Abstract
Two-photon microscopy (2PM) has become a gold standard for deep-tissue observations in the living animal as well as on thick samples. Using 2PM, the endofluorescence properties of biomolecules have shown an interesting potential for the imaging of tissues without any staining. In this short communication, we report a method to observe the different layers of mouse small intestine explants with subcellular resolution and without any staining or clearing. This method allows rapid observations of samples with little to no preparation thanks to the endofluorescence properties of biomolecules such as NAD(P)H or flavins and second-harmonic generation. Finally, we show different three-dimensional reconstructions of the mouse small intestine anatomy obtained with this approach to show the potential of this method in morphological studies.
Keywords: endofluorescence imaging, intestine histology, second-harmonic generation, two-photon microscopy
Introduction
Over the past 20 years, two-photon microscopy (2PM) has become a powerful tool for the investigation of biological phenomenon in and ex vivo (Denk et al. 1990; Helmchen & Denk, 2005). Its high spatial (< 1 μm) and temporal (less than a second to acquire a 512 × 512 pixels image) resolution enable subcellular observations on various samples from tissue slices to whole living organisms.
One of the biggest challenges during the microscopical examination of biological samples is to obtain the best contrast between the different parts of an organ or the various compartments of a cell. With 2PM, such contrast enhancement can be obtained using three different approaches: (i) with fluorophores that specifically bind structures of interest or fill the lumen of an organ [e.g. rhodamine-conjugated dextrans to label the blood stream (Ricard et al. 2009)]; (ii) using second-harmonic generation (SHG), a non-linear phenomenon allowing the detection of non-centrosymmetric molecules such as type I and III collagen or myosin (Mohler et al. 2003); (iii) using the fluorescent properties of endogenous biomolecules such as NADP(H) or flavins (endofluorescence phenomenon) (Zipfel et al. 2003; Zoumi et al. 2004). These last two approaches are of particular interest for histology as they require no staining of the samples and can be accomplished in a short time.
The small intestine is responsible of the absorption of nutrients and acts as a barrier in between the environment and the organism. Its wall is made of four different layers. Starting from the lumen is the mucosa, a columnar epithelium organized in villi and crypts. The villi are made of polarized enterocytes with a dense brush border at their apical side and mucus-secretory cells called goblet-cells. At the bottom of the crypts, granule-rich cells and stem-cells are present. The next layer is the submucosa, a collagen-rich connective tissue, followed by the muscularis, a circular and longitudinal layer of muscle fibers, and, finally, the serosa (Junqueira & Carneiro, 1980).
Many papers have described the advantages of the two-photon/endofluorescence approach for various organs (Zoumi et al. 2004; Li et al. 2009; Parra et al. 2010) but to our knowledge high-resolution three-dimensional imaging of small intestine morphology using the endofluorescence properties of biomolecules and SHG without any clearing treatment have never been reported. Clearing protocols aim to make tissues transparent to allow a better penetration of photons and to reduce light scattering using various chemical compounds such as 1 : 2 benzyl alcohol to benzyl benzoate (BABB) or SCALE (Parra et al. 2010; Hama et al. 2011). However, these protocols require long processing times, which can cause significant delays.
In the present short communication we report the setup and parameters to image mouse intestinal ring morphology with subcellular spatial resolution and without any staining. A special emphasis is placed on the discrimination of the various cell types encountered from the bottom of an intestinal crypt to the top of an intestinal villus. Finally, we show that this method is suitable for making three-dimensional reconstructions of intestinal structures based on endofluorescence and SHG image stacks.
Materials and methods
Microscopy setup
Experiments were conducted on an inverted Zeiss LSM510 confocal microscope equipped with a Maï-Taï/DeepSee femtosecond laser (with prechirp). All observations were done with a non-descanned configuration. Briefly, the incident laser beam was reflected on an HFT KP 650, passed through an NDD KP 685 and the objective. The emitted light was epi-collected and reflected by the NDD KP 685. Two different setups are then available: (i) whole signal, where the whole emitted light is collected on a Photo-Multiplier Tube (PMT) (wavelengths ranging from 370 to 685 nm) and (ii) endofluorescence and SHG, where the emitted light is split by a dichroic mirror (R405; Semrock, Rochester, MN, USA) and collected on two PMTs (wavelengths of 399–410 nm for the SHG signal, with a FF01-406/15 filter in front of the PMT, and 420–685 nm for the endofluorescence signal).
