Abstract
Understanding the levator ani complex architecture is of major clinical relevance. The aim of this study was to determine the feasibility of magnetic resonance (MR) fiber tractography with diffusion tensor imaging (DTI) as a tool for the three-dimensional (3D) representation of normal subdivisions of the levator ani. Ten young nulliparous female volunteers underwent DTI at 1.5 T MR imaging. Diffusion-weighted axial sequence of the pelvic floor was performed with additional T2-weighted multiplanar sequences for anatomical reference. Fiber tractography for visualization of each Terminologia Anatomica-listed major levator ani subdivision was performed. Numeric muscular fibers extracted after tractography were judged as accurate when localized within the boundaries of the muscle, and inaccurate when projecting out of the boundaries of the muscle. From the fiber tracking of each subdivision the number of numeric fibers (inaccurate and accurate) and a score (from 3 to 0) of the adequacy of the 3D representation were calculated. All but two volunteers completed the protocol. The mean number of accurate fibers was 17 ± 2 for the pubovisceralis, 14 ± 6 for the puborectalis and 1 ± 1 for the iliococcygeus. The quality of the 3D representation was judged as good (score = 2) for the pubovisceralis and puborectalis, and inaccurate (score = 0) for the iliococcygeus. Our study is the first step to a 3D visualization of the three major levator ani subdivisions, which could help to better understand their in vivo functional anatomy.
Keywords: anatomy, diffusion tensor imaging, fiber tractography, levator ani, magnetic resonance imaging, pelvic floor
Introduction
Understanding the basic anatomy of the levator ani complex architecture is essential when formulating a clinical opinion as damage may be implicated in the pathophysiology of pelvic organ prolapse (Jelovsek et al. 2007). Standardized anatomical terminology identifies three major subdivisions of the levator ani muscle: the pubovisceralis (= pubococcygeus), which is further subdivided into pubovaginalis, puboperinealis and puboanalis; as well as the puborectalis and the iliococcygeus muscles (Kearney et al. 2004). Each subdivision has a unique origin-insertion pair and, thus, a unique line of action. Recent work has described the normal in vivo appearance of each of the different levator ani subdivisions on magnetic resonance imaging (MRI) (Margulies et al. 2006), making it possible to visualize defects (Margulies et al. 2007) and to explore the hypothesis that dysfunction of any subdivisions would cause respective clinical findings (Kearney et al. 2004; DeLancey et al. 2007). In their studies, Margulies et al. (2006, 2007) raised the limits of conventional multiplanar MRI studies to estimate the fiber direction of levator ani subdivisions and to hypothesize about their line of action. A three-dimensional (3D) MRI reconstruction of the levator ani from manual segmentation on MRI morphological sequences gives a more volumetric than anatomical analysis, and can not accurately visualize the orientation and the insertions of the subdivisions (Fielding et al. 2000; Margulies et al. 2006).
Recently diffusion tensor imaging (DTI) has emerged as a unique non-invasive tool to describe the direction of the internal micro-structure within anisotropic tissues (Basser & Jones, 2002). This technique is known as MR fiber tractography. It is known that in anisotropic tissues water diffusion is higher along the direction of an internal microstructure and, conversely, more restricted along its perpendicular axis. The directional dependence of water diffusion in tissue can be evaluated by determining in a voxel, the three orthogonal directions called eigenvectors and their intensities, called eigenvalues. Fiber tractographic analysis of DT MRI data is based on the assumption that the main eigenvector (corresponding to the largest eigenvalue) of the DT coincides with the local fiber orientation (Bammer et al. 2003). By combining the diffusion tensor data from multiple voxels, fiber tracts can be reconstructed that correlate with the principal diffusion direction of water molecules in a tissular microstructure. DTI and tractography have already found clinical applications in brain imaging, but their use in the musculoskeletal field is still emerging (Van Donkelaar et al. 1999) and has potential for probing the in vivo architecture of striated muscle (Budzik et al. 2007; Deux et al. 2008). Few authors have studied pelvic floor muscular structure using DTI (Zijta et al. 2011) and, to the best of our knowledge, none has performed a focused study on the levator ani subdivisions that could provide a new assessment of their in vivo anatomy to better understand their function.
The aim of this study was to determine the feasibility of MR fiber tractography with DTI as a tool for the 3D representation of normal subdivisions of the levator ani in healthy volunteers.
