Abstract
Objectives
Resting metabolic rate (RMR) is the largest component of total energy expenditure. It has not been studied in old-old adults living in the community, though abnormalities in RMR may play a critical role in the development of the clinical syndrome of frailty. The objective was to measure RMR and examine the association of measured RMR with frailty status and compare it to expected RMR generated by a predictive equation.
Design
Physiologic sub-study conducted as a home visit within an observational cohort study.
Setting
Baltimore City and County, Maryland.
Participants
77 women age 83–93 years enrolled in the Women’s Health and Aging Study II.
Measurements
RMR with indirect calorimetry; frailty status; fat-free mass; ambient and body temperature; expected RMR via the Mifflin-St. Jeor equation.
Results
Average RMR was 1119 kcal/d (s.d.± 205; range 595–1560). Agreement between observed and expected RMR was biased and very poor (between-subject coefficient of variation 38.0%, 95%CI: 35.1–40.8). Variability of RMR was increased in frail subjects (heteroscedasticity F test P value=0.02). Both low and high RMR were associated with being frail (Odds Ratio 5.4, P value=0.04) and slower self-selected walking speed (P value<0.001) after adjustment for covariates.
Conclusion
Equations to predict RMR that are not validated in old-old adults appear to correlate poorly with measured RMR. RMR values are highly variable among old-old women, with deviations from the mean predicting clinical frailty. These exploratory findings suggest a pathway to clinical frailty through either high or low RMR.
Keywords: resting metabolic rate, frailty, older adults
INTRODUCTION
Resting metabolic rate (RMR) is the largest component of total energy expenditure, which also includes thermogenesis from feeding and activity energy expenditure.(1) On average, RMR declines in old age(2) but there is a paucity of information regarding the distribution of RMR in old-old adults, aged 80 and over, living in the community. The modest amount of average decline seen with increasing old age in longitudinal studies of healthy older adults has led to the observation that group averages may hide inherent heterogeneity, with some subjects experiencing an increase and others a decrease in RMR.(3)
Changes in RMR in old age may play a critical role in the pathogenesis of the clinical syndrome of frailty.(4–6) Declines in subjective energy levels and increased fatigue are important signs and symptoms of frailty.(7) Changes in energy metabolism may explain the associations among weakness, weight loss, inactivity and decreased food intake components of frailty. If RMR is increased in frail older adults, compared to non-frail, then it can cause cachexia-like muscle loss. This model of altered energetics accompanied by weakness has been shown in some inflammatory diseases and cancer.(8, 9) Alternatively, if RMR is decreased it is likely to be the consequence but not the cause of muscle loss. This alternative model of altered energetics accompanied by weakness suggests frailty could be a form of accelerated aging.(10) It is currently not known whether RMR is decreased or increased in frail old-old adults, despite the presence of frailty in 23% of adults aged 90 or older,(5, 6) because of challenges related to study recruitment and retention of frail old-old adults. (11) There are also clinical implications to assessing RMR in older people, as equations used to estimate RMR have not been validated in older populations, despite their widespread use in creating nutritional plans for old-old adults.(12) This issue affects a substantial and important population, as old-old adults made up 13% of Medicare admissions in 2008.(13)
We developed and implemented a home visitation protocol to characterize RMR in old-old women, including frail women. We hypothesized that RMR was altered in frail, compared to non-frail, older women after adjusting for body composition and confounders. We also characterized the association between RMR and a cardinal feature of frailty, self-selected walking speed.(14, 15) Finally, we examined agreement between expected and observed RMR.
METHODS
Design and Setting
The Women’s Health and Aging Study II (WHAS II) is a longitudinal cohort designed to understand the onset of disability in women in eastern Baltimore City and County, Maryland.(16) At the time of inception, WHAS II eligibility criteria included self-reported task difficulty in ≤1 out of 4 physical domains, Mini-Mental State Examination score ≥24 (19), and ability to participate in a clinic examination. The 436 women enrolled were aged 70–79 and represented the 2/3 least disabled community-dwelling women. Subjects were enrolled in 1994–1995 and followed every 18–36 months.