Animal care guidelines
All experimental procedures were performed in accordance with the French Government guidelines for the care and use of laboratory animals (licenses 380702 and D-13-055-21).
Sample preparation and observations
Mice were sacrificed by cervical dislocation and the small intestine was removed, washed in phosphate-buffered saline (PBS; Gibco) and stored in a 3% paraformaldehyde solution. Prior to observations, 2–3-mm-thick transversal sections of the intestine were cut with a blade. The intestine rings obtained were placed in a glass-bottom Petri dish and immersed in PBS. Image acquisition was performed with the Zeiss lsm 4.2 SP1 software and a 40X/1.2NA water-immersion objective (Zeiss), spectra collection was done with a dedicated macro (Excitation Finger Printing). Post-treatment was done with Zeiss lsm image browser version 4.2.0.121, Zeiss Zen2009 and imagej version 1.42. For acquisitions on fresh samples, the same protocol was used except the fixation step in 3% paraformaldehyde solution was not performed. Acquisition parameters are displayed in the legend of the figures.
Results
To determine the optimal excitation parameters, the endofluorescence excitation spectrum of the intestinal mucosa was measured under various two-photon excitation wavelengths ranging from 720 to 960 nm. Characteristic excitation spectra of fixed and fresh tissues are depicted in Fig. 1 for measurements made on a villus (Fig. 1B). Similar spectra were obtained on crypts (data not shown). As the power delivered by the femtosecond laser is not constant and varies as a function of the wavelength used, the endofluorescence excitation spectrum of the intestinal villus was normalized to a constant excitation laser power (Fig. 1A,C). On fixed samples, the excitation peak is centered on 760–770 nm but there is an intense signal observed at 730–810 nm. On fresh tissues, the excitation peak is centered on 730–740 nm but there is an intense signal at 720–780 nm.
Fig. 1.
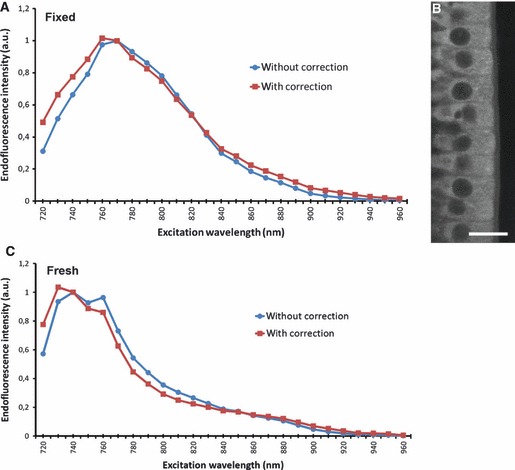
Excitation spectra for endofluorescence acquisition. (A) The intensity of the endofluorescence signal of enterocytes on a fixed intestinal villus was measured at various excitation wavelengths ranging from 720 to 960 nm (blue line and circles). The endofluorescence intensity was normalized to a constant laser excitation power (red line and squares). (B) Representative image of a region of interest where the spectral measurements were performed. (C) Intensity of the endofluorescence signal on a fresh intestinal villus. Scale bar: 20 μm. Laser power: 0.92 mW at 810 nm; pixel time: 1.77 μs; average: 8; setup#1 whole signal.
The different parts of the intestinal wall were observed on fixed tissues under an 810-nm excitation wavelength allowing the collection of SHG signals around 405 nm with our filter set. High-resolution images of the different layers of the intestinal wall were obtained (Fig. 2). The acquisition of z-stacks allowed the three-dimensional reconstruction of the structure of intestinal crypts (Fig. 3A, Supporting Information Video S1) and intestinal villi (Fig. 3B, Supporting Information Video S2). With a constant laser excitation power (around 1 mW at the objective, see figure legends), a maximum depth of 100 μm can be achieved. However, deeper structures may be observable by increasing laser power.
Fig. 2.
