Abstract
A “spindle assembly” checkpoint has been described that arrests cells in G1 following inappropriate exit from mitosis in the presence of microtubule inhibitors. We have here addressed the question of whether the resulting tetraploid state itself, rather than failure of spindle function or induction of spindle damage, acts as a checkpoint to arrest cells in G1. Dihydrocytochalasin B induces cleavage failure in cells where spindle function and chromatid segregation are both normal. Notably, we show here that nontransformed REF-52 cells arrest indefinitely in tetraploid G1 following cleavage failure. The spindle assembly checkpoint and the tetraploidization checkpoint that we describe here are likely to be equivalent. Both involve arrest in G1 with inactive cdk2 kinase, hypophosphorylated retinoblastoma protein, and elevated levels of p21WAF1 and cyclin E. Furthermore, both require p53. We show that failure to arrest in G1 following tetraploidization rapidly results in aneuploidy. Similar tetraploid G1 arrest results have been obtained with mouse NIH3T3 and human IMR-90 cells. Thus, we propose that a general checkpoint control acts in G1 to recognize tetraploid cells and induce their arrest and thereby prevents the propagation of errors of late mitosis and the generation of aneuploidy. As such, the tetraploidy checkpoint may be a critical activity of p53 in its role of ensuring genomic integrity.
INTRODUCTION
Aneuploidy is common among tumor cells and frequently follows after an intermediate tetraploid state (Shackney et al., 1989; Galipeau et al., 1996). The presence of aneuploidy in tumors is correlated with metastatic progression and poor prognosis (Sandberg, 1977; Rabinovitch et al., 1989). A carefully studied progression toward tumor status in Barrett's esophagus cells has shown that the precancerous state is characterized by loss of p53 function, followed by tetraploidy, and then aneuploidy (Galipeau et al., 1996). Subversion of the capacity of the cell to evade the consequences of tetraploidization, which inevitably leads to aneuploidy, may be a common intermediate in the multistep process by which tumor cells arise in situ.
Tetraploidy can arise by exit of a cell from mitosis following failures of spindle assembly, of chromosome segregation, or of cytokinesis (Andreassen et al., 1996). Mammalian cells have a checkpoint that maintains cdc2-cyclin B activity and induces mitotic arrest in the presence of inhibitors of microtubule assembly (Kung et al., 1990; Andreassen and Margolis, 1994). This mechanism can logically be expected to ensure the assembly of a functioning mitotic spindle before exit from mitosis. But in actuality, cells have a widely varied capacity to arrest in mitosis in the presence of microtubule inhibitors (Kung et al., 1990; Schimke et al., 1991; Cahill et al., 1998), and many nontransformed cells undergo only a transient mitotic arrest, and then exit mitosis without chromosome segregation and become tetraploid (Minn et al., 1996; Lanni and Jacks, 1998). However, a p53-dependent backup mechanism induces G1 arrest in cells that have evaded mitotic arrest imposed by inhibitors of microtubule assembly (Minn et al., 1996; Lanni and Jacks, 1998).
This “spindle assembly” checkpoint does not influence mitotic arrest but instead mediates arrest in G1 following evasion of mitotic arrest by inhibitors of microtubule assembly (Minn et al., 1996; Lanni and Jacks, 1998). There could be several reasons for why spindle failure would lead to G1 arrest, including signals generated by failure of spindle function. Our goal here was to determine whether tetraploidy per se determines G1 arrest. Here, we have inhibited cell cleavage in REF-52 cells with dihydrocytochalasin B (DCB), an inhibitor of actin assembly that is required for cytokinesis (Aubin et al., 1981; Martineau et al., 1995), and demonstrate that tetraploidy yields G1 arrest even when mitotic spindle formation and chromosome segregation have proceeded normally. We show that cells that become tetraploid, either by failure of mitotic spindle assembly or by failure of cytokinesis, arrest at an equivalent point in G1 in a p53-dependent manner. We therefore propose the existence of a general checkpoint that induces G1 arrest in response to the polyploid state of the cell, and suggest that this checkpoint can act independently of the nature of the mitotic defect by which the cell becomes polyploid. Our results suggest that the spindle assembly checkpoint is a subset of a more general phenomenon, a “tetraploidy” checkpoint.
The importance of p53 in maintaining genomic stability is well established (Hartwell, 1992; Donehower et al., 1995), and it is known that p53 is critical for G1 arrest in response to DNA damage (Kastan et al., 1992; Cox and Lane, 1995). In addition to the finding that p53 is required for the arrest of polyploid cells following evasion of mitotic arrest with inhibitors of microtubule assembly (Minn et al., 1996; Lanni and Jacks, 1998), other evidence suggests that p53 may play a role in the maintenance of euploidy. For example, mouse embryonic fibroblasts from which the p53 gene is deleted become highly aneuploid without showing chromosomal structural abnormalities (Harvey et al., 1993). We show here that REF-52 cells in which p53 is inactive do not arrest in G1 following tetraploidization and rapidly progress to aneuploidy. Given the rapid progression to aneuploidy in vivo of tetraploid cells that are deficient for p53 function (Ornitz et al., 1987; Galipeau et al., 1996), our results suggest that the tetraploidy checkpoint, which arrests in G1 cells that become tetraploid through a variety of mitotic errors, may be an essential function of p53 in ensuring genomic integrity.
MATERIALS AND METHODS
Cell Culture and Transfection
REF-52 (primary rat embryo fibroblasts) and their simian virus-40 large T antigen-transformed derivatives (TAG) (Perry et al., 1992) were a kind gift of G.R. Stark (Cleveland Clinic, Cleveland, OH). REF-52 primary cells were used at <35 passages. NIH3T3 cells were obtained from American Type Culture Collection (Manassus, NJ) and IMR-90 from Coriell Cell Repositories (Camden, NJ). All cell lines were cultured as monolayers in DMEM (Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel), except NIH3T3 were supplemented with 10% bovine calf serum (Hyclone Laboratories, Logan, UT). Cell doubling times at mid-log phase were 24 h for REF-52, NIH3T3, and IMR-90 and 18 h for TAG.
Retrovirus packaging cells that produced viruses expressing either p53DD (LXSN-p53DD) or the neomycin resistance gene only (LXSN; control) (Gottlieb et al., 1994) were obtained from M. Oren (Weizmann Institute of Science, Rehovot, Israel). These cells were cultured as monolayers in DMEM supplemented with 10% fetal bovine serum and 200 μg/ml G418 (Life Technologies, Rockville, MD). At 24 h before infection, target REF-52 cells were seeded at a density sufficient to reach ∼70% confluency at the time of infection. For infection, virus-containing culture medium from packaging cells was filtered through a 0.45-μm acetate cellulose filter and added to target cells. Infection was allowed to proceed for 5 h, after which virus-containing medium was washed out and replaced by fresh medium. Selection was initiated either 5 or 24 h after infection, and performed for 5–6 d in the continued presence of 1 mg/ml G418, at which time all control uninfected cells had died. Identical results were obtained with cells that were used immediately or stored frozen and rethawed before use. All cells were maintained in a humid incubator at 37°C in a 5% CO2 environment.
Synchronization and Cell Treatment
To synchronize cells at mitosis, subconfluent cultures were treated with 5 μM aphidicolin 2 d after replating to presynchronize cells in S phase, and they were released into 0.04 μg/ml nocodazole to accumulate cells in mitosis. At 7–8 h following release from aphidicolin, when the mitotic index was peak, cells were selectively detached. To inhibit cell cleavage and generate tetraploid cells, cells were washed with drug-free medium following selective detachment and then replated in the presence of 10 μM DCB. After 5 h, the cells had exited mitosis and spread. Cells were then either harvested immediately or washed with drug-free medium and continued in culture. Controls were prepared by washing detached mitotic cells with drug-free medium following synchronization and replating. Similar results were obtained if cells were synchronized only by treatment with 0.04 μg/ml nocodazole, and not presynchonized with aphidicolin, before selective detachment.
To focus on cleavage failure as the cause of G1 arrest, randomly cycling REF-52 cells were exposed to 10 μM DCB for 5 h without prior presynchronization, and then DCB was washed out and cells were exposed to 10 μM bromodeoxyuridine (BrdU) for 25 h in DMEM with 10% fetal calf serum.
