 days or required pharmacologic treatments or electric cardioversion. Atrial fibrillation that failed cardioversion or was allowed to continue was defined as permanent.
Familial AF was defined as the presence of one or more first- or second-degree family members, age >18 years, and affected with AF as documented by ECG, rhythm strips or Holter monitor. Permission to talk to the family members was allowed by the proband and a more detailed pedigree was generated by history and review of the relatives' medical records.
Genetic screening
Whole blood was collected for genomic DNA extraction and analysis from all subjects. We resequenced the 5′UTR, 3′UTR, coding regions, and splice sites of high priority candidate ion channel genes [KCNJ8, KCNQ1 (potassium voltage-gated channel, KQT-like subfamily, member 1), KCNE1–5 (potassium voltage-gated channel, Isk-related family, member 1-4 and member 1-like), KCNJ2 (potassium inwardly-rectifying channel, subfamily J, member 2), KCNA5 (potassium voltage-gated channel, shaker-related subfamily, member 5), SCN5A (sodium channel, voltage-gated, type V, alpha subunit), SCN1B (sodium channel, voltage-gated, type I, beta subunit), SCN2B (sodium channel, voltage-gated, type II, beta subunit), CACNA1C (calcium channel, voltage-dependent, L type, alpha 1C subunit), and CACNA2D2 (calcium channel, voltage-dependent, alpha 2/delta subunit 2) as well as non-ion channel candidates including gap junction protein, alpha 5 and natriuretic peptide precursor A (that encodes atrial natriuretic peptide, ANP). Non-synonymous variants in sodium and potassium channels have been identified in ∼10% of the subjects.13 These co-segregate in extended kindreds (where they are available) and those studied to date show abnormal electrophysiology in vitro.14,15
Among 325 lone AF probands, 311 underwent genotying. Fourteen probands were excluded due to inadequate DNA sample volume. The coding and splice junctions of KCNJ8 were amplified by polymerase chain reaction (PCR) using primers designed to obtain fragments of appropriate size. The PCR-amplified DNA fragments were analysed using the Reveal® Discovery System (based on temperature gradient capillary electrophoresis) to identify aberrant conformers, which were then directly sequenced by Sanger sequencing (ABI 3730xl DNA Analyzer, Vanderbilt DNA Sequencing Facility). Additionally, 391 controls were sequenced for the KCNJ8-S422L variant with Applied Biosystem's TaqMan. Thirty-eight case samples and 23 control samples failed genotyping due to poor DNA quality.
Results
Cohort demographics
The average age of diagnosis of AF was 41.5 years and the cohort was predominantly male (73%) and of European descent (95%). A total of 133 (40%) patients were found to have a family history of AF and 22 (7%) were found to have ER on their ECG.
Prevalence of KCNJ8 variants in lone atrial fibrillation cohort
The non-synonymous variant S422L was identified in two probands resulting in a minor allele frequency of 0.37% (Figure 1). The variant was absent in all 368 controls. Furthermore, both of the S422L carriers were found to have ER on the ECG. No additional variants in KCNJ8 were identified among the probands.
Figure 1.
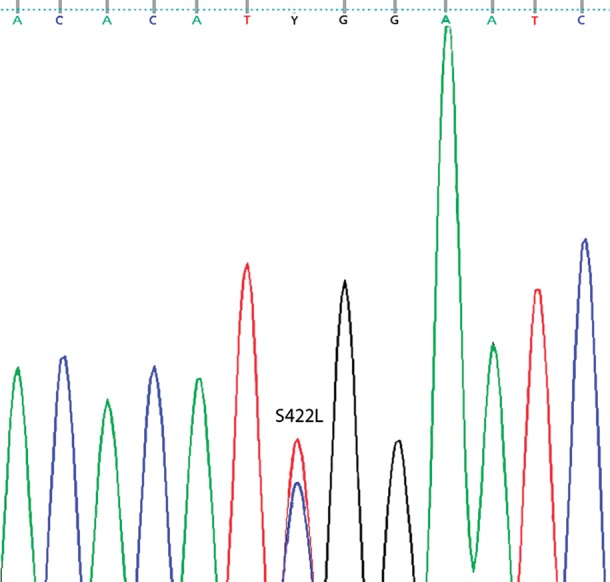
Proband DNA sequence chromatogram from Exon 3b of KCNJ8 with the heterozygous substitution of wild-type allele C->T at position 1265 resulting in missense mutation S422L.