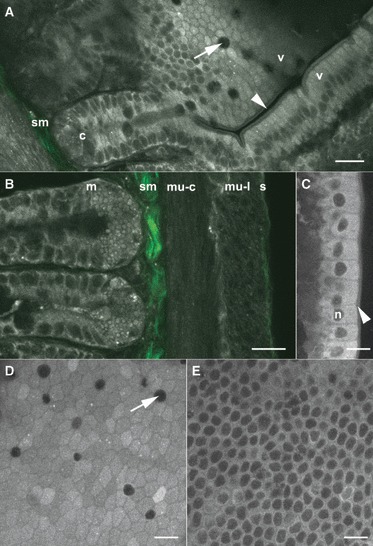
Morphological images of the small intestine. (A) Junction of an intestinal crypt (c) and two intestinal villi (v). Endofluorescence signal (grey level) allows the individualization of the cells (e.g. goblet cells, arrow) and the observation of subcellular structures such as the brush-border (arrowhead). The SHG signal (green) enables the visualization of the submucosa (sm). (B) The four layers of the intestinal wall were observed with endofluorescence signal (grey level) and SHG imaging (green). Two intestinal crypts with many zymogen-rich granules can be seen at the base of the mucosa (m), an intense SHG signal arises from the submucosa (sm). The two parts of the muscularis can be clearly defined [the circular (mu-c) and the longitudinal (mu-l) layers, respectively]. Finally, the thin serosa (s) shows both endofluorescence and SHG signals. (C) Endofluorescence image of enterocytes on an intestinal villus. Note the brush-border (arrowhead), the nuclei of cells (n) and the lower endofluorescence intensity between cells. (D,E) xy sections of enterocytes on an intestinal villus at different levels. At the apical side (D), the junctions in-between cells show a low endofluorescence signal. Goblet cells (arrow) can also be observed. On the basal side (E), nuclei of enterocytes can be seen but the borders between the cells is not obvious. Scale bar: 20 μm. Laser power: 1.1 mW (A), 0.71 mW (B), 0.92 mW (C–E); pixel time: 0.89 μs (A), 1.35 μs (B), 3.2 μs (C–E); average: 8 (A), 4 (B–E); setup#2 endofluorescence and SHG (A,B), setup#1 whole signal (C–E).
Fig. 3.
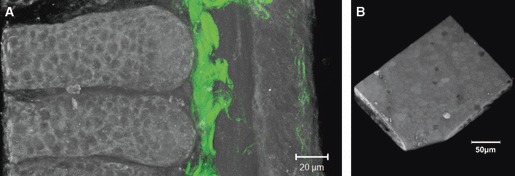
3D imaging of an intestinal crypt and a villus. (A) 3D reconstruction of two intestinal crypts (greyscale: endofluorescence; green: SHG) made from a stack of 51 images with a z-spacing of 1 μm. (B) 3D reconstruction of the topography of an intestinal villus made from a stack of 61 images with a z-spacing of 1 μm. A and B are images extracted from Videos S1 and S2, respectively. Laser power: 0.71 mW (A), 0.92 mW (B); pixel time: 1.35 μs (A), 3.2 μs (B); average: 4 (A, B); setup#2 endofluorescence and SHG (A), setup#1 whole signal (B) (Video S1, Quicktime, 1.6 MB; Video S2, Quicktime, 2.2 MB).
Discussion
Two-photon microscopy is widely used in the field of gastro-intestinal physiology (Tutsch et al. 2004; Bao et al. 2009) and many papers have enlisted the use of the endofluorecence properties of biomolecules in epithelia to obtain contrasted images without any staining (Zheng et al. 2008; Li et al. 2009; Parra et al. 2010). Rogart et al. (2008) reported that the mucosa of different parts of the gut can be imaged with this method using specimens from unfixed human biopsies. They have also shown an excitation peak centered around 735 nm (Rogart et al. 2008). This excitation peak is similar to the one we have found on fresh intestinal tissue. However, the peak moves to 765 nm when the sample is fixed with paraformaldehyde. As a consequence, endofluorescence imaging can be performed on both fresh and fixed tissues, but the excitation wavelength has to be modified according to the status of the sample. Intravital imaging may be possible provided the development of surgical protocols. Parra et al. (2010) reported observation depths of 1.7 mm with a 4×, 0.28 NA objective after a clearing protocol involving fixation, dehydration and incubation with 1 : 2 benzyl alcohol to benzyl benzoate (BABB). To make their observations, they used an excitation wavelength of 740 nm. The imaging depth reached with this method is deeper than ours; however, the clearing approach requires a longer preparation of the sample (more than a day vs. 10 min for our protocol).
In the present paper, we have shown that 3D imaging and topographic reconstructions of mouse small intestine crypts and villi are possible with a submicrometric resolution and using only the endofluorecence and SHG signals. Up to now, 3D imaging of the small intestine with 2PM was mainly reported after staining of structures with dyes (e.g. Phalloidin-TexasRed; Appleton et al. 2009) or after clearing (Parra et al. 2010). To the best of our knowledge, 3D images of intestinal mucosa using endofluorescence and without any clearing were only reported in term of orthogonal sections with poor spatial resolution. Topographic images were not shown.
Our approach combines numerous advantages: low laser power excitation, no staining, no clearing, reduced procedures for sample preparation, high spatial and temporal resolution and may be applied to many areas from fundamental studies on cell polarity to biomedical applications.
Acknowledgments
This project is supported through Coordination Theme 1 (Health) of the European Community's FP7, Grant agreement number HEALTH-F2-2008-200234. The authors warmly thank Dr André Le Bivic for the critical reading of the manuscript, Dr Keith Fenrich for the editing of the manuscript, the IBDML imaging facility and colleagues from Le Bivic and Rougon team. Le Bivic's group is ‘Equipe labélisée de la Ligue Nationale Contre le Cancer’.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Video S1 and S2. 3D imaging of an intestinal crypt and a villus.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Appleton PL, Quyn AJ, Swift S, et al. Preparation of wholemount mouse intestine for high-resolution three-dimensional imaging using two-photon microscopy. J Microsc. 2009;234:196–204. doi: 10.1111/j.1365-2818.2009.03163.x. [DOI] [PubMed] [Google Scholar]
- Bao H, Boussioutas A, Reynolds J, et al. Imaging of goblet cells as a marker for intestinal metaplasia of the stomach by one-photon and two-photon fluorescence endomicroscopy. J Biomed Opt. 2009;14 doi: 10.1117/1.3269681. 064031. [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Hama H, Kurokawa H, Kawano H, et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 2011;14:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Carneiro J. Basic Histology. Los Altos, CA: LANGE Medical Publications; 1980. [Google Scholar]
- Li D, Zheng W, Qu JY. Imaging of epithelial tissue in vivo based on excitation of multiple endogenous nonlinear optical signals. Opt Lett. 2009;34:2853–2855. doi: 10.1364/OL.34.002853. [DOI] [PubMed] [Google Scholar]
- Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29:97–109. doi: 10.1016/s1046-2023(02)00292-x. [DOI] [PubMed] [Google Scholar]
- Parra SG, Chia TH, Zinter JP, et al. Multiphoton microscopy of cleared mouse organs. J Biomed Opt. 2010;15 doi: 10.1117/1.3454391. 036017. [DOI] [PubMed] [Google Scholar]
- Ricard C, Fernandez M, Gastaldo J, et al. Short-term effects of synchrotron irradiation on vasculature and tissue in healthy mouse brain. J Synchrotron Radiat. 2009;16:477–483. doi: 10.1107/S0909049509015428. [DOI] [PubMed] [Google Scholar]
- Rogart JN, Nagata J, Loeser CS, et al. Multiphoton imaging can be used for microscopic examination of intact human gastrointestinal mucosa ex vivo. Clin Gastroenterol Hepatol. 2008;6:95–101. doi: 10.1016/j.cgh.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutsch E, Griesemer D, Schwarz A, et al. Two-photon analysis of calcium signals in T lymphocytes of intact lamina propria from human intestine. Eur J Immunol. 2004;34:3477–3484. doi: 10.1002/eji.200425265. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wu Y, Li D, et al. Autofluorescence of epithelial tissue: single-photon versus two-photon excitation. J Biomed Opt. 2008;13 doi: 10.1117/1.2975866. 054010. [DOI] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Christie R, et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003;100:7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoumi A, Lu X, Kassab GS, et al. Imaging coronary artery microstructure using second-harmonic and two-photon fluorescence microscopy. Biophys J. 2004;87:2778–2786. doi: 10.1529/biophysj.104.042887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