To assay inappropriate mitotic exit in the presence of a microtubule inhibitor, detached mitotic cells, synchronized by release from aphidicolin into nocodazole as described above, were replated in the continued presence of 0.04 μg/ml nocodazole. Cells had exited mitosis and had respread by 5 h following replating. These cells were either harvested or washed with drug-free medium for the continued culture of the resultant tetraploid cells. REF-LXSNp53DD, and REF-LXSN cells were irradiated by a 137Cs gamma irradiator at ∼2 Gy/min (4 Gy total).
Flow Cytometry
To analyze exit from mitosis and determine cell cycle profiles, cells were prepared for two-dimensional flow cytometry by using antibodies to mitotic phosphoepitope marker-2, a mitotic marker (Davis et al., 1983), and propidium iodide, a marker of DNA content. Cells were collected by centrifugation, resuspended in phosphate-buffered saline (PBS), and fixed by the addition of 90% methanol at −20°C for 10 min. Cells were then pelleted and resuspended and stored at 4°C in PBS with 0.04% sodium azide. Incubation with MPM-2 antibodies, washes, incubation with fluorescein isothiocyanate-conjugated donkey anti-mouse IgG secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), and incubation with 50 μg/ml propidium iodide in 4 mM sodium citrate containing RNase A were as previously described (Andreassen and Margolis, 1994).
For quantification of entry into S phase, 10 μM BrdU was added to cells from a frozen 10 mM stock for 30 min. Cells were then collected by trypsinization, centrifuged, and resuspended in 1× PBS and fixed by the addition of 90% methanol for 10 min at −20°C. Cells were then kept at 4°C in PBS containing 0.04% sodium azide pending preparation for analysis. To prepare cells for analysis, they were resuspended in 50 μl PBS to which 1 ml of PBS containing 2 N HCl and 0.5% Triton X-100 was added for 30 min at room temperature (RT). Following centrifugation, 1 ml of 0.1 M sodium tetraborate, pH 8.5, was added. Cells were then centrifuged and 100 μl FITC-conjugated anti-BrdU antibody (Becton Dickinson, San Jose, CA) diluted fourfold in PBS/0.5% Tween 20/1% bovine serum albumin was added for 30 min at RT. Cells were washed with 1 ml of PBS, and DNA was stained by the addition of 50 μg/ml propidium iodide in PBS. Data were collected using a FACScan flow cytometer (Becton Dickinson). For each sample, 10,000 events were collected, and aggregated cells were gated out.
Microscopy
In all cases, cells prepared for immunofluorescence microscopy were grown on poly-d-lysine-coated glass coverslips before drug treatment. To assay spindle formation and chromosome segregation in the presence of DCB, cells were synchronized in mitosis by treatment with aphidicolin and then nocodazole, as described above. Cells were then released from mitotic arrest by washing with drug-free medium and were subsequently treated with 10 μM DCB. At 30 min or 45 min following release into mitosis, cells were fixed with 2% paraformaldehyde in PBS for 20 min at 37°C and were permeabilized 3 min with 0.2% Triton X-100 in PBS. Incubation with primary and secondary antibodies, washes, counterstaining with propidium iodide, and mounting of coverslips were as previously described (Andreassen and Margolis, 1994). Anti-β-tubulin antibody from Sigma Chemical, St. Louis, MO (Tub 2.1) was used at a 400-fold dilution, and FITC-conjugated donkey anti-mouse IgG antibody (Jackson ImmunoResearch Laboratories) was used at 2.5 μg/ml. For experiments where REF-52 cells were briefly treated with DCB (without presynchronization) and then exposed to BrdU for 25 h, cells were fixed by the addition of 90% methanol for 10 min at −20°C. Cells were then prepared and incubated with FITC-coupled anti-BrdU antibody as described above for the detection of BrdU by flow cytometry but without the centrifugation. Samples were observed using an Optiphot II microscope (Nikon, Melville, NY) attached to an MRC-600 laser scanning confocal apparatus (Bio-Rad Microscience Division, Herts, England). Images were treated with Adobe Photoshop and printed on an Inkjet Epson Stylus Color 900 printer (Seiko Instruments, Nagano-Ken, Japan).
To determine that DCB induced failure of cell cleavage, cells grown on culture dishes were examined by phase contrast microscopy (Nikon Diaphot), and photographed using a Nikon F-601 M camera.
Cell Counts
REF-52 cells were synchronized in mitosis by treatment with aphidicolin and nocodazole, as described above. Following selective detachment, cells were washed in drug-free medium and replated in 10-cm dishes containing 10 μM DCB. At 5 h after replating, cells were either harvested (time zero) or washed free of DCB with fresh drug-free medium. Control cells were prepared by replating in drug-free medium after selective detachment. Every 24 h, cells were harvested, resuspended in PBS, and counts were taken using a Neubauer hemacytometer. Figures shown are a mean value of eight counts for each sample.
Chromosome Counts
TAG cells were synchronized in mitosis as described above by treatment with aphidicolin, and then nocodazole, and then were selectively detached. Cells were washed with drug-free medium and then replated either in drug-free medium (controls) or the presence of 10 μM DCB for 5 h. At 24 h following replating, when cells had progressed through a complete cell cycle, they were treated with 0.5 μg/ml nocodazole to collect mitotic cells. After 12 h, mitotic cells were selectively detached and were swollen in 0.8% sodium citrate for 10 min at RT. Cells were then fixed 30 min at RT in methanol/acetic acid (3:1) and then spread on poly-d-lysine-coated coverslips by drying at 37°C. The preparation was then washed with PBS, stained for 5 min with 0.5 μg/ml propidium iodide, washed again twice with PBS, and mounted as for immunofluorescence microscopy.
For chromosome enumeration, samples were observed using a Nikon Optiphot microscope. Chromosomes were counted in cells in which chromosomes formed a discrete, individual set. For each sample, 40 mitotic cells were counted.
Assay of cdk2 Activity
REF-52 cells were synchronized with aphidicolin and then accumulated in mitosis by treatment with 0.04 μg/ml nocodazole and were selectively detached. Cells were either replated in the presence of nocodazole for 5 h to undergo inappropriate mitotic exit, or were replated in the presence of 10 μM DCB for 5 h, and were harvested at successive time points. Cell lysates were prepared in 50 mM Tris-HCl, pH 7.4, 250 mM NaCl, 5 mM EGTA, 0.1% NP-40 containing 0.1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 60 mM β-glycerophosphate, 50 mM NaF, and 0.5 mM sodium vanadate as previously described (Andreassen and Margolis, 1994). Immunoprecipitation with cdk2-specific antibodies and radioimmune assay for the phosphorylation of histone H1 was performed as previously described (Trielli et al., 1996).
Immunoblotting
Cells were lysed as described above. For the detection of p21WAF1 and cyclin E, lysates were resolved on 12% polyacrylamide gels and gel-separated proteins were then transferred to nitrocellulose sheets by using a semidry blotting apparatus, blocked with 5% nonfat milk, and incubated overnight with anti-p21WAF1 antibody (C19; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 2000-fold in TNT buffer (25 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, and 0.05% Tween 20) and anti-cyclin E (Brénot-Bosc et al., 1995) diluted 3000-fold in TNT containing 2.5% nonfat milk, respectively. Nitrocellulose sheets containing transferred proteins were then washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibodies diluted in TNT (also containing 2.5% nonfat milk for detection of cyclin E), as previously described (Andreassen and Margolis, 1994). For the detection of retinoblastoma protein (pRb), protein was transferred to Immobilon and incubated with antibody specific to pRb (Pharmigen, San Diego, CA) diluted 500-fold in TNT containing 1% nonfat milk and detected by incubation with secondary HRP-conjugated goat anti mouse IgG antibody (KPL, Gaithersburg, MD) diluted 300-fold in TNT. Protein–antibody complex was detected by enhanced chemiluminescence (Pierce, Rockford, IL).
