Abstract
Components found within the extracellular matrix (ECM) have emerged as an essential subset of biomaterials for tissue engineering scaffolds. Collagen, glycosaminoglycans, bioceramics, and ECM-based matrices are the main categories of “raw materials” used in a wide variety of tissue engineering strategies. The advantages of raw materials include their inherent ability to create a microenvironment that contains physical, chemical, and mechanical cues similar to native tissue, which prove unmatched by synthetic biomaterials alone. Moreover, these raw materials provide a head start in the regeneration of tissues by providing building blocks to be bioresorbed and incorporated into the tissue as opposed to being biodegraded into waste products and removed. This article reviews the strategies and applications of employing raw materials as components of tissue engineering constructs. Utilizing raw materials holds the potential to provide both a scaffold and a signal, perhaps even without the addition of exogenous growth factors or cytokines. Raw materials contain endogenous proteins that may also help to improve the translational success of tissue engineering solutions to progress from laboratory bench to clinical therapies. Traditionally, the tissue engineering triad has included cells, signals, and materials. Whether raw materials represent their own new paradigm or are categorized as a bridge between signals and materials, it is clear that they have emerged as a leading strategy in regenerative medicine. The common use of raw materials in commercial products as well as their growing presence in the research community speak to their potential. However, there has heretofore not been a coordinated or organized effort to classify these approaches, and as such we recommend that the use of raw materials be introduced into the collective consciousness of our field as a recognized classification of regenerative medicine strategies.
Introduction
As the intertwined fields of tissue engineering and regenerative medicine continue to grow and evolve, the search for a “perfect” scaffold inevitably continues. This ongoing quest to search for new materials and fabrication techniques has led researchers anywhere from insect cuticle1,2 to precious metals and minerals3 over the past decade. Researchers are continuously finding new materials and technology for fabricating scaffolds with heightened mechanical integrity, porosity, biocompatibility, and biodegradability. Hollister4 described biomaterials used in tissue engineering scaffolds as the distinct “lynch pin” for finding effective regenerative solutions. Most attribute the lack of efficacy of biomaterials to the inability of materials to mimic the extracellular matrix (ECM) when compared with natural tissues and organs of the body.5 Recent trends in the field suggest that it may be appropriate to ask the question, “Have we looked too far for the ideal, synthetic biomaterial and missed the actual building blocks needed for scaffolds in this process?” Utilization of materials that occur naturally within the human body, such as collagen, chondroitin sulfate (CS), and calcium phosphates, has gained immense attention within the tissue engineering community.
This review seeks to indicate the emergence of raw materials as components of tissue engineering scaffolds. For the purpose of this review, we define raw materials as those found naturally within the human body, such as collagen, glycosaminoglycans (GAGs), bioceramics, and ECM-based matrices. Several comprehensive reviews of nonmammalian, natural polysaccharides, such as alginate, chitosan, dextran, and gelatin, have been detailed extensively in the literature.5–9 In this review, we intend to instead highlight the most widely used mammalian raw materials and the strategies behind using these materials as building blocks for tissue engineering scaffolds. In addition, we seek to review the connection made to formulate scaffolds based upon components of native extracellular matrix, which has been used as a strategy by many in the field, but has not been collectively brought to the attention of our field as a classification of strategies, but which perhaps should become part of our collective consciousness.
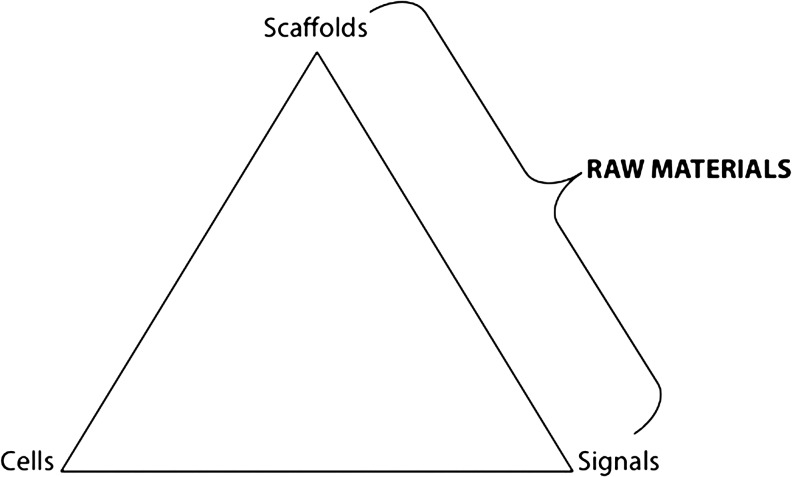
Traditionally, the most common strategy to develop a tissue-engineered construct is through a combination of the factors described in the tissue engineering triad (Fig. 1): scaffolds, signals, and cells. Scaffold development has attracted immense attention among researchers to design biomaterials with highly specific properties. The primary objective of a tissue engineering scaffold is to emulate the natural environmental conditions of the target tissue, while contributing to the synthesis of new tissue.8,10 Sokolsky-Papkov et al.11 outlined the ideal criteria for tissue engineering constructs: (a) sufficient mechanical properties, (b) low toxicity, (c) mimic the native extracellular matrix, (d) support cell adhesion and migration, and (e) degradation rate that is approximately equal to the growth rate of new tissue. Selection of the appropriate biomaterial(s) to construct a scaffold must take into consideration the differences that exist between the components, types, and organization of both the cells and the surrounding ECM of the tissue.12 One of the main advantages of raw materials is the ability of the body to recognize and metabolize these scaffold components in the local microenvironment, which mitigates toxicity or chronic inflammatory response that may be observed with synthetic polymers.13 Ultimately, the scaffold materials will influence multiple interactions in the microenvironment surrounding an implanted scaffold, which is crucial to the success or failure of tissue regeneration. Investigations of biomaterial-based “physical” signals propose that cell–biomaterial components and orientation at the micro/nanoscale level may affect cell survival, differentiation, and motility through interactions between cell surface receptors and ECM molecules.12,14 Toh et al.15 as well as Marklein and Burdick16 have suggested the importance of optimizing scaffold materials and fabrication processes to modulate these interactions. The researchers noted that physical cues, such as the scaffold formulation and/or geometry, and mechanical cues, such as matrix elasticity, should be controlled to aid in the proliferation and differentiation of stem cells.15,16 In addition, inherent adhesive cues or peptides and immobilized cues can also be incorporated into the biomaterial to manipulate the cell–matrix interface.15,16 Adopting a methodology that focuses on cell–scaffold interactions provides an effective strategy for utilizing material selection and fabrication to bridge two components of the tissue engineering triad—scaffolds and signals (Fig. 1). For example, selecting a collagen microparticle scaffold could affect three of these cues through high surface area and porosity (scaffold and geometric cue),14 soft matrix rigidity (mechanical cue), and inherent adhesive Arg-Gly-Asp (RGD) sequences.17 All of these scaffold attributes can collectively influence the local scaffold microenvironment before the addition of growth factors or cytokines, which is a common theme in many tissue engineering strategies.18 Raw materials, such as collagen, can effectively deliver microenvironmental cues without additional materials or fabrication steps that may be needed in a synthetic polymer scaffold. Therefore, the selection of raw materials as scaffold components could potentially bridge the gap between scaffolds and signals in the traditional tissue engineering triad, suggesting that the two are not modulated as separate components, but rather as integrated factors that contribute to the local scaffold microenvironment (Fig. 1). Employing raw materials that are natural components of tissue's ECM within scaffolds can act not only as a substrate for cell proliferation and attachment, but also as a physical signaling environment for differentiation.
FIG. 1.
Schematic of the traditional tissue engineering triad illustrating the potential of raw materials to bridge the gap between scaffolds and signals.
The following sections will highlight four main categories of raw materials that are commonly used in recent tissue engineering scaffold strategies: collagen, GAGs, bioceramics, and ECM-based materials. Within each section, the most frequently used materials for tissue repair and regeneration purposes will be highlighted. For organizational purposes, although raw materials have been grouped by material type in the following sections, due to the overlap of multiple raw materials in several approaches, tables are arranged by the target tissue application. Specifically, raw materials used in bone tissue engineering applications in vitro and in vivo can be found in Tables 1 and 2, respectively. Strategies employed in cartilage tissue engineering in vitro and in vivo can be found in Tables 3 and 4, respectively. Additional target tissue applications can be found in Table 5 and strategies utilizing small intestinal submucosa (SIS) can be found in Table 6.
Table 1.
Recent Applications of Raw Material Strategies for In Vitro Bone Tissue Engineering Applications
| Reference(s) | Raw material(s) | Additional material(s) | Scaffold formulation | Cell type | Growth factor supplementation |
|---|---|---|---|---|---|
| Chan et al.44 | Collagen type I (rat) | – | Microspheres | Human and murine MSCs | – |
| Koegh et al.46 | Collagen type I (bovine), CS | – | Porous composite | Human fetal osteoblasts | TGF-β1 |
| Kruger et al.34 | Collagen type I (bovine) | PLGA | Porous matrix | Human MSCs | – |
| Shen et al.42 | Collagen type I (porcine), HAp | – | Nanocomposite hydrogel | – | – |
| Sionkowska and Kozlowska29 | Collagen type I, HAp | – | Nanocomposite hydrogel | – | – |
| Thein-Han and Xu35 | Collagen type I (rat) | CaP cement, alginate | Injectable microbead hydrogel | Human UCMSCs | – |
| Wang and Stegemann40 | Collagen type I (bovine) | Chitosan | Composite hydrogel | Human MSCs | – |
| Akkouch et al.27 | Collagen type Ib, HAp | PLCL | Porous composite | Human osteosarcoma | HEGF |
| Chicatun et al.57 | Collagen type I (rat) | Chitosan | Dense collagen composite hydrogel | MC3T3-E1 | – |
| Marelli et al.43 | Collagen type I (rat) | Bioactive glass | Composite hydrogel | MC3T3-E1 | – |
| Bae et al.80 | HA | – | Hydrogel | MC3T3-E1 | Simvastatina |
| Chen et al.81 | HA, collagen type Ib | PCL | Porous matrix | hMSC-TERT | – |
| Chen et al.82 | HA, collagen type I (bovine) | Bioactive glass, PS | Porous composite | MC3T3-E1 | – |
| Liao et al.83 | HA | HA-CPN | Injectable, thermoresponsive hydrogel | Canine MSCs | TGF-β1 |
| Li et al.104 | HAp | Chitosan, PLLA | Porous composite | MC3T3-E1 cells | – |
| Liu et al.109 | nHAp, collagen type Ib | PLA | Porous composite | Rabbit DPSCs | BMP-2 |
| Peng et al.107 | HAp | PLLA | Nanofibrous composite | Rat osteosarcoma cells | – |
| Prosecka et al.110 | HAp, collagen type I (bovine) | – | Porous composite | Porcine MSCs | – |
| Haimi et al.122 | TCP | PLA, bioactive glass | Porous composite | Human ASCs | – |
| Lee et al.117 | TCP, collagen type I (porcine) | PCL | Porous composite | MG63 | – |
| Lin et al.131 | TCP | – | Porous matrix | – | – |
| Rai et al.118 | TCP | PCL | Porous composite | hMSCs | – |
| Yanoso-Scholl et al.123 | TCP | PLA | Porous composite | None | BMP-2, VEGFa |
| Yeo et al.120 | TCP, collagen type I (porcine) | PCL | Nanofibrous composite | MG63 | – |
| Zhang et al.126 | TCP, collagen type Ib | – | Microfibrous composite | MG63 | – |
| Honsawek et al.141 | SIS, human DBM | – | Composite matrix | Human periosteal cells | – |
| Supronowicz et al.155 | Human DBM | – | Porous matrix | Human ASPSCs | – |
| Thomas et al.160 | Bovine DBM | PL | Composite matrix | Murine MSCs | – |
| Lee et al.156 | Human DBM, HAp | – | Porous composite | Human MSCs | – |
| Liu et al.152 | pDBM | – | Porous matrix | UCB-BMSCs | TGF-β1 |
| Chen et al.153 | Bovine DBM | Heparin | Porous matrix | HUVECs | VEGFa |
| Jayasuriya et al.162 | Human DBM | PLGA | Composite film | Murine MSCs | – |
| Kang et al.158 | Human DBM | Fibrin glue | Composite glue | Porcine SDMSCs | – |
Denotes incorporation of the molecules into the scaffold. All other entries indicate the addition of the growth factor to culture medium; dexamethasone, β-glycerophosphate, and ascorbic acid were considered standard osteogenic medium components and not factored in for growth factor supplementation.
Collagen species not specified.
CS, chondroitin sulfate; HAp, hydroxyapatite; HA, hyaluronic acid; TCP, β-tricalcium phosphate; SIS, porcine small intestinal submucosa; DBM, demineralized bone matrix; pDBM, partially demineralized porcine trabecular bone; PLGA, poly(lactic-co-glycolic acid); CaP, calcium phosphate; PLCL, poly(lactide-co-ɛ-caprolactone); HA-CPN, hyaluronic acid-g-chitosan-g-poly(N-isopropylacrylamide); PCL, poly(ɛ-caprolactone); PLA, poly(L-lactic acid); PLLA, poly(L-lactic acid); PL, polylactide; MSCs, mesenchymal stem cells; UCMSCs, umbilical cord mesenchymal stem cells; MC3T3-E1, murine calvarial osteoblasts; hMSC-TERT, human mesenchymal stem cell–telomerase reverse transcriptase gene-transduced; DPSCs, dental pulp stem cells; ASCs, adipose stem cells; MG63, human osteoblast-like cells; ASPSCs, adipose-derived side population stem cells; UCB-BMSCs, human umbilical cord blood–derived mesenchymal stem cells; HUVECs, human umbilical vein endothelial cells; SDMSCs, skin-derived mesenchymal stem cell–like cells; TGF-β1, transforming growth factor beta-1; HEGF, human epidermal growth factor; BMP-2, bone morphogenetic protein-2; VEGF, vascular endothelial growth factor.
Table 2.
