Abstract
The rare earth element scandium has weak antibacterial potency. We identified a mutation responsible for a scandium-resistant phenotype in Bacillus subtilis. This mutation was found within the uppS gene, which encodes undecaprenyl pyrophosphate synthase, and designated uppS86 (for the Thr-to-Ile amino acid substitution at residue 86 of undecaprenyl pyrophosphate synthase). The uppS86 mutation also gave rise to increased resistance to bacitracin, which prevents cell wall synthesis by inhibiting the dephosphorylation of undecaprenyl pyrophosphate, in addition to enhanced amylase production. Conversely, overexpression of the wild-type uppS gene resulted in increased susceptibilities to both scandium and bacitracin. Moreover, the mutant lacking undecaprenyl pyrophosphate phosphatase (BcrC) showed increased susceptibility to all rare earth elements tested. These results suggest that the accumulation of undecaprenyl pyrophosphate renders cells more susceptible to rare earth elements. The availability of undecaprenyl pyrophosphate may be an important determinant for susceptibility to rare earth elements, such as scandium.
INTRODUCTION
Rare earth elements consist of 17 elements, including scandium, yttrium, and the lanthanides (15 elements from lanthanum to lutetium in the periodic table). As rare earth elements have useful physical and chemical properties, these elements are of considerable importance in various industries. Although there have been many studies concerning their useful features, little is known about their biological effects in living cells. We recently reported that rare earth elements, especially scandium, activate secondary metabolism and extracellular enzyme production in certain microorganisms (12, 15, 31). In the Gram-positive model bacterium Bacillus subtilis, the addition of scandium to the growth medium stimulates production of an extracellular enzyme, amylase, and the dipeptide antibiotic, bacilysin (12). This effect was found to be exerted at the transcriptional level during the late stationary growth phase. These previous results suggest that the rare earth elements (especially scandium) have remarkable biological effects on microorganisms.
Rare earth elements have long been known to have weak antimicrobial potency. For example, lanthanum has been reported to inhibit the growth of Streptococcus faecalis due to depletion of phosphate from the medium (34). It has also been reported that scandium and indium complexes of enterochelin have bacteriostatic effects on both Klebsiella pneumoniae (25) and Escherichia coli (26). However, there have been no reports addressing mutations conferring rare earth element resistance. In the present study, we isolated a scandium-resistant B. subtilis mutant and successfully identified the mutation responsible for its phenotype. The results presented here suggest that undecaprenyl pyrophosphate (C55-PP) is involved in susceptibility to rare earth elements, including scandium. This is the first report describing a key molecule involved in rare earth element susceptibility.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. The spontaneous scandium-resistant mutant SC26 was derived from B. subtilis strain 168. L medium (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl per liter) and NG medium (10 g of nutrient broth, 10 g of glucose, 2 g of NaCl, 5 mg of CuSO4 · 5H2O, 7.5 mg of FeSO4 · 7H2O, 3.6 mg of MnSO4 · 5H2O, 15 mg of CaCl2 · 2H2O, and 9 mg of ZnSO4 · 7H2O per liter) were used in this study. Scandium chloride (ScCl3 · 6H2O; purity, 99.9%) and other rare earth elements (all chloride salts) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Erythromycin (0.5 μg/ml), neomycin (3 μg/ml), and chloramphenicol (4 μg/ml) were used for selection of B. subtilis transformants. Ampicillin (100 μg/ml) was used for selection of E. coli transformants.
Table 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| 168 | trpC2 | Laboratory stock |
| YO-005 | hisC101 | 11 |
| SC26 | trpC2 yufQ38 uppS86 Scr | This study |
| TI367 | trpC2 yufQ∷pMutinT3 | This study |
| TI389 | hisC101 yufQ∷pMutinT3 | This study |
| TI391 | trpC2 yufQ38 | This study |
| TI392 | trpC2 uppS86 | This study |
| TI393 | trpC2 bcrC∷pMutinT3 | This study |
| TI403 | trpC2 ΔyubB∷neo | This study |
| TI408 | trpC2 uppS∷pHASH120 (PS10-uppS) | This study |
| TI409 | trpC2 yubB∷pHASH120 (PS10-yubB) | This study |
Plasmids and DNA procedures.