RESULTS
Nontransformed REF-52 Cells Arrest in Tetraploid G1, Whereas Transformed Cells Continue to Cycle
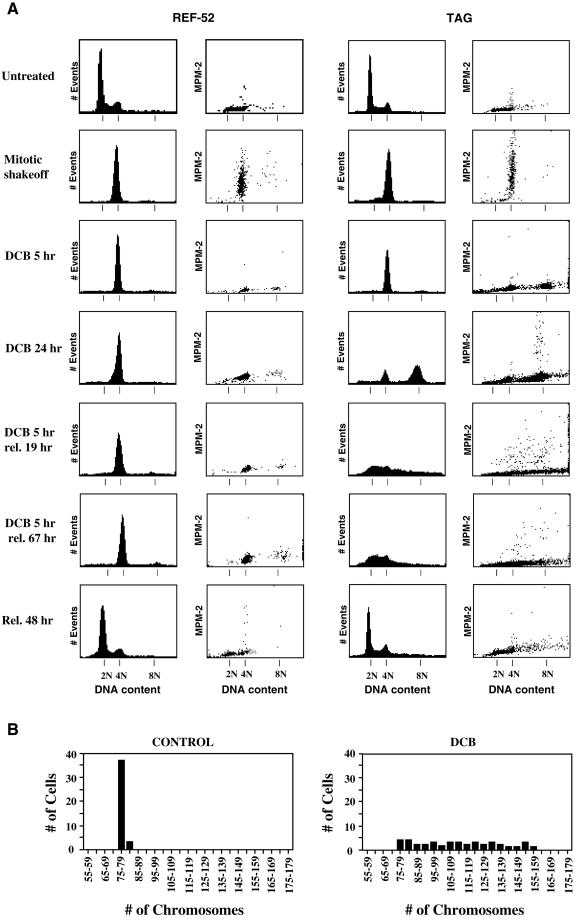
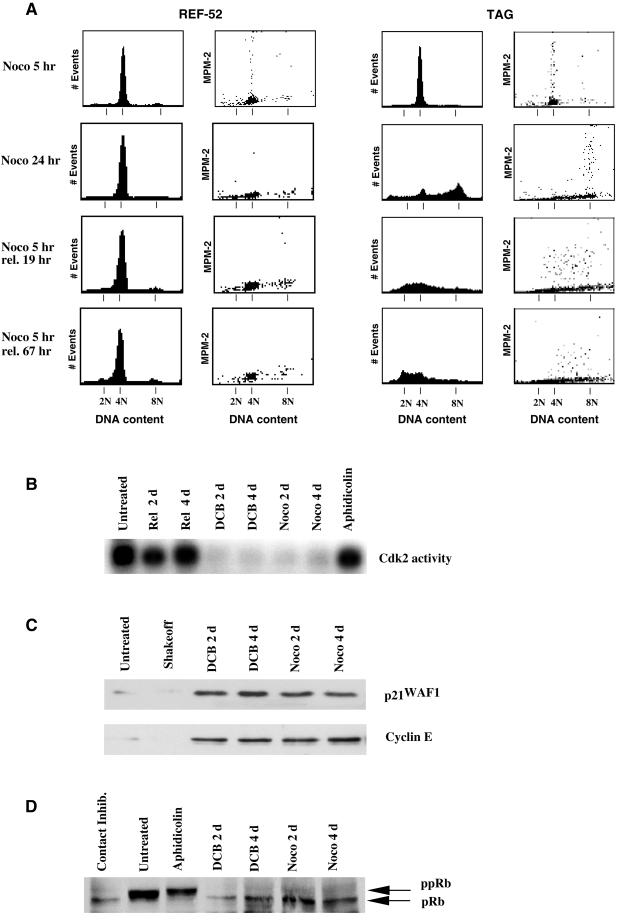
To determine whether the tetraploid state itself might induce G1 arrest, we induced cleavage failure in nontransformed REF-52 cells and their T-antigen–transformed derivatives, TAG cells, by transient exposure to DCB. To this end, both REF-52 and TAG cells were presynchronized in S phase with aphidicolin, subsequently arrested in mitosis by release into nocodazole, and mitotic cells were harvested by selective detachment. At the time of replating (mitotic shakeoff, Figure 1), FACscan analysis showed that cells had uniform 4N DNA content and contained abundant MPM-2, a specific phospho-epitope antigen marker of mitotic cells (Davis et al., 1983).
Figure 1.
REF-52 cells, but not transformed TAG cells, arrest in G1 following tetraploidization by treatment with DCB. (A) Both untreated REF-52 (left) and TAG (right) cells had similar cell cycle distributions, as shown by histograms of DNA content determined by flow cytometry. Mitotically synchronized (mitotic shakeoff) REF-52 and TAG cells were in mitosis as determined by 4N DNA content (1st and 3rd columns) and elevated MPM-2 signals (2nd and 4th columns). Both REF-52 and TAG cells resumed cycling and had DNA content profiles similar to untreated cells 48 h following release from mitotic synchronization (Rel. 48 h). REF-52 and TAG cells exited mitosis, as determined by minimal MPM-2 signals (2nd and 4th columns, respectively), following release of mitotically synchronized cells into medium containing 10 μM DCB, an inhibitor of the actin assembly required for cytokinesis, for 5 h (DCB 5 h). Tetraploid REF-52 cells remained arrested in G1with 4N DNA content (1st column) and minimal MPM-2 signal (2nd column) either when maintained in DCB for 24 h following mitotic synchronization, or when released from 5 h treatment with DCB for either 19 or 67 h. In contrast, transformed TAG cells continued cycling and developed 8N DNA content with elevated MPM-2 signal when maintained in DCB for 24 h following mitotic synchronization. When released from 5 h treatment with DCB following mitotic synchronization for either 19 or 67 h, TAG cells became aneuploid with elevated MPM-2 signal and a corresponding DNA content ranging from 2N to 8N. (B) As determined by chromosome counts, transformed TAG cells rapidly become aneuploid following tetraploidization by inhibition of cytokinesis with DCB. Control cells had 78 ± 0.9 chromosomes, whereas chromosome counts prepared from chromosome spreads of DCB-treated cells ranged from 76 to 156. Control cells were released from mitotic synchronization into drug-free medium for 24 h and were then arrested in mitosis by treatment with 0.5 μg/ml nocodazole for 12 h. DCB-treated cells were made tetraploid by 5 h exposure to 10 μM DCB following mitotic synchronization. These cells were then also released into drug-free medium for 19 h and then accumulated in mitosis with 0.5 μg/ml nocodazole for 12 h. A total of 40 cells was counted both for controls and DCB-treated cells.
These cells were then released from nocodazole upon replating and exposed to 10 μM DCB, a concentration sufficient to completely suppress cell cleavage. After 5 h exposure to DCB, all cells exited mitosis to G1 as evidenced both by low MPM-2 signal and by tetraploid DNA content (Figure 1A), as determined by flow cytometry (Andreassen and Margolis, 1994). REF-52 cells that were maintained in DCB for 24 h following mitotic synchronization remained arrested with 4N DNA content and a minimal MPM-2 signal, whereas TAG cells did not arrest but accumulated with 8N DNA content and included mitotic cells with elevated MPM-2 signal.
Alternatively, cells were released from DCB after 5 h of exposure and were later harvested for flow cytometric analysis. This allowed determination of the behavior of both REF-52 and TAG cells to a transient exposure to DCB that was just sufficient to induce tetraploidy. Nineteen hours after DCB release, REF-52 cells remained 4N with a minimal MPM-2 signal, whereas tetraploid TAG cells had proceeded through a full cell cycle and had become highly aneuploid, with DNA contents ranging from <2N to >4N. Elevated MPM-2 signal, associated with a range of DNA contents, indicated that many of the aneuploid cells were undergoing mitosis (Andreassen et al., 1996). Sixty-seven hours after DCB release, the REF-52 cells remained 4N with no cells in mitosis, and the highly aneuploid status of the TAG cells remained unchanged. These results thus suggest that REF-52 cells, but not TAG cells, arrest indefinitely in G1 when made tetraploid by failure of cytokinesis induced by transient exposure to DCB.
It is important to note that the synchronization procedure alone had no lasting effect on the cell cycle. In contrast to the behavior resulting from tetraploidization, control REF-52 or TAG cells, synchronized with aphidicolin and nocodazole, and then released from the nocodazole block immediately after shakeoff, reentered the next cell cycle normally and 48 h later displayed flow cytometry profiles indistinguishable from untreated cells (rel. 48 h, Figure 1A).
Flow cytometric analysis of DNA content suggested that TAG cells that failed to arrest in G1 became aneuploid during the cell cycle that followed tetraploidization. We confirmed this by counting the number of chromosomes in mitotic spreads (Figure 1B). Control cells arrested with nocodazole had 78 ± 0.9 chromosomes, whereas synchronous TAG cells, made tetraploid by treatment for 5 h with DCB, and then harvested in mitosis 31 h after release from DCB treatment (following accumulation in mitosis by treatment with nocodazole for the last 12 h) had a chromosome distribution ranging from 76 to 156.