Recent Applications of Raw Material Strategies for In Vivo Bone Tissue Engineering Applications
| Reference(s) | Raw material(s) | Additional material(s) | Scaffold formulation | Animal model | Highlighted finding |
|---|---|---|---|---|---|
| Bae et al.80 | HA | Simvastatin | Hydrogel | New Zealand white rabbits, parietal bone defect model | Hydrogels loaded with simvastatin significantly increased new bone formation after 9 weeks |
| Chen et al.82 | HA, collagen type I (bovine) | Bioactive glass, phosphatidylserine | Porous composite | New Zealand white rabbits, radial defect model | Composite scaffold promoted new bone formation and new blood vessel formation after 12 weeks compared to the control (sham) which displayed little new bone formation |
| Xu et al.78 | HA, collagen type I (bovine) | Bioactive glass, phosphatidylserine | Porous composite | Sprague-Dawley rat, femoral defect model | Composite scaffolds seeded with MSCs enhanced greater new bone formation compared with control scaffolds with no seeded cells after 6 weeks |
| Patterson et al.79 | HA | HA-GMA | Hydrogel | Sprague-Dawley rat, critical-sized calvarial defect model | Codelivery of BMP-2 and VEGF from hydrogels showed improved new bone formation after 6 weeks compared with either of the proteins when delivered alone |
| Rentsch et al.99 | CS, collagen type I (porcine) | PCL | Porous composite | Athymic nude mice, critical-sized femoral defect model | PCL meshes coated with CS/collagen type I and seeded with rat MSCs showed significantly increased new bone formation and new blood vessel formation after 12 weeks |
| Lee et al.156 | HAp, human DBM | – | Porous composite | Athymic nude rat, intramuscular abdominal pouch model | DBM/HA putty induced ectopic mineralized bone formation after 8 weeks, HA granules alone (control) showed limited mineralization |
| Liu et al.109 | nHAp, collagen type Ia | PLA | Porous composite | New Zealand white rabbits, segmental critical-sized alveolar bone defect model | nHAp–collagen type I–PLA scaffolds seeded with rabbit DPSCs and cultured with BMP-2 prior to implantation promoted new bone formation after 12 weeks |
| Teixeira et al.102 | HAp, collagen type I (bovine) | – | Porous matrix | Immune-deficient mice, subcutaneous implantation model | HAp scaffolds seeded with human MSCs showed that those with a collagen coating tended to have a negative effect on bone formation regardless of collagen crosslinking method after 6 weeks |
| Yeo et al.119 | TCP | PCL, NaOH | Porous composite | New Zealand white rabbits, calvarial defect model | PCL-TCP scaffolds treated with NaOH for 48 hours to increase surface roughness showed superior bone formation after 8 weeks |
| Yanoso-Scholl et al.123 | TCP | PLA | Porous composite | C57BL/6 mice, intramuscular quadriceps implantation | Scaffolds loaded with BMP-2 and VEGF promoted new blood vessel formation but limited mineralization after 8 weeks |
| Rojbani et al.174 | TCP, HAp | – | Porous matrix | Wistar rat, calvarial defect model | TCP promoted greater bone regeneration after 8 weeks, the addition of simvastatin increased bone formation in all groups |
| Rai et al.118 | TCP | PCL | Porous composite | CBH/Rnu rats, critical-sized femoral defect model | Composites seeded with human MSCs showed new bone formation after 3 weeks compared with limited bone formation in scaffolds seeded with no cells |
| Hao et al.125 | TCP, collagen type I (bovine) | PLGA | Composite hydrogel | Japanese white rabbits, critical-sized segmental radial defect | Higher cell numbers of rabbit ASCs encapsulated within the scaffold led to enhanced osteogenesis and bone union after 24 weeks |
| Ghanaati et al.115 | TCP | – | Granules with varying size and porosity | Wistar rat, subcutaneous implantation model | Higher porosity led to greater new blood vessel formation near the center of the construct over 60 days |
| Ghanaati et al.129 | TCP, HA | Methylcellulose | Injectable bone paste | Wistar rat, subcutaneous implantation model | The addition of HA and methylcellulose resulted in a formable material to fill in bone defects and led to higher vascularization after 60 days |
| Cao et al.124 | TCP, HAp | PGA | Porous composite | Sprague-Dawley rat, critical-sized femoral defect | PGA–TCP scaffolds in a 1:3 ratio provided the greatest new bone formation after 90 days |
| Eleftheriadis et al.157 | TCP, human DBM | Hydroxyl sulfate | Porous composite | New Zealand white rabbits, mandibular defect model | TCP–hydroxyl sulfate scaffolds resorbed more slowly than DBM putty over 8 weeks, making them a potential candidate for larger, critical-sized defects |
| Fujita et al.127 | TCP | Gelatin | Sponge | Nihon white rabbits, segmental bone defect model | No significant difference was found in new bone regeneration between gelatin–TCP sponge and the BMP-2-loaded gelatin–TCP sponge after 8 weeks |
| Tadokoro et al.128 | TCP | Gelatin | Sponge | Fisher rats, subcutaneous implantation model | TCP–gelatin sponges loaded with BMP-2 and seeded with MSCs showed significant new bone formation compared with nonloaded scaffolds and scaffolds with no cells after 4 weeks |
| Abbah et al.116 | TCP | PCL | Porous composite | Yorkshire pigs, spinal interbody fusion model | PCL–TCP scaffolds seeded with autogenous MSCs showed new bone formation after 3 months and fusion was observed after 6 months, no fusion occurred in control samples with no seeded cells |
| Chen et al.153 | Bovine DBM | – | Porous matrix | Sprague-Dawley rat, subcutaneous implantation model | Heparin-crosslinked DBM loaded with VEGF promoted new blood vessel formation superior to unloaded and noncrosslinked scaffolds after 3 weeks |
| Kang et al.158 | Human DBM | Fibrin glue | Composite glue | Miniature pig, maxillary sinus floor implantation model | Enhanced new bone activity was observed in the cell-seeded scaffold sites compared with the scaffold-only regions after 4 weeks |
| Rhee et al.154 | Human DBM | PLA | Porous composite | Sprague-Dawley rat, critical-sized calvarial defect model | DBM seeded with SVF cells promoted greater new bone formation than groups containing PLA and those without cells after 8 weeks |
| Supronowicz et al.155 | Human DBM | – | Porous matrix | Athymic nude rat, intramuscular abdominal pouch model | DBM seeded with human ASPSCs provided significantly greater new bone formation after 14 days |
Collagen species not specified.
nHAp, nanohydroxyapatite; HA-GMA, glycidyl methacrylate modified hyaluronic acid; NaOH, sodium hydroxide; PGA, poly(glycolic acid); SVF, human stromal vascular fraction.
Table 3.
Recent Applications of Raw Material Strategies for In Vitro Cartilage Tissue Engineering Applications
| Reference(s) | Raw material(s) | Additional material(s) | Scaffold formulation | Cell type | Growth factor supplementation |
|---|---|---|---|---|---|
| Li et al.45 | Collagen type I (rat) | – | Microspheres | Human MSCs | TGF-β3 |
| Lu et al.26 | Collagen type I (porcine) | – | Sponge | Bovine chondrocytes | – |
| Lu et al.33 | Collagen type I (porcine) | PLGA | Porous composite | Bovine chondrocytes | – |
| Ng et al.32 | Collagen type I, collagen type II (porcine) | – | Porous matrix | Porcine MSCs and murine ECCs | TGF-β1 |
| Ohyabu et al.31 | Collagen type I,a HAp, CS | – | Porous composite sponge | Rabbit MSCs | TGF-β3 |
| Yan et al.28 | Collagen type I (bovine), chitosan | Chitosan | Composite hydrogel | Rabbit chondrocytes | – |
| Berendsen et al.36 | Collagen type I (rat), collagen type II (chicken sternum) | – | Hydrogel | Goat articular chondrocytes | – |
| Zhang et al.67 | Collagen type I (bovine), HA, CS | – | Composite hydrogel | Rabbit articular cartilage | – |
| Mueller-Rath et al.59 | Collagen type I (rat) | – | Dense collagen hydrogel | Human articular chondrocytes | – |
| Chang et al.60 | Collagen type II,a CS | PCL | Coated porous mesh | Rat chondrocytes | – |
| Francioli et al.61 | Collagen type II (porcine) | – | Porous matrix | Human articular chondrocytes | TGF-β1, TGF-β3, FGF-2 |
| Vickers et al.22 | Collagen type II (porcine), GAG | – | Composite hydrogel | Carpine MSCs | FGF-2, TGF-β1 |
| Wu et al.62 | Collagen type II (bovine) | Exogenous GAGs | Composite hydrogel | Human articular chondrocytes | – |
| Park et al.77 | HA | Fibrin | Composite hydrogel | Rabbit MSCs | TGF-β1 |
| Fan et al.76 | HA, CS | PLGA, gelatin | Porous composite | Rabbit MSCs | TGF-β3b |
| Correia et al.66 | HA | Chitosan | Porous composite | Bovine chondrocytes | TGF-β3 |
| Nguyen et al.54 | CS, HA | PEG, MMP-pep | Multilayered hydrogel | Murine MSCs | TGF-β1 |
| Coburn et al.97 | CS | PCL, PVAMA, CSMA, PEGDA | Fiber–hydrogel composite | Goat MSCs | – |
| Liang et al.56 | Concentrated CS, collagen type I (bovine) | – | Porous composite | Human MSCs | TGF-β1, FGF-2 |
| Kinneberg et al.53 | CS, collagen type I (bovine) | – | Sponge | Rabbit MSCs | – |
| Wang et al.159 | Human DBM | Gelatin, fibrin glue | Composite sponge | Rabbit articular chondrocytes | – |
Collagen species not specified.
Denotes incorporation of the protein into the scaffold. All other entries indicate the addition of the growth factor to culture medium; nonessential amino acids, ascorbic acid, and dexamethasone were considered standard chondrogenic medium components and were not factored in for growth factor supplementation.
GAG, glycosaminoglycan; PEG, poly(ethylene glycol); MMPs, matrix metalloproteinase–sensitive peptides; PVAMA, poly-(vinyl alcohol)-methacrylate; CSMA, chondroitin sulfate-methacrylate; PEGDA, poly(ethylene glycol)-diacrylate; ECCs, P19 embryonal carcinoma cells; FGF-2, fibroblast growth factor-2.
Table 4.
Recent Applications of Raw Material Strategies For In Vivo Cartilage Tissue Engineering Applications
| Reference(s) | Raw material(s) | Additional material(s) | Scaffold formulation | Animal model | Highlighted finding |
|---|---|---|---|---|---|
| Chang et al.37 | Collagen type I (porcine) | – | Hydrogel | Lee-Sung miniature pigs, osteochondral defect model | Undifferentiated collagen gels seeded with porcine MSCs were superior to those that were differentiated using TGF-β3 prior to implantation based on gross appearance and histological evaluation after 6 months |
| Lu et al.26 | Collagen type I (porcine) | – | Funnel-like sponge | Athymic nude mice, subcutaneous dorsa model | After 3 weeks, funnel-like collagen sponges outperformed control collagen sponges in cell number and GAG production |
| Lu et al.33 | Collagen type I (porcine) | PLGA | Funnel-like hybrid sponge | Athymic nude mice, subcutaneous dorsa model | Funnel-like hybrid sponges (collagen type I–PLGA) outperformed collagen-only sponges in the expression of collagen type II and aggrecan genes after 7 weeks of implantation |
| Fan et al.76 | HA, CS | PLGA, gelatin with immobilized TGF-β3 | Porous composite sponge | New Zealand white rabbits, full-thickness osteochondral defect model | After 8 weeks, TGF-β3-immobilized scaffolds seeded with autologous MSCs promoted significant cartilage formation when compared with control (no TGF-β3) |
| Yagihashi et al.164 | Bovine DDM | – | Powder | New Zealand white rabbits, full-thickness osteochondral defect model | After 9 weeks, defects filled with 100 mg of DDM had filled in with hyaline-like cartilage, with incomplete cartilage formation in the control (sham) group |
DDM, demineralized dentin matrix.
Table 5.
Recent Applications of Raw Material Strategies for Additional Tissue Engineering Applications
| Target tissue | Reference(s) | Raw material(s) | Additional material(s) | Scaffold formulation | Biological model(s) |
|---|---|---|---|---|---|
| Skin | Gaspar et al.25 | Collagen type I (bovine) | Agarose | Composite sponge | In vitro: composite sponges seeded with L929 mouse fibroblasts and cultured for 48 hours |
| Hartwell et al.38 | Collagen type I (rat), CS | PVA | Composite hydrogel | In vitro: primary human fibroblasts were encapsulated within the hydrogel and cultured for 10 days | |
| Wang et al.50 | Collagen type I (bovine), HA, CS | – | Porous composite | In vitro: 9 different ratios of collagen type I, HA, and CS were tested and seeded with rat dermal fibroblasts | |
| In vivo: composite seeded with rat dermal fibroblasts and cultured for 7 days before implantation into full-thickness skin defect model in Sprague-Dawley rats for 6 weeks | |||||
| Wong et al.39 | Collagen type I (rat) | Pullulan | Composite hydrogel | In vitro: murine MSCs and human foreskin fibroblasts were seeded onto separate hydrogel scaffolds and cultured for 7 days | |
| In vivo: hydrogel implanted into subcutaneous wound model and stented excisional wound model in C57BL/6 mice for 21 days | |||||
| Ghezzi et al.58 | Collagen type I (bovine) | Silk fibroin | Dense collagen multilayered composite | In vitro: composites seeded with rat MSCs and cultured for 7 days | |
| Zhang et al.75 | HA | Gelatin | Composite hydrogel | In vitro: hydrogels seeded with L929 mouse fibroblasts and cultured for 7 days | |
| Emami et al.98 | CS | Gelatin, chitosan | Composite hydrogel | In vitro: hydrogels seeded with L929 mouse fibroblast and cultured for 14 days | |
| Liang et al.56 | CS, collagen type I (bovine) | – | Porous composite (concentrated CS) | In vitro: composite seeded with human foreskin keratinocytes and cultured for 7 days | |
| Osteochondral | Harley et al.63 | Collagen type I (bovine), collagen type II (porcine), CS, CaP | – | Multilayered porous composite | – |
| Sundararaghavan and Burdick92 | HA | RGD peptide | Gradient fibrous composite | In vitro: scaffolds seeded with aortic arch explants from chick embryos and cultured for 7 days | |
| Zhou et al.103 | HAp, collagen type Ia | – | Porous, layered composite | In vitro: scaffold seeded with human MSCs and cultured in both osteogenic and chondrogenic medium for 14 days | |
| Haaparanta et al.121 | TCP | PLA | Porous composite | – | |
| Niyama et al.130 | TCP | – | Porous matrix with chondrocyte cell sheet | In vitro: porcine articular chondrocyte attached to TCP block and cultured for 21 days | |
| Nucleus Pulposus | Calderon et al.21 | Collagen type II (bovine), HA | – | Composite hydrogel | In vitro: rat MSCs injected into hydrogels and cultured for 21 days |
| Park et al.90 | HA | Silk fibroin | Composite hydrogel | In vitro: human chondrocytes encapsulated in hydrogel and cultured for 28 days | |
| Nerve | Suri et al.41 | Collagen type Ia, HA | Laminin | Hydrogel | In vitro: rat neonatal Schwann cells were encapsulated in hydrogel and cultured for 14 days |
| Tendon, meniscus, ligament | Caliari et al.47 | Collagen type I (bovine), CS | – | Core-shell composite | In vitro: composites seeded with horse tendon cells and cultured for 14 days |
| Freymann et al.88 | HA | PGA | Porous composite | In vitro: composites seeded with human adult meniscus-derived cells and cultured for 21 days | |
| Vascular | Duffy et al.55 | Collagen type I,a CS | – | Porous composite | In vitro: composites seeded with rat MSCs or rat aortic endothelial cells and cultured for 28 days |
| Perng et al.30 | Collagen type I (bovine), HA | – | Porous composite | In vivo: composites implanted into inferior epigastric skin flap of nude mice as angiogenesis model for 28 days | |
| Ekaputra et al.84 | Collagen type I (bovine), HA | PCL | Fiber–hydrogel composite | In vitro: composites seeded with HUVECs and IMR90 human lung fibroblast cells and cultured for 14 days | |
| Seidlits et al.85 | HA | Fibronectin | Composite hydrogel | In vitro: HUVECs encapsulated in hydrogels and cultured for 6 days | |
| Tedder et al.150 | Adult swine pericardium | – | Trilayered construct with adhesive | In vivo: constructs implanted subdermally into Sprague-Dawley rats for 35 days | |
| Urogenital | Engelhardt et al.51 | Collagen type I (rat) | PLAC | Porous composite (dense collagen) | In vitro: human SMCs and urothelial cells encapsulated within composite and cultured for 14 days |
| In vivo: precultured constructs (14 days) were implanted subcutaneously on the backs of nude mice for 6 months |
Collagen species not specified.