Participants
At the seventh round WHAS II visit, subjects were invited to participate in a home sub-study in which RMR and glucose tolerance testing were performed (May, 2008 – March, 2009). There were no exclusion criteria for RMR measurement. Analyses include all 77 women in whom RMR was measured. RMR was not measured in two participants due to equipment malfunction or discomfort using the equipment mouthpiece. The Johns Hopkins Medical Institutions Institutional Review Board approved the research protocols. Informed consent was obtained from all participants.
Measurement of Outcome
Metabolic rate was measured in kilocalories/day (kcal/d) with indirect calorimetry through a standardized protocol for the home.(17, 18) Participants were called the day before to inquire about acute illness and remind them to fast at least 12 hours and avoid all non-essential activity before the arrival of the research team between 8 and 9 a.m. Participants were placed in a comfortable position in a semi-recumbent chair or similar position and asked to rest for ≥15 min. Lighting was dimmed. Participants were reminded to stay “as quiet as possible and avoid moving around.” Trained research staff recorded any deviations from protocol. The Medgem® by HealtheTech portable indirect calorimeter with a mouthpiece and nose-clip obtained two readings without delay between readings.(19, 20) The device was held by research staff. Calibration was performed before each measurement and analyses use the average of the two readings, each of which was obtained after plateau of oxygen consumption.
Measurement of Main Independent Variables
Frailty status was assessed at the seventh round and was defined as originally operationalized in the Cardiovascular Health Study(7) and validated in the WHAS studies.(14) Five criteria for frailty were used: shrinking (BMI <18.5 kg/m2 or 5% annual weight loss), weakness (grip strength equivalent to the lowest quintile in CHS, by gender and BMI strata), poor endurance (self-report of exhaustion), slowness (average of self-selected “usual” walking speed, equivalent to the lowest quintile in CHS), and low activity (activity level in kcal/week equivalent to the lowest quintile in CHS). Walking speed was obtained as the time (to 0.1 second) needed to cover a 4-m course. Participants were told, ‘‘Now we are going to observe how you normally walk. If you use a cane or a walking aid and would feel more comfortable with it, you may use it.’’ Time began with a verbal command to start walking (‘‘Ready, Begin’’) from a standing start with both feet touching a starting line and ended when a foot broke the plane of the finishing line. Two trials were performed with no structured delay. Those with none of the characteristics were categorized as non-frail, those with 1 or 2 pre-frail and those with 3, 4, or 5 as frail. The Mifflin-St. Jeor equation, which has been proposed to have superior validity in older adults,(21) was used to calculate expected RMR.(22)
Measurement of Covariates
Ambient temperature and barometric pressure were measured to the first decimal place for degrees Farenheit (oF) and millibar (mbar), respectively, at the beginning and end of the visit with a Linseis® SAM700BAR ambient thermometer/hygrometer. The instrument was at the level of the participant’s torso on a table or similar item. Body temperature has been shown to affect measured RMR(23) and was measured to the first decimal place at the beginning and end of the visit with an Omron® MC-106 digital oral thermometer placed as far back as possible under the tongue on one side. Height and weight were measured using a standardized protocol. Fat-free mass (FFM) was determined at the seventh round under standardized conditions by measurement of bioelectric impedance with all subjects in a recumbent position (Danninger Medical Technology Body Composition Analyzer TVI-10). Impedance measurements were performed with an alternating single frequency 50kHz current and an amplitude of 800 mA. The FFM in kg was calculated from equations based on resistance in ohms.(24) RMR was not divided by a body size measure to avoid the systematic bias such a step introduces for comparisons across groups.(25)
Analytic Methods
We examined RMR values graphically according to frailty status. Pre-specified analyses examined the association between RMR and frailty status using simple and adjusted multinomial regression models. A linear regression model examined the association of RMR with self-selected walking speed. The multivariable fractional polynomial models algorithm(26) developed a non-linear equation for this model, which was evaluated through examination of residuals. Agreement between observed and expected RMR was evaluated with a plot and calculation of between-subject coefficient of variation. Adjusted models included ambient temperature, body temperature height and weight. FFM was available as a sensitivity analyses instead of height and weight for 64 subjects who did not have a contraindication (e.g. pacemaker). An alpha level of 0.05, two-sided, was considered statistically significant. Two post-hoc analyses were: a) the F-test for heteroscedasticity comparing pre-frail and frail to the non-frail group and multinomial model for the deviation from mean RMR (i.e. |RMR|i - mean RMR, scaled to s.d.) as a predictor of frailty status. Analyses were performed using Stata, version 11, Stata Corporation, College Station, TX.