Oligonucleotides used for PCR are listed in Table 2. To disrupt the yufQ gene in B. subtilis, the DNA fragment containing a partial yufQ gene was amplified using the primers HindIII-yufQF and BamHI-yufQR, digested with HindIII and BamHI, and cloned into the corresponding sites of pMutinT3 (19). The resulting plasmid, pMutinT3-yufQ, was used for transformation of B. subtilis 168 with selection for erythromycin resistance, generating the strain TI367 (yufQ∷pMutinT3).
Table 2.
Oligonucleotides used in this study
| Primer | Oligonucleotide sequence (5′ → 3′)a |
|---|---|
| HindIII-yufQ F | CCCAAGCTTGTCCCTGCCACACTCG |
| BamHI-yufQ R | CGCGGATCCGCTGACTGTCTGGTCG |
| HindIII-bcrC F | CCCAAGCTTGTCGCCTATGCCCTTATCC |
| BamHI-bcrC R | CGCGGATCCCAAAAATGACAAGCGGC |
| yubB-F | CGGTTGCCAAAGTCATTACG |
| yubB-R | GCCTTAAGAAGAAACGGACG |
| pHASH120-uppSF | ATGCTCAACATACTCAAAAATTGGAAG |
| pHASH120-uppSR | GATCATTCCGTCGTTTTGCG |
| pHASH120-yubBF | ATGACTCTATGGGAATTGTTTGTAGC |
| pHASH120-yubBR | GTGTCTGTCGCTGTTTTCCG |
| RT-qPCR 16S-F | ATCTTCCGCAATGGACGA |
| RT-qPCR 16S-R | GCCGTGGCTTTCTGGTTA |
| RT-qPCR uppS-F | CAAACTGCGGCTTCCAAC |
| RT-qPCR uppS-R | CGGCCATTTCCATCCATG |
| RT-qPCR bcrC-F | ATACGCAGGCATCACAGG |
| RT-qPCR bcrC-R | CACCTGTCGTATGGTCAC |
| RT-qPCR yubB-F | AAATTGCCGTCGGACTCG |
| RT-qPCR yubB-R | GACCCAGTCAGCAAAAAGC |
HindIII and BamHI restriction sites are underlined.
The amino acid auxotrophic marker genes, hisC and trpC, are cotransformed at high frequency (approximately 70%). Utilizing these selectable markers, the strains TI391 (yufQ38) (where yufQ38 refers to the Asn-to-Asp amino acid substitution at residue 38 of YufQ protein encoded by yufQ) and TI392 (uppS86) (where uppS86 refers to the Thr-to-Ile amino acid substitution at residue 86 of undecaprenyl pyrophosphate synthase encoded by uppS) were obtained as follows. To yield the strain TI391, the histidine auxotrophic recipient strain TI389 (hisC101 yufQ∷pMutinT3) was constructed by transforming chromosomal DNA from TI367 into strain YO-005, followed by selection for erythromycin resistance. The resulting recipient strain TI389 was transformed with genomic DNA of SC26 with selection for histidine prototrophy. Of 100 His+ Trp− transformants, several erythromycin-sensitive transformants were selected. It was confirmed by DNA sequencing analysis that all erythromycin-sensitive transformants contained the expected yufQ38 mutation. One of these transformants was designated TI391. To generate TI392 (uppS86), the recipient strain YO-005 was transformed with genomic DNA of SC26 with selection for histidine prototrophy. Of 100 His+ Trp− transformants, scandium-resistant transformants were selected. DNA sequencing analysis showed that all scandium-resistant transformants carried the uppS86 mutation. One of these transformants was designated TI392. To generate TI393 (bcrC∷pMutinT3), the DNA fragment containing a partial coding region of the bcrC gene was amplified using the primers HindIII-bcrCF and BamHI-bcrCR, digested with HindIII and BamHI, and cloned into the corresponding sites of pMutinT3. The resulting plasmid, pMutinT3-bcrC, was used for transformation with selection for erythromycin resistance.