Materials and methods
Study population
In this study, approved by our institutional review board, 10 healthy nulliparous women gave written informed consent. Volunteers were recruited via fliers without any offer of financial inducement from the hospital staff. All volunteers with a history of pregnancy or any symptom of pelvic floor disease and/or previous pelvic surgery were excluded, as were contra-indications to undergoing MRI (pacemakers, claustrophobia and pregnancy). Volunteers were also asked to fill out the Urogenital Distress Inventory, Defecation Distress Inventory and Incontinence Impact Questionnaire to exclude prolapse, micturition and/or defecation dysfunction (Uebersax et al. 1995; van Brummen et al. 2006). None received a clinical examination.
Volunteers had a mean age of 26 years (range 23–30 years) and a mean body mass index of 21.3 (range 18–25) {body mass index was calculated as weight [kg]/[height (m)]2}. All were Caucasian.
MRI data acquisition
Magnetic resonance imaging was performed using 1.5 T MRI (Achieva, Philips Medical Systems, Best, the Netherlands). A five-channel surface coil wrapped around the pelvis allowed coverage of the entire pelvic floor area. Volunteers were in the supine position with their legs parallel, and were asked to empty their bladder before the examination. No contrast agent was administered.
The MRI protocol was as follows. A Turbo Spin Echo (TSE) T2-weighted (T2w) sequence was first performed on the pelvic floor with the following parameters: field of view (FOV): 230 × 230 mm2; voxel size 0.45 × 0.45 × 3 mm3; slice thickness: 3 mm without spacing; TR/TE: 6500/130 ms; NSA = 2; acquisition time: 3 min 49 s. This sequence was performed in the axial, sagittal and coronal planes for anatomical reference. A Spin Echo- Echo-Planar-Imaging (SE-EPI) was acquired for DTI imaging in the same geometry and location as the axial TSE sequence. The following parameters were used: FOV: 230 × 230 mm2; isotropic voxel of 3 mm3; 30 axial slices of the pelvic floor (corresponding slices position-matched with axial T2w slices); TR/TE: 8653/49 ms; 16 diffusion-weighed directions; NSA: 4; b = 0 s mm−2 and b = 600 s mm−2; EPI factor = 37; Spectral Adiabatic Inversion Recovery (SPAIR) for fat suppression, parallel imaging with an acceleration factor 2; acquisition time: 18 min 2 s.
Data analysis
All but two volunteers were analyzed, as their images were excluded because of motion (n = 1) and aliasing artifacts (n = 1). Two radiologists (PR, a fourth-year fellow specialized in female pelvic imaging with an additional 4 years experience as a teaching assistant at the Department of Anatomy and Embryology; and JNB with 30 years experience in female pelvic imaging) performed and analyzed all measurements in consensus. Before analysis the investigators were instructed in the anatomy of the levator ani muscle by an academic anatomist (VD, an urologist and academic anatomist with 40 years experience) with extensive experience in the anatomy of the pelvic floor. A separate group of 40 pelvic MRI was also evaluated to gain familiarity with the morphological appearance of the levator ani subdivisions, as previously described by Kearney et al. (2004).
The three Terminologia Anatomica-listed major subdivisions of the levator ani muscle, namely the pubovisceralis (= pubococcygeus) that is further subdivided into pubovaginalis, puboperinealis and puboanalis; as well as the puborectalis and the iliococcygeus muscles, were studied (Kearney et al. 2004). In addition, measurements were performed to track the major striated muscular component of the pelvic wall, i.e. the obturator internus, which was chosen as the reference muscle of the region. For each of the analyzed eight volunteers, the left and right muscles were studied.
Fractional anisotropy (FA) maps
The FA maps were calculated from DTI imaging using a dedicated software program (Philips Pride Fiber Tracking). FA is a quantitative index of DTI used to characterize the degree of tissue anisotropy, and FA maps were calculated from the eigenvalues on the basis of standard formulas (Jellison et al. 2004). The eigenvector associated with the largest eigenvalue was used to represent the local fiber direction. Color maps were created on the basis of the three vector elements for each voxel. On the FA map, the per voxel absolute vector values were color-coded: red (medio-lateral); blue (cranio-caudal); and green (antero-posterior direction).