RESULTS
The average participant age was 86.9 years (range 83–93). Among participants, 17% were black and 83% were white. Average BMI was 26.8 kg/m2 (range17.8–42.6). With respect to protocol attainment, 2 participants were fasting <10 hours (1 hour since cup of coffee, 8 hours since meal). These participants are indicated in figures with special symbols. The test-repeat coefficient of variation for RMR was 12.5% (95% CI: 7.1–16.2) and the means of the first and repeat measurement were 1125 and 1116 kcal/d, respectively (P-value 0.86).
Average RMR was 1119 kcal/d (s.d.± 205 ; range 595–1560). Fifty subjects were pre-frail, 17 were non-frail and 10 were frail. Mean values (standard deviations) of covariates were: height 1.54 (0.074) meters; weight 63.3 (12.8) kg; fat-free mass 38.3 (5.27) kg; body temperature 96.6 (0.71) oF; ambient temperature 72.0 (4.29) oF.
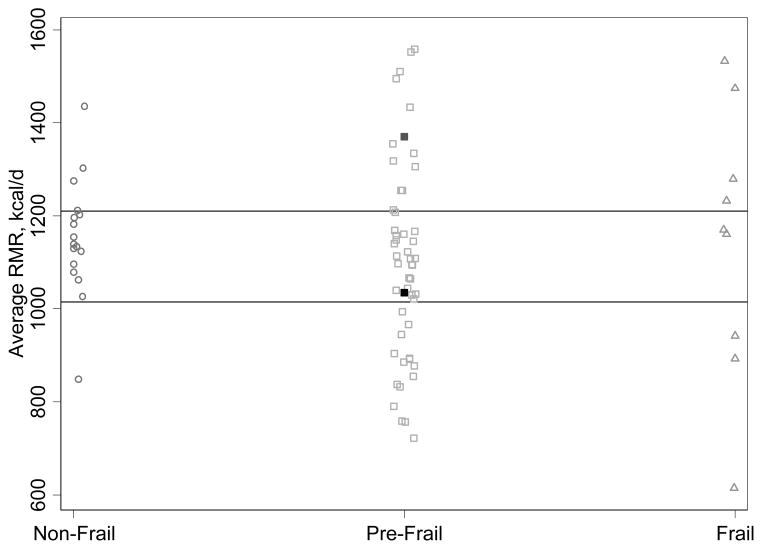
Figure 1 displays the distribution of RMR according to frailty status. Among non-frail participants the distribution was more compact compared to the wider distribution among pre-frail and frail people. In the frail group, only 2 of 10 had RMR values within the intra-quartile range for the entire sample. There was a higher degree variability of RMR in pre-frail or frail subjects, compared to non-frail (unadjusted F-test pre-frail vs. non-frail P value=0.06; frail vs. non-frail P value=0.02).
Figure 1. Distribution of Resting Metabolic Rate, According to Frailty Status.
RMR is resting metabolic rate. Two people identified by filled squares were fasting <10 hours. Horizontal lines are 25th and 75th percentiles for the sample.
RMR was not linearly associated with frailty status (P >0.05), whereas deviation from mean RMR was a predictor of frail status (OR of frail status 6.2 and 5.0 for simple and adjusted models; P values=0.01 and 0.04, respectively) but not a strong predictor of pre-frail status (OR of pre-frail status 2.2 and 2.4 for simple and adjusted models; P values=0.07 and 0.18, respectively). Adjusting for FFM instead of height and weight in in models with body temperature and ambient temperature did not change these results substantially.