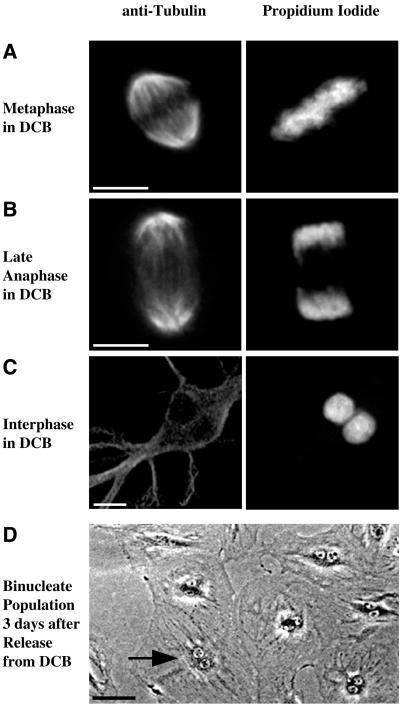
It is unlikely that DCB-dependent arrest in G1 arose through DNA damage or a checkpoint provoked by defective spindle function. DCB affects neither the formation of the mitotic spindle nor chromosome segregation. As shown in Figure 2A, a metaphase REF-52 cell, in the presence of 10 μM DCB, had a normal mitotic spindle and normal chromosome alignment. During late anaphase, spindle elongation and chromosome segregation had occurred normally (Figure 2B). Following treatment with DCB well spread binucleate cells, as shown in the example in Figure 2C, were ubiquitous. A field of such cells, as observed by phase contrast microscopy 72 h after exposure to DCB, is shown in Figure 2D. By contrast, control cells released from mitotic synchronization without DCB treatment divided and maintained a normal mononucleate population (our unpublished observations). Normal chromosome segregation was universal at DCB concentrations sufficient to completely block cleavage, as previously observed (Aubin et al., 1981; Martineau et al., 1995).
Figure 2.
REF-52 cells form a normal mitotic spindle and undergo normal chromosome segregation, but become binucleate, in the presence of DCB. (A) Metaphase REF-52 cells in 10 μM DCB formed a normal mitotic spindle, as determined by immunofluorescence microscopy with anti-tubulin antibodies (left column). Chromosomes aligned normally, indicating that the spindle functioned normally, as determined by counterstaining of chromosomes with propidium iodide (right column). The chromosomes are positioned equidistant between the poles of the mitotic spindle. (B) At late anaphase, the mitotic spindle detected by anti-tubulin antibodies (left column) elongated normally and chromosomes detected by staining with propidium iodide (right column) were completely segregated. (C) At 5 h treatment with 10 μM DCB following mitotic synchronization cells became binucleate, as determined by counterstaining with propidium iodide (right column). The anti-tubulin image (left) shows that the two nuclei were present in a single cell. (D) A phase contrast image of a field of REF-52 cells 3 d following mitotic synchronization and tetraploidization with 10 μM DCB shows that cells were binucleate. An arrow indicates a typical binucleate cell. Bars, 10 μm (A–C) and 50 μm (D).
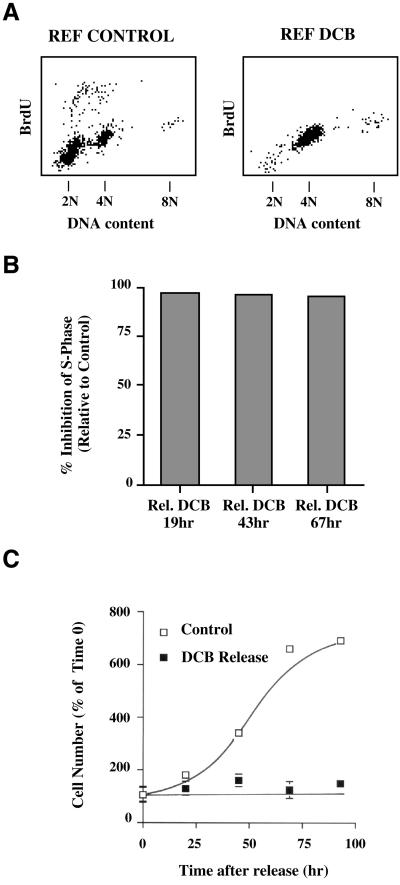
To further demonstrate that tetraploid REF-52 cells remain blocked in G1 following transient exposure to DCB, we assayed for their level of incorporation of BrdU relative to control cells synchronized with nocodazole, harvested by selective detachment, and then released from mitotic arrest. Whereas control cells released for 24 h from nocodazole synchronization showed an active S phase, BrdU incorporation was greatly suppressed 19 h after release from DCB (Figure 3A).
Figure 3.
REF-52 cells are inhibited from entering S phase and do not proliferate following tetraploidization induced by exposure to DCB. (A) REF-52 cells, released from mitotic synchronization into DCB for 5 h, and then released into drug-free medium for 19 h, did not enter S phase by 24 h after mitosis, as determined by BrdU incorporation measured by two-dimensional flow cytometry. By contrast, controls released into drug-free medium for 24 h following mitotic synchronization reentered S phase and incorporated BrdU. (B) REF-52 cells made tetraploid by 5 h treatment with DCB following mitotic synchronization showed >95% inhibition of S phase entry through 67 h following release from DCB, relative to controls, measured 24 h following release from mitotic synchronization. Inhibition of S phase was quantitated for 10,000 cells each from two-dimensional FACS histograms to obtain the number of cells incorporating BrdU. (C) Counts of cell number show that REF-52 cells did not proliferate following tetraploidization induced by treatment with DCB. Cells were sychronized in mitosis, selectively detached, and then released from mitotic synchronization into me dium containing 10 μM DCB for 5 h. Cells were then fixed (0 time) or were released in drug-free medium for up to 96 h. Cells were fixed at the time points indicated and cell numbers counted using a hemacytometer. By contrast, mitotically synchronized cells replated in drug-free medium resumed proliferation. Each point is an average of eight counts, and error bars represent SDs.
Relative S phase levels were quantitated by FACscan analysis (Figure 3B). At 19, 43, and 67 h after release from 5 h DCB exposure the level of BrdU incorporation was <5% that exhibited by controls in a half-hour pulse (Figure 3B). The arrest of tetraploid REF-52 cells in G1 following treatment with DCB is further demonstrated by the fact that cell counts remained constant following release from DCB (Figure 3C). In comparison, control cells released from nocodazole synchronization resumed normal proliferation (Figure 3C).
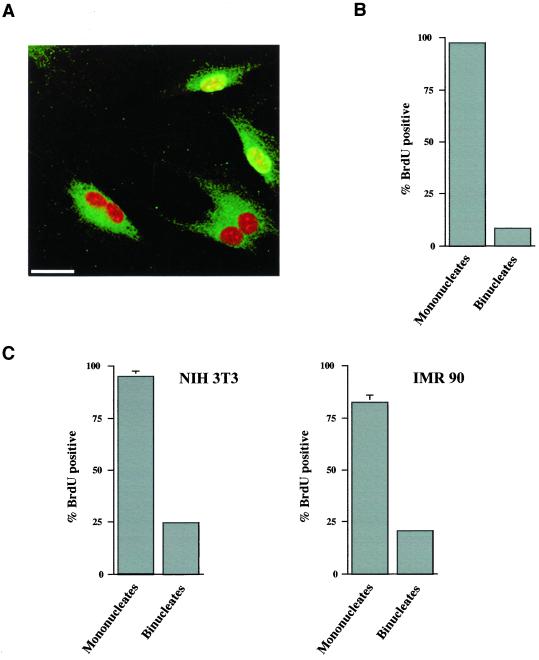
To perform the FACscan analysis shown above, extensive synchronization procedures were necessary. Whereas controls demonstrated that the synchronization procedures alone did not influence cell cycle recovery, they could not eliminate the possibility that the combination of synchronization protocols and exposure to DCB might cause arrest in the tetraploid state. For this reason we performed an assay of S phase entry on randomly cycling cells that were briefly exposed to 10 μM DCB without presynchronization. After 5 h exposure to DCB and subsequent release into drug-free medium, randomly cycling cells were given 10 μM BrdU for 25 h and analyzed for DNA replication by immunofluorescence microscopy. In the experiment shown, 18.6 ± 1% of the DCB-treated cells were binucleate, compared with 1 ± 0.3% of control cells in untreated populations. The results clearly demonstrate that DNA replication was almost completely suppressed in binucleate cells, whereas almost all mononucleate cells continued to cycle. A field of cells is shown wherein mononucleate cells are uniquely BrdU positive (Figure 4A). Quantitative analysis of cells for BrdU incorporation relative to nuclear number confirms that a nearly absolute suppression of DNA replication (8.4 ± 0.2% BrdU positive) correlates with binucleate (tetraploid) status (Figure 4B). Of the mononucleate cells in the population, 97.3 ± 0.9% incorporated BrdU. These results demonstrate that it is the tetraploid state, and not short-term exposure to DCB, that inhibits subsequent cell cycle progression.