Muscular fiber tractography
To allow the 3D visualization of the predefined muscles, up to two seed regions of interest (ROI) were manually drawn in the axial plane on the b0 image at the site where the muscle was best visualized on the corresponding axial T2w image (Figs 1A,B, 2A,B and 3A,B). For the obturator internus, only its endopelvic boundary was drawn. For the iliococcygeus and the obturator internus, a single additional manual drawing was performed in the coronal plane where the muscle was best visualized. To avoid minimal distortions due to field heterogeneity, the manual drawing for all muscles was preferentially made on the b0 image rather than on the corresponding T2w image.
Fig. 1.
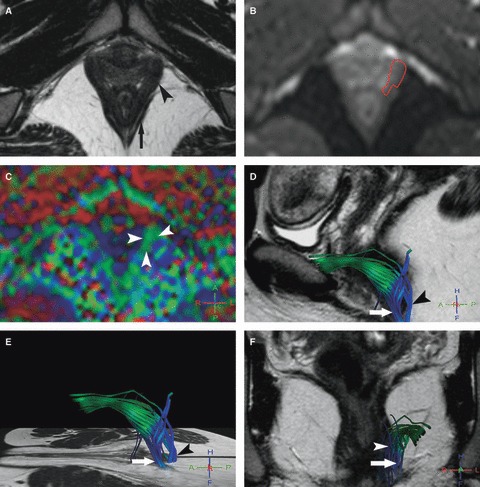
Left pubovisceralis MR tractography of a 26-year-old volunteer (score = 3). (A) Axial T2w image; and (B) corresponding axial b0 image show how the manual drawing of the left pubovisceralis (A, arrowhead) was performed on the b0 image (B, red line), excluding the external anal sphincter (A, arrow) with help of the T2w image. (C) The FA map shows a predominantly green color in projection of the pubovisceralis (arrowheads) due to the mainly antero-posterior direction of fibers. (D–F) 3D representations of some fibers in the left pubovisceralis (D, E) in the sagittal and (F) coronal planes with projection on T2w images show accurate green fibers extending posteriorly with a blue encoding in the perineal body (puboperinealis; arrow) and to the lateral canal anal wall (puboanalis; arrowhead). Note also the presence of some blue inaccurate fibers in projection in the ischio-anal adipose tissue.
Fig. 2.
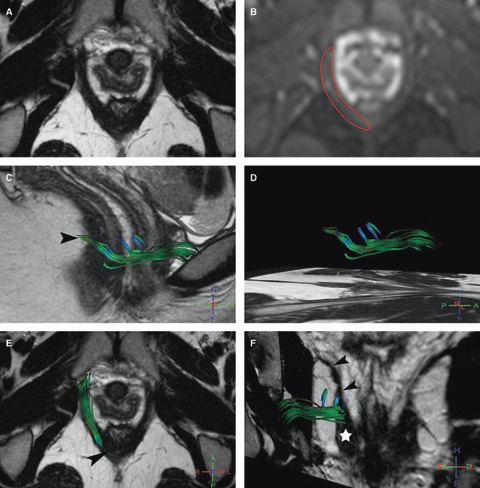
Right puborectalis MR tractography of a 22-year-old volunteer (score = 2.5). (A) Axial T2w image; and (B) corresponding axial b0 image show how the manual drawing (B, red line) was performed. (C–F) 3D representation of some fibers in the right puborectalis (C, D) in the sagittal, (E) axial and (F) coronal oblique planes with projection on T2w images show green accurate fibers and the presence of some blue inaccurate fibers. On the coronal view (F) it lies at its characteristic anatomic location lateral and slightly above the pubovisceralis (star), and medial and below the iliococcygeus (arrowheads). Note that fibers around the anorectal junction (C, E, arrowhead) were not tracked.
Fig. 3.
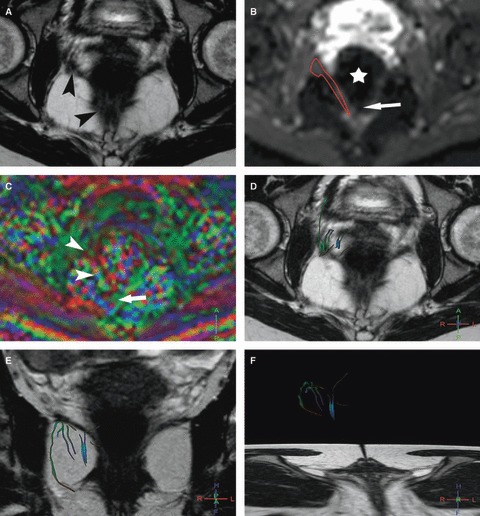
Right iliococcygeus MR tractography of a 24-year-old volunteer (score = 0). (A) Axial T2w image; and (B) corresponding axial b0 image show the difficulty of the manual drawing of the right iliococcygeus (arrowheads) particularly at its posterior part with susceptibility artifact (arrow) due to the presence of air within the rectum (star). (C) The FA map shows a random coloration in the area of the iliococcygeus (arrowheads) with distortion artifact at its posterior part (arrow). (D–F) 3D representation of some fibers in the right iliococcygeus (D) in the axial and (E, F) coronal planes with projection on T2w images show sparse and multicolored inaccurate fibers.