To disrupt the yubB gene, the DNA fragment containing a partial yubB gene was amplified using the primers yubB-F and yubB-R. The amplified DNA was cloned into pCR2.1 (Invitrogen, Carlsbad, CA), generating pCR2.1-yubB. The neomycin resistance cassette derived from pBEST501 (14) was inserted into the region between SspI sites within the yubB gene on pCR2.1-yubB. The resulting plasmid, pCR2.1-ΔyubB∷neo, was linearized with HindIII and transformed into B. subtilis 168 with selection for neomycin resistance, generating the strain TI403 (ΔyubB∷neo).
For overexpression of uppS, the 456-bp fragment encoding the N-terminal part of UppS was synthesized by PCR with the primers pHASH120-uppSF and pHASH120-uppSR and cloned into the EcoRV site of pHASH120 by the TA cloning method as described by Ohashi et al. (21), resulting in pHASH120-uppS. For overexpression of yubB, the 452-bp fragment encoding the N-terminal part of YubB was amplified by PCR with primers pHASH120-yubBF and pHASH120-yubBR and cloned into pHASH120, resulting in pHASH120-yubB. The resulting plasmids, pHASH120-uppS and pHASH120-yubB, were used for transformation of B. subtilis 168 with selection for chloramphenicol resistance, creating the strains TI408 (PS10-uppS) and TI409 (PS10-yubB), in which the promoter of uppS or yubB has been replaced by the S10 promoter (PS10).
RT-qPCR.
Cells were grown in L medium at 37°C until the optical density at 650 nm (OD650) reached 0.5 (exponential growth phase). Then, the total RNAs were prepared as described previously (13). A PrimeScript RT reagent kit with a genomic DNA (gDNA) Eraser (TaKaRa Bio Inc., Otsu, Japan) was used for the reverse transcriptase reaction according to the manufacturer's instructions. Samples were analyzed using a 7300 real-time PCR system and Thunder Bird SYBR qPCR Mix (Toyobo, Osaka, Japan). Amplification of the 16S rRNA gene was used as an internal control. Oligonucleotides used for reverse transcription-quantitative PCR (RT-qPCR) are listed in Table 2.
Determination of susceptibility to rare earth elements and bacitracin.
Cells were grown in L medium at 37°C for 4 h (approximately 5 × 108 cells/ml) and appropriately diluted with distilled water. Then, aliquots of 2 μl of the cell suspension were spotted onto half-strength solid L medium (agar concentration, 1.5%) containing each rare earth element at various concentrations, followed by incubation at 37°C for 18 h. To determine bacitracin susceptibility, aliquots of 2 μl of cell suspension were spotted onto solid L medium containing ZnSO4 (40 μg/ml) and bacitracin (80 or 100 μg/ml). Bacitracin (71,100 IU/g) was purchased from Nacalai Tesque (Kyoto, Japan).
Amylase assay.
B. subtilis strains were grown in NG medium at 30°C with vigorous shaking. Amylase activity was measured as described previously (12).
RESULTS
Isolation of mutants resistant to scandium.
B. subtilis cells were more susceptible to scandium than to other rare earth elements. In addition, we found that the scandium resistance was dependent on the growth conditions. The MIC of scandium (ScCl3 · 6H2O) on half-strength L agar medium was approximately 2-fold lower (i.e., increased susceptibility) than that on full-strength L medium (data not shown). We therefore used half-strength L medium for determination of B. subtilis susceptibility to rare earth elements. The MIC value of scandium was 300 μg/ml, whereas MICs of other rare earth elements were in the range of 500 to 800 μg/ml. To understand the physiological action of scandium, we isolated a number of scandium-resistant mutants that had developed spontaneously on half-strength L agar plates containing 300 μg/ml of scandium. All mutants (we tested 100 mutants) had only a slight resistance to scandium (MIC of 350 μg/ml). As addition of scandium to the growth medium stimulates amylase production in B. subtilis (12), we examined whether a scandium resistance mutation also activates amylase production. Of 30 mutants tested for their capability to produce amylase, one scandium-resistant mutant strain (SC26) showed enhanced amylase production. Therefore, we further characterized this mutant. The SC26 mutant grew normally in NG medium and sporulated well in sporulation medium (data not shown). The amylase activity in the culture broth of this mutant was approximately 2-fold higher than that in its parent strain 168 (data not shown). We previously reported that the expression level of the amylase-coding gene amyE in cells grown in scandium-supplemented medium was 2.3-fold higher than levels in cells grown in scandium-free medium, eventually leading to amylase overproduction (12). RT-qPCR analysis, however, revealed no significant difference in the levels of amyE expression between the mutant SC26 and 168 (data not shown), suggesting that the effect of the scandium resistance mutation on amylase overproduction was exerted at the posttranscriptional level. Unlike amylase production, extracellular protease and antibiotic bacilysin were produced at the same levels in the SC26 mutant as in its parent strain 168 (data not shown).