Family phenotype evaluation among S422L carriers
Proband AF558 presented with symptomatic, early onset AF at the age of 17 underwent direct current cardioversion to restore sinus rhythm. The proband underwent a standard electrophysiology study to evaluate for supraventricular arrhythmias as a possible trigger for AF. In both the resting state and with isoproterenol, the conduction intervals and refractory periods were within normal range. The atrial effective refractory period (ERP) was 190 ms when pacing at 800 ms (normal: 150–360 ms) and the ventricular ERP was 240 ms when pacing at 550 ms on isoproterenol (normal: 170–290 ms). Atrial fibrillation was induced during the study with atrial extrastimuli, but there was no evidence of a substrate for additional supraventricular arrhythmias. A standard ventricular pacing protocol with incremental and extrastimulus testing was performed and no ventricular arrhythmias were induced.
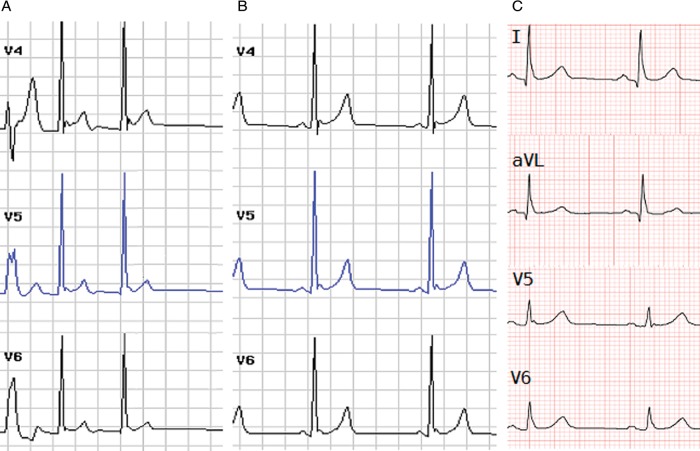
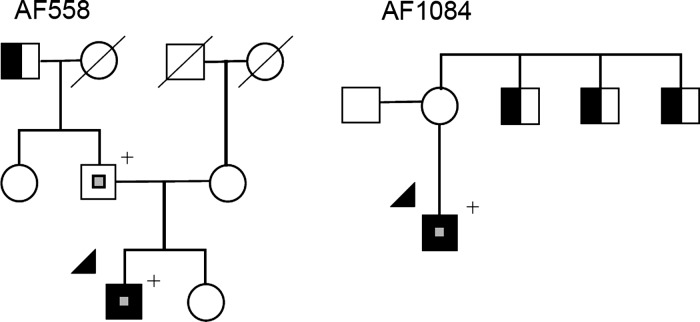
Electrocardiograms performed both while in AF and sinus rhythm showed ER in the right precordial and lateral leads with maximal elevation in lead V4 measuring 0.2 mV (Figure 2). Over 5 years of follow-up the patient had no further episodes of AF. A family history for the AF558 kindred is significant for a paternal grandfather having ‘a chronic arrhythmia’; however no ECG was available for evaluation (Figure 3). The patient's father gave no history of symptomatic arrhythmias; however, his ECG showed ER in the lateral leads of 0.1 mV and he was found to be heterozygous for the KCNJ8-S422L missense mutation. The proband's mother gave a history of palpitations but her work up, including a holter monitor, was inconclusive and she denies any further episodes. She was screened for the KCNJ8-S422L mutation and was found to be wild-type.