To control the relevance of the muscular fiber tractography, an additional ROI (size of 100 mm2) was systematically taken in the urine, which is considered as an isotropic medium, as well as the left adipose tissue of the ischio-anal fossa, which is considered as an anisotropic medium without organized anisotropic microstructure.
Fiber tractography was performed using a line propagation technique from a dedicated Philips software program (Philips Pride Fiber Tracking). Tracking was launched from the seed ROI, and propagated in both the retrograde and anterograde directions through successive local searches according to the main eigenvector at each voxel. Fiber tracking was performed with a continuous fiber track assignment algorithm. The 3D fiber track was allowed to continue unless it entered a region of FA < 0.15 or turned at an angle > 45 ° between two consecutive voxels (Heemskerk et al. 2009). All possible continuous fiber tracks above a threshold of 10 mm were reconstructed from voxels. The global color assigned to each fiber tracked was the same color code as the FA map.
Muscular fiber tractography analysis
The total number of fibers tracked for each muscle was automatically calculated by the DT MRI software at the end of the tractography and noted for each muscle. The inaccurate numeric fibers were identified and their number also counted. Thanks to the image fusion software, a numeric fiber was judged inaccurate when it was projecting out of the boundaries of the corresponding muscle on the fused anatomical T2w multiplanar images with DTI tractography. An inaccurate fiber was also identified if it was multicolored. The remaining fibers were considered as accurate fibers, and their number was calculated from the subtraction of the inaccurate number from the total number of fibers.
To determine whether the accurate fibers orientation and muscle shape were an adequate representation of the expected anatomical appearance, a qualitative score of adequacy of the 3D representation was proposed. The 3D representation of each muscle was judged as excellent (score = 3), good (score = 2), acceptable (score = 1) or inaccurate (score = 0). This score was based on the presence of different criteria and up to three points were given: an homogenous repartition of the accurate fibers within the boundaries on corresponding T2w images in the three orthogonal planes (one point); a good correlation between the predominant color orientation of the muscle on FA maps (corresponding with the global color assigned to these fibers) and the anatomical expected orientation (Kearney et al. 2004) (one point); the visualization of the proximal insertion (half point); and the distal insertion for pubovisceralis and iliococcygeus or distal portion for puborectalis and obturator internus (half point). For the pubovisceralis, visualization of one of the three distal insertions (pubovaginalis, puboperinealis or puboanalis) was considered as sufficient to attribute a half point.
Signal-to-noise ratio (SNR)
The technique described by Kaufman et al. (1989) was used to calculate the SNR. For each volunteer, ROI with a round surface of 200 mm2 were placed in the air in front of the anterior pelvic wall and in the left obturator internus on the axial b = 0 and b = 600 images. SNR was then calculated using the following equation: SNR = SI muscle/SD SI air, where SI muscle is the signal intensity (SI) of the left obturator internus and SD SI air is the standard deviation (SD) of signal in air (equal to noise).
Results
Muscular fiber tractography
The mean quantitative and qualitative data are reported in Table 1. MR tractography was able to assess the global muscle shape and the orientation of the pubovisceralis and puborectalis fibers (score of adequacy = 2.1 ± 0.6 and 1.9 ± 0.5, respectively; Figs 1 and 2), but was not satisfactory for the iliococcygeus (score of adequacy = 0; Fig. 3). The pubovisceralis and puborectalis demonstrated a predominantly green color on the FA map (due to their mainly anterior-posterior direction). The iliococcygeus could not be tracked in the available data sets and was therefore rated as inaccurate (score = 0) in all volunteers. All fibers were located out of the muscle on T2w multiplanar images and judged inaccurate in nine out of the 16 iliococcygeus (56%). For the seven other iliococcygeus (n = 7/16, 44%), only one (n = 3/7) or up to two (n = 4/7) fibers were judged to be accurate among the fibers tracked. In addition, all FA maps had a random coloration in the area of the iliococcygeus, and most of the fibers tracked had a multicolor encoding.
