Abstract
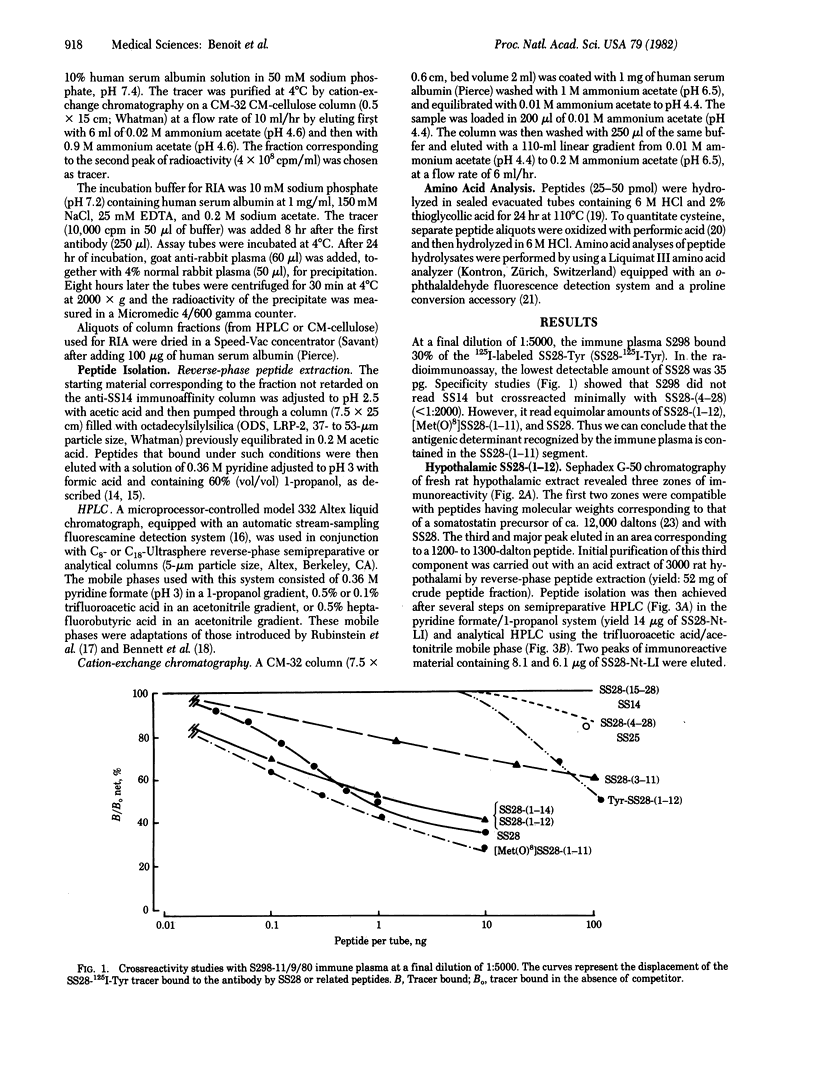
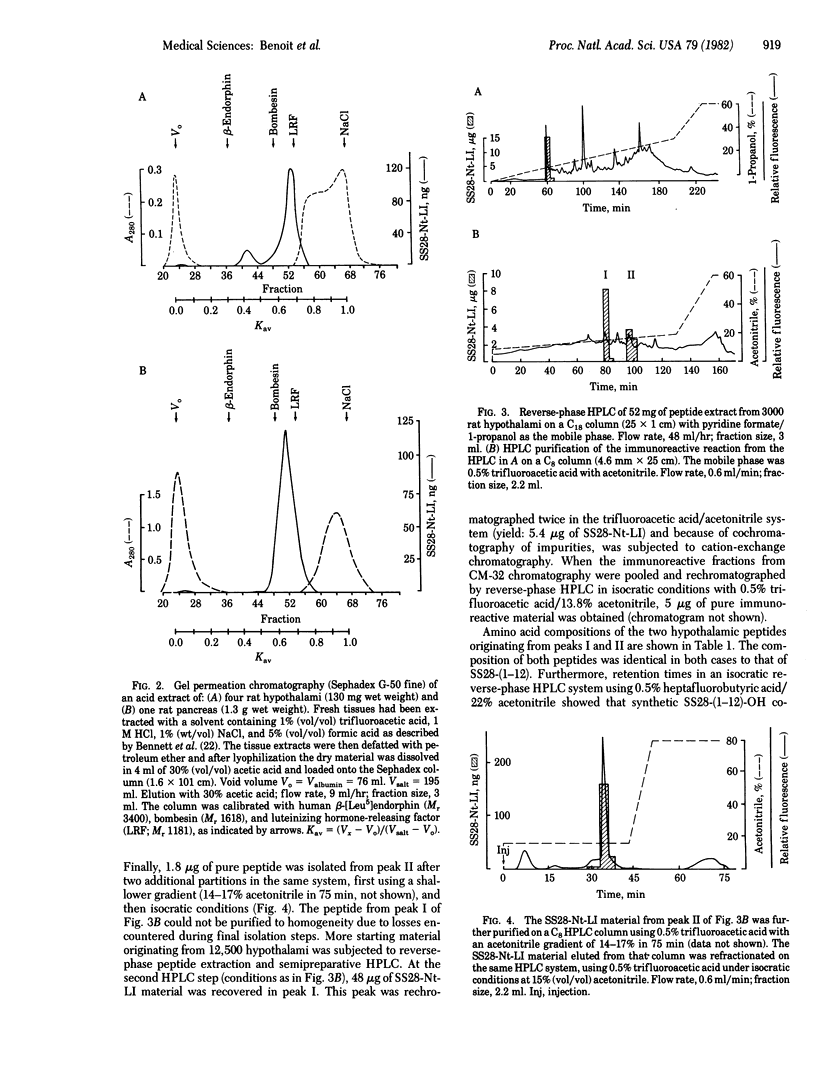
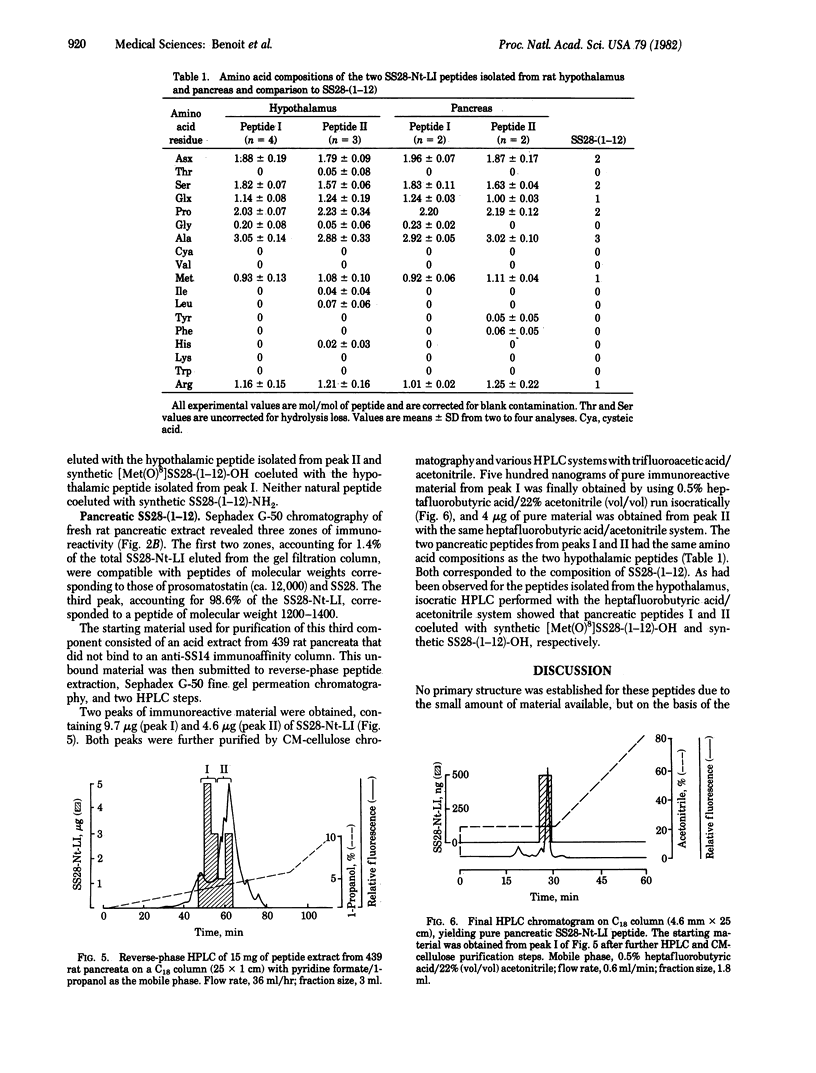
Acid extracts from rat pancreas and hypothalamus were analyzed for the presence of the antigenic determinant corresponding to the NH2 terminus of somatostatin-28 (SS28), using an antiserum directed against amino acids 1 to less than or equal to 11 of the SS28 molecule. On gel permeation chromatography the majority of the immunoreactive material from each tissue extract eluted in one zone compatible with a peptide of 1250 daltons. Purification of this immunoreactive material by reverse-phase HPLC and cation-exchange chromatography yielded two immunoreactive peptides from each tissue extract. The amino acid compositions of both peptides in pancreas and hypothalamus correspond to the fragment 1-12 of SS28. The more hydrophobic peptide from each tissue coeluted with synthetic SS28-(1-12) on HPLC, while the other one coeluted with synthetic SS28-(1-12)-amide. We conclude that the prosomatostatin fragment Ser-Ala-Asn-Ser-Asn-Pro-Ala-Met-Ala-Pro-Arg-Glu-OH is present in both rat hypothalamus and rat pancreas.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoit R., Böhlen P., Brazeau P., Ling N., Guillemin R. Isolation and characterization of rat pancreatic somatostatin. Endocrinology. 1980 Dec;107(6):2127–2129. doi: 10.1210/endo-107-6-2127. [DOI] [PubMed] [Google Scholar]
- Bohlen P., Benoit R., Ling N., Guillemin R., Brazeau P. Isolation and characterization of rat hypothalamic somatostatin-14. Endocrinology. 1981 May;108(5):2008–2010. doi: 10.1210/endo-108-5-2008. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Brazeau P., Esch F., Ling N., Guillemin R. Isolation and chemical characterization of somatostatin-28 from rat hypothalamus. Regul Pept. 1981 Nov;2(6):359–369. doi: 10.1016/0167-0115(81)90018-5. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Castillo F., Ling N., Guillemin R. Purification of peptides: an efficient procedure for the separation of peptides from amino acids and salt. Int J Pept Protein Res. 1980 Oct;16(4):306–310. doi: 10.1111/j.1399-3011.1980.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Mellet M. Automated fluorometric amino acid analysis: the determination of proline and hydroxyproline. Anal Biochem. 1979 Apr 15;94(2):313–321. doi: 10.1016/0003-2697(79)90366-x. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Stone J., Udenfriend S. Automatic Monitoring of primary amines in preparative column effluents with fluorescamine. Anal Biochem. 1975 Aug;67(2):438–445. doi: 10.1016/0003-2697(75)90316-4. [DOI] [PubMed] [Google Scholar]
- Esch F., Böhlen P., Ling N., Benoit R., Brazeau P., Guillemin R. Primary structure of ovine hypothalamic somatostatin-28 and somatostatin-25. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6827–6831. doi: 10.1073/pnas.77.11.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Esch F., Davis D., Mercado M., Regno M., Bohlen P., Brazeau P., Guillemin R. Solid phase synthesis of somatostatin-28. Biochem Biophys Res Commun. 