Identification of a scandium resistance mutation.
To identify the mutation conferring scandium resistance in the mutant SC26, we performed microarray-based mutation mapping analysis (Roche NimbleGen) and DNA sequencing. The results successfully identified two mutations at different loci in the SC26 genome: a single base substitution (112A to G, designated yufQ38) within the yufQ gene, which encodes an uncharacterized protein; and a single base substitution (257C to T, designated uppS86) within the uppS gene, which encodes an undecaprenyl pyrophosphate synthase (UppS). To investigate the possible roles of these gene products in scandium resistance, we attempted to disrupt each gene by insertional mutagenesis with the plasmid pMutinT3. Disruption of the yufQ gene affected neither scandium resistance nor amylase production (data not shown). Attempts to disrupt the uppS gene were unsuccessful, suggesting that the uppS gene is essential. In fact, the uppS gene has been reported to be essential in Streptococcus pneumoniae (1). To confirm the causal relationship between the identified mutations and scandium resistance, we constructed the strains TI391 (yufQ38) and TI392 (uppS86) by transformation using a histidine auxotrophic marker (see Materials and Methods). Similar to the original mutant SC26, the uppS86 transformant showed increased resistance to scandium (Fig. 1), while the yufQ38 transformant did not (data not shown). Furthermore, the levels of amylase production by the uppS86 transformant TI392, as well as SC26, were approximately 2-fold greater than the level in the wild-type strain (Table 3), whereas the yufQ38 transformant showed no effect on amylase production (data not shown). These results indicate that the uppS86 mutation (but not the yufQ38 mutation) was responsible for both increased scandium resistance and enhanced amylase production. Although other scandium-resistant mutants without amylase overproduction (10 strains were tested) also had the same level of scandium resistance as the SC26 mutant, these mutants had no mutation in the uppS gene (data not shown). Unexpectedly, the enhanced amylase production in the scandium-resistant uppS86 mutant was no longer increased by addition of scandium to the culture medium (data not shown).
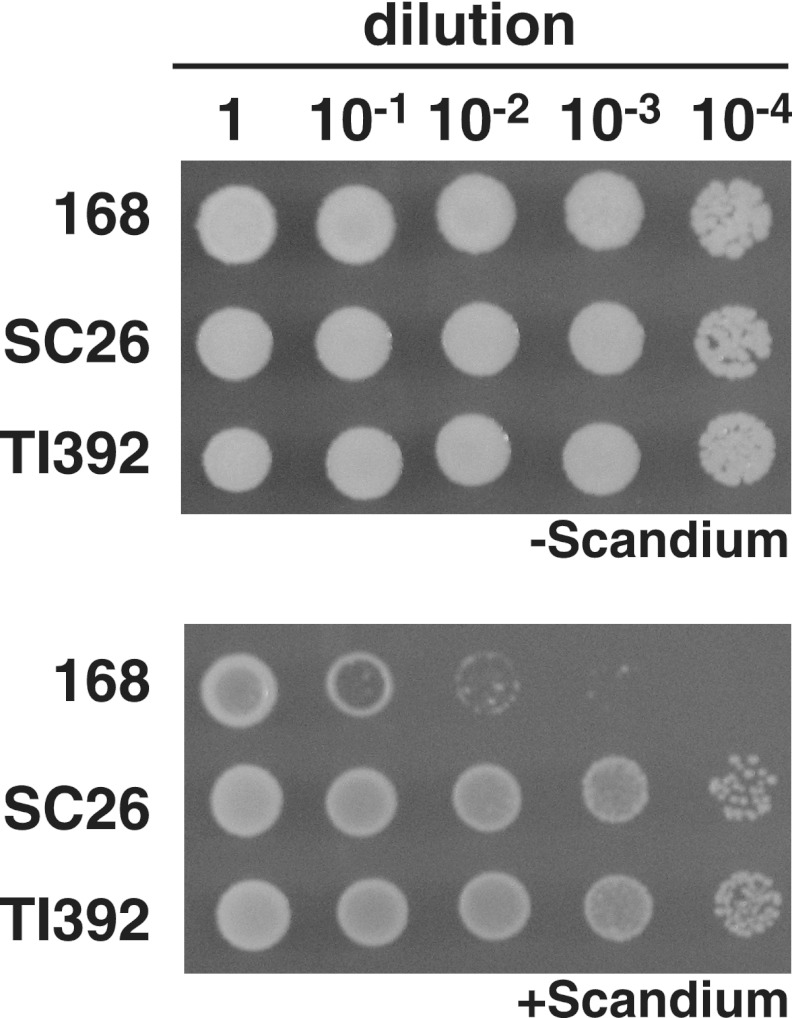
Fig 1.