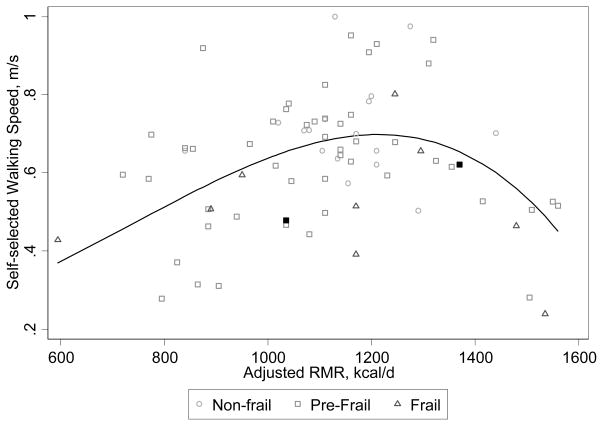
RMR was a non-linear predictor of self-selected walking speed as shown in Figure 2. Both low and high RMR were associated with slower self-selected walking speed after adjustment for covariates (P value<0.001). The non-linear coefficient increased the model’s adjusted R2 value from 0.0455 to 0.222 and decreased the root mean squared error from 0.1648 to 0.1488. Average self-selected walking speed was nonlinearly related to a 100 kcal/d difference in RMR, seen as the model fit line in Figure 2. Examination of model residuals demonstrated good nonlinear model fit to the data. Adjusting for FFM instead of height and weight did not change these results substantially.
Figure 2. Association Between Resting Metabolic Rate and Self-Selected Walking Speed, a Cardinal Feature of Frailty.
RMR is resting metabolic rate and was adjusted for height, weight, body temperature and ambient temperature. Two people identified by filled squares were fasting <10 hours. Line is fitted from model adjusting for height, weight, ambient temperature and body temperature (equation y=0.684+RMR*0.00248+RMR*-0.00875).
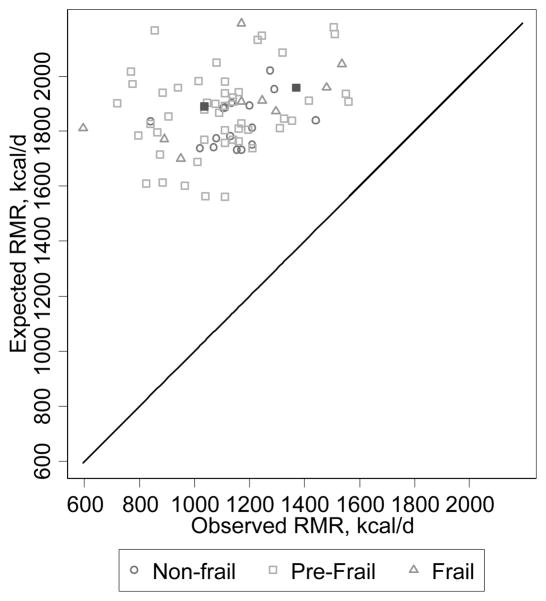
Figure 3 shows that agreement between observed and expected RMR was markedly biased such that expected values were higher than observed values by a factor of 1.5–3. There was also little correlation (comparison to a 45-degree line). Where perfect agreement would be zero, the within-subject coefficient of variation between observed and expected RMR was 38.0% (95%CI: 35.1–40.8) indicating very poor agreement.
Figure 3. Agreement Between Observed and Expected Resting Metabolic Rate. RMR is resting metabolic rate.
Two people identified by filled squares were fasting <10 hours.
CONCLUSION
This study characterizes RMR in a group of high clinical importance for whom very little information is available, women older than 80 including frail women living in the community. RMR displayed a large degree of variability of RMR among old-old women and deviation from the sample average value was associated with being clinically frail. The likelihood of frailty did not increase monotonically with increasing RMR. Instead, either a low or high RMR was associated with an increase in the odds of frailty status after adjusting for body composition, body temperature and ambient temperature, compared to the sample average RMR. The relationship between RMR and self-selected walking speed, a key constituent feature of frailty, confirmed the findings for the frailty status indicator as either very low or high RMR was associated with slower average self-selected walking speeds compared to the sample average. This main finding, the increased variability of RMR in old-old women, particularly among the frail, has implications for both clinical practice and understanding the clinical syndrome of frailty if it is replicated.
There may be limited clinical utility to the practice of using population-based equations to predict energy requirements and create nutritional recommendations for old-old adults. The equation thought to be best suited for prediction of energy requirements in older adults correlated very poorly with measured RMR and was markedly biased toward a higher RMR. Difficulty predicting energy requirement may be even more pronounced for hospitalized old-old adults who are acutely ill than for these subjects, who were examined at home. Mean RMR and variability similar to this study were found in an examination of women in a nursing home in Sweden, which reported a mean reliable RMR of 1174 kcal/d ±175 (range 810–1560) among 33 women with an age range of 67–102.(27) The findings here are also consistent with increased between-subject variability in total energy expenditure previously noted in old-old community-dwelling adults compared to younger adults.(28) Until more information is available for old-old adults, clinical practice may be better suited by examination of clinical indicators such as oral intake and loss of muscle than by an estimate of energy requirement.