PVA, poly(vinyl alcohol); RGD, (arginine-glycine-aspartic acid); PLAC, poly(lactic acid-co-ɛ-caprolactone); SMCs, smooth muscle cells.
Table 6.
Recent Tissue Engineering Strategies Utilizing Porcine Small Intestinal Submucosa
| Target tissue | Reference(s) | Additional material(s) | Scaffold formulation | Biological model(s) |
|---|---|---|---|---|
| Bone | Kim et al.142 | – | Sponge | In vitro: sponges seeded with rat MSCs and cultured for 14 days |
| In vivo: sponges seeded with cells and implanted into a cranial defect model in Fisher rats for 28 days | ||||
| Honsawek et al.141 | Human DBM | Tissue/composite matrix | In vitro: scaffolds seeded with human periosteal cells and cultured for 10 days | |
| In vivo: composites were implanted intramuscularly into Wistar rats for 42 days | ||||
| Zhao et al.143 | – | Hydrated SIS matrix | In vivo: SIS scaffolds seeded with rabbit MSCs and implanted into radial bone defects of critical size in New Zealand white rabbits for 12 weeks | |
| Skin | Zhou et al.145 | – | Hydrated SIS matrix | In vitro: scaffolds seeded with murine ADSCs and cultured for 7 days before digestion |
| In vivo: scaffolds seeded with murine ADSCs and cultured for 1 week and then implanted into cutaneous and subcutaneous wound models in C57 mice for 28 days | ||||
| Nerve | Kang et al.144 | PLGA | Porous composite | In vivo: composite scaffolds seeded with rat ADSCs and implanted into complete spinal cord transaction in Fisher rat model for 8 weeks |
| Vascular | Liu et al.86 | Collagen type I–HA–CS (comparison study between SIS and polymer composite) | Tissue scaffold and polymer composite | In vivo: SIS and polymer composite scaffolds seeded with murine ADSCs and implanted into full-thickness cutaneous defects in C57BL/6 mice for 21 days |
| Mondalek et al.87 | HA-PLGA nanoparticles | Porous composite | In vivo: composite scaffolds implanted into canine bladder model of Beagle dogs for 10 weeks to evaluate angiogenic potential | |
| Crapo et al.137 | – | Gel | In vitro: SIS gel seeded with rat neonatal cardiomyocytes and cultured for 13 days | |
| Okada et al.138 | – | Gel | In vivo: SIS gel injected into infarct cardiac tissue in NON-SCID mice for 6 weeks | |
| Peng et al.139 | – | Hydrated SIS matrix | In vitro: SIS tissue seeded with lamb hair follicle MSCs and cultured for 14 days under uniaxial strain conditions | |
| Tan et al.140 | – | Hydrated SIS matrix | In vivo: SIS sheets seeded with rabbit MSCs and implanted to patch infarct myocardial tissue model in New Zealand White rabbits for 28 days | |
| Urogenital | Heise et al.146 | – | Hydrated SIS matrix | In vitro: SIS sheets seeded with rat MSCs and subjected to a period of static culture for 7 days followed by dynamic culture with cyclic strain for an additional 7 days |
| Qin et al.147 | – | Hydrated SIS matrix | In vitro: SIS sheets seeded with rat intestinal SMCs and implanted into jejunal interposition model of adult Lewis rats for 8 weeks | |
| Wu et al.148 | – | Hydrated SIS matrix | In vitro: SIS sheets seeded with human UDSCs and cultured under static and dynamic conditions for 14 days. Cultured sheets were sectioned for in vitro characterization and implantation | |
| In vivo: precultured SIS sheets were implanted subcutaneously into the flanks of athymic nude mice for 1 month | ||||
| Zhang et al.149 | – | Hydrated SIS matrix | In vivo: SIS sheets implanted into abdominal wall defect model in adult Sprague-Dawley rats for 8 weeks |
ADSCs, adipose-derived stem cells; NON-SCID, nonobese diabetic severe combined immunodeficiency; UDSCs, urine-derived stem cells.
Collagen
Collagen is the most prevalent protein in the body, making up approximately 30 percent of proteins in mammals, and is responsible for both tensile strength and structural support in the ECM of many tissues.19 Collagen type I is the most universal type—found in bone, skin, tendons, ligaments, and other tissues—and its ubiquity has made it one of the most frequently used raw materials in tissue engineering over the past decade.20 Hyaline cartilage and nucleus pulposus are the main tissues that contain little collagen type I in their native ECM, but are rich in collagen type II.10,21,22 The main advantages of utilizing collagen as a part of a tissue engineering scaffold include its intrinsic cell adhesion motif RGD, biocompatibility, and bioresorbability.17,23 Questions concerning immunogenicity are considered negligible with the development of enzymatic digestion procedures to remove telopeptides.23 Poor mechanical properties and rapid degradation are the main drawbacks when considering collagen as a scaffold component.17,20,23 The following sections will discuss the use of collagens type I and II in tissue engineering scaffolds and applications of collagen as a component of constructs (Tables 1–5). The reader is also directed to an extensive review on the use of collagen scaffolds in tissue engineering23 and collagen nanofibers for bone tissue engineering applications.24 Strategies for overcoming limitations associated with collagen biomaterials will be highlighted along with raw material scaffold concepts used in several areas of tissue engineering.
Collagen type I
Collagen type I scaffold formulations have included sponges,25–33 fibers,19,34,35 hydrogels,36–43 and microspheres.44,45 Applications of collagen type I span target areas of bone,27,29,34,35,40,42,44,46 tendon,19,47 peripheral nerves,41,48 cartilage,26,28,31–33,37,45 skin,25,39,49,50 and bladder tissue engineering.51 To address limitations associated with collagen, researchers have often chosen to use different crosslinking agents and/or composites of collagen with other materials.20 In many approaches, blends of collagen I with CS, hyaluronic acid (HA), bioceramics, and synthetic polymers have been utilized to enhance mechanical properties, reduce susceptibility to degradation, and encourage mineralization.7 Seo et al.52 provided a comprehensive review of the reinforcement of collagen and other raw materials by synthetic polymers. Akkouch et al.27 presented an interesting approach of employing a reinforced natural material scaffold composed of collagen–hydroxyapatite (HAp)–poly(lactide-co-ɛ-caprolactone) (PLCL) for bone tissue engineering (Table 1). In this case, PLCL offered a solution to enhance the inherent poor mechanical stability that collagen and HAp lacked when used without a reinforcing material.27 This composite material showed the innovative use of both a synthetic and bioceramic material additives to a collagen type I matrix to overcome limitations associated with each of the materials when used alone.
Other strategies have combined collagen with GAGs for additional applications. For example, scaffolds of type I collagen and chondroitin-6-sulfate, termed in the literature more generally as collagen–GAG or CG scaffolds, represent a common raw material blend for bone,46 cartilage,53,54 tendon,47 and skin38,55,56 tissue engineering. One particularly innovative raw material technique used a CG core-shell fabrication strategy to enhance mechanical integrity while maintaining a highly porous structure.47 The scaffold consisted of a high-density CG shell to promote tensile strength and a low-density CG core scaffold with high porosity (Table 5). This study was representative of a scaffold that combined an innovative formulation approach and raw materials for tendon tissue engineering.
Another method for overcoming the inherent poor mechanical properties of collagen included plastic compression of collagen type I hydrogels to produce dense collagen.43,51,57–59 This approach has been employed for applications in bone,43,57 cartilage,59 and bladder51 tissue engineering with favorable outcomes. Chicatun et al.57 fabricated a dense collagen and chitosan scaffold that retained an open, interconnected pore structure that attempted to mimic the osteoid of native bone (Table 1). This strategy demonstrated an excellent example of the use of a raw material to mimic not only a component of native bone tissue but also the inherent pore structure and ECM structure. The ubiquity of collagen type I in the body and the versatility of scaffold formulations have promoted widespread use in tissue engineering scaffolds. Relatively new fabrication methods, such as dense collagen techniques, help to mitigate mechanical limitations without the need for additional materials. However, crosslinking and composite strategies still remain the most common approach for enhancing construct properties, while maintaining the benefits associated with cell adhesion capability of collagen.
Collagen type II
Collagen type II has been used much less frequently in raw material strategies for tissue engineering constructs, mostly likely due to its presence in considerably fewer extracellular matrices of tissues in the body. Scaffold formulations reported recently in the literature of collagen type II include hydrogels,21,36 sponges,22,60–62 and microspheres.14 These scaffolds have been mainly utilized for cartilage22,36,60–63 and nucleus pulposus21 tissue engineering. Hyaline cartilage and the nucleus pulposus have the greatest amount of collagen type II present in their ECM with little-to-no collagen type I, so this material strategy may be beneficial for these limited applications. One group utilized a collagen type I/calcium phosphate layered with an interfacial layer connecting to a collagen type II/CS layer to mimic native constituents involved in the transition of tissue types at the osteochondral interface.63 Calderon et al.21 utilized a similar strategy to formulate a scaffold for nucleus pulposus tissue engineering that consisted entirely of raw materials. They used collagen type II and HA in a ratio equivalent to the native tissue ECM of the nucleus pulposus and noted that with sufficient crosslinking, this raw material scaffold would be a potential candidate for regeneration of the nucleus pulposus (Table 5).21 Far fewer approaches utilize collagen type II in raw material scaffolds; however, the strategy of mimicking native ECM composition has increased its utility in hyaline cartilage and nucleus pulposus applications.
Summary
Overall, collagen type I has been explored in numerous areas of tissue engineering with growing interest in areas of new fabrication techniques and composite strategies. Collagen type II, however, has been utilized much less frequently and may require more in-depth studies to verify its potential. It is unclear whether the limited use of collagen II is due more to its high cost and limited availability, the absence of compelling data thus far to support its use, a limited awareness of the idea to use collagen II, or a combination of the above. There is no question, however, that using collagen I or collagen II can allow for scaffold bioresorbability and cell adhesion unmatched by synthetic polymers, which will most likely continue to propagate its use as a raw material component in tissue engineering scaffolds.
Glycosaminoglycans
Over the past decade, GAGs have emerged as an additional raw material strategy for multiple tissue engineering applications. Two of the most widely used GAGs include HA and CS. HA is well known for its role in the regulation of cell behaviors, such as adhesion, proliferation, differentiation, and migration.64 However, limitations including water solubility, fast resorption, and negative charge have caused researchers to adopt specific concentration limits and fabrication methods.65,66 CS functions as a structural component of native ECM and strategies have utilized CS in tissue-engineered constructs often with additional raw materials, such as HA and collagen, respectively.53,67 The main motivation for blending CS with additional raw materials or synthetic polymers lies in its innate capability to be readily water soluble.9 Some of the approaches used to overcome weaknesses and incorporate these raw materials will be discussed in the following sections. For more in-depth reviews of all natural polysaccharides used in tissue engineering, the reader is directed to articles by Baldwin et al.6 and Oliveira et al.9 An exceptional review of HA is also available from Murano et al.64 Hydrogels that are fabricated from biopolymers have also been reviewed extensively, and the reader is directed to articles by Van Vlierberghe et al.,68 Slaughter et al.,69 Spiller et al.,70 Hunt et al.,71 and Burdick and Prestwich.72
Hyaluronic acid
HA is the only nonsulfated GAG and is found in the ECM of many tissues in the body. HA is well known for its viscoprotective capabilities and has been used in ophthalmology applications for over 30 years.73 Supplementation of HA for synovial fluid viscosity in arthritic joints has also been used for over a decade.73 In addition, HA interacts with specific protein receptors on the surface of cells, such as CD44 and RHAMM, to modulate cell adhesion, proliferation, motility, and other signaling cascades.74 For these reasons, HA has been utilized in recent tissue engineering strategies for skin,50,75 cartilage,14,66,76–78 bone,79–83 angiogenesis,30, 84–87 meniscus,88 nerve,41,89 and nucleus pulposus21,90 applications. Methacrylated HA that is crosslinked to form hydrogels21,41,72,79,80,83,89–91 has been the most common formulation as a tissue engineering construct; however, electrospun fibers,92 porous composite coatings, and sponges have also been tested.91 For an exceptional review on the use of HA in cartilage tissue engineering, the reader is directed to Kim et al.91
Many different strategies have been employed to overcome the fast resorption, mechanical integrity, and water solubility of HA. An approach most frequently employed for formulating tissue engineering constructs consists of crosslinking HA by photopolymerization79,80,85,92–94 or thermal76,83,90 mechanisms to form hydrogels in which cells can be encapsulated.89,91 Crosslinking can function to increase mechanical strength, while also prolonging degradation of HA.65 Zhang et al.67 engineered a hydrogel scaffold by thermal crosslinking for cartilage tissue engineering that comprised solely of components found in the ECM of cartilage tissue using bovine collagen type I, HA, and CS (Table 3). Freeze drying is a common fabrication method to form composite porous matrices containing HA and other materials for tissue engineering constructs.66,75,78,81,82,88,95 Zhang et al.75 assembled highly macroporous composite scaffolds of HA and gelatin for soft tissue engineering applications using a freeze drying technique (Table 5). It is also important to note that HA must be utilized in relatively low concentrations to avoid limited cell adhesion that can occur at higher concentrations due to its negative charge.66 Fabricating composites with HA and neutral or positively charged materials can help mitigate this charge limitation. One specific example of a composite HA strategy by Sundararaghavan and Burdick92 created dual-gradient, electrospun fiber scaffolds incorporating HA with RGD peptide sequences to promote cell adhesion. This example demonstrated both an exceptional raw material and scaffold formulation approach, while also providing a recent example of a gradient scaffold that incorporated a raw material.92
The versatility and biocompatibility of HA has attracted attention for the delivery of growth factors and other biological molecules in tissue engineering scaffolds.18 Recent approaches have included the delivery of signaling molecules, such as simvastatin,80 vascular endothelial growth factor,79,84 platelet-derived growth factor,84 transforming growth factor beta-1 (TGF-β1),77 TGF-β3,76 bone morphogenetic protein-2 (BMP-2),79 phosphatidylserine,78 and fibronectin.85 Bae et al.80 fabricated HA hydrogels loaded with simvastatin prior to photocrosslinking to entrap the molecule within the entangled gel matrix. Most researchers utilized the ability to control molecule delivery within HA scaffolds by modulating properties, such as molecular weight, crosslinking, and scaffold formulation, accordingly. Overall, the ubiquity of HA in the body has been mirrored by tissue engineers in a wide variety of applications. Chemical modifications, crosslinking, and blending of HA with other materials are the most common methods used to apply this raw material for regenerative constructs and innovative approaches continue to be developed for several different applications.