Figure 4.
DCB induces cell cycle arrest only in cells that become binucleate following short-term exposure. (A) REF-52 cells were exposed to 10 μM DCB for 5 h without pre-synchronization, and they were then cultured for 25 h in medium containing 10 μM BrdU but not DCB. As shown by an immunofluorescence image, mononucleate, but not binucleate, cells incorporate BrdU (green) following 5 h treatment with DCB. Mononucleate cells are distinguishable from binucleate cells by a counterstain of nuclei with propidium iodide (red). FITC background was enhanced to better distinguish between mononucleate and binucleate cells. Bar, 25 μm. (B) Quantitation of the percentage of mononucleate and binucleate REF-52 cells that incorporate BrdU in 25 h following a 5 h exposure to DCB. (C) Similar analysis of random cycling NIH3T3 and IMR-90 cells was conducted following exposure to DCB for 10 h and then BrdU for 24 h. Incorporation is specifically suppressed in binucleate cells, indicating cell cycle arrest in cells that become tetraploid due to inhibition of cytokinesis by DCB. For each condition, at least 300 cells were counted. Values shown are mean values of three counts. SDs were as stated in the text.
The observed arrest in tetraploid G1 was not unique to REF-52 cells. Both nontransformed human IMR-90 and mouse NIH3T3 cells exhibited similar BrdU incorporation profiles following brief exposure to DCB (Figure 4C). Randomly cycling NIH3T3 and IMR-90 cells were given DCB for 10 h, and then exposed continuously to BrdU for 24 h following DCB release. As shown, whereas 94.7 ± 2.7% of mononucleate NIH3T3 cells were labeled, 24.6 ± 0.9% of binucleates had label. Similarly, 20.8 ± 1.4% of binucleate IMR-90 cells were labeled, whereas 82.3 ± 3.7% of mononucleate cells were labeled. As determined by flow cytometric analysis, a stably arrested tetraploid population was maintained over several days (our unpublished results). These results show a strong influence of tetraploidy on G1 arrest, although it is somewhat less stringent in both NIH3T3 and IMR-90 cells than that observed in REF-52 cells.
G1 Arrest of Tetraploid Cells following Spindle Failure Is Indistinguishable from Arrest following Cleavage Failure
Exposure to 0.04 μg/ml nocodazole, which caused a failure of spindle function resulted in an arrest in tetraploid G1 similar to that yielded by treatment with DCB. For these experiments REF-52 and TAG cells were synchronized as described above with aphidicolin, exposed to nocodazole, and then harvested by selective detachment and replated in the continued presence of drug. At the time of replating, the harvested cells were mitotic (Figure 1).
Five hours after replating in the continued presence of nocodazole both REF-52 and TAG cells remained 4N but had exited mitosis, as demonstrated by the loss of MPM-2 marker (Figure 5A). Microscopic examination confirmed that the REF-52 and TAG populations had both reconstituted interphase nuclei by this time (our unpublished obser-vations). After 24 h in the continuous presence of nocodazole, REF-52 cells retained 4N DNA content, whereas TAG cells had largely progressed to 8N DNA content. The TAG cells thus rereplicated their DNA following mitotic failure, whereas the REF-52 cells did not.
Figure 5.
Aberrant exit from mitosis in the presence of nocodazole yields an arrest similar to that obtained by cleavage failure in DCB. (A) Both REF-52 and TAG cells exited mitosis in the presence of 0.04 μg/ml nocodazole for 5 h following mitotic synchronization (noco 5 h) and displayed minimal MPM-2 signal with 4N DNA content. REF-52 cells remained arrested in interphase as shown by a continued 4N DNA content (1st column) and minimal MPM-2 signal (2nd column) both in 0.04 μg/ml nocodazole for 24 h or following release from 5 h treatment with nocodazole for 19 or 67 h after aberrant exit from mitosis. By contrast, TAG cells did not arrest, and in the presence of 0.04 μg/ml nocodazole for 24 h had 8N DNA content with elevated MPM-2 signal. But when released from 5-h treatment with nocodazole for either 19 or 67 h cells developed an aneuploid DNA content yielding mitotic cells that continued to cycle. Tetraploidization by either aberrant exit frommitosis in the presence of nocodazole or inhibition of cytokinesis by DCB results in identical profiles of effectors of G1 progression. Cells were treated with either 10 μM DCB or 0.04 μg/ml nocodazole for 5 h following mitotic synchronization, and then following tetraploidization were released in drug-free medium for the indicated period of time. (B) Cdk2 activity, which is required for G1-S progression (Pagano et al., 1993; Tsai et al., 1993), was suppressed by tetraploidization of REF-52 cells with either DCB or nocodazole relative to untreated cells, and also suppressed relative to cells arrested in S phase by treatment with 5 μM aphidicolin for 24 h, or to cells released from mitotic synchronization for 2 or 4 d. (C) Both the cdk2 inhibitor p21WAF1 and the cdk2 activator cyclin E were similarly present at elevated levels in cells made tetraploid by exposure to either nocodazole or DCB relative to untreated cells or mitotic cells synchronized by selective detachment (mitotic shakeoff). (D) pRb was in a hypophosphorylated state during G1 arrest following tetraploidization of cells by either nocodazole or DCB, as it was in cells arrested in G1 by contact inhibition. By contrast, pRb was in a hyperphosphorylated state in untreated cells and those arrested in S phase by treatment with aphidicolin.
The tetraploid block persisted even in the absence of nocodazole. The REF-52 and TAG cells that had exited mitosis by 5 h after shakeoff in the continued presence of nocodazole were then released from the drug. Nineteen hours after release, the TAG cells had become highly aneuploid, and 3 d after release had a DNA content ranging from <2N to >4N and had a broadly distributed MPM-2 signal, indicating entry into mitosis with an aneuploid content of DNA (Andreassen et al., 1996). By contrast, REF-52 cells remained arrested with 4N DNA content and no significant MPM-2 signal. In contrast, control REF-52 or TAG cells, synchronized identically with aphidicolin and nocodazole, but released from the nocodazole block immediately after shakeoff, had normal cell cycle profiles 48 h later (Figure 1). Similar results were obtained with taxol, another spindle inhibitor with a different mechanism of action (our unpublished results).
We next compared for several biochemical markers the arrest of cells made tetraploid by exit from mitosis either without spindle function or without cytokinesis. It has been reported that cdk2 protein kinase activity is suppressed in human colon carcinoma cells following exit from mitosis in the absence of spindle function (Stewart et al., 1999). In accord with this, we find that cdk2 activity in REF-52 cells was suppressed following aberrant exit from mitosis in the presence of nocodazole (Figure 5B). Cdk2 activity was similarly suppressed in REF-52 cells following failure of cell cleavage in the presence of DCB (Figure 5B). This suppression thus correlates with tetraploidization, rather than failure of spindle function per se.
Additionally, p21WAF1 inhibits cdk2 activity following spindle failure in human colon carcinoma cells (Stewart et al., 1999). In accord with this, we find that p21WAF1 is induced following aberrant exit from mitosis in the presence of nocodazole (Figure 5C). Importantly, elevated levels of p21WAF1 were maintained even though nocodazole was removed from the medium following mitotic exit, thus correlating with the tetraploid status of the cells in the absence of drug. p21WAF1 was similarly induced and maintained in REF-52 cells following failure of cell cleavage by exposure to DCB, suggesting that p21WAF1 was also generally induced by tetraploidy status in G1.
In accord with a previous report (Lanni and Jacks, 1998), we find that cyclin E, which activates cdk2 (Koff et al., 1992), is present at elevated levels following failure of spindle function, relative to levels in randomly cycling or mitotically synchronized cells (Figure 5C). Cyclin E levels are elevated to a similar degree in REF-52 cells made tetraploid by failure of cytokinesis induced by DCB (Figure 5C).
pRb, which antagonizes cell cycle progression by binding to members of the E2F transcription factor family (Weintraub et al., 1992), is hypophosphorylated during G1 (Buchkovich et al., 1989; Chen et al., 1989). We find that pRb is hypophosphorylated following inappropriate exit from mitotic arrest with nocodazole (Figure 5D), as previously reported for another cell line (Lanni and Jacks, 1998). A similar hypophosphorylated state accompanies tetraploidization following inhibition of cytokinesis by treatment with DCB (Figure 5D).