The exploratory findings here that relate deviations from average of RMR to frailty suggest the possibility of distinct pathways that converge on the clinical phenotype of frailty.(5, 6, 29, 30) One is characterized by a relatively hypermetabolic state, which may be disease-related.(31, 32) In another pathway, acceleration of age-associated decline in metabolic rate may occur, leading to a low resting metabolic rate. The nonlinearity of the relationship between RMR and walking speed is consistent with numerous studies showing an inverse u-shaped association between activity energy efficiency and walking speed (as examples, see Weyand et al.(33) or Bastien et al.(34)). Indeed, more generally it appears that for physiologic variables reflecting homeostasis (arterial blood pressure; serum glucose concentration; serum cholesterol concentration; body mass index; etc.) values at either extreme are an indicator of impaired physiologic function.
Our data suggest this occurs even after controlling for loss of muscle mass, and may represent physiologic compensation to preserve precious energy stores. The causal mechanisms behind alterations in RMR are likely to involve highly complex and redundant neurologic and endocrine pathways. That metabolic regulation is highly complex is suggested by the resilience in good health of both body temperature and body composition to fluctuations in ambient temperature and caloric intake across immediate, short-term and long-term time intervals. Specifically, sympathetic nervous system activity is a fundamental modulator of metabolism that has been shown to be positively associated both with physical activity and muscle mass(35) and with dysfunction of the cardiac left ventricle.(36) Among endocrine organs affecting metabolism, the thyroid plays a central role as a modulator of metabolism, perhaps partly through an influence on sympathetic nervous system activity.(37) However, a substantial line of research suggests the thyroid may also be essential to adaptive changes that occur in the face of reduced energy intake. Caloric restriction studies have identified changes in mitochondrial membrane content, as well as function of uncoupling proteins ,governed by thyroid hormone that create thermogenesis through a mitochondrial proton leak.(38, 39) Studies in young healthy humans have repeatedly shown that a negative energy balance leads to a lowering of thyroid hormone levels that is accompanied by a lower resting metabolic rate (RMR) not explained by changes in body composition.(40–43) The causal link between thyroid hormone levels and RMR was further been strengthened by an experimental study displaying how small changes in the dose of thyroid hormone replacement therapy led directly to corresponding increases or decreases in RMR.(44) An attempt to replicate the previously-reported associations between sympathetic nervous system activity, thyroid hormone and RMR is planned in this old-old sample.
These data, if they are confirmed, support the hypothesis that there is more than one point of entry into the frailty spiral, and the importance of ongoing efforts to identify subtypes of frailty that point to subtype-specific preventive interventions. Previous work in older adults mostly younger than 80 years has also suggested that body composition alone does not explain differences in RMR between older and younger adults.(45)
The study findings should be considered with its limitations. These results were obtained from a small sample of mostly urban, community-dwelling women. While filling an important void, these results are preliminary and larger studies will be required to examine RMR among men and for subgroups defined by chronic diseases and medication use. The equipment used to measure RMR did not measure ventilation rate. The external validity of the findings is strongly limited even if it is supported indirectly by the literature noted above. The post-hoc nature of some analyses warrants that P values should not be interpreted as confirmatory and detailed longitudinal examination of RMR is necessary to establish causal pathways.
In summary, in exploratory analyses RMR displayed a large degree of variability of RMR among old-old women and deviation from the sample average value was associated with being clinically frail. Equations to predict RMR that are not validated in old-old adults appear to have little ability to inform the clinical management of nutritional status in old-old adults. Frailty in late life may be the result of more than one type of derangement of metabolism.
Acknowledgments
Funding sources: Carlos O. Weiss was supported by the Robert Wood Johnson Harold Amos Medical Faculty Development Program.