Scandium susceptibility of B. subtilis mutants. B. subtilis strains 168 (wild type), SC26 (scandium-resistant mutant), and a transformant TI392 (uppS86) were grown in L medium at 37°C for 4 h. Cells (approximately 5 × 108 cells/ml) were appropriately diluted with distilled water. Then, aliquots of 2 μl of cell suspension were spotted onto the scandium-free (−) and scandium-containing (+) (320 μg/ml of ScCl3 · 6H2O) medium, followed by incubation at 37°C for 18 h.
Table 3.
Amylase production by various mutant strains
| Strain | Amylase productiona |
|---|---|
| 168 (wt)b | 1.99 ± 0.26 |
| TI392 (uppS86) | 3.36 ± 0.42* |
| TI408 (PS10-uppS) | 2.48 ± 0.53 |
| TI403 (yubB) | 2.88 ± 0.48* |
| TI409 (PS10-yubB) | 2.12 ± 0.32 |
| TI393 (bcrC) | 2.73 ± 0.45* |
B. subtilis strains were grown in NG medium at 30°C. After 72 h of cultivation, the culture supernatant obtained after centrifugation was used for amylase assay. Amylase production is expressed as amylase activity (units) as described previously (12). The asterisks denote significant differences (P < 0.05).
wt, wild type.
Undecaprenyl pyrophosphate participates in the scandium susceptibility.
C55-PP is the precursor for the carrier lipid, undecaprenyl phosphate (C55-P), which is required for cell wall synthesis (5). C55-PP synthase catalyzes eight consecutive condensation reactions of isopentenyl pyrophosphate (IPP) with farnesyl pyrophosphate (FPP) to form C55-PP (1). Bacitracin, a mixture of related cyclic peptide antibiotics, binds to C55-PP in the presence of divalent cations such as Zn2+ and prevents the dephosphorylation of C55-PP (28, 29). In E. coli, the overproduction of C55-PP phosphatase, BacA, accelerates the conversion of C55-PP to C55-P, resulting in an increase in cellular resistance to bacitracin (8). Accordingly, the bacitracin susceptibilities of the uppS86 mutants were compared to those of the parent strain in the presence of Zn2+ (Fig. 2). As expected, both of the uppS86 mutant strains (SC26 and TI392) showed cross-resistance to bacitracin, suggesting that the uppS86 mutation affects the available pool size of C55-PP. To further investigate the involvement of C55-PP in scandium resistance, we constructed a strain overexpressing uppS, in which the uppS promoter was replaced with a powerful promoter derived from the S10 ribosomal protein gene cluster (17). During the exponential growth phase, the uppS expression level in the resulting strain TI408 (PS10-uppS) was approximately 16-fold higher than that in wild-type strain 168 as determined by RT-qPCR analysis (data not shown). As expected, the uppS-overexpressing strain TI408 was apparently more susceptible to both scandium and bacitracin (Fig. 3), thus indicating that the increase in free C55-PP results in increased susceptibility.