Table 1.
Mean quantitative and qualitative data for each muscle of eight volunteers
| Muscle (n = 16) | Total number of fibers | Number of inaccurate fibers | Percentage of inaccurate fibers (%) | Number of accurate fibers | Score of adequacy |
|---|---|---|---|---|---|
| Pubovisceralis | 24 ± 3 (19–31) | 7 ± 2 (5–10) | 29 | 17 ± 2 (13–23) | 2.1 ± 0.6 (1.5–3) |
| Puborectalis | 23 ± 8 (10–39) | 8 ± 5 (3–17) | 35 | 14 ± 6 (7–23) | 1.9 ± 0.5 (1–2.5) |
| Iliococcygeus | 15 ± 3 (7–19) | 14 ± 3 (7–17) | 93 | 1 ± 1 (0–2) | 0 |
| Obturator internus | 133 ± 28 (89–208) | 9 ± 5 (3–22) | 7 | 124 ± 27 (84–199) | 2.9 ± 0.2 (2–3) |
Each value is presented as mean ± standard deviation (extreme values).
Regarding proximal insertion and distal insertion or portion of muscles, anterior proximal retro-pubic insertion of all pubovisceralis (n = 16) and all but two (n = 14/16) puborectalis were seen. For the pubovisceralis, distal insertion extending to the perineal body corresponding to the puboperinealis was seen in eight out of the 16 pubovisceralis (50%). Distal insertion extending to the lateral canal anal wall corresponding to the puboanalis was seen in all cases. Distal insertion extending to the vaginal wall corresponding to the pubovaginalis was not seen in all cases. For the puborectalis, no case was seen at the distal portion, which wrapped around the anorectal junction.
In all volunteers, fiber tractography of the obturator internus resulted in a very satisfactory representation of the global muscle morphology and fiber orientation (Fig. 4), with a mean score of 2.9 (Table 1).
Fig. 4.
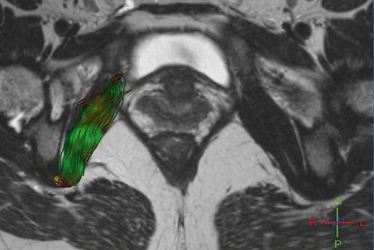
Right obturator internus MR tractography of a 23-year-old volunteer (score = 3). 3D representation of some fibers in the right obturator internus in the oblique axial plane with projection on T2w image shows green accurate fibers and the distal red encoding color of the fiber passing below the ischial spine.
The relevance of the muscular tractography was judged correct. There was no fiber tracked in the urine; in the adipose tissue of ischio-anal fossa there was a mean number of fibers of 6 ± 1 with a random coloration on the FA map in the corresponding area of the seed region. These thick, tortuous and multicolored fibers were easily recognized as inaccurate, with no anatomical relevance.
SNR
The mean values of the signal were 306 ± 33.5 and 121 ± 12.7, respectively, for b0 and b600 images, i.e. a decrease of 60%. Mean values of SNR were 46 ± 2.9 and 34 ± 3.5, respectively, for b0 and b600 images, i.e. a decrease of 26%.
Discussion
In this feasibility study we reported that MR tractography provided a good approach for visualizing the shape and fiber orientation, with a satisfactory 3D representation of the pubovisceralis and puborectalis. The study of the iliococcygeus was more difficult. The pubovisceralis was seen lying medial to the puborectalis, arising on the pubis forming an antero-posterior single mass that divided in distal supero-inferior insertions. Due to their small size, these distal insertions (the pubovaginalis, puboperinealis and puborectalis) were not always clearly visible. The puborectalis appeared as an antero-posterior sling joining the anorectal junction. Our 3D representation showing the orientation of the fibers and most insertions may help to better hypothesize about the pubovisceralis and puborectalis line of action and functionality. One could better understand how the pubovisceralis can elevate the pelvic floor with a compressor effect on the vagina and the anal canal, and how the puborectalis can reduce the antero-posterior dimension of the ano-urogenital hiatus. MR tractography may provide a better understanding of the in vivo levator ani comparative to both conventional MRI techniques (Fielding et al. 2000; Margulies et al. 2006) where fiber direction can not be extrapolated as well as ex vivo studies (Strohbehn et al. 1996; Shobeiri et al. 2008) where the levator ani has lost its tone and has a different shape from that of a living woman.