Anne R. Cappola, Ravi Varadhan, Linda P. Fried were supported by the National Institute on Aging grant R01 AG11703 and by NIH-NCRR Grant M01-RR000052 at the Johns Hopkins School of Medicine as well as by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Sponsor's Role: The sponsor played no role in the design, methods, subject recruitment, data collections, analysis and preparation of the paper.
Footnotes
Author Contributions: Carlos O. Weiss, Anne R. Cappola, Ravi Varadhan, Linda P. Fried contributed to the study concept and design, acquisition of subjects and data, analysis and interpretation of data and preparation of the manuscript.
References
- 1.Levine JA. Measurement of energy expenditure. Public Health Nutr. 2005;8:1123–1132. doi: 10.1079/phn2005800. [DOI] [PubMed] [Google Scholar]
- 2.Elia M, Ritz P, Stubbs RJ. Total energy expenditures in the elderly. Eur J Clin Nutr. 2000;54:s77–s91. doi: 10.1038/sj.ejcn.1601030. [DOI] [PubMed] [Google Scholar]
- 3.Henry CJK. Mechanisms of changes in basal metabolism during ageing. Eur J Clin Nutr. 2000;54:s77–s91. doi: 10.1038/sj.ejcn.1601029. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP. Introduction Aging (Milano); Conference on the physiologic basis of frailty; April 28, 1992; Baltimore, Maryland, U.S.A. 1992. pp. 251–252. [PubMed] [Google Scholar]
- 5.Fried LP, Walston J. Frailty and Failure to Thrive. In: Hazzard WR, Blass JP, Halter JB, et al., editors. Principles of Geriatric Medicine and Gerontology. New York: McGraw-Hill Companies; 2003. pp. 1487–502. [Google Scholar]
- 6.Weiss CO. Chronic diseases and frailty. Clin Geriatr Med. 2010;27:39–52. doi: 10.1016/j.cger.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Roubenoff R. Molecular basis of inflammation: relationships between catabolic cytokines, hormones, energy balance, and muscle. J Parenter Enteral Nutr. 2008;32:630–632. doi: 10.1177/0148607108324875. [DOI] [PubMed] [Google Scholar]
- 9.Axelrod L, Halter JB, Cooper DS, et al. Hormone levels and fuel flow in patients with weight loss and lung cancer. Evidence for excessive metabolic expenditure and for an adaptive response mediated by a reduced level of 3,5,3'-triiodothyronine. Metabolism. 1983;32:924–937. doi: 10.1016/0026-0495(83)90208-1. [DOI] [PubMed] [Google Scholar]
- 10.Ruggiero C, Ferrucci L. The endeavor of high maintenance homeostasis: Resting metabolic rate and the legacy of longevity. J Gerontol A Biol Sci Med Sci. 2006;61:466–471. doi: 10.1093/gerona/61.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci L, Guralnik J, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A Consensus Report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 12.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Curr Opin Clin Nutr Metab Care. 2004;7:599–605. doi: 10.1097/00075197-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 13.MEPSnet Query Tool. [Accessed May 13, 2011];Agency for Healthcare Research and Quality (online) Available at: http://www.meps.ahrq.gov/mepsweb/data_stats/meps_query.jsp.