Chondroitin sulfate
CS is a GAG that is found mainly attached to proteoglycans in connective tissue matrices or conjugated to proteins, such as aggrecan, in articular cartilage.6 The different forms of CS depend on the sulfation site, typically at either the 4 or 6 carbon; however, chondroitin-6-sulfate is used in tissue engineering most frequently.6 The presence of CS in native tissues has led to its use in cartilage,31,53,54,56,60,76,96,97 skin,38,50,56,98 bone,46,99 and blood vessel55,86 tissue engineering scaffolds. In addition to the aforementioned CG scaffolds, CS has been blended with many synthetic polymers and raw materials. A study by Kinneberg et al.53 employed CS within a collagen hydrogel to investigate a potential increase in the linear stiffness of the gel constructs by helping to link discontinuous collagen fibrils in the gel network. Nguyen et al.96 designed a three-layer hydrogel scaffold with varying compositions of CS, HA, and polyethylene glycol (PEG) to simulate the mechanical properties of each zone of articular cartilage. This triphasic construct demonstrated another approach for mimicking native tissue using raw materials and synthetic polymers in a spatially varying scaffold architecture. Additionally, Coburn et al.97 pioneered a fiber–hydrogel composite fabricated with methacrylated poly(vinyl-alcohol) and CS fibers encapsulated within a PEG hydrogel. The fibers were hypothesized to mimic the nature of native protein networks, while the hydrogel served to simulate the polysaccharide-based ground substance that are both characteristic of the ECM of tissue.97 Liang et al.56 investigated the differences in scaffold properties with varying concentrations of collagen and CS in CG scaffolds for both cartilage and skin tissue engineering. This strategy showed the tunability of CG scaffolds with respect to water uptake, pore size, and elastic modulus to tailor properties for necessary properties for each target tissue.56 A combination of HA, CS, and gelatin was fabricated into tri-co-polymer sponges and incorporated into a poly(lactic-co-glycolic acid) (PLGA) framework.76 Additionally, the scaffolds were loaded with immobilized TGF-β3 and implanted in full-thickness cartilage defects in New Zealand white rabbits (Table 4).76 Wang et al.50 employed a strategy of using solely raw materials to mimic the ECM of the dermis for skin tissue engineering grafts. The scaffold matrix consisted of collagen, CS, and HA with different ratios of each component, and was tested for optimal construct properties (Table 5).50 This study, along with several others, embodied the emerging raw material approach for tissue engineering scaffolds. Overall, CS can be used to enhance mechanical integrity of a scaffold while also helping to mimic native ECM in connective tissues as well as articular cartilage. Skin, cartilage, and bone tissue engineering have utilized CS most frequently; however, this raw material is poised to become an effective scaffold component in many other target tissue applications.
Summary
The use of GAGs in tissue engineering strategies continues to become more sophisticated in fabrication techniques and raw material approaches. The combination of HA and CS has recently became evident as a conceivable raw material approach in both cartilage and skin tissue engineering applications. As the use of these native molecules continues to spread to additional applications, the potential of achieving clinical success using these raw materials appears limitless.
Bioceramics
Mineralization of scaffolds plays a major role in bone as well as osteochondral interface tissue engineering. Calcium phosphate ceramics are biocompatible and their ability to be bioactive in the body stems from their similarity in composition and structure to the mineral phase of bone.100 Some of the advantages of using bioceramics as part of a tissue engineering scaffold include increased mechanical strength, biocompatibility, and osteoconductivity.3,100 However, the brittle nature and slow degradation times of these ceramics can prove unattractive for tissue engineering constructs.3 Researchers have blended synthetic polymers and/or several of the aforementioned raw materials with bioceramics to help to overcome the limitations of calcium phosphate materials for bone and cartilage tissue engineering constructs. Additionally, advances in fabrication methods to produce highly macroporous bioceramic scaffolds have helped to facilitate faster degradation rates. Two of the most widely used bioceramic materials in tissue engineering scaffolds, HAp and beta-tricalcium phosphate (β-TCP), will be highlighted in the following sections. For comprehensive reviews on ceramic materials and their use in tissue engineering, the reader is directed to articles by Dorozhkin et al.,100 Li et al.,3 and Porter et al.101
Hydroxyapatite
HAp is the main inorganic phase of bone and these crystals bind to collagen type I fibers in the ECM of native tissue.101 Since collagen regulates the size and orientation of the HAp crystals, the structural relationship of this organic–inorganic matrix contributes largely to the mechanical properties of bone.3,101 In its nonporous and highly crystalline form, HA is known to remain unchanged for 5–7 years in the body with little-to-no resorption.100 However, most tissue engineering strategies have incorporated synthetic HAp into porous scaffolds along with raw materials and/or synthetic polymers to best mimic the native ECM and properties of bone. The need for blends of polymeric materials with HAp stems from the brittle nature of HAp as a macroporous scaffold, and biopolymer incorporation can help to tune the elasticity of the scaffold as well as the degradation properties.101 Teixeira et al.102 employed a raw material blend consisting of a collagen type I coating on a porous HAp matrix to mimic native bone composition and aid in cell adhesion. As a composite matrix, this material combination provided a microstructure that attempted to mimic native bone and provided a suitable microenvironment for new bone formation in vivo (Table 2).102 Zhou et al.103 demonstrated a similar strategy by formulating bilayered osteochondral scaffolds that consisted of a collagen type I layer on the top of the construct with a collagen/HAp layer on the bottom to imitate the transition from cartilage to bone tissue structure at this interface. The biphasic scaffolds were seeded with human mesenchymal stem cells (MSCs) and cultured separately in chondrogenic and osteogenic medium (Table 5).103 Li et al.104 constructed a composite of poly(L-lactic) acid (PLLA), chitosan, and HAp microspheres as a hybrid bone tissue engineering composite and studied the cellular response to these constructs in vitro using murine calvarial osteoblasts (Table 1). Approaches by each of these groups demonstrated the growing tendency of raw materials to be utilized as building blocks in bone tissue engineering scaffolds.
A longstanding debate in the bone tissue engineering literature is the use of micro- versus nanoscale HAp in constructs.3,17 Employing a nanoscale HAp approach is hypothesized to allow the scaffold to better mimic the nanostructure of bone and encourage the differentiation of stem cells.105,106 Peng et al.107 investigated the use of microscale versus nanoscale HAp powders incorporated with PLLA electrospun fibers. After a 10-day culture period, the composite scaffolds containing microscale HAp particles showed the best cell performance, but both particle sizes exhibited satisfactory cell viability and signaling.107 Nanoscale HAp formulations have included nanoparticles108–110 or nanofibers111,112 in combination with other materials. Zhang et al.112 created a nanofibrous composite scaffold of HAp, collagen type I, and chitosan to mimic the nanostructure of native bone. A similar nanocomposite approach was employed by Liu et al.109 for treatment of periodontal bone defects using nanoscale HAp, collagen type I, and poly(lactic acid) (PLA). Overall, collagen type I has been one of the most widely utilized raw materials for creating HAp composites due to its ability to promote cell adhesion, which is limited in pure HAp constructs (Table 1). An exceptional review by Wahl et al.113 detailed collagen–HAp composites for bone regeneration. The results of the debate between microscale and nanoscale HAp formulations may suggest the need for additional studies to examine multiple size ranges simultaneously or differences that exist between fabrication methods that can help enhance mechanical integrity while also modulating cell differentiation.
Beta-tricalcium phosphate
The tunability of resorption rates of β-TCP has attracted great attention within the bone and osteochondral interface tissue engineering communities.101,114 While β-TCP can be resorbed too quickly for some applications in vivo, the ability to blend the material with polymers and control the granule size115 offers methods to modulate resorption rate while utilizing the advantage for tissue in-growth when compared to the prolonged degradation of crystalline HAp. Synthetic polymers, such as poly(ɛ-caprolactone) (PCL),116–120 PLA,121–123 poly(glycolic acid) (PGA),124 and PLGA,125 are used most often to fabricate composite scaffolds with β-TCP. The main drawback of composites with β-TCP and synthetic polymers is poor cell attachment and proliferation. However, collagen,117,120,126 gelatin,127,128 and HA129 have also been employed with β-TCP and/or synthetic polymers to aid in cell adhesion and viability. Yeo et al.120 presented an innovative approach composed of a PCL–β-TCP composite embedded in collagen nanofibers to create a hierarchical structure similar to native bone. Niyama et al.130 formulated an osteochondral scaffold using a β-TCP porous block covered with a scaffold-free chondrocyte matrix to induce both types of tissue formation. Tadokoro et al.128 utilized a gelatin and a β-TCP sponge loaded with BMP-2 in an in vivo subcutaneous model and observed the presence of new bone formation.
The microscale versus nanoscale debate has been investigated using powders of β-TCP, although the issue is much less controversial than that of HAp. Lin et al.131 found that nanoscale β-TCP ceramics degraded slower than those fabricated from microscale powders. Further, ceramics made from nanoscale β-TCP had twice the mechanical strength of those fabricated from microscale powder, and the nanoscale β-TCP ceramic reached a compressive strength in the upper range of native cancellous bone.131 The combination of mechanical properties and fast resorption of β-TCP made from nanoscale powder provides tissue engineers another attractive bioceramic formulation option. Another group investigated granule size and morphology of β-TCP granules in a subcutaneous rat model (Table 2) and found that the greatest vascularization occurred in the group with polygonal morsel-shaped granules ranging from 63 to 250 microns in size.115 Depending on defect size, healing time, and/or target application of the bone tissue engineering construct, the size and shape of β-TCP particles used in the raw material strategy must be considered and characterized.
Summary
Overall, the raw material approach to the use of bioceramics in bone tissue engineering constructs appears to be shifting more away from HAp and more toward β-TCP due to the ability to finely tune resorption rates to match newly forming bone and allow for incorporation of the scaffold into new bone tissue (summarized more in-depth in the Discussion section). HAp may still be an effective raw material strategy in cases where new bone formation is expected to take more time. Advances in particle size and formulations of each bioceramic material have allowed for many new insights into considerations for fabricating bone tissue engineering scaffolds.
ECM-Based Materials
In addition to native ECM components, raw materials include those derived from mammalian tissue, which have been used in several tissue engineering applications from skin to heart valves.132–135 Decellularized matrices, such as SIS, as well as heart valves and arteries, are additional sources of collagen and endogenous proteins.132 Demineralized bone matrix (DBM) and decellularized cartilage are additional ECM-based strategies for retaining organic components of native tissue, while removing cells and/or mineralized crystals. Both decellularizing and demineralizing strategies can potentially weaken mechanical integrity of the matrix. However, many approaches have been employed to modulate mechanical stability of SIS and DBM. The following sections will review the use of SIS, DBM, and decellularized cartilage as components of tissue engineering scaffolds and strategies to blend each with additional materials or cells for enhanced properties.
Small intestinal submucosa
Of all the potential sources, porcine SIS has been one of the most studied and utilized ECM-based raw materials in a wide variety of applications.132,135 Studies have shown that SIS contains over 90% collagen by dry weight, with a majority being collagen type I.136 Depending on the type of decellularization method used, SIS can maintain GAGs and growth factors present in the native tissue.132,135 In addition to these native ECM molecules, the collagen fiber orientation that is maintained after the decell process has also attracted attention.132 Both of these inherent properties have sparked strategies employing SIS as scaffolds in the fields of cardiovascular,137–140 bone,141–143 nerve,144 soft tissue,86,87,145 and urogenital146–149 tissue engineering (Table 6). Currently, SIS is Food and Drug Administration (FDA) approved for several urogenital applications, including hernia repair.132 The presence of aligned collagen fibers and endogenous growth factors remaining in the acellular SIS matrix has sparked interest within the bone tissue engineering community as well. Kim et al.142 and Honsawek et al.141 showed that SIS scaffolds promoted new bone formation in a rat model. Zhao et al.143 found similar results in a rabbit model when SIS was seeded with MSCs. Composite scaffolds fabricated with SIS and synthetic polymers or other raw materials have also been employed. Mondalek et al.87 utilized all three types of materials by fabricating an SIS scaffold combined with HA-PLGA nanoparticles to enhance angiogenesis in the implanted scaffold when compared with SIS only (Table 6).
Urinary bladder matrix as well as heart valves and arteries from both xenogeneic and allogeneic sources have also been used in several other applications.150 For a more comprehensive review on decellularized matrices and their role in tissue engineering, the reader is directed to articles by Badylak et al.,132 Hoshiba et al.,135 and Piterina et al.136 Overall, utilizing SIS may offer a new dimension to raw material scaffolding by inherently combining aligned collagen fibers with remaining GAG molecules and growth factors. This complex tissue arrangement presents a suitable option for many different tissue engineering applications.
Demineralized bone matrix
DBM mimics the strategy behind SIS, and has been studied for over 3 decades for use in bone grafting procedures.151 DBM is formulated through acidic washing and defatting of human allograft cortical bone, which leaves an acellular organic matrix that mimics the microstructure of bone tissue.151 Native concentrations of organic materials as well as mechanical integrity following the demineralization process are directly proportional to the extent of acidic washing.152 Therefore, as more mineral is removed, the mechanical properties weaken and the presence of organic components decreases.152 Nevertheless, the presence of organic components and proteins has led to the use of DBM in both bone and cartilage tissue engineering solutions. After the demineralization process, the remaining acellular matrix is composed mainly of collagen with associated BMPs and GAGs, which is an osteoinductive network that can aid in cell attachment, migration, and differentiation.153 However, the inherently poor mechanical performance of DBM, along with the variance in quality and concentration of the organic materials from donor to donor, presents barriers for utilizing DBM as a single-component construct.151 To address these limitations and construct DBM composite constructs, studies have seeded DBM with stem cell sources or blended DBM with both synthetic and raw materials. Researchers have used DBM as a sole scaffold component in conjunction with seeded umbilical cord blood-derived MSCs152 and adipose-derived stem cells.154,155 Combination of DBM with additional raw materials, such as SIS,141 HAp,156 and β-TCP,157 as well as fibrin glue158,159 and heparin,153 has been employed in bone and cartilage tissue engineering. In addition, blends of DBM with synthetic PLA,160 reverse thermoresponsive polymers,161 and PLGA162 allow for increased stability and modulation of mechanical properties.163 Demineralized dentin matrix (DDM) has also gained attention for use in osteochondral tissue engineering. As an example, Yagihashi et al.164 investigated the potential of DDM to promote osteochondral regeneration in full-thickness cartilage defects of New Zealand white rabbits and observed the formation of hyaline-like cartilage and new bone (Table 4). Both DBM and DDM can serve as effective raw materials to be incorporated into both bone and tissue engineering scaffolds without the need for additional exogenous growth factors or cytokines. Endogenous organic components allow these raw materials to signal surrounding cells and tissue in ways unmatched by purely synthetic scaffolds.