Thus, G1 arrest activated by tetraploidization, whether it follows aberrant exit from mitosis in the presence of inhibitors of spindle assembly, or inhibition of cytokinesis, is indistinguishable with respect to each of the several G1 cell cycle markers that we have examined.
Arrest in Tetraploid G1 State Is p53-dependent
The previously described “spindle assembly checkpoint” that arrests cells in G1 following evasion of mitotic arrest has been reported to depend upon p53 (Minn et al., 1996; Lanni and Jacks, 1998). We asked whether checkpoint arrest following failure of cytokinesis is similarly p53-dependent. For this, we inactivated p53 in REF-52 cells with a retrovirus expressing a truncated dominant-negative mutant of p53 (p53DD) (Gottlieb et al., 1994). As controls, we infected cells with the retroviral vector alone. Untreated populations of p53DD and control cells had similar cell cycle profiles, as determined by flow cytometry (Figure 6B).
Figure 6.
p53 is required for tetraploid arrest whether induced by inhibition of cytokinesis or by exit from mitosis without the formation of a mitotic spindle. p53 was inactivated in REF-52 (p53DD) by expression of a dominant-negative mutant (Shaulian et al., 1992). (A) Inactivation of p53 in cells expressing p53DD resulted in their inability to induce p21WAF1 following exposure to gamma irradiation, as demonstrated by Western blots. By contrast, control REF-52 cells (p53+/+) induced p21WAF1 following irradiation. (B) FACSscan analysis showed that both p53+/+ and p53DD cells had similar cell cycle profiles when untreated and had the 4N DNA content expected of cells following mitotic synchronization (mitotic shakeoff) and subsequent tetraploidization by exposure to 10 μM DCB for 5 h (DCB 5 h). Control p53+/+ cells remained arrested with 4N DNA content following exposure to DCB, whether released into drug-free medium for 19 or 67 h, or released into 0.04 μg/ml nocodazole for 19 h. By contrast, p53DD cells continued cycling when made tetraploid by exposure to DCB. Cells accumulated with 8N DNA content when released from DCB into nocodazole and became aneuploid when released from DCB into drug-free medium for 19 or 67 h. (C) Similarly, p53DD cells continued cycling when made tetraploid by aberrant exit from mitosis in the presence of 0.04 μg/ml nocodazole, whereas control p53+/+ cells arrested with 4N DNA content. p53DD cells accumulated a subpopulation of 8N cells when exposed to 0.04 μg/ml nocodazole for 24 h following mitotic synchronization, and they became aneuploid when mitoticallysynchronized, then treated with 0.04 μg/ml nocodazole for 5 h, and finally released into drug-free medium for either 19 or 67 h. (D) p21WAF1 was induced in a p53-dependent manner in cells made tetraploid with DCB, relative to controls or actively cycling cells 2 d after release from mitotic synchronization. Western blots show p21WAF1 was induced in p53+/+, but not p53DD cells following mitotic synchronization and then treatment with DCB for 5 h to obtain a tetraploid G1 population.
The p53DD mutant oligomerizes with wild-type p53, abrogating sequence-specific DNA binding (Shaulian et al., 1992) and thus blocking p53-dependent transactivation. To confirm that p53DD effectively blocked p53-dependent transcription in infected REF-52 cells, we exposed control and p53DD cells to γ-irradiation (Figure 6A), which induces p53-dependent transcription of p21WAF1 (El-Deiry et al., 1993; Dulic et al., 1994). p21WAF1 was induced in control cells, but not p53DD cells, at 3 and 6 h postirradiation.
p53DD cells failed to arrest in a tetraploid G1 state following DCB treatment. Both control REF-52- and p53DD-expressing populations had 4N DNA content following mitotic synchronization and selective detachment (mitotic shakeoff, Figure 6B). However, when the mitotically synchronized p53DD cells were treated with DCB for 5 h to induce cleavage failure and then treated for 19 h with nocodazole, they exhibited a largely 8N DNA content, indicating cell cycle progression to the next mitosis. By contrast, control cells infected with the retroviral vector alone maintained 4N DNA content following identical treatment (Figure 6B). This result indicates that p53DD abrogates G1 arrest following tetraploidization obtained by cleavage failure. When p53DD cells were similarly exposed to DCB for 5 h during release from mitotic synchronization, but permitted to pass through the next mitosis, they developed an aneuploid DNA distribution, evident both at 19 and 67 h after release from DCB (Figure 6B). This result is comparable to observations in similarly treated TAG cells (Figure 1). By contrast, control cells similarly exposed to DCB maintained G1 arrest with 4N DNA content when assayed at both 19 and 67 h following release from DCB (Figure 6B).
p53DD REF-52 cells also failed to arrest in G1 following evasion of nocodazole-dependent mitotic arrest (Figure 6C) and had 8N DNA content following 24 h treatment with nocodazole. Furthermore, cells made tetraploid by 5 h treatment with nocodazole following mitotic synchronization exhibited aneuploidy 19 h after release from nocodazole. By comparison, control cells retained 4N DNA content following evasion of mitotic arrest in the presence of nocodazole (Figure 6C). Thus, maintenance of G1 arrest following tetraploidization, whether caused by evasion of mitotic arrest or by failure of cytokinesis, is similarly dependent upon p53.
p21WAF1 is transcribed in a p53-dependent manner in response to certain stimuli (El-Deiry et al., 1993; Dulic et al., 1994). To determine whether this is the case after induction of a tetraploid state, we assayed the status of p21WAF1 in control and p53DD cells, and found that p21WAF1 was induced in a p53-dependent manner following failure of cytokinesis (Figure 6D). In control cells, p21WAF1 was present at elevated levels relative to controls at 1, 2, and 4 d following tetraploidization by cleavage failure.
By contrast, p21WAF1 is not induced in p53DD cells following cleavage failure. Thus, we conclude that the tetraploidization checkpoint requires p53-dependent transcriptional activity regardless of whether it is induced by failure of chromosome segregation or failure of cytokinesis, and it is associated with sustained levels of the p53-dependent transcriptional product p21WAF1.
DISCUSSION
We have demonstrated that nontransformed mammalian cells arrest in G1 following failure of any late mitotic event that generates a tetraploid condition. The mechanism of arrest is dependent on the tumor suppressor p53. A “spindle assembly” checkpoint has been previously described that arrests cells in G1 in a p53-dependent manner when they inappropriately exit mitosis in the presence of inhibitors of microtubule assembly (Minn et al., 1996; Lanni and Jacks, 1998). In these previous studies, arrest could have been triggered by a checkpoint that responds to mitotic exit in the absence of a functioning mitotic spindle, or it could have resulted from the induction of tetraploidy itself. Here, we have used DCB, an inhibitor of actin assembly, to inhibit cytokinesis and have generated tetraploid cells that exit mitosis following normal mitotic spindle formation and normal chromosome segregation. We are thus able to conclude that induction of tetraploidy itself blocks cell cycle progression.
The tetraploid arrest that results from cleavage failure is very similar in its molecular signature to the G1 arrest generated by inappropriate mitotic exit in the presence of spindle inhibitors. Both are p53-dependent and are accompanied by the inhibition of the cdk2 protein kinase activity that is required for G1-S progression (Pagano et al., 1993; Tsai et al., 1993). Furthermore, both are characterized by the induction of the cdk inhibitor p21WAF1 (Dulic et al., 1994), by elevated levels of the cdk2 activator cyclin E (Koff et al., 1992), and by hypophosphorylation of the pRb. These similarities lead us to conclude that nontransformed cells possess a common G1 checkpoint that induces arrest in response to tetraploidization. This checkpoint operates regardless of the means by which the cell becomes tetraploid, and is not specifically due to exit from mitosis in the absence of spindle function.
It appears that the tetraploidy checkpoint may be essential to prevent the formation of aneuploid cells from tetraploid intermediates. When a cell fails in mitosis and becomes tetraploid, it reenters G1 with double the normal number of G1 centrosomes. Centrosome duplication is coupled to cell cycle progression, and continued cycling of such a tetraploid cell would inevitably result in a multipolar spindle at the next mitosis, resulting in random chromosome segregation. Indeed, we find that inactivation of the tetraploidy checkpoint uniformly results in aneuploidization following passage through the next cell cycle (Figure 1B), and consequent induction of multipole spindles (our unpublished observations). Thus, the tetraploidy checkpoint prevents aneuploidization by inducing the tetraploid cell to withdraw from the cell cycle before DNA replication.
The tumor suppressor protein p53 has a well established role in maintaining the integrity of the genome (Hartwell, 1992; Donehower et al., 1995). Given that inactivation of p53 in tetraploid cells results in progression toward aneuploidy, it is evident that tetraploid arrest may be an important means by which p53 preserves genomic integrity. Because aneuploidization is tightly correlated with tumor formation (Rabinovitch et al., 1989, Li et al., 1997; Duesberg et al., 1998; Lengauer et al., 1998), we conclude that the tetraploidy checkpoint function of p53 may be essential to its role as a tumor suppressor.
Nontransformed Cells Have a G1 Checkpoint That Prevents Rereplication following Tetraploidization
Initial results suggested that the spindle assembly checkpoint, defined as suppression of mitotic exit in the presence of mitotic spindle inhibitors, is dependent upon p53 (Cross et al., 1995). More recent results have demonstrated, however, that it is not the timing of mitotic exit, but arrest in the subsequent G1, that is p53-dependent (Minn et al., 1996; Lanni and Jacks, 1998), because paired cells that are either competent or deficient for p53 function have been shown to exit mitosis at similar rates in the presence of inhibitors of microtubule assembly. Because expression of a dominant-negative p53 protein in REF-52 cells abrogates the G1 arrest of cells that inappropriately exit mitosis in nocodazole, our results are in accord with this conclusion. Furthermore, using nontransformed REF-52 cells and their transformed derivatives (TAG), we show that cellular transformation is not a determinant of the ability to sustain mitotic arrest in the presence of inhibitors of microtubule assembly.
During mitosis, spindle function checkpoints depend upon proteins such as Bub1, BubR1, and Mad2 to delay progression from metaphase to anaphase if microtubule attachment or tension upon kinetochores is impaired (Taylor and McKeon, 1997; Gorbsky et al., 1998; Chan et al., 1999). These checkpoint controls are independent of p53 (Minn et al., 1996; Lanni and Jacks, 1998). Despite these controls, most cells have limited capacity to sustain mitotic arrest. If a cell does exit mitosis in the presence of inhibitors of microtubule assembly, there is a subsequent and independent checkpoint in G1 that arrests cells in a p53-dependent manner (Minn et al., 1996; Lanni and Jacks, 1998). Thus, these two checkpoints appear to act in concert; the first acting to ensure accurate cell division and the second acting to protect the cell from aneuploidy if cell division has not been completed accurately.
Various mechanisms could account for the arrest of cells containing functional p53 in G1 following inappropriate exit from mitosis in the presence of inhibitors of microtubule assembly. These include DNA damage during aberrant mitosis (Lanni and Jacks, 1998), failure to decatenate DNA during anaphase, induction of signal mechanisms by inhibition of spindle formation and function (Lanni and Jacks, 1998), or induction of controls that arise from tetraploid status. From our results we conclude that the G1 arrest is attributable to tetraploidization per se. Using DCB we have inhibited cytokinesis following chromosome segregation (Aubin et al., 1981; Martineau et al., 1995). Both spindle function and chromosome segregation are normal in the presence of DCB. It is thus apparent that both decatenation and separation of sister chromatids occur as in control cells. DCB acts upon the actin assembly that initiates in the cell cortex following chromosome segregation, and cells become tetraploid without DNA damage. Given that both spindle failure and failure of cytokinesis yield a similar outcome following mitotic exit our results permit us to conclude that any late mitotic failure, rendering a cell tetraploid, will induce a p53-dependent arrest in the subsequent G1.
Possible Mechanisms of Tetraploidy Arrest
Among the reasonable possibilities that may account for the induction of G1 arrest in tetraploid cells, G1 cells have double the normal number of centrosomes following aberrant mitotic exit. The importance of the centrosome to cell cycle control has been demonstrated recently in experiments where the ablation of the centrosome during the prior cell cycle in African Green Monkey BSC-1 cells resulted in G1 arrest following mitosis (Hinchcliffe et al., 2001). These results demonstrate that cell cycle progression is very sensitive to the presence of centrioles in G1, and suggest that the arrest we observe could be dependent upon the number of centrosomes inherited by tetraploid G1 cells.
An alternative possibility is that tetraploid arrest is induced by ploidy-specific regulation of gene expression. Interestingly, ploidy-specific alteration in G1 gene regulation has been reported for isogenic budding yeast (Galitski et al., 1999). The Galitski et al. (1999) result is of substantial importance in light of our results, because it demonstrates that, like mammalian cells, yeast have an unknown mechanism of responding to their ploidy status.
We find that cells that become tetraploid either through inhibition of cytokinesis or by aberrant exit from mitosis in the presence of inhibitors of spindle assembly display an arrest in G1 that is indistinguishable both with respect to the point of arrest and dependency upon p53. Although inhibition of cytokinesis results in a binucleate state in which each nucleus has an identical genetic complement, aberrant exit from mitosis in the presence of inhibitors of spindle assembly results in a micronucleated state in which the individual micronuclei do not have an identical genetic complement. A sensing mechanism based upon the number of centrosomes or upon the total DNA content could generate a tetraploid arrest independent of whether the tetraploid cell is mononucleate, binucleate, or micronucleated. It will now be important to determine the mechanism by which mammalian cells read their tetraploid state.
It has been previously reported that arrest in G1 following exit from mitosis in the presence of inhibitors of microtubule assembly is accompanied by the suppression of cdk2 protein kinase activity (Stewart et al., 1999). Here, we find that G1 arrest following inhibition of cytokinesis leads to an equivalent inhibition of cdk2 activity. Cdk2 activity is required for progression from G1 to S (Pagano et al., 1993; Tsai et al., 1993), and one possible mechanism for the inhibition of cdk2 activity following tetraploidization is inhibition by the cdk2 inhibitor p21WAF1. Consistent with such a role, we find that p21WAF1 is induced by tetraploidization whether it results from inhibition of cytokinesis or from failure of mitotic arrest by spindle inhibitors.
A scenario of events consistent with our results is that tetraploidization stimulates p53-dependent transcription of p21WAF1, which leads to the inhibition of cdk activity and to the hypophosphorylation of pRb, which can independently block cell cycle progression (Kato et al., 1993; Dynlacht et al., 1994). pRb has been demonstrated to be required for G1 arrest following inappropriate mitotic exit in the presence of inhibitors of microtubule assembly (Di Leonardo et al., 1997). Given that pRb is hypophosphorylated following tetraploidization, either by exit from mitosis in the presence of inhibitors of spindle assembly or by inhibition of cytokinesis, pRb may be similarly required for the G1 tetraploidy checkpoint.
p53 is activated by ataxia telangiectasia mutated-dependent phosphorylation following ionizing radiation (Banin et al., 1998; Canman et al., 1998), but it is presently unknown whether tetraploidization activates p53 through the same or alternate pathways. Thus, it remains possible that although both DNA damage and tetraploidization induce p53-dependent G1 arrest, the cell might read these events independently as distinct insults to the genome.
Role of Tetraploidy Checkpoint in Maintenance of Genomic Integrity
p53 is well characterized as a tumor suppressor (Baker et al., 1990; Donehower et al., 1992), but how p53 serves this function is not clear. It has well-documented roles as a transactivator involved in many different processes, including the induction of cell cycle arrest following DNA damage (Kastan et al., 1992; Cox and Lane, 1995) and induction of apoptosis (Yonish-Rouach et al., 1991; Lowe et al., 1993). p53 also has a well-documented role in maintaining genomic stability (Hartwell, 1992; Donehower et al., 1995). Cell cycle arrest following DNA damage could be expected to promote genomic stability by allowing DNA repair before reactivation of the cell cycle machinery, whereas apoptosis would serve to eliminate cells with genomic damage. Most human solid tumors are genetically unstable, and the loss or gain of complete chromosomes is the predominant form of genetic instability (Lengauer et al., 1998). Aneuploidy is an important element of tumorigenesis (Li et al., 1997), and inactivation of p53 is probably an important element of this process (Harvey et al., 1993). Because we show here that the inactivation of p53 in tetraploid cells results in aneuploidization following the completion of a single tetraploid cell cycle, and because the tetraploid state is a frequent precursor to aneuploidization in solid human tumors (Shackney et al., 1989), we propose that the prevention of aneuploidy by blocking the rereplication of tetraploid cells that result from failures in mitosis may be a vital function of p53.
ACKNOWLEDGMENTS
We thank Dr. George R. Stark (Cleveland Clinic Foundation) for providing REF-52 and TAG cell lines, Dr. Moshe Oren (Weizmann Institute of Science) for providing packaging cells for the production of retroviruses expressing p53DD, and Dr. Rati Fotedar (Institut de Biologie Structurale) for supplying antibodies to cdk2 and cyclin E. This work was supported by grants from La Ligue nationale contre le Cancer (Laboratoire Labelisé) and from ARC (#9830). Olivier Lohez was supported by le Commissariat à l'Energie Atomique.
Abbreviations used:
- BrdU
bromodeoxyuridine
- DCB
dihydrocytochalasin B
- REF
rat embryo fibroblast
- TAG
simian virus-40 T-antigen transformed REF-52 cell
REFERENCES
- Andreassen PR, Margolis RL. Microtubule dependency of p34cdc2 inactibation and mitotic exit in mammalian cells. J Cell Biol. 1994;127:789–802. doi: 10.1083/jcb.127.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen PR, Martineau SN, Margolis RL. Chemical induction of mitotic checkpoint override in mammalian cells results in aneuploidy following a transient tetraploid state. Mutat Res. 1996;372:181–194. doi: 10.1016/s0027-5107(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Aubin JE, Osborn M, Weber K. Inhibition of cytokinesis and altered contractile ring morphology induced by cytochalasins in synchronized PtK2 cells. Exp Cell Res. 1981;136:63–79. doi: 10.1016/0014-4827(81)90038-0. [DOI] [PubMed] [Google Scholar]
- Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- Brénot-Bosc F, Gupta S, Margolis RL, Fotedar R. Changes in the subcellular localization of the replication initiation proteins and cell cycle proteins during G1- to S-phase transition in mammalian cells. Chromosoma. 1995;103:517–527. doi: 10.1007/BF00355316. [DOI] [PubMed] [Google Scholar]
- Buchkovich K, Duffy LA, Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989;58:1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Cahill DP, Lengauer C, Yu Y, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- Chan GK, Jablonski SA, Sudakin V, Hittle JC, Yen TJ. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J Cell Biol. 1999;146:941–954. doi: 10.1083/jcb.146.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PL, Scully P, Shew JY, Wang JY, Lee WH. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989;58:1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Cox LS, Lane DP. Tumor suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. Bioessays. 1995;17:501–508. doi: 10.1002/bies.950170606. [DOI] [PubMed] [Google Scholar]
- Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leonardo A, Kahn SH, Linke SP, Greco V, Seidita G, Wahl GM. DNA rereplication in the presence of mitotic spindle inhibitors in human and mouse fibroblasts lacking either p53 or pRb function. Cancer Res. 1997;57:1013–1019. [PubMed] [Google Scholar]
- Donehower LA, Godley LA, Aldaz CM, Pyle R, Shi YP, Pinkel D, Gray J, Bradley A, Medina D, Varmus HE. Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev. 1995;9:882–895. doi: 10.1101/gad.9.7.882. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P, Rausch C, Rasnick D, Hehlmann R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc Natl Acad Sci USA. 1998;95:13692–13697. doi: 10.1073/pnas.95.23.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ, Reed SI. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Dynlacht BD, Flores O, Lees JA, Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Galipeau PC, Cowan DS, Sanchez CA, Barrett MT, Emond MJ, Levine DS, Rabinovitch PS, Reid BJ. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett's esophagus. Proc Natl Acad Sci USA. 1996;93:7081–7084. doi: 10.1073/pnas.93.14.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galitski T, Saldanha AJ, Styles CA, Lander ES, Fink GR. Ploidy regulation of gene expression. Science. 1999;285:251–254. doi: 10.1126/science.285.5425.251. [DOI] [PubMed] [Google Scholar]
- Gorbsky GJ, Chen RH, Murray AW. Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J Cell Biol. 1998;141:1193–1205. doi: 10.1083/jcb.141.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E, Haffner R, von Ruden T, Wagner EF, Oren M. Down-regulation of wild-type p53 activity interferes with apoptosis of IL-3-dependent hematopoietic cells following IL-3 withdrawal. EMBO J. 1994;13:1368–1374. doi: 10.1002/j.1460-2075.1994.tb06390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instablility in cancer cells. Cell. 1992;71:543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- Harvey M, Sands AT, Weiss RS, Hegi ME, Wiseman RW, Pantazis P, Giovanella BC, Tainsky MA, Bradley A, Donehower LA. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene. 1993;8:2457–2467. [PubMed] [Google Scholar]
- Hinchcliffe EH, Miller FH, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–1550. doi: 10.1126/science.1056866. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Kung AL, Sherwood SW, Schimke RT. Cell-line specific differences in the control of cell cycle progression in the absence of mitosis. Proc Natl Acad Sci USA. 1990;87:9553–9557. doi: 10.1073/pnas.87.24.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni JS, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- Li R, Yerganian G, Duesberg P, Kraemer A, Willer A, Rausch C, Hehlmann R. Aneuploidy correlated 100% with chemical transformation of Chinese hamster cells. Proc Natl Acad Sci USA. 1997;94:14506–14511. doi: 10.1073/pnas.94.26.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Martineau SN, Andreassen PR, Margolis RL. Delay of HeLa cell cleavage into interphase using dihydrocytochalasin B: retention of a postmitotic spindle and telophase disc correlates with synchronous cleavage recovery. J Cell Biol. 1995;131:191–205. doi: 10.1083/jcb.131.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Boise LH, Thompson CB. Expression of Bcl-x and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996;10:2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Hammer RE, Messing A, Palmiter RD, Brinster RL. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987;238:188–193. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Lukas J, Baldin V, Ansorge W, Bartek J, Draetta G. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J Cell Biol. 1993;121:101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ME, Commane M, Stark GR. Simian virus 40 large tumor antigen alone or two cooperating oncogenes convert REF52 cells to a state permissive for gene amplification. Proc Natl Acad Sci USA. 1992;89:8112–8116. doi: 10.1073/pnas.89.17.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch PS, Reid BJ, Haggitt RC, Norwood TH, Rubin CE. Progression to cancer in Barrett's esophagus is associated with genomic instability. Lab Invest. 1989;60:65–71. [PubMed] [Google Scholar]
- Sandberg AA. Chromosome markers and progression in bladder cancer. Cancer Res. 1977;37:2950–2956. [PubMed] [Google Scholar]
- Schimke RT, Kung AL, Rush DF, Sherwood SW. Differences in mitotic control among mammalian cells. Cold Spring Harbor Symp Quant Biol. 1991;56:417–425. doi: 10.1101/sqb.1991.056.01.049. [DOI] [PubMed] [Google Scholar]
- Shackney SE, Smith CA, Miller BW, Burholt DR, Murtha K, Giles HR, Ketterer DM, Pollice AA. Model for the genetic evolution of human solid tumors. Cancer Res. 1989;49:3344–3354. [PubMed] [Google Scholar]
- Shaulian E, Zauberman A, Ginsberg D, Oren M. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol. 1992;12:5581–5592. doi: 10.1128/mcb.12.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ZA, Leach SD, Pietenpol JA. P21Waf1/Cip1 inhibition of cyclin E and Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol. 1999;19:205–215. doi: 10.1128/mcb.19.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, McKeon F. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell. 1997;89:727–735. doi: 10.1016/s0092-8674(00)80255-x. [DOI] [PubMed] [Google Scholar]
- Trielli MO, Andreassen PR, Lacroix FB, Margolis RL. Differential taxol-dependent arrest of transformed and nontransformed cells in the G1 phase of the cell cycle, and specific-related mortality of transformed cells. J Cell Biol. 1996;135:689–700. doi: 10.1083/jcb.135.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L-H, Lees E, Faha B, Harlow E, Riabowol K. The cdk2 kinase is required for the G1-S transition in mammalian cells. Oncogene. 1993;8:1593–1602. [PubMed] [Google Scholar]
- Weintraub SJ, Prater CA, Dean DC. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992;358:259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukemic cells that is inhibited by interleukin-6. Nature. 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]