- 14.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 15.Purser JL, Kuchibhatla MN, Fillenbaum, et al. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Young Y, Rubin G, et al. Self-reported preclinical disability identifies older women with early declines in performance and early disease. J Clin Epidemiol. 2001;54:889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- 17.Bullough RC, Melby CL. Effect of inpatient versus outpatient measurement protocol on resting metabolic rate and respiratory exchange ratio. Ann Nutr Metab. 1993;37:24–32. doi: 10.1159/000177745. [DOI] [PubMed] [Google Scholar]
- 18.Compher C, Frankenfield D, Keim N, et al. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J Am Diet Assoc. 2006;106:881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Compher C, Hise M, Sternberg A, et al. Comparison between Medgem and Deltatrac resting metabolic rate measurements. Eur J Clin Nutr. 2005;59:1136–1141. doi: 10.1038/sj.ejcn.1602223. [DOI] [PubMed] [Google Scholar]
- 20.Melanson EL, Coelho LB, Tran ZV, et al. Validation of the BodyGem hand-held calorimeter. Int J Obes Relat Metab Disord. 2004;28:1479–1484. doi: 10.1038/sj.ijo.0802643. [DOI] [PubMed] [Google Scholar]
- 21.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: A systematic review. J Am Diet Assoc. 2005;105:775–789. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Mifflin MD, St Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 23.Munn MW. Body temperature effects on basal energy expenditure (BEE): Proposed protocols for experiments and application of “average” equations for prediction of BEE for individuals. Am J Phys Med Rehab. 2006;85:244. doi: 10.1097/01.phm.0000200402.42084.c7. [DOI] [PubMed] [Google Scholar]
- 24.Roubenoff R, Baumgartner RN, Harris TB, et al. Application of bioelectrical impedance analysis to elderly populations. J Gerontol A Biol Sci Med Sci. 1997;52:M129–136. doi: 10.1093/gerona/52a.3.m129. [DOI] [PubMed] [Google Scholar]
- 25.Poehlman ET, Toth MJ. Mathematical ratios lead to spurious conclusions regarding age- and sex-related differences in resting metabolic rate. Am J Clin Nutr. 1995;61:482–485. doi: 10.1093/ajcn/61.3.482. [DOI] [PubMed] [Google Scholar]
- 26.Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology--with an emphasis on fractional polynomials. Methods Inf Med. 2005;44:561–571. [PubMed] [Google Scholar]
- 27.Lammes E, Akner G. Resting metabolic rate in elderly nursing home patients with multiple diagnoses. J Nutr Health Aging. 2006;10:263–270. [PubMed] [Google Scholar]
- 28.Fuller NJ, Sawyer MB, Laskey MA, et al. Prediction of body composition in elderly men over 75 years of age. Ann Hum Biol. 1996;23:127–147. doi: 10.1080/03014469600004352. [DOI] [PubMed] [Google Scholar]
- 29.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: Research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 30.Ferrucci L, Windham BG, Fried L. Frailty in older persons. GENUS LXI. :39–53. [Google Scholar]
- 31.Singh J, Musialek P. Increased resting metabolic rate in congestive heart failure. Ann Intern Med. 1995;122:800. doi: 10.7326/0003-4819-122-10-199505150-00014. [DOI] [PubMed] [Google Scholar]
- 32.Nawata K, Sohmiya M, Kawaguchi M, et al. Increased resting metabolic rate in patients with type 2 diabetes mellitus,accompanied by advanced diabetic nephropathy. Metabolism. 2004;53:1395–1398. doi: 10.1016/j.metabol.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Weyand PG, Smith BR, Puyau MR, et al. The mass-specific energy cost of human walking is set by stature. J Exp Biol. 2010;213:3972–3979. doi: 10.1242/jeb.048199. [DOI] [PubMed] [Google Scholar]
- 34.Bastien GJ, Willems PA, Schepens B, et al. Effect of load and speed on the energetic cost of human walking. Eur J Appl Physiol. 2005;94:76–83. doi: 10.1007/s00421-004-1286-z. [DOI] [PubMed] [Google Scholar]
- 35.Bell C, Day DS, Jones PP, et al. High energy flux mediates the tonically augmented beta-adrenergic support of resting metabolic rate in habitually exercising older adults. J Clin Endocrinol Metab. 2004;89:3573–3578. doi: 10.1210/jc.2003-032146. [DOI] [PubMed] [Google Scholar]
- 36.Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 37.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 38.Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002;34:1340–1354. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 39.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 40.Fontana L, Klein S, Holloszy JO, et al. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 41.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loucks AB, Heath EM. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol. 1994;266:R817–823. doi: 10.1152/ajpregu.1994.266.3.R817. [DOI] [PubMed] [Google Scholar]
- 43.De Souza MJ, Hontscharuk R, Olmsted M, et al. Drive for thinness score is a proxy indicator of energy deficiency in exercising women. Appetite. 2007;48:359–367. doi: 10.1016/j.appet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 44.al-Adsani H, Hoffer LJ, Silva JE. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab. 1997;82:1118–1125. doi: 10.1210/jcem.82.4.3873. [DOI] [PubMed] [Google Scholar]
- 45.Krems C, Luhrmann PM, Strassburg A, et al. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur J Clin Nutr. 2005;59:255–262. doi: 10.1038/sj.ejcn.1602066. [DOI] [PubMed] [Google Scholar]