Decellularized cartilage
One scarcely explored tissue in the area of ECM-based materials is the notion of decellularizing hyaline cartilage. In theory, acellular hyaline cartilage would be expected to provide a scaffold rich in collagen type II, aggrecan, and endogenous growth factors following the decellularization process. Some groups have attempted to render hyaline cartilage acellular as an intact explant,165,166 while others have sliced or shattered explanted cartilage prior to this process due to the compact nature of cartilage tissue that does not allow complete penetration of decellularization solutions.165,167–169 Once the tissue had all of the cellular components removed, the remaining cartilage powder or solution was freeze dried to obtain an acellular, porous matrix.167,168 Gong et al. made a sandwich model of porcine acellular cartilage sheets with porcine chondrocytes seeded in between each layer of cartilage sheets.169 This raw material strategy appears to have potential in the area of ECM-based materials, but will warrant future investigation both in vitro and in animal models.
Summary
ECM-based matrices offer a distinct advantage of retaining the composition of native materials and proteins as well as their inherent spatial arrangements in some cases. Both SIS and human DBM are FDA approved for clinical applications.132,170 Composites utilizing ECM-based materials may also have the potential to translate into the clinical setting considering all of the current research attempting to develop these raw material hybrids.
Bioactive Signaling of Raw Materials
In addition to providing building blocks for fabricating tissue engineering scaffolds, raw materials also hold the potential to present signals to cells. As previously mentioned, biomaterial-based signaling can arise from physical, chemical, adhesive, and mechanical properties of the construct. While many have exploited the inherent adhesive RGD peptide present in collagen, many others have examined the signaling potential of other raw materials used in tissue engineering constructs. Park et al.77 investigated the chondrogenic potential of HA–fibrin glue composite hydrogels with encapsulated rat MSCs when treated with or without TGF-β1. Results suggested that treatment with exogenous growth factors was not essential for chondrogenic differentiation of rat MSCs in the HA–fibrin glue gel.77 The authors hypothesized that the chondroinductive signaling potential of this composite gel most likely stemmed from the interaction of cells with the scaffold ECM via integrins on the cell surface.77 This interaction was thought to induce intracellular signaling for regulation of many cell functions, including differentiation and matrix synthesis.77 Another study aimed to elucidate the osteoinductive potential of collagen type I–HAp scaffolds for bone regeneration.110 The porous composite constructs were seeded with porcine MSCs and cultured for 28 days. Results demonstrated osteogenic differentiation of seeded MSCs by relative gene expression analysis using common osteogenic markers.110 These studies suggested that mimicking the ECM components of native tissue may be a suitable alternative for the promotion of bioactive signaling without the addition of exogenous proteins. Similarly, ECM-based materials also offer evidence of bioactive signaling potential that stems from inherent native materials and growth factors. For example, Kim et al.142 compared the regenerative potential of rat MSCs seeded on either a PGA mesh or an SIS sponge to repair full-thickness bilateral bone defects in rat crania. SIS sponges showed significantly greater new bone regeneration when compared with PGA meshes 4 weeks after implantation.142 Additionally, DBM–fibrin glue scaffolds have been investigated for osteoinductive capability with skin-derived MSC–like cells.171 After 4 weeks of culture, osteogenic differentiation was confirmed by relative gene expression and flow cytometry.171
Overall, raw materials offer bioactive signaling potential that is unmatched by synthetic biomaterials. Optimization of raw material components and fabrication methods may alleviate the need to supplement tissue engineering scaffolds with immobilized or solubilized growth factors.
Discussion
Integration of two components of the tissue engineering triad—scaffolds and signals—can be accomplished by utilizing raw material strategies in tissue engineering constructs. Raw materials can present physical, chemical, adhesive, and mechanical cues to cells without the addition of immobilized or solubilized bioactive molecules. Moreover, collagen, GAGs, and bioceramics can be blended into composites using additional synthetic polymers and/or other raw materials based on the desired scaffold properties. Kruger et al.34 characterized the ability of type I collagen to mineralize in comparison to PLGA when seeded with human MSCs subjected to osteogenic media. Collagen scaffolds mineralized within 8 weeks of culture, while PLGA scaffolds displayed mineralization after 12 weeks.34 Time differences were ultimately attributed to degradation of PLGA, which ultimately changed the matrix rigidity, porosity, scaffold architecture, and pH balance that can disrupt cell signaling in the local microenvironment. These results highlight an important distinction between bioresorbable and biodegradable tissue engineering constructs. Bioresorbable scaffold materials are generally raw materials that the body is able to recognize and incorporate into surrounding tissue. However, biodegradable scaffolds tend to break down in the body over time, creating alterations in the local microenvironment and microstructure of the scaffold that may adversely affect cell–biomaterial interactions. Arguably, the ability of a scaffold to integrate into surrounding tissue is one of the most crucial interaction that governs the success of the implanted construct.172 While both synthetic polymers and the aforementioned raw materials possess distinct strengths and weaknesses, bioresorbability of scaffolds in vivo is certainly a crucial aspect of scaffold fabrication and development.
Additionally, selection of the most appropriate raw materials for the target tissue remains another important, yet controversial, issue. While most researchers tend to utilize raw materials that are present in the native ECM, cartilage tissue engineering solutions tend to conflict between the selection of type I and type II collagen (Table 3). Several raw material approaches utilize collagen type I to regenerate articular cartilage,26,28,31,33,37,45,53,56,78 despite the well-known fact that the collagen of hyaline cartilage is predominately type II rather than type I. Studies by Berendsen et al.36 and Ng et al.32 attempted to address this raw material debate. Berendesen et al.36 found that chondrocyte-mediated contraction occurred only on collagen type I gels but not on collagen type II gels, allowing chondroctyes to maintain their phenotype on collagen type II gels, which was confirmed by relative gene expression of matrix proteins and matrix metalloproteinases. Contraction seemed to be a contributing factor to the dedifferentiation of chondrocytes in the case of collagen type I gels.36 The authors acknowledged that their results pointed toward collagen type II as the material of choice for cartilage tissue engineering; however, whether this outcome occurred because type II collagen presented a superior cell–biomaterial response or a catabolic response of cells to reorganize and produce their own collagen type II has yet to be determined.36 However, raw material strategies using collagen type II to mimic the native ECM have been employed by other groups with similar success.22,53,61 Contrasting data were obtained in a study by Ng et al.,32 where no difference was found between the effects of collagen type I and type II gels on mesenchymal stem cell proliferation and contraction. It is important to note, however, that differences in cell type, seeding density, seeding technique, and crosslinking method could all contribute to the discrepancy between these studies. An additional study examining two-dimensional culture of chondrocytes on collagen type II versus aggrecan-coated polystyrene found that aggrecan-coated surfaces best retained chondrogenic phenotype over four passages and collagen type II surfaces tended to induce loss of chondrogenic phenotype.173 Logically, collagen type II would appear as the raw material of choice for articular cartilage scaffolds, but future studies examining both collagen type II and aggrecan will be necessary to confirm the most appropriate chondroinductive raw material for cartilage applications.
A similar debate exists in the bone tissue engineering community involving the choice between HAp and β-TCP as scaffold components. Rojbani et al.174 examined the differences in osteoconductivity of HAp and β-TCP microparticles. The particles were loaded into calvarial defects in rats and supplemented with and without simvastatin. Results concluded that β-TCP proved to be a superior osteoconductive scaffold, resulting in greater bone formation compared with HAp, and the addition of simvastatin tended to increase bone regeneration in both of the bioceramic scaffolds. The authors attributed the success of β-TCP to faster degradation, which allows for a synchronized equilibrium between particle degradation and new bone formation.174 No composite scaffolds incorporating either material were used in this study; however, investigation of hybrid materials containing both HAp and β-TCP would be needed to resolve the conflicting strategies of these materials, since these ceramics are not frequently used as sole scaffold components. In addition, exploration of each material's osteoconductive potential in a nanoparticle format would also be necessary. Ultimately, the ability of β-TCP to resorb much more quickly than HAp can provide an appealing solution for hastened bone in-growth.
Finally, trends in FDA-approved tissue engineering scaffolds suggest that many areas of tissue engineering have failed to conquer the translational barrier from laboratory benches to clinical solutions. Healon® and Synvisc® are examples of HA formulations used clinically for ophthalmologic and orthopedic applications, respectively.73 Human allograft DBM products, such as Allomatrix®,170 DBX®,170 Puros®,175 and Grafton®,171 are also commercially available. Healos FX®, Collapat II®, and Biostite® are collagen type I–based medical products used clinically for various applications.136 However, most attribute the failure of many other tissue engineering strategies to lie in the distinction between medical devices and combination products, respectively.176,177 Combination products often employ the use of biologics—cells, drugs, or growth factors—and must be proven in animal studies and a series of three clinical trials, likely spanning over 8 years before approval.177–179 Medical devices do not contain biologics and can often be classified as a Class II device or under 510k approval (depending on application), alleviating the need for the three phases of clinical trials.176,178 Raw materials, such as collagen, SIS, and human DBM, contain endogenous growth factors and adhesive cues to aid in signaling, without the addition of biologics to the scaffold. Therefore, raw materials could provide a method for translating effective tissue engineering scaffolds to the clinic without all of the additional associated cost and time associated with combination products.
In summary, raw materials present a crucial subset of biomaterials for tissue engineering scaffolds. It is no coincidence that industry has already been using raw materials, such as HA, collagen, and DBM, in their regenerative medicine products. Quite simply, industry employs these materials because they produce results, although academia may be able to contribute more sophisticated and more effective designs by being more in tune to this classification of materials in our design strategies. Collagen, GAGs, and bioceramics can modulate cell–biomaterial interactions and provide building blocks to give tissues a jump start in the regeneration process. Many strategies have incorporated raw materials in constructs with exact ratios of these components in native tissue. However, a much larger subset of tissue engineering approaches rely on the tunability and predictability of synthetic polymer scaffolds. Studies suggest that composite materials may be the best method for combining both schools of thought. In the ongoing quest to find “perfect” tissue engineering scaffolds, it is essential that researchers look to the composition and structure of native tissue for material selection and design inspiration.
Acknowledgments
The authors would like to acknowledge the Madison & Lila Self Graduate Fellowship for supporting A.N. Renth. We would also like to acknowledge support from the NIH/NIAMS (R01 AR056347).
Disclosure Statement
No competing financial interests exist.