Figure 2.
(A) AF588 proband's electrocardiogram with both atrial fibrillation and early repolarization in the lateral leads. The arrows in leads V4–V6 indicate the leads with the highest ST-segment (segment of ECG connecting QRS complex and the T wave) elevation. (B) The proband's electrocardiogram during sinus rhythm shows early repolarization in the lateral leads. (C) The probands (AF588) father's electrocardiogram with early repolarization in the lateral leads, indicated by the arrows in lead I, AVL (augmented vector left), V5, and V6.
Figure 3.
Pedigrees of families with atrial fibrillation and KCNJ8 mutations and early repolarization. Squares and circles indicate male and female members, respectively. Symbols with a slash indicate deceased family members. Arrows indicate probands. Open symbols indicate unaffected family members. Solid black symbols indicate the presence of atrial fibrillation. Half-shaded symbols indicate individuals with atrial fibrillation by history only. Small central grey squares indicate early repolarization on electrocardiogram. + symbol indicates the presence of the KCNJ8-S422L variant.
Proband AF1084 presented with recurrent symptomatic AF, experiencing yearly episodes since his mid-20s all requiring direct current cardioversion. A family history for AF1084 is significant for the proband's mother having a history of supraventricular arrhythmias and three maternal uncles who have a history of AF and two of them underwent AF ablations; however, no ECGs were available to document these episodes (Figure 2). The DNA samples from family members were not available for analyses.
Discussion
The KCNJ8-S422L variant was identified in two lone AF probands, one showed evidence of ER on ECG. For these affected probands, we believe the S422L variant to be the atrial phenotype of a channelopathy previously associated with ER syndrome. As KCNJ8 is expressed in both atria and ventricles the findings of an AF phenotype in individuals carrying the mutation is perhaps not completely surprising. A large proportion of the mutations that have been identified in cases of familial AF have been identified in ion channel genes.16,17 Additionally, for many channelopathies, including Brugada syndrome and the Long QT syndrome, the prevalence of AF is greater than in the general population and can imply a worse prognosis.18
Originally identified from cDNA isolated from rat pancreatic cells, KCNJ8 encodes for the Kir6.1 subunit of the KATP inward rectifying potassium.19 The in vitro studies with Xenopus oocytes or COS-1 cells indicate sulphonylurea receptor (SUR) co-expression essential for ATP sensitivity.20 The Katp channel is responsive to the metabolic state of the cell by tying potassium influx to cellular ATP stores.21 Metabolic stress, measured by a change in the ATP/ADP ratio is believed to activate the channels, thereby shortening the AP duration. A previous study in which KCNJ8-S422L with SUR2A was co-expressed in COS-1 cells found a 60% increase in the potassium current when compared with wild-type; this is consistent with a gain-of-function mechanism.5 Additional studies in TSA201 cells found an almost two-fold increase in potassium current and the mechanism for this is thought to be due to incomplete closing of the channel as a result of ATP insensitivity.6 Further support for the KACH channel in AF generation comes from the identification of the SUR2A T1547I missense mutation in a patient with adrenergic AF.22 Co-expression of SUR2A with Kir6.2 in Xenopus oocytes was found to result in reduced ADP-dependent channel opening and hence reduced potassium current. Although the effect on repolarization with this mutation would most likely be opposite to that for the KCNJ8-S422L mutation, it highlights the association of mutations within the Kir6.x–SUR complex and AF, albeit by differing electrophysiological effects.
Shortening of the atrial AP duration is a common mechanism leading to propagation of AF.23 Additionally, in cases of familial AF with known mutations in ion channel genes, KCNQ1, KCNE2, and KCNJ2, shortening of the AP duration has been identified as the pathogenic mechanism. The Kir6.1 subunit is present in both atrial and ventricular tissues, a mutation that results in a change of function of this channel would be expected to lead to arrhythmia susceptibility in not just ventricular tissue, but also the atrial tissue. Although not previously associated with AF, it is possible that in both patients, the KCNJ8-S422L missense mutation contributed not only to ECG findings of ER, but also to his early presentation of AF.