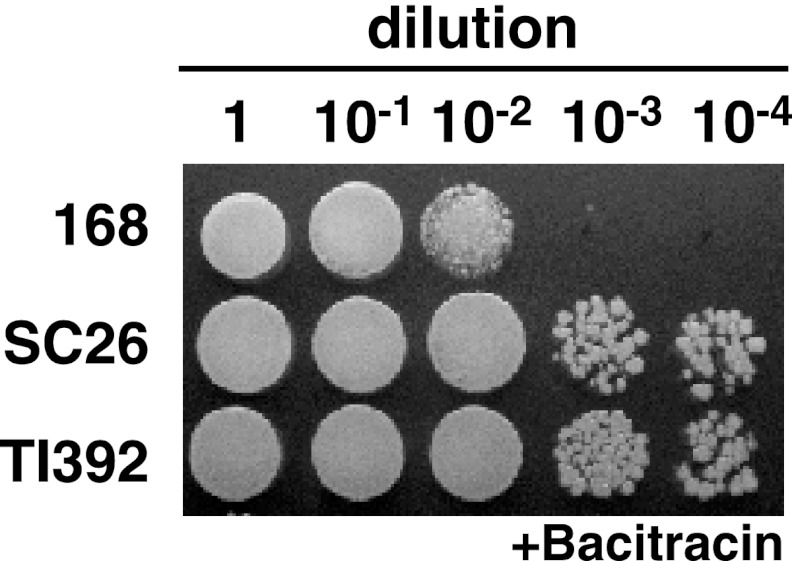
Fig 2.
Bacitracin susceptibility of B. subtilis mutants. B. subtilis strains 168 (wild type), SC26 (scandium-resistant mutant), and a transformant TI392 (uppS86) were grown in L medium at 37°C for 4 h. Cells (approximately 5 × 108 cells/ml) were appropriately diluted with distilled water. Then, aliquots of 2 μl of cell suspension were spotted onto solid L medium (1.5% agar) containing ZnSO4 (40 μg/ml) and bacitracin (+) (100 μg/ml [7.11 units/ml]), followed by incubation at 37°C for 18 h.
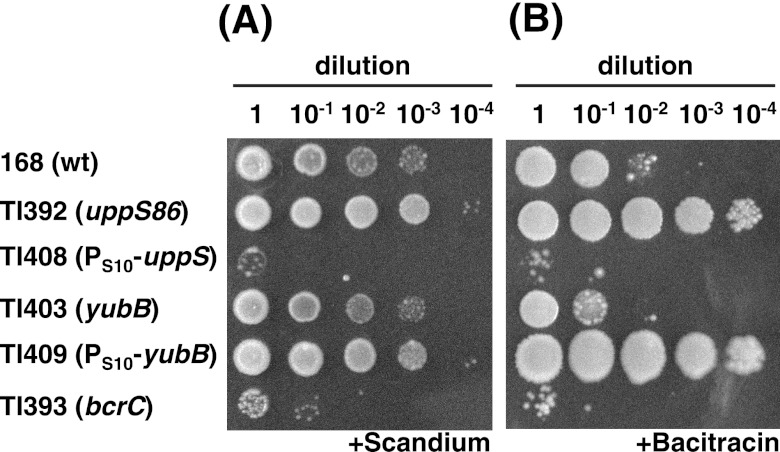
Fig 3.
Scandium and bacitracin susceptibilities of various B. subtilis strains. B. subtilis strains 168, TI392 (uppS86), TI408 (PS10-uppS), TI403 (yubB), TI409 (PS10-yubB), and TI393 (bcrC) were grown in L medium at 37°C for 4 h. The susceptibilities to scandium (A) and bacitracin (B) were tested as described in the legends to Fig. 1 and 2, except that a scandium concentration of 300 μg/ml was used. wt, wild type.
Effects of bcrC and yubB disruption on susceptibility.
As the reduction in C55-PP phosphatase activity is also thought to result in accumulation of available C55-PP, we next examined whether the disruption of C55-PP phosphatase affects scandium susceptibility. B. subtilis possesses at least two C55-PP phosphatases (3). The bcrC gene product BcrC, which is similar to the BcrC component of the bacitracin immunity system of Bacillus licheniformis (22), has been reported to have C55-PP phosphatase activity (3). In addition, the yubB gene product YubB, which is 46% identical to E. coli BacA, is also a putative C55-PP phosphatase in B. subtilis. We disrupted each of these genes to examine the possible roles of these proteins in scandium susceptibility. As expected, the bcrC disruptant mutant TI393 showed higher levels of susceptibility to both scandium and bacitracin (Fig. 3). In contrast, the disruption of yubB had no substantial effect on scandium resistance although it slightly decreased the bacitracin resistance. Moreover, the bcrC yubB double mutant exhibited the same susceptibility to scandium as the single bcrC mutant (data not shown). We assumed that the phosphatase activity of YubB is much lower than that of BcrC. To test this hypothesis, we constructed the yubB-overexpressing strain by replacing the yubB promoter with an S10 promoter. RT-qPCR analysis showed that the relative amount of yubB transcript in the yubB-overexpressing strain TI409 (PS10-yubB) was 80-fold higher than that in the wild-type strain 168 (data not shown). As expected, overexpression of yubB resulted in increased resistance to both scandium and bacitracin (Fig. 3). These results indicate that both BcrC and YubB can function as C55-PP phosphatase in B. subtilis.
As the uppS86 mutation resulted in increased amylase production, we examined whether C55-PP is involved in amylase overproduction. The disruption of bcrC or yubB stimulated the amylase production to some extent, while the effects of uppS or yubB overexpression were marginal (Table 3), thus showing a lack of clear correlation between these gene expression levels and amylase production.
Susceptibilities to other rare earth elements.
We examined the effects of the uppS86 mutation on susceptibilities to other rare earth elements. The mutant TI392 (uppS86) was more resistant to ytterbium but not to other rare earth elements tested (Fig. 4). In contrast, the bcrC disruption mutant TI393 showed higher susceptibilities to all rare earth elements tested.
Fig 4.
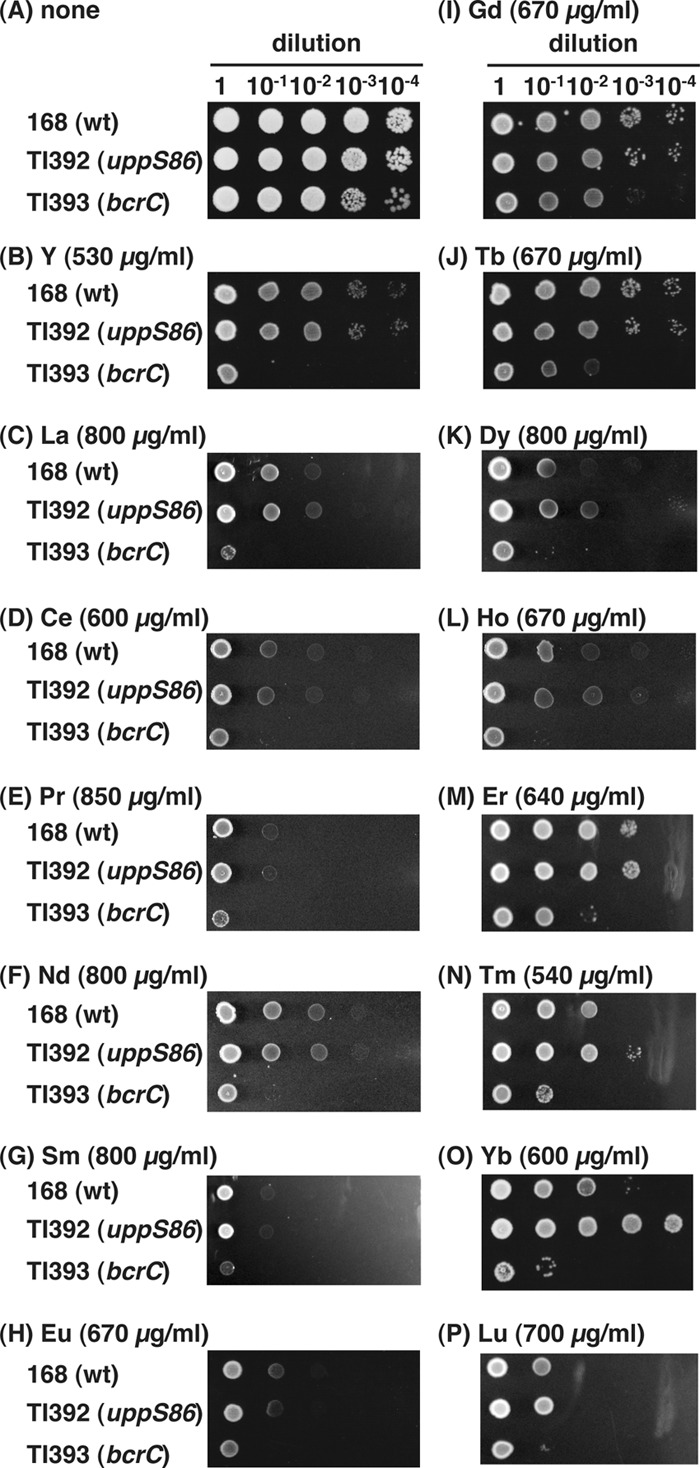
Rare earth element susceptibilities of B. subtilis strains. B. subtilis strains 168 (wild type), TI392 (uppS86), and TI393 (bcrC) were grown in L medium at 37°C for 4 h. Then, aliquots of 2 μl of cell suspension were spotted onto rare earth element-free medium (A) and medium containing each rare earth element at the indicated concentration (B to P), followed by incubation at 37°C for 18 h. The rare earth elements used were all chloride salts. Abbreviations: Y, yttrium; La, lanthanum; Ce, cerium; Pr, praseodymium; Nd, neodymium; Sm, samarium; Eu, europium; Gd, gadolinium; Tb, terbium; Dy, dysprosium; Ho, holmium; Er, erbium; Tm, thulium; Yb, ytterbium; Lu, lutetium.
DISCUSSION
The ability of bacterial surfaces to bind metal cations has been studied extensively (4). Rare earth elements have also been reported to be accumulated on the cell envelope in both the Gram-positive bacterium B. subtilis and the Gram-negative bacterium E. coli (2, 30). Recent work clarified that rare earth elements form complexes with multiple phosphate sites at the bacterial cell surface (30). More recently, Moriwaki et al. showed that adsorption of rare earth ion onto wild-type cell powder was greater than that of lipoteichoic acid-defective strain, suggesting that rare earth ions can be adsorbed to lipoteichoic acid in Gram-positive bacteria (18). Another possible candidate as a rare earth element-binding site on the cell envelope is the carrier lipid precursor C55-PP, which is required for the synthesis of peptidoglycan and a variety of other cell wall polysaccharide components, such as lipopolysaccharides, the enterobacterial common antigen, capsule polysaccharides, and teichoic acids (20, 23, 24, 32, 33). Consistent with these previous studies, we demonstrated that the C55-PP synthase mutation conferred resistance to scandium in B. subtilis. Moreover, we found that a mutant lacking the C55-PP phosphatase BcrC was more susceptible to all rare earth elements than the wild-type strain (Fig. 3 and 4). Our findings suggest that the accumulation of C55-PP renders the cell more susceptible to rare earth elements although the mode of action remains unknown. As C55-PP can function as a binding site for rare earth elements on the cell envelope, we propose that the availability of C55-PP is an important determinant of susceptibility to rare earth elements. Although the uppS86 mutation had no significant effect on resistance to rare earth elements other than scandium and ytterbium, accumulation of available C55-PP is assumed to increase the susceptibility of B. subtilis to various rare earth elements.
We found a scandium resistance mutation (uppS86 mutation) in the uppS gene, which encodes C55-PP synthase. This mutation results in a Thr-to-Ile amino acid substitution at residue 86 of the C55-PP synthase. As the forced expression of the wild-type uppS gene using a powerful promoter caused increased susceptibility to scