Abstract
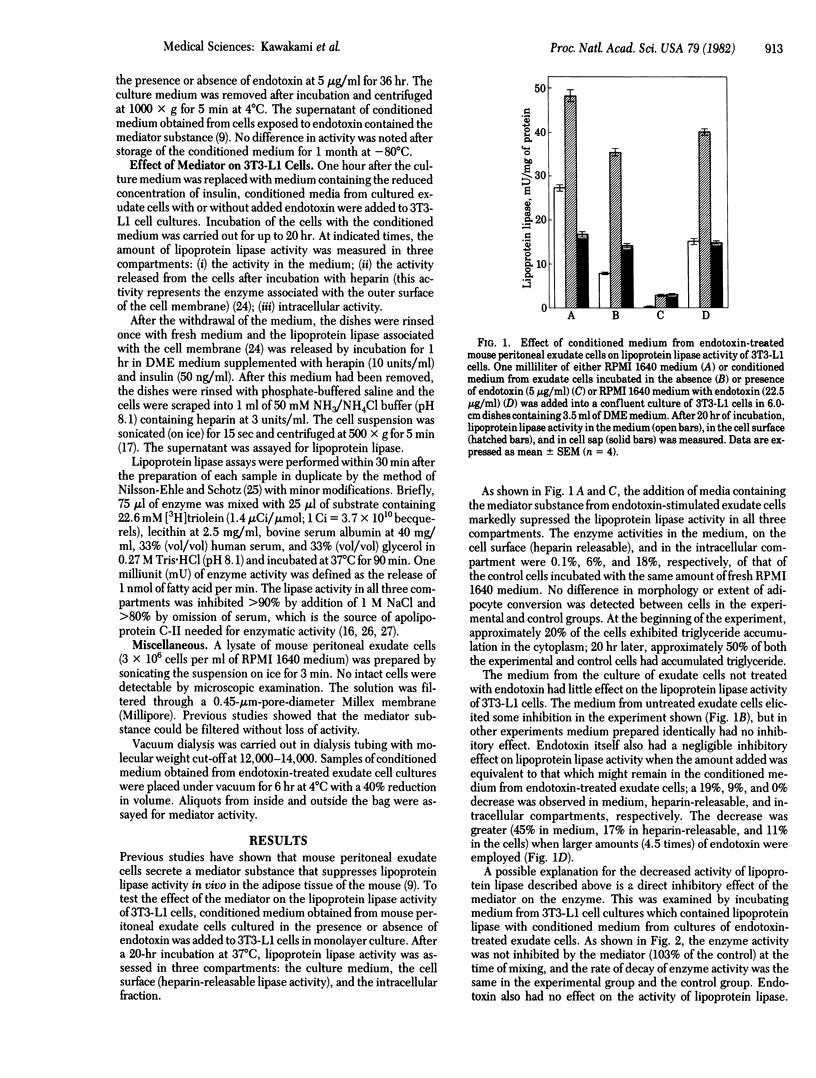
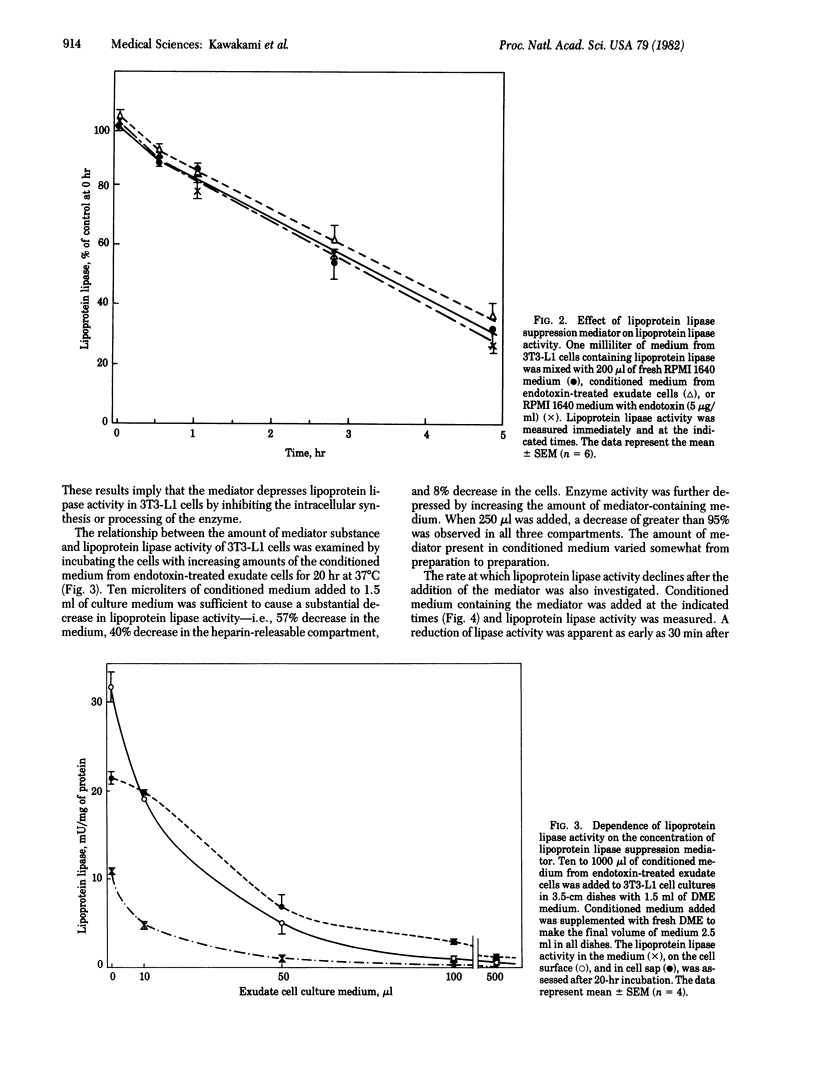
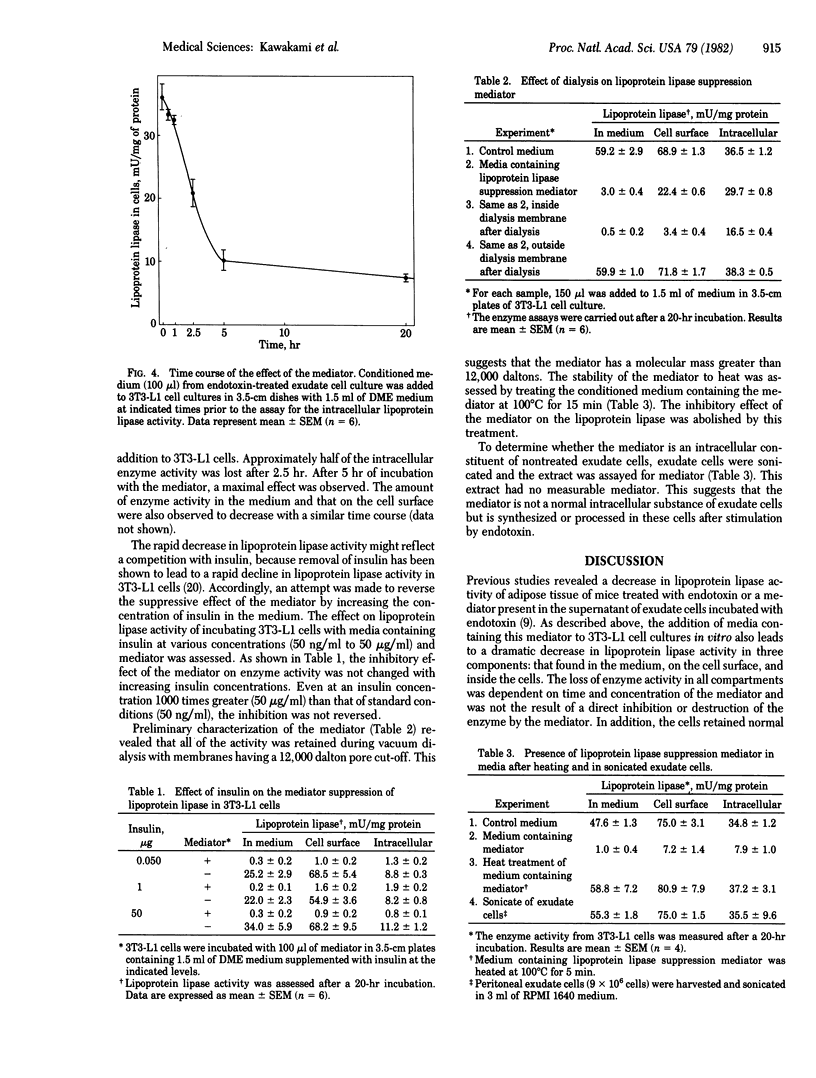
Conditioned medium from cultures of mouse peritoneal exudate cells incubated wih endotoxin contains a mediator that markedly suppresses (greater than 90%) lipoprotein lipase (triacylglycero-protein acylhydrolase, EC 3.1.1.34) activity in differentiating 3T3-L1 mouse preadipocytes. The effect is dependent upon the amount of mediator and is evident as early as 30 min after the addition of the mediator-containing medium to 3T3-L1 cell cultures. Neither endotoxin nor conditioned medium from cultures of exudate cells not exposed to endotoxin shows the presence of the mediator. Lysates of the exudate cells are also unable to suppress the lipase activity. Increasing the amount of insulin does not reverse this suppression, even at 1000 times the concentration used for standard experiments. The lipoprotein lipase suppression mediator present in the conditioned medium of endotoxin-treated exudate cells is heat labile and has an apparent molecular weight of at least 12,000. The mediator does not inhibit lipoprotein lipase activity directly nor does it affect the half-life of enzyme activity released in the medium. The present study demonstrates that endotoxin promotes the release of a mediator from exudate cells that suppresses the activity of lipoprotein lipase in 3T3-L1 preadipocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby G. J., Spitzer J. A. Lipoprotein lipase activity in rat heart and adipose tissue during endotoxic shock. Am J Physiol. 1980 Mar;238(3):H325–H330. doi: 10.1152/ajpheart.1980.238.3.H325. [DOI] [PubMed] [Google Scholar]
- Barclay M., Skipski V. P. Lipoproteins in relation to cancer. Prog Biochem Pharmacol. 1975;10:76–111. [PubMed] [Google Scholar]