Additionally, our findings contribute to the association of S422L and ER syndrome as diagnosed from the pathogenomic finding on ECG. Prior reports did not include linkage information; however within our cohort, we were able to acquire data from a first degree relative with ER on the baseline ECG who was also found to be mutation positive. Taken together with the in vitro studies, this further supports KCNJ8 as an important determinant of repolarization in ventricular tissue.5
Conclusion
The KCNJ8-S422L mutation has been shown to shorten repolarization in ventricular tissue, yet with the identification of this mutation among patients with lone AF it is possible that expression of this mutation in the atrium could also shorten repolarization to increase AF susceptibility. Further evidence for KCNJ8 as a candidate gene for ER syndrome was provided by the finding of ER on the ECG of a mutation-positive proband and a mutation-positive first-degree relative. More study is needed to elucidate the relationship between the KCNJ8 variant S422L and its effect in the atrial myocyte.
Funding
This work was supported by National Institutes of Health HL65962, HL092217 and an American Heart Association Established Investigator Award.
Conflict of interest: none declared.
References
- 1.Mehta MC, Jain AC. Early repolarization on scalar electrocardiogram. Am J Med Sci. 1995;309:305–11. doi: 10.1097/00000441-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–37. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C, Yan G-X. J wave syndromes. Heart Rhythm. 2010;7:549–58. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haïssaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, et al. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–8. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros-Domingo A, Tan B-H, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–71. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barajas-Martínez H, Hu D, Ferrer T, Onetti CG, Wu Y, Burashnikov E, et al. Molecular genetic and functional association of Brugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2011;9:14–22. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–53. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y-H, Xu S-J, Bendahhou S, Wang X-L, Wang Y, Xu W-Y, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia M, Jin Q, Bendahhou S, He Y, Larroque M-M, Chen Y, et al. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–19. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Darbar D, Motsinger AA, Ritchie MD, Gainer JV, Roden DM. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart Rhythm. 2007;4:743–9. doi: 10.1016/j.hrthm.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Body SC, Collard CD, Shernan SK, Fox AA, Liu K-Y, Ritchie MD, et al. Variation in the 4q25 chromosomal locus predicts atrial fibrillation after coronary artery bypass graft surgery. Circ Cardiovasc Genet. 2009;2:499–506. doi: 10.1161/CIRCGENETICS.109.849075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, et al. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–35. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang T, Yang P, Roden DM, Darbar D. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010;7:1246–52. doi: 10.1016/j.hrthm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham RL, Yang T, Blair M, Roden DM, Darbar D. Augmented potassium current is a shared phenotype for two genetic defects associated with familial atrial fibrillation. J Mol Cell Cardiol. 2010;48:181–90. doi: 10.1016/j.yjmcc.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darbar D. Genetics of atrial fibrillation: rare mutations, common polymorphisms, and clinical relevance. Heart Rhythm. 2008;5:483–6. doi: 10.1016/j.hrthm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatkin D, Otway R, Vandenberg JI. Genes and atrial fibrillation: a new look at an old problem. Circulation. 2007;116:782–92. doi: 10.1161/CIRCULATIONAHA.106.688889. [DOI] [PubMed] [Google Scholar]
- 18.Bigi MAB, Aslani A, Shahrzad S. Clinical predictors of atrial fibrillation in Brugada syndrome. Europace. 2007;9:947–50. doi: 10.1093/europace/eum110. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, et al. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270:5691–4. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- 20.Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–62. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- 21.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–48. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 22.Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–6. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts JD, Gollob MH. Impact of genetic discoveries on the classification of lone atrial fibrillation. J Am Coll Cardiol. 2010;55:705–12. doi: 10.1016/j.jacc.2009.12.005. [DOI] [PubMed] [Google Scholar]