1980 Aug 14;95(3):945–951. doi: 10.1016/0006-291x(80)91564-8. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Pradayrol L., Jörnvall H., Mutt V., Ribet A. N-terminally extended somatostatin: the primary structure of somatostatin-28. FEBS Lett. 1980 Jan 1;109(1):55–58. doi: 10.1016/0014-5793(80)81310-x. [DOI] [PubMed] [Google Scholar]
- Rubinstein M., Stein S., Gerber L. D., Udenfriend S. Isolation and characterization of the opioid peptides from rat pituitary: beta-lipotropin. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3052–3055. doi: 10.1073/pnas.74.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally A. V., Dupont A., Arimura A., Redding T. W., Nishi N., Linthicum G. L., Schlesinger D. H. Isolation and structure of somatostatin from porcine hypothalami. Biochemistry. 1976 Feb 10;15(3):509–514. doi: 10.1021/bi00648a009. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Huang W. Y., Chang R. C., Arimura A., Redding T. W., Millar R. P., Hunkapiller M. W., Hood L. E. Isolation and structure of pro-somatostatin: a putative somatostatin precursor from pig hypothalamus. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4489–4493. doi: 10.1073/pnas.77.8.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Vale W. Multiple forms of somatostatin-like activity in rat hypothalamus. Biochemistry. 1980 Jun 24;19(13):2861–2866. doi: 10.1021/bi00554a007. [DOI] [PubMed] [Google Scholar]
- Spiess J., Villarreal J., Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981 Mar 31;20(7):1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]