- Beisel W. R. Metabolic response to infection. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Mathur S. N., Spector A. A. Characterization of the hyperlipidemia in mice bearing the Ehrlich ascites tumor. Eur J Cancer. 1975 Apr;11(4):225–230. doi: 10.1016/0014-2964(75)90002-x. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H., Sayeed M. M., Baue A. E. Insulin resistance in experimental shock. Arch Surg. 1974 Sep;109(3):412–415. doi: 10.1001/archsurg.1974.01360030064017. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Reed B. C., Mackall J. C., Student A. K., Lane M. D., Bell R. M. Selective changes in microsomal enzymes of triacylglycerol phosphatidylcholine, and phosphatidylethanolamine biosynthesis during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1978 Oct 25;253(20):7256–7261. [PubMed] [Google Scholar]
- Dinarello C. A. Production of endogenous pyrogen. Fed Proc. 1979 Jan;38(1):52–56. [PubMed] [Google Scholar]
- Eckel R. H., Fujimoto W. Y., Brunzell J. D. Development of lipoprotein lipase in cultured 3T3-L1 cells. Biochem Biophys Res Commun. 1977 Sep 9;78(1):288–293. doi: 10.1016/0006-291x(77)91252-9. [DOI] [PubMed] [Google Scholar]
- Eckel R. H., Fujimoto W. Y., Brunzell J. D. Insulin regulation of lipoprotein lipase in cultured 3T3-L1 cells. Biochem Biophys Res Commun. 1978 Oct 30;84(4):1069–1075. doi: 10.1016/0006-291x(78)91692-3. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Zwiebel R., Cohn Z. A. The pinocytic rate of activated macrophages. J Exp Med. 1975 Nov 1;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn K. N. Effects of burn injury on insulin secretion and on sensitivity to insulin in the rat in vivo. Eur J Clin Invest. 1975 Jul 29;5(4):331–337. doi: 10.1111/j.1365-2362.1975.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Kaye D., O'Leary W. M. Serum lipids in infection. N Engl J Med. 1969 Nov 13;281(20):1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- HIRSCH R. L., MCKAY D. G., TRAVERS R. I., SKRALY R. K. HYPERLIPIDEMIA, FATTY LIVER, AND BROMSULFOPHTHALEIN RETENTION IN RABBITS INJECTED INTRAVENOUSLY WITH BACTERIAL ENDOTOXINS. J Lipid Res. 1964 Oct;5:563–568. [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. II. Substrate specificity and activation of coconut oil. J Biol Chem. 1955 Jul;215(1):15–26. [PubMed] [Google Scholar]
- Kampschmidt R. F. Leukocytic endogenous mediator. J Reticuloendothel Soc. 1978 Apr;23(4):287–297. [PubMed] [Google Scholar]
- Kawakami M., Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981 Sep 1;154(3):631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Nilsson-Ehle P., Schotz M. C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976 Sep;17(5):536–541. [PubMed] [Google Scholar]
- Rouzer C. A., Cerami A. Hypertriglyceridemia associated with Trypanosoma brucei brucei infection in rabbits: role of defective triglyceride removal. Mol Biochem Parasitol. 1980 Oct;2(1):31–38. doi: 10.1016/0166-6851(80)90046-8. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Sakaguchi O., Sakaguchi S. Alterations of lipid metabolism in mice injected with endotoxin. Microbiol Immunol. 1979;23(2):71–85. doi: 10.1111/j.1348-0421.1979.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Vogel S. N., Ryan J. L., McAdam K. P., Rosenstreich D. L. Detection of a mediator derived from endotoxin-stimulated macrohpages that induces the acute phase serum amyloid A response in mice. J Exp Med. 1979 Sep 19;150(3):597–606. doi: 10.1084/jem.150.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner P. M., Chernick S. S., Garrison M. M., Scow R. O. Development of lipoprotein lipase activity and accumulation of triacylglycerol in differentiating 3T3-L1 adipocytes. Effects of prostaglandin F2alpha, 1-methyl-3-isobutylxanthine, prolactin, and insulin. J Biol Chem. 1979 Feb 25;254(4):1305–1311. [PubMed] [Google Scholar]
- Spooner P. M., Chernick S. S., Garrison M. M., Scow R. O. Insulin regulation of lipoprotein lipase activity and release in 3T3-L1 adipocytes. Separation and dependence of hormonal effects on hexose metabolism and synthesis of RNA and protein. J Biol Chem. 1979 Oct 25;254(20):10021–10029. [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Wise L. S., Green H. Studies of lipoprotein lipase during the adipose conversion of 3T3 cells. Cell. 1978 Feb;13(2):233–242. doi: 10.1016/0092-8674(78)90192-7. [DOI] [PubMed] [Google Scholar]