References
- 1.Costa-Pinto A.R. Reis R.L. Neves N.M. Scaffolds based bone tissue engineering: the role of chitosan. Tissue Eng Part B-Rev. 2011;17:331. doi: 10.1089/ten.teb.2010.0704. [DOI] [PubMed] [Google Scholar]
- 2.Lomakin J. Huber P.A. Eichler C. Arakane Y. Kramer K.J. Beernan R.W. Kanost M.R. Gehrke S.H. Mechanical properties of the beetle elytron, a biological composite material. Biomacromolecules. 2011;12:321. doi: 10.1021/bm1009156. [DOI] [PubMed] [Google Scholar]
- 3.Li Z.X. Kawashita M. Current progress in inorganic artificial biomaterials. J Artif Organs. 2011;14:163. doi: 10.1007/s10047-011-0585-5. [DOI] [PubMed] [Google Scholar]
- 4.Hollister S.J. Hierarchical bioactive materials for tissue reconstruction: integrated design and manufacturing challenges. Jom. 2011;63:56. [Google Scholar]
- 5.Barrere F. Mahmood T.A. de Groot K. van Blitterswijk C.A. Advanced biomaterials for skeletal tissue regeneration: instructive and smart functions. Mater Sci Eng R-Rep. 2008;59:38. [Google Scholar]
- 6.Baldwin A.D. Kiick K.L. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers. 2010;94:128. doi: 10.1002/bip.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gloria A. De Santis R. Ambrosio L. Polymer-based composite scaffolds for tissue engineering. J Appl Biomater Biomech. 2010;8:57. [PubMed] [Google Scholar]
- 8.Kim M.S. Kim J.H. Min B.H. Chun H.J. Han D.K. Lee H.B. Polymeric scaffolds for regenerative medicine. Polym Rev. 2011;51:23. [Google Scholar]
- 9.Oliveira J.T. Reis R.L. Polysaccharide-based materials for cartilage tissue engineering applications. J Tissue Eng Regen Med. 2011;5:421. doi: 10.1002/term.335. [DOI] [PubMed] [Google Scholar]
- 10.Huang B. Li C.Q. Zhou Y. Luo G. Zhang C.Z. Collagen II/hyaluronan/chondroitin-6-sulfate tri-copolymer scaffold for nucleus pulposus tissue engineering. J Biomed Mater Res Part B. 2010;92B:322. doi: 10.1002/jbm.b.31518. [DOI] [PubMed] [Google Scholar]
- 11.Sokolsky-Papkov M. Agashi K. Olaye A. Shakesheff K. Domb A.J. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:187. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Singh M. Berkland C. Detamore M.S. Strategies and applications for incorporating physical and chemical signal gradients in tissue engineering. Tissue Eng Part B Rev. 2008;14:341. doi: 10.1089/ten.teb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mano J.F. Silva G.A. Azevedo H.S. Malafaya P.B. Sousa R.A. Silva S.S. Boesel L.F. Oliveira J.M. Santos T.C. Marques A.P. Neves N.M. Reis R.L. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4:999. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang S.J. Kuo S.M. Manousakas I. Niu G.C. Chen J.P. Preparation and characterization of hyaluronan/collagen II microspheres under an electrostatic field system with disc electrodes. Acta Biomater. 2009;5:101. doi: 10.1016/j.actbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Toh W.S. Spector M. Lee E.H. Cao T. Biomaterial-mediated delivery of microenvironmental cues for repair and regeneration of articular cartilage. Mol Pharm. 2011;8:994. doi: 10.1021/mp100437a. [DOI] [PubMed] [Google Scholar]
- 16.Marklein R.A. Burdick J.A. Controlling stem cell fate with material design. Adv Mater. 2010;22:175. doi: 10.1002/adma.200901055. [DOI] [PubMed] [Google Scholar]
- 17.Wang H. Leeuwenburgh S. Yubao L. Jansen J. The use of micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng Part B Rev. 2012;18:24. doi: 10.1089/ten.teb.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uebersax L. Merkle H.P. Meinel L. Biopolymer-based growth factor delivery for tissue repair: from natural concepts to engineered systems. Tissue Eng Part B Rev. 2009;15:263. doi: 10.1089/ten.TEB.2008.0668. [DOI] [PubMed] [Google Scholar]
- 19.Kew S.J. Gwynne J.H. Enea D. Abu-Rub M. Pandit A. Zeugolis D. Brooks R.A. Rushton N. Best S.M. Cameron R.E. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 2011;7:3237. doi: 10.1016/j.actbio.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Cunniffe G.M. O'Brien F.J. Collagen scaffolds for orthopedic regenerative medicine. Jom. 2011;63:66. [Google Scholar]
- 21.Calderon L. Collin E. Velasco-Bayon D. Murphy M. O'Halloran D. Pandit A. Type II collagen-hyaluronan hydrogel—a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 22.Vickers S.M. Gotterbarm T. Spector M. Cross-linking affects cellular condensation and chondrogenesis in type II collagen-GAG scaffolds seeded with bone marrow-derived mesenchymal stem cells. J Orthop Res. 2010;28:1184. doi: 10.1002/jor.21113. [DOI] [PubMed] [Google Scholar]
- 23.Glowacki J. Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 24.Zheng W.F. Zhang W. Jiang X.Y. Biomimetic collagen nanofibrous materials for bone tissue engineering. Adv Eng Mater. 2010;12:B451. [Google Scholar]
- 25.Gaspar A. Moldovan L. Constantin D. Stanciuc A.M. Sarbu Boeti P.M. Efrimescu I.C. Collagen-based scaffolds for skin tissue engineering. J Med Life. 2011;4:172. [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H. Ko Y.G. Kawazoe N. Chen G. Cartilage tissue engineering using funnel-like collagen sponges prepared with embossing ice particulate templates. Biomaterials. 2010;31:5825. doi: 10.1016/j.biomaterials.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Akkouch A. Zhang Z. Rouabhia M. A novel collagen/hydroxyapatite/poly(lactide-co-epsilon-caprolactone) biodegradable and bioactive 3D porous scaffold for bone regeneration. J Biomed Mater Res Part A. 2011;96A:693. doi: 10.1002/jbm.a.33033. [DOI] [PubMed] [Google Scholar]
- 28.Yan L.P. Wang Y.J. Ren L. Wu G. Caridade S.G. Fan J.B. Wang L.Y. Ji P.H. Oliveira J.M. Oliveira J.T. Mano J.F. Reis R.L. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J Biomed Mater Res A. 2010;95:465. doi: 10.1002/jbm.a.32869. [DOI] [PubMed] [Google Scholar]
- 29.Sionkowska A. Kozlowska J. Characterization of collagen/hydroxyapatite composite sponges as a potential bone substitute. Int J Biol Macromol. 2010;47:483. doi: 10.1016/j.ijbiomac.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Perng C.-K. Wang Y.-J. Tsi C.-H. Ma H. In vivo angiogenesis effect of porous collagen scaffold with hyaluronic acid oligosaccharides. J Surg Res. 2011;168:9. doi: 10.1016/j.jss.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 31.Ohyabu Y. Adegawa T. Yoshioka T. Ikoma T. Uemura T. Tanaka J. Cartilage regeneration using a porous scaffold, a collagen sponge incorporating a hydroxyapatite/chondroitinsulfate composite. Mater Sci Eng B-Adv Funct Solid-State Mater. 2010;173:204. [Google Scholar]
- 32.Ng K.K. Thatte H.S. Spector M. Chondrogenic differentiation of adult mesenchymal stem cells and embryonic cells in collagen scaffolds. J Biomed Mater Res A. 2011;99A:275. doi: 10.1002/jbm.a.33163. [DOI] [PubMed] [Google Scholar]
- 33.Lu H. Ko Y.G. Kawazoe N. Chen G. Culture of bovine articular chondrocytes in funnel-like collagen-PLGA hybrid sponges. Biomed Mater. 2011;6:045011. doi: 10.1088/1748-6041/6/4/045011. [DOI] [PubMed] [Google Scholar]
- 34.Kruger E.A. Im D.D. Bischoff D.S. Pereira C.T. Huang W. Rudkin G.H. Yamaguchi D.T. Miller T.A. In vitro mineralization of human mesenchymal stem cells on three-dimensional type I collagen versus PLGA scaffolds: a comparative analysis. Plast Reconstr Surg. 2011;127:2301. doi: 10.1097/PRS.0b013e318213a004. [DOI] [PubMed] [Google Scholar]
- 35.Thein-Han W. Xu H.H. Collagen-Calcium Phosphate Cement Scaffolds Seeded with Umbilical Cord Stem Cells for Bone Tissue Engineering. Tissue Eng Part A. 2011;17:2943. doi: 10.1089/ten.tea.2010.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berendsen A.D. Vonk L.A. Zandieh-Doulabi B. Everts V. Bank R.A. Contraction-induced Mmp13 and −14 expression by goat articular chondrocytes in collagen type I but not type II gels. J Tissue Eng Regen Med. 2011 doi: 10.1002/term.477. [DOI] [PubMed] [Google Scholar]
- 37.Chang C.H. Kuo T.F. Lin F.H. Wang J.H. Hsu Y.M. Huang H.T. Loo S.T. Fang H.W. Liu H.C. Wang W.C. Tissue engineering-based cartilage repair with mesenchymal stem cells in a porcine model. J Orthop Res. 2011;29:1874. doi: 10.1002/jor.21461. [DOI] [PubMed] [Google Scholar]
- 38.Hartwell R. Leung V. Chavez-Munoz C. Nabai L. Yang H. Ko F. Ghahary A. A novel hydrogel-collagen composite improves functionality of an injectable extracellular matrix. Acta Biomater. 2011;7:3060. doi: 10.1016/j.actbio.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Wong V.W. Rustad K.C. Galvez M.G. Neofyotou E. Glotzbach J.P. Januszyk M. Major M.R. Sorkin M. Longaker M.T. Rajadas J. Gurtner G.C. Engineered pullulan-collagen composite dermal hydrogels improve early cutaneous wound healing. Tissue Eng Part A. 2011;17:631. doi: 10.1089/ten.tea.2010.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L.M. Stegemann J.P. Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomater. 2011;7:2410. doi: 10.1016/j.actbio.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suri S. Schmidt C.E. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16:1703. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 42.Shen X.Y. Chen L. Cai X.A. Tong T. Tong H. Hu J.M. A novel method for the fabrication of homogeneous hydroxyapatite/collagen nanocomposite and nanocomposite scaffold with hierarchical porosity. J Mater Sci Mater Med. 2011;22:299. doi: 10.1007/s10856-010-4199-x. [DOI] [PubMed] [Google Scholar]
- 43.Marelli B. Ghezzi C.E. Mohn D. Stark W.J. Barralet J.E. Boccaccini A.R. Nazhat S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials. 2011;32:8915. doi: 10.1016/j.biomaterials.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Chan B.P. Hui T.Y. Wong M.Y. Yip K.H. Chan G.C. Mesenchymal stem cell-encapsulated collagen microspheres for bone tissue engineering. Tissue Eng Part C Methods. 2010;16:225. doi: 10.1089/ten.tec.2008.0709. [DOI] [PubMed] [Google Scholar]
- 45.Li C.H. Chik T.K. Ngan A.H.W. Chan S.C.H. Shum D.K.Y. Chan B.P. Correlation between compositional and mechanical properties of human mesenchymal stem cell-collagen microspheres during chondrogenic differentiation. Tissue Eng Part A. 2011;17:777. doi: 10.1089/ten.TEA.2010.0078. [DOI] [PubMed] [Google Scholar]
- 46.Keogh M.B. FJ O.B. Daly J.S. A novel collagen scaffold supports human osteogenesis—applications for bone tissue engineering. Cell Tissue Res. 2010;340:169. doi: 10.1007/s00441-010-0939-y. [DOI] [PubMed] [Google Scholar]
- 47.Caliari S.R. Ramirez M.A. Harley B.A. The development of collagen-GAG scaffold-membrane composites for tendon tissue engineering. Biomaterials. 2011;32:8990. doi: 10.1016/j.biomaterials.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhatyar V. Karumbaiah L. Yeh J. Bellamkonda R. Tissue engineering strategies designed to realize the endogenous regenerative potential of peripheral nerves. Adv Mater. 2009;21:4670. [Google Scholar]
- 49.Huang S. Fu X.B. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J Control Release. 2010;142:149. doi: 10.1016/j.jconrel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Wang W. Zhang M. Lu W. Zhang X. Ma D. Rong X. Yu C. Jin Y. Cross-linked collagen-chondroitin sulfate-hyaluronic acid imitating extracellular matrix as scaffold for dermal tissue engineering. Tissue Eng Part C Methods. 2010;16:269. doi: 10.1089/ten.TEC.2009.0161. [DOI] [PubMed] [Google Scholar]
- 51.Engelhardt E.M. Micol L.A. Houis S. Wurm F.M. Hilborn J. Hubbell J.A. Frey P. A collagen-poly(lactic acid-co-varepsilon-caprolactone) hybrid scaffold for bladder tissue regeneration. Biomaterials. 2011;32:3969. doi: 10.1016/j.biomaterials.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 52.Seo Y.K. Park J.K. Tissue Engineered Scaffold Utilizing the Reinforced Technique. Biotechnol Bioprocess Eng. 2010;15:527. [Google Scholar]
- 53.Kinneberg K.R.C. Nirmalanandhan V.S. Juncosa-Melvin N. Powell H.M. Boyce S.T. Shearn J.T. Butler D.L. Chondroitin-6-Sulfate Incorporation and Mechanical Stimulation Increase MSC-Collagen Sponge Construct Stiffness. J Orthop Res. 2010;28:1092. doi: 10.1002/jor.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen L.H. Kudva A.K. Saxena N.S. Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Duffy G.P. McFadden T.M. Byrne E.M. Gill S.L. Farrell E. O'Brien F.J. Towards in vitro vascularization of collagen-GAG scaffolds. Eur Cells Mater. 2011;21:15. doi: 10.22203/ecm.v021a02. [DOI] [PubMed] [Google Scholar]
- 56.Liang W.H. Kienitz B.L. Penick K.J. Welter J.F. Zawodzinski T.A. Baskaran H. Concentrated collagen-chondroitin sulfate scaffolds for tissue engineering applications. J Biomed Mater Res Part A. 2010;94A:1050. doi: 10.1002/jbm.a.32774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chicatun F. Pedraza C.E. Ghezzi C.E. Marelli B. Kaartinen M.T. McKee M.D. Nazhat S.N. Osteoid-mimicking dense collagen/chitosan hybrid gels. Biomacromolecules. 2011;12:2946. doi: 10.1021/bm200528z. [DOI] [PubMed] [Google Scholar]
- 58.Ghezzi C.E. Marelli B. Muja N. Hirota N. Martin J.G. Barralet J.E. Alessandrino A. Freddi G. Nazhat S.N. Mesenchymal stem cell-seeded multilayered dense collagen-silk fibroin hybrid for tissue engineering applications. Biotechnol J. 2011;6:1198. doi: 10.1002/biot.201100127. [DOI] [PubMed] [Google Scholar]
- 59.Mueller-Rath R. Gavenis K. Andereya S. Mumme T. Albrand M. Stoffel M. Weichert D. Schneider U. Condensed cellular seeded collagen gel as an improved biomaterial for tissue engineering of articular cartilage. Biomed Mater Eng. 2010;20:317. doi: 10.3233/BME-2010-0645. [DOI] [PubMed] [Google Scholar]
- 60.Chang K.Y. Hung L.H. Chu I.M. Ko C.S. Lee Y.D. The application of type II collagen and chondroitin sulfate grafted PCL porous scaffold in cartilage tissue engineering. J Biomed Mater Res Part A. 2010;92A:712. doi: 10.1002/jbm.a.32198. [DOI] [PubMed] [Google Scholar]
- 61.Francioli S.E. Candrian C. Martin K. Heberer M. Martin I. Barbero A. Effect of three-dimensional expansion and cell seeding density on the cartilage-forming capacity of human articular chondrocytes in type II collagen sponges. J Biomed Mater Res Part A. 2010;95A:924. doi: 10.1002/jbm.a.32917. [DOI] [PubMed] [Google Scholar]
- 62.Wu C.H. Ko C.S. Huang J.W. Huang H.J. Chu I.M. Effects of exogenous glycosaminoglycans on human chondrocytes cultivated on type II collagen scaffolds. J Mater Sci Mater Med. 2010;21:725. doi: 10.1007/s10856-009-3889-8. [DOI] [PubMed] [Google Scholar]
- 63.Harley B.A. Lynn A.K. Wissner-Gross Z. Bonfield W. Yannas I.V. Gibson L.J. Design of a multiphase osteochondral scaffold III: fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res Part A. 2010;92:1078. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 64.Murano E. Perin D. Khan R. Bergamin M. Hyaluronan: from biomimetic to industrial business strategy. Nat Prod Commun. 2011;6:555. [PubMed] [Google Scholar]
- 65.Puppi D. Chiellini F. Piras A.M. Chiellini E. Polymeric materials for bone and cartilage repair. Prog Polym Sci. 2010;35:403. [Google Scholar]
- 66.Correia C.R. Moreira-Teixeira L.S. Moroni L. Reis R.L. van Blitterswijk C.A. Karperien M. Mano J.F. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng Part C-Methods. 2011;17:717. doi: 10.1089/ten.tec.2010.0467. [DOI] [PubMed] [Google Scholar]
- 67.Zhang L. Li K.F. Xiao W.Q. Zheng L. Xiao Y.M. Fan H.S. Zhang X.D. Preparation of collagen-chondroitin sulfate-hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr Polym. 2011;84:118. [Google Scholar]
- 68.Van Vlierberghe S. Dubruel P. Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387. doi: 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- 69.Slaughter B.V. Khurshid S.S. Fisher O.Z. Khademhosseini A. Peppas N.A. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spiller K.L. Maher S.A. Lowman A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev. 2011;17:281. doi: 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunt N.C. Grover L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010;32:733. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 72.Burdick J.A. Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volpi N. Schiller J. Stern R. Soltes L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009;16:1718. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- 74.Turley E.A. Noble P.W. Bourguignon L.Y. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 75.Zhang F. He C. Cao L. Feng W. Wang H. Mo X. Wang J. Fabrication of gelatin-hyaluronic acid hybrid scaffolds with tunable porous structures for soft tissue engineering. Int J Biol Macrom. 2011;48:474. doi: 10.1016/j.ijbiomac.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Fan H.B. Tao H.R. Wu Y.N. Hu Y.Y. Yan Y.N. Luo Z.J. TGF-beta 3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration. J Biomed Mater Res Part A. 2010;95A:982. doi: 10.1002/jbm.a.32899. [DOI] [PubMed] [Google Scholar]
- 77.Park S.H. Choi B.H. Park S.R. Min B.H. Chondrogenesis of rabbit mesenchymal stem cells in fibrin/hyaluronan composite scaffold in vitro. Tissue Eng Part A. 2011;17:1277. doi: 10.1089/ten.TEA.2010.0337. [DOI] [PubMed] [Google Scholar]
- 78.Xu C. Su P. Wang Y. Chen X. Meng Y. Liu C. Yu X. Yang X. Yu W. Zhang X. Xiang A.P. A novel biomimetic composite scaffold hybridized with mesenchymal stem cells in repair of rat bone defects models. J Biomed Mater Res Part A. 2010;95:495. doi: 10.1002/jbm.a.32877. [DOI] [PubMed] [Google Scholar]
- 79.Patterson J. Siew R. Herring S.W. Lin A.S. Guldberg R. Stayton P.S. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31:6772. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bae M.S. Yang D.H. Lee J.B. Heo D.N. Kwon Y.D. Youn I.C. Choi K. Hong J.H. Kim G.T. Choi Y.S. Hwang E.H. Kwon I.K. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a bone tissue regeneration scaffold. Biomaterials. 2011;32:8161. doi: 10.1016/j.biomaterials.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 81.Chen M. Le D.Q. Baatrup A. Nygaard J.V. Hein S. Bjerre L. Kassem M. Zou X. Bunger C. Self-assembled composite matrix in a hierarchical 3-D scaffold for bone tissue engineering. Acta Biomater. 2011;7:2244. doi: 10.1016/j.actbio.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 82.Chen X. Meng Y. Wang Y. Du C. Yang C. A Biomimetic Material with a High Bio-responsibility for Bone Reconstruction and Tissue Engineering. J Biomater Sci Polym Ed. 2011;22:153. doi: 10.1163/092050609X12583524936191. [DOI] [PubMed] [Google Scholar]
- 83.Liao H.T. Chen C.T. Chen J.P. Osteogenic differentiation and ectopic bone formation of canine bone marrow-derived mesenchymal stem cells in injectable thermo-responsive polymer hydrogel. Tissue Eng Part C Methods. 2011;17:1139. doi: 10.1089/ten.tec.2011.0140. [DOI] [PubMed] [Google Scholar]
- 84.Ekaputra A.K. Prestwich G.D. Cool S.M. Hutmacher D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (varepsilon-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials. 2011;32:8108. doi: 10.1016/j.biomaterials.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 85.Seidlits S.K. Drinnan C.T. Petersen R.R. Shear J.B. Suggs L.J. Schmidt C.E. Fibronectin-hyaluronic acid composite hydrogels for three-dimensional endothelial cell culture. Acta Biomater. 2011;7:2401. doi: 10.1016/j.actbio.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Liu S. Zhang H. Zhang X. Lu W. Huang X. Xie H. Zhou J. Wang W. Zhang Y. Liu Y. Deng Z. Jin Y. Synergistic angiogenesis promoting effects of extracellular matrix scaffolds and adipose-derived stem cells during wound repair. Tissue Eng Part A. 2011;17:725. doi: 10.1089/ten.TEA.2010.0331. [DOI] [PubMed] [Google Scholar]
- 87.Mondalek F.G. Ashley R.A. Roth C.C. Kibar Y. Shakir N. Ihnat M.A. Fung K.M. Grady B.P. Kropp B.P. Lin H.K. Enhanced angiogenesis of modified porcine small intestinal submucosa with hyaluronic acid-poly(lactide-co-glycolide) nanoparticles: from fabrication to preclinical validation. J Biomed Mater Res Part A. 2010;94:712. doi: 10.1002/jbm.a.32748. [DOI] [PubMed] [Google Scholar]
- 88.Freymann U. Endres M. Neumann K. Scholman H.J. Morawietz L. Kaps C. Expanded human meniscus-derived cells in 3-D polymer-hyaluronan scaffolds for meniscus repair. Acta Biomater. 2011;8:677. doi: 10.1016/j.actbio.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Perale G. Rossi F. Sundstrom E. Bacchiega S. Masi M. Forloni G. Veglianese P. Hydrogels in Spinal Cord Injury Repair Strategies. ACS Chem Neurosci. 2011;2:336. doi: 10.1021/cn200030w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park S.H. Cho H. Gil E.S. Mandal B.B. Min B.H. Kaplan D.L. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng Part A. 2011;17:2999. doi: 10.1089/ten.tea.2010.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim I.L. Mauck R.L. Burdick J.A. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2011;32:8771. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sundararaghavan H.G. Burdick J.A. Gradients with depth in electrospun fibrous scaffolds for directed cell behavior. Biomacromolecules. 2011;12:2344. doi: 10.1021/bm200415g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moller L. Krause A. Dahlmann J. Gruh I. Kirschning A. Drager G. Preparation and evaluation of hydrogel-composites from methacrylated hyaluronic acid, alginate, and gelatin for tissue engineering. Int J Artif Organs. 2011;34:93. doi: 10.5301/ijao.2011.6397. [DOI] [PubMed] [Google Scholar]
- 94.Hanson S.E. King S.N. Kim J. Chen X. Thibeault S.L. Hematti P. The effect of mesenchymal stromal cell-hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng Part A. 2011;17:2463. doi: 10.1089/ten.tea.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Griffon D.J. Abulencia J.P. Ragetly G.R. Fredericks L.P. Chaieb S. A comparative study of seeding techniques and three-dimensional matrices for mesenchymal cell attachment. J Tissue Eng Regen Med. 2011;5:169. doi: 10.1002/term.302. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen L.H. Kudva A.K. Guckert N.L. Linse K.D. Roy K. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials. 2011;32:1327. doi: 10.1016/j.biomaterials.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Coburn J. Gibson M. Bandalini P.A. Laird C. Mao H.Q. Moroni L. Seliktar D. Elisseeff J. Biomimetics of the extracellular matrix: an integrated three-dimensional fiber-hydrogel composite for cartilage tissue engineering. Smart Struct Syst. 2011;7:213. doi: 10.12989/sss.2011.7.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Emami S.H. Abad A.M.A. Bonakdar S. Tahriri M.R. Samadikuchaksaraei A. Bahar M.A. Preparation and evaluation of chitosan-gelatin composite scaffolds modified with chondroitin-6-sulphate. Int J Mater Res. 2010;101:1281. [Google Scholar]
- 99.Rentsch C. Rentsch B. Breier A. Spekl K. Jung R. Manthey S. Scharnweber D. Zwipp H. Biewener A. Long-bone critical-size defects treated with tissue-engineered polycaprolactone-co-lactide scaffolds: a pilot study on rats. J Biomed Mater Res Part A. 2010;95:964. doi: 10.1002/jbm.a.32878. [DOI] [PubMed] [Google Scholar]
- 100.Dorozhkin S.V. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31:1465. doi: 10.1016/j.biomaterials.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 101.Porter J.R. Ruckh T.T. Popat K.C. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25:1539. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 102.Teixeira S. Fernandes H. Leusink A. van Blitterswijk C. Ferraz M.P. Monteiro F.J. de Boer J. In vivo evaluation of highly macroporous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res Part A. 2010;93:567. doi: 10.1002/jbm.a.32532. [DOI] [PubMed] [Google Scholar]
- 103.Zhou J. Xu C. Wu G. Cao X. Zhang L. Zhai Z. Zheng Z. Chen X. Wang Y. In vitro generation of osteochondral differentiation of human marrow mesenchymal stem cells in novel collagen-hydroxyapatite layered scaffolds. Acta Biomater. 2011;7:3999. doi: 10.1016/j.actbio.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 104.Li L.H. Kommareddy K.P. Pilz C. Zhou C.R. Fratzl P. Manjubala I. In vitro bioactivity of bioresorbable porous polymeric scaffolds incorporating hydroxyapatite microspheres. Acta Biomater. 2010;6:2525. doi: 10.1016/j.actbio.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 105.Ngiam M. Nguyen L.T.H. Liao S. Chan C.K. Ramakrishna S. Biomimetic nanostructured materials—potential regulators for osteogenesis? Ann Acad Med Singap. 2011;40:213. [PubMed] [Google Scholar]
- 106.Swetha M. Sahithi K. Moorthi A. Srinivasan N. Ramasamy K. Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macrom. 2010;47:1. doi: 10.1016/j.ijbiomac.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 107.Peng F. Yu X.H. Wei M. In vitro cell performance on hydroxyapatite particles/poly(L-lactic acid) nanofibrous scaffolds with an excellent particle along nanofiber orientation. Acta Biomater. 2011;7:2585. doi: 10.1016/j.actbio.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 108.Zhou H. Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7:2769. doi: 10.1016/j.actbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 109.Liu H.C. E L.L. Wang D.S. Su F. Wu X. Shi Z.P. Lv Y. Wang J.Z. Reconstruction of Alveolar Bone Defects Using Bone Morphogenetic Protein 2 Mediated Rabbit Dental Pulp Stem Cells Seeded on Nano-Hydroxyapatite/Collagen/Poly(L-lactide) Tissue Eng Part A. 2011;17:2417. doi: 10.1089/ten.TEA.2010.0620. [DOI] [PubMed] [Google Scholar]
- 110.Prosecka E. Rampichova M. Vojtova L. Tvrdik D. Melcakova S. Juhasova J. Plencner M. Jakubova R. Jancar J. Necas A. Kochova P. Klepacek J. Tonar Z. Amler E. Optimized conditions for mesenchymal stem cells to differentiate into osteoblasts on a collagen/hydroxyapatite matrix. J Biomed Mater Res A. 2011;99A:307. doi: 10.1002/jbm.a.33189. [DOI] [PubMed] [Google Scholar]
- 111.Venugopal J. Prabhakaran M.P. Zhang Y.Z. Low S. Choon A.T. Ramakrishna S. Biomimetic hydroxyapatite-containing composite nanofibrous substrates for bone tissue engineering. Philos Trans R Soc Math Phys Eng Sci. 2010;368:2065. doi: 10.1098/rsta.2010.0012. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y. Reddy V.J. Wong S.Y. Li X. Su B. Ramakrishna S. Lim C.T. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng Part A. 2010;16:1949. doi: 10.1089/ten.TEA.2009.0221. [DOI] [PubMed] [Google Scholar]
- 113.Wahl D.A. Czernuszka J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43. doi: 10.22203/ecm.v011a06. [DOI] [PubMed] [Google Scholar]
- 114.Kretlow J.D. Young S. Klouda L. Wong M. Mikos A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21:3368. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghanaati S. Barbeck M. Orth C. Willershausen I. Thimm B.W. Hoffmann C. Rasic A. Sader R.A. Unger R.E. Peters F. Kirkpatrick C.J. Influence of beta-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010;6:4476. doi: 10.1016/j.actbio.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 116.Abbah S.A. Lam C.X.F. Ramruttun K.A. Goh J.C.H. Wong H.K. Autogenous bone marrow stromal cell sheets-loaded mPCL/TCP scaffolds Induced osteogenesis in a porcine model of spinal interbody fusion. Tissue Eng Part A. 2011;17:809. doi: 10.1089/ten.TEA.2010.0255. [DOI] [PubMed] [Google Scholar]
- 117.Lee H. Kim G. Three-dimensional plotted PCL/beta-TCP scaffolds coated with a collagen layer: preparation, physical properties and in vitro evaluation for bone tissue regeneration. J Mater Chem. 2011;21:6305. [Google Scholar]
- 118.Rai B. Lin J.L. Lim Z.X.H. Guldberg R.E. Hutmacher D.W. Cool S.M. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials. 2010;31:7960. doi: 10.1016/j.biomaterials.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 119.Yeo A. Wong W.J. Khoo H.H. Teoh S.H. Surface modification of PCL-TCP scaffolds improve interfacial mechanical interlock and enhance early bone formation: An in vitro and in vivo characterization. J Biomed Mater Res Part A. 2010;92A:311. doi: 10.1002/jbm.a.32366. [DOI] [PubMed] [Google Scholar]
- 120.Yeo M. Lee H. Kim G. Three-dimensional hierarchical composite scaffolds consisting of polycaprolactone, beta-tricalcium phosphate, and collagen nanofibers: fabrication, physical properties, and in vitro cell activity for bone tissue regeneration. Biomacromolecules. 2011;12:502. doi: 10.1021/bm1013052. [DOI] [PubMed] [Google Scholar]
- 121.Haaparanta A.M. Haimi S. Ella V. Hopper N. Miettinen S. Suuronen R. Kellomaki M. Porous polylactide/beta-tricalcium phosphate composite scaffolds for tissue engineering applications. J Tissue Eng Regen Med. 2010;4:366. doi: 10.1002/term.249. [DOI] [PubMed] [Google Scholar]
- 122.Haimi S. Suuriniemi N. Haaparanta A.M. Ella V. Lindroos B. Huhtala H. Raty S. Kuokkanen H. Sandor G.K. Kellomaki M. Miettinen S. Suuronen R. growth and osteogenic differentiation of adipose stem cells on PLA/bioactive glass and PLA/beta-TCP scaffolds. Tissue Eng Part A. 2009;15:1473. doi: 10.1089/ten.tea.2008.0241. [DOI] [PubMed] [Google Scholar]
- 123.Yanoso-Scholl L. Jacobson J.A. Bradica G. Lerner A.L. O'Keefe R.J. Schwarz E.M. Zuscik M.J. Awad H.A. Evaluation of dense polylactic acid/beta-tricalcium phosphate scaffolds for bone tissue engineering. J Biomed Mater Res Part A. 2010;95:717. doi: 10.1002/jbm.a.32868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao H. Kuboyama N. A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone. 2010;46:386. doi: 10.1016/j.bone.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 125.Hao W. Pang L. Jiang M. Lv R. Xiong Z. Hu Y.Y. Skeletal repair in rabbits using a novel biomimetic composite based on adipose-derived stem cells encapsulated in collagen I gel with PLGA-beta-TCP scaffold. J Orthop Res. 2010;28:252. doi: 10.1002/jor.20969. [DOI] [PubMed] [Google Scholar]
- 126.Zhang S. Zhang X. Cai Q. Wang B. Deng X.L. Yang X.P. Microfibrous beta-TCP/collagen scaffolds mimic woven bone in structure and composition. Biomed Mater. 2010;5 doi: 10.1088/1748-6041/5/6/065005. [DOI] [PubMed] [Google Scholar]
- 127.Fujita N. Matsushita T. Ishida K. Sasaki K. Kubo S. Matsumoto T. Kurosaka M. Tabata Y. Kuroda R. An analysis of bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein 2 from a biodegradable sponge composed of gelatin and beta-tricalcium phosphate. J Tissue Eng Regen Med. 2012;6:291. doi: 10.1002/term.432. [DOI] [PubMed] [Google Scholar]
- 128.Tadokoro M. Matsushima A. Kotobuki N. Hirose M. Kimura Y. Tabata Y. Hattori K. Ohgushi H. Bone morphogenetic protein-2 in biodegradable gelatin and beta-tricalcium phosphate sponges enhances the in vivo bone-forming capability of bone marrow mesenchymal stem cells. J Tissue Eng Regen Med. 2011;6:253. doi: 10.1002/term.427. [DOI] [PubMed] [Google Scholar]
- 129.Ghanaati S. Barbeck M. Hilbig U. Hoffmann C. Unger R.E. Sader R.A. Peters F. Kirkpatrick C.J. An injectable bone substitute composed of beta-tricalcium phosphate granules, methylcellulose and hyaluronic acid inhibits connective tissue influx into its implantation bed in vivo. Acta Biomater. 2011;7:4018. doi: 10.1016/j.actbio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 130.Niyama K. Ide N. Onoue K. Okabe T. Wakitani S. Takagi M. Construction of osteochondral-like tissue graft combining beta-tricalcium phosphate block and scaffold-free centrifuged chondrocyte cell sheet. J Orthop Sci. 2011;16:613. doi: 10.1007/s00776-011-0120-9. [DOI] [PubMed] [Google Scholar]
- 131.Lin K.L. Chen L. Qu H.Y. Lu J.X. Chang J. Improvement of mechanical properties of macroporous beta-tricalcium phosphate bioceramic scaffolds with uniform and interconnected pore structures. Ceram Int. 2011;37:2397. [Google Scholar]
- 132.Badylak S.F. Freytes D.O. Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 133.Pankajakshan D. Agrawal D.K. Scaffolds in tissue engineering of blood vessels. Can J Physiol Pharmacol. 2010;88:855. doi: 10.1139/y10-073. [DOI] [PubMed] [Google Scholar]
- 134.Prabhakaran M.P. Venugopal J. Kai D. Ramakrishna S. Biomimetic material strategies for cardiac tissue engineering. Mater Sci Eng C-Mater Biol Appl. 2011;31:503. [Google Scholar]
- 135.Hoshiba T. Lu H. Kawazoe N. Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10:1717. doi: 10.1517/14712598.2010.534079. [DOI] [PubMed] [Google Scholar]
- 136.Piterina A.V. Cloonan A.J. Meaney C.L. Davis L.M. Callanan A. Walsh M.T. McGloughlin T.M. ECM-based materials in cardiovascular applications: inherent healing potential and augmentation of native regenerative processes. Int J Mol Sci. 2009;10:4375. doi: 10.3390/ijms10104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crapo P.M. Wang Y.D. Small intestinal submucosa gel as a potential scaffolding material for cardiac tissue engineering. Acta Biomater. 2010;6:2091. doi: 10.1016/j.actbio.2009.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okada M. Payne T.R. Oshima H. Momoi N. Tobita K. Huard J. Differential efficacy of gels derived from small intestinal submucosa as an injectable biomaterial for myocardial infarct repair. Biomaterials. 2010;31:7678. doi: 10.1016/j.biomaterials.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 139.Peng H.F. Liu J.Y. Andreadis S.T. Swartz D.D. Hair follicle-derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng Part A. 2011;17:981. doi: 10.1089/ten.tea.2010.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tan M.Y. Zhi W. Wei R.Q. Huang Y.C. Zhou K.P. Tan B. Deng L. Luo J.C. Li X.Q. Xie H.Q. Yang Z.M. Repair of infarcted myocardium using mesenchymal stem cell seeded small intestinal submucosa in rabbits. Biomaterials. 2009;30:3234. doi: 10.1016/j.biomaterials.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 141.Honsawek S. Bumrungpanichthaworn P. Thanakit V. Kunrangseesomboon V. Muchmee S. Ratprasert S. Tangchainavaphum P. Dechprapatsorn S. Prajuabtanyachat S. Suksamran A. Rojchanawatsirivech A. Osteoinductive potential of small intestinal submucosa/demineralized bone matrix as composite scaffolds for bone tissue engineering. Asian Biomed. 2010;4:913. [Google Scholar]
- 142.Kim K.S. Lee J.Y. Kang Y.M. Kim E.S. Kim G.H. Rhee S.D. Cheon H.G. Kim J.H. Min B.H. Lee H.B. Kim M.S. Small intestine submucosa sponge for in vivo support of tissue-engineered bone formation in the presence of rat bone marrow stem cells. Biomaterials. 2010;31:1104. doi: 10.1016/j.biomaterials.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 143.Zhao L. Zhao J. Wang S. Wang J. Liu J. Comparative study between tissue-engineered periosteum and structural allograft in rabbit critical-sized radial defect model. J Biomed Mater Res B Appl Biomater. 2011;97:1. doi: 10.1002/jbm.b.31768. [DOI] [PubMed] [Google Scholar]
- 144.Kang K.N. Lee J.Y. Kim da Y. Lee B.N. Ahn H.H. Lee B. Khang G. Park S.R. Min B.H. Kim J.H. Lee H.B. Kim M.S. Regeneration of completely transected spinal cord using scaffold of poly(D,L-lactide-co-glycolide)/small intestinal submucosa seeded with rat bone marrow stem cells. Tissue Eng Part A. 2011;17:2143. doi: 10.1089/ten.TEA.2011.0122. [DOI] [PubMed] [Google Scholar]
- 145.Zhou Y. Yan Z. Zhang H. Lu W. Liu S. Huang X. Luo H. Jin Y. Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng Part A. 2011;17:2981. doi: 10.1089/ten.tea.2010.0707. [DOI] [PubMed] [Google Scholar]
- 146.Heise R.L. Ivanova J. Parekh A. Sacks M.S. generating elastin-rich small intestinal submucosa-based smooth muscle constructs utilizing exogenous growth factors and cyclic mechanical stimulation. Tissue Eng Part A. 2009;15:3951. doi: 10.1089/ten.tea.2009.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qin H.H. Dunn J.C. Small intestinal submucosa seeded with intestinal smooth muscle cells in a rodent jejunal interposition model. J Surg Res. 2011;171:e21. doi: 10.1016/j.jss.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu S.F. Liu Y. Bharadwaj S. Atala A. Zhang Y.Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang J. Wang G.Y. Xiao Y.P. Fan L.Y. Wang Q. The biomechanical behavior and host response to porcine-derived small intestine submucosa, pericardium and dermal matrix acellular grafts in a rat abdominal defect model. Biomaterials. 2011;32:7086. doi: 10.1016/j.biomaterials.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 150.Tedder M.E. Simionescu A. Chen J. Liao J. Simionescu D.T. Assembly and testing of stem cell-seeded layered collagen constructs for heart valve tissue engineering. Tissue Eng Part A. 2011;17:25. doi: 10.1089/ten.tea.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Iwata H. Sakano S. Itoh T. Bauer T.W. Demineralized bone matrix and native bone morphogenetic protein in orthopaedic surgery. Clin Orthop Relat Res. 2002;99:100. doi: 10.1097/00003086-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 152.Liu G. Li Y. Sun J. Zhou H. Zhang W. Cui L. Cao Y. In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood-derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng Part A. 2010;16:971. doi: 10.1089/ten.TEA.2009.0516. [DOI] [PubMed] [Google Scholar]
- 153.Chen L. He Z. Chen B. Yang M. Zhao Y. Sun W. Xiao Z. Zhang J. Dai J. Loading of VEGF to the heparin cross-linked demineralized bone matrix improves vascularization of the scaffold. J Mater Sci Mater Med. 2010;21:309. doi: 10.1007/s10856-009-3827-9. [DOI] [PubMed] [Google Scholar]
- 154.Rhee S.C. Ji Y.H. Gharibjanian N.A. Dhong E.S. Park S.H. Yoon E.S. In vivo evaluation of mixtures of uncultured freshly isolated adipose-derived stem cells and demineralized bone matrix for bone regeneration in a rat critically sized calvarial defect model. Stem Cells Dev. 2011;20:233. doi: 10.1089/scd.2009.0525. [DOI] [PubMed] [Google Scholar]
- 155.Supronowicz P. Gill E. Trujillo A. Thula T. Zhukauskas R. Ramos T. Cobb R.R. Human adipose-derived side population stem cells cultured on demineralized bone matrix for bone tissue engineering. Tissue Eng Part A. 2011;17:789. doi: 10.1089/ten.TEA.2010.0357. [DOI] [PubMed] [Google Scholar]
- 156.Lee J.H. Lee K.M. Baek H.R. Jang S.J. Ryu H.S. Combined effects of porous hydroxyapatite and demineralized bone matrix on bone induction: in vitro and in vivo study using a nude rat model. Biomed Mater. 2011;6:015008. doi: 10.1088/1748-6041/6/1/015008. [DOI] [PubMed] [Google Scholar]
- 157.Eleftheriadis E. Leventis M.D. Tosios K.I. Faratzis G. Titsinidis S. Eleftheriadi I. Dontas I. Osteogenic activity of beta-tricalcium phosphate in a hydroxyl sulphate matrix and demineralized bone matrix: a histological study in rabbit mandible. J Oral Sci. 2010;52:377. doi: 10.2334/josnusd.52.377. [DOI] [PubMed] [Google Scholar]
- 158.Kang E.J. Byun J.H. Choi Y.J. Maeng G.H. Lee S.L. Kang D.H. Lee J.S. Rho G.J. Park B.W. In vitro and in vivo osteogenesis of porcine skin-derived mesenchymal stem cell-like cells with a demineralized bone and fibrin glue scaffold. Tissue Eng Part A. 2010;16:815. doi: 10.1089/ten.TEA.2009.0439. [DOI] [PubMed] [Google Scholar]
- 159.Wang Z.H. He X.J. Yang Z.Q. Tu J.B. Cartilage tissue engineering with demineralized bone matrix gelatin and fibrin glue hybrid scaffold: an in vitro study. Artif Organs. 2010;34:161. doi: 10.1111/j.1525-1594.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- 160.Thomas C.B. Maxson S. Burg K.J.L. Preparation and characterization of a composite of demineralized bone matrix fragments and polylactide beads for bone tissue engineering. J Biomater Sci Polym Ed. 2011;22:589. doi: 10.1163/092050610X488232. [DOI] [PubMed] [Google Scholar]
- 161.Kurkalli B.G. Gurevitch O. Sosnik A. Cohn D. Slavin S. Repair of bone defect using bone marrow cells and demineralized bone matrix supplemented with polymeric materials. Curr Stem Cell Res Ther. 2010;5:49. doi: 10.2174/157488810790442831. [DOI] [PubMed] [Google Scholar]
- 162.Champa Jayasuriya A. Ebraheim N.A. Evaluation of bone matrix and demineralized bone matrix incorporated PLGA matrices for bone repair. J Mater Sci Mater Med. 2009;20:1637. doi: 10.1007/s10856-009-3738-9. [DOI] [PubMed] [Google Scholar]
- 163.Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: review. Prog Polym Sci. 2011;36:1254. [Google Scholar]
- 164.Yagihashi K. Miyazawa K. Togari K. Goto S. Demineralized dentin matrix acts as a scaffold for repair of articular cartilage defects. Calcif Tissue Int. 2009;84:210. doi: 10.1007/s00223-008-9205-7. [DOI] [PubMed] [Google Scholar]
- 165.Elder B.D. Kim D.H. Athanasiou K.A. Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery. 2010;66:722. doi: 10.1227/01.NEU.0000367616.49291.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kheir E. Stapleton T. Shaw D. Jin Z. Fisher J. Ingham E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J Biomed Mater Res Part A. 2011;99:283. doi: 10.1002/jbm.a.33171. [DOI] [PubMed] [Google Scholar]
- 167.Yang Z. Shi Y. Wei X. He J. Yang S. Dickson G. Tang J. Xiang J. Song C. Li G. Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng Part C Methods. 2010;16:865. doi: 10.1089/ten.TEC.2009.0444. [DOI] [PubMed] [Google Scholar]
- 168.Yang Q. Peng J. Guo Q. Huang J. Zhang L. Yao J. Yang F. Wang S. Xu W. Wang A. Lu S. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 169.Gong Y.Y. Xue J.X. Zhang W.J. Zhou G.D. Liu W. Cao Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32:2265. doi: 10.1016/j.biomaterials.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 170.Bormann N. Pruss A. Schmidmaier G. Wildemann B. In vitro testing of the osteoinductive potential of different bony allograft preparations. Arch Orthop Trauma Surg. 2010;130:143. doi: 10.1007/s00402-009-0908-7. [DOI] [PubMed] [Google Scholar]
- 171.Kang S.H. Chung Y.G. Lee Y.G. Kim Y.S. Kim J.M. Park S.W. The effect of demineralized bone matrix on bone regeneration. Tissue Eng Regen Med. 2010;7:373. [Google Scholar]
- 172.Moroni L. Elisseeff J.H. Biomaterials engineered for integration. Mater Today. 2008;11:44. [Google Scholar]
- 173.Darling E.M. Athanasiou K.A. Retaining zonal chondrocyte phenotype by means of novel growth environments. Tissue Eng. 2005;11:395. doi: 10.1089/ten.2005.11.395. [DOI] [PubMed] [Google Scholar]
- 174.Rojbani H. Nyan M. Ohya K. Kasugai S. Evaluation of the osteoconductivity of alpha-tricalcium phosphate, beta-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J Biomed Mater Res Part A. 2011;98A:488. doi: 10.1002/jbm.a.33117. [DOI] [PubMed] [Google Scholar]
- 175.Moore S.T. Katz J.M. Zhukauskas R.M. Hernandez R.M. Lewis C.S. Supronowicz P.R. Gill E. Grover S.M. Long N.S. Cobb R.R. Osteoconductivity and osteoinductivity of Puros(R) DBM putty. J Biomater Appl. 2011;26:151. doi: 10.1177/0885328210366061. [DOI] [PubMed] [Google Scholar]
- 176.Hollister S.J. Murphy W.L. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev. 2011;17:459. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ratcliffe A. the translation of product concept to bone products: a partnership of therapeutic effectiveness and commercialization. Tissue Eng Part B Rev. 2011;17:443. doi: 10.1089/ten.TEB.2011.0236. [DOI] [PubMed] [Google Scholar]
- 178.Lee M.H. Arcidiacono J.A. Bilek A.M. Wille J.J. Hamill C.A. Wonnacott K.M. Wells M.A. Oh S.S. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng Part B Rev. 2010;16:41. doi: 10.1089/ten.TEB.2009.0449. [DOI] [PubMed] [Google Scholar]
- 179.Evans C.H. Barriers to the clinical translation of orthopedic tissue engineering. Tissue Eng Part B Rev. 2011;17:437. doi: 10.1089/ten.teb.2011.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]