Most DTI studies assessing striated muscles are limited to perfectly geometrically arranged striated muscles of the lower extremity (Budzik et al. 2007; Deux et al. 2008). However, the pelvic floor is a more challenging region for DTI as it contains thin muscles providing a weaker tracking signal and has numerous artifactual interfaces between air, urine or adipose tissue (Mukherjee et al. 2008). Despite these limitations, Zijta et al. (2011) have recently reported the imaging of several pelvic floor muscular structures using 3 T DTI with MR tractography but without a focused analysis of levator ani subdivisions. Our DTI imaging of the pubovisceralis and puborectalis was viable because they are thick and have little surrounding adipose tissue, and thus provide a high qualitative score and a low inaccurate fibers count. Similarly, we reported a very satisfactory representation of the obturator internus, suggesting that DTI fiber tractography may be reliable in this region. In contrast, for several reasons we had difficulties in analyzing the iliococcygeus subdivision. It has a complex shape and a very thin network of fibers (Shobeiri et al. 2008), which provided few fibers and could make it difficult to analyze their accuracy. For instance, in this work we have considered as inaccurate a multicolored fiber with great changes in direction. By so doing it may be possible that we have excluded some ‘real’ accurate fibers with significant change in direction. Moreover, the iliococcygeus is surrounded by sub-peritoneal and ischio-anal adipose tissue, while the presence of air in the rectum responsible for susceptibility artifact could make manual contouring more difficult. Therefore, voxels at the boundaries of the manual contouring might contain signals originating from both muscle and fat leading to partial volume effects, which could lead to inaccurate fiber tractography. This phenomenon was potentially magnified by our choice of tractography parameters (Heemskerk et al. 2009) used to maximize the muscle fibers tracking in this feasibility study.
Our study had a number of limitations. First, we investigated only a small number of young volunteers without symptoms, so further studies are required to confirm the normal levator ani anatomy and its variations before proceeding in patients. We also limited our study to female subjects as pelvic floor dysfunction primarily concerns women. Second, as our primary aim was to assess the feasibility of visualizing the levator ani subdivisions, both reproducibility and the inter-observer reliability were not assessed. Thirdly, we were limited to using a 1.5 T MRI system for image acquisition. Hence, to obtain tractography of muscles on a 1.5 T magnet, we had to optimize several parameters at the cost of a longer acquisition time. Nevertheless, in our study the decrease in signal and the increase of background noise between the b0 and b600 images resulted in a 26% decrease of the SNR, which is in line with previous reports (Budzik et al. 2007). Imaging at a higher field strength (3 T) would have afforded the opportunity for increased signal relative to 1.5 T imaging (Saupe et al. 2008), which might allow a more detailed fiber tractography of smaller muscular structure and a less compromising acquisition time (Zijta et al. 2011). However, artifacts are more numerous on 3 T MRI (Bolog et al. 2006), which could be a limiting factor in the pelvic region. Indeed, Zijta et al. (2011) using a 3 T MRI DTI showed a nice global appearance of the pubovisceralis (which include the puborectalis in their definition), but without any clear visualization of its insertions. Nevertheless, our 1.5 T MRI DTI study should give encouragement to repeat with a higher field strength.
In conclusion, our study demonstrated the feasibility of providing a 3D representation of the pubovisceralis and puborectalis levator ani subdivisions using 1.5 T MRI DTI with fiber tractography. The study of the iliococcygeus was more challenging. Pelvic floor specialists should be aware of this new development as it can provide complementary information to better understand the in vivo levator ani functional anatomy. Further research is open to optimize fiber tractography, and to define its potential role in providing new insights into the levator ani subdivisions and their role in pelvic organ support as well as demonstrating alterations in pelvic floor dysfunction.
Author contributions
Concept/design: Pascal Rousset, Vincent Delmas, Jean-François Deux. Acquisition data: Pascal Rousset, Jean-François Deux, Dominique Vadrot. Data analysis/interpretation: Pascal Rousset, Jean-Noël Buy. Drafting the manuscript: Pascal Rousset, Jean-François Deux, Alain Rahmouni. Critical revision: all authors. Approval of the article: all authors.
References
- Bammer R, Acar B, Moseley ME. In vivo MR tractography using diffusion imaging. Eur J Radiol. 2003;45:223–234. doi: 10.1016/s0720-048x(02)00311-x. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis – a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Bolog N, Nanz D, Weishaupt D. Muskuloskeletal MR imaging at 3.0 T: current status and future perspectives. Eur Radiol. 2006;16:1298–1307. doi: 10.1007/s00330-006-0184-7. [DOI] [PubMed] [Google Scholar]
- van Brummen HJ, Bruinse HW, van de Pol G, et al. Defecatory symptoms during and after the first pregnancy: prevalences and associated factors. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:224–230. doi: 10.1007/s00192-005-1351-0. [DOI] [PubMed] [Google Scholar]
- Budzik JF, Le Thuc V, Demondion X, et al. In vivo MR tractography of thigh muscles using diffusion imaging: initial results. Eur Radiol. 2007;17:3079–3085. doi: 10.1007/s00330-007-0713-z. [DOI] [PubMed] [Google Scholar]
- DeLancey JO, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- Deux JF, Malzy P, Paragios N, et al. Assessment of calf muscle contraction by diffusion tensor imaging. Eur Radiol. 2008;18:2303–2310. doi: 10.1007/s00330-008-1012-z. [DOI] [PubMed] [Google Scholar]
- Fielding JR, Dumanli H, Schreyer AG, et al. MR-based three-dimensional modeling of the normal pelvic floor in women: quantification of muscle mass. Am J Roentgenol. 2000;174:657–660. doi: 10.2214/ajr.174.3.1740657. [DOI] [PubMed] [Google Scholar]
- Heemskerk AM, Sinha TK, Wilson KJ, et al. Quantitative assessment of DTI-based muscle fiber tracking and optimal tracking parameters. Magn Reson Med. 2009;61:467–472. doi: 10.1002/mrm.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. Am J Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369:1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- Kaufman L, Kramer DM, Crooks LE, et al. Measuring signal-to-noise ratios in MR imaging. Radiology. 1989;173:265–267. doi: 10.1148/radiology.173.1.2781018. [DOI] [PubMed] [Google Scholar]
- Kearney R, Sawhney R, DeLancey JO. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet Gynecol. 2004;104:168–173. doi: 10.1097/01.AOG.0000128906.61529.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies RU, Hsu Y, Kearney R, et al. Appearance of the levator ani muscle subdivisions in magnetic resonance images. Obstet Gynecol. 2006;107:1064–1069. doi: 10.1097/01.AOG.0000214952.28605.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies RU, Huebner M, DeLancey JO. Origin and insertion points involved in levator ani muscle defects. Am J Obstet Gynecol. 2007;196:251–255. doi: 10.1016/j.ajog.2006.10.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Chung SW, Berman JI, et al. Diffusion tensor MR imaging and fiber tractography: technical considerations. Am J Neuroradiol. 2008;29:843–852. doi: 10.3174/ajnr.A1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe N, White LM, Sussman MS, et al. Diffusion tensor magnetic resonance imaging of the human calf: comparison between 1.5 T and 3.0 T – preliminary results. Invest Radiol. 2008;43:612–618. doi: 10.1097/RLI.0b013e31817e909f. [DOI] [PubMed] [Google Scholar]
- Shobeiri SA, Chesson RR, Gasser RF. The internal innervation and morphology of the human female levator ani muscle. Am J Obstet Gynecol. 2008;199:681–686. doi: 10.1016/j.ajog.2008.07.057. [DOI] [PubMed] [Google Scholar]
- Strohbehn K, Ellis JH, Strohbehn JA, et al. Magnetic resonance imaging of the levator ani with anatomic correlation. Obstet Gynecol. 1996;87:277–285. doi: 10.1016/0029-7844(95)00410-6. [DOI] [PubMed] [Google Scholar]
- Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14:131–139. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- Van Donkelaar CC, Kretzers LJ, Bovendeerd PH, et al. Diffusion tensor imaging in biomechanical studies of skeletal muscle function. J Anat. 1999;194:79–88. doi: 10.1046/j.1469-7580.1999.19410079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijta FM, Froeling M, van der Paardt MP, et al. Feasibility of diffusion tensor imaging (DTI) with fibre tractography of the normal female pelvic floor. Eur Radiol. 2011;21:1243–1249. doi: 10.1007/s00330-010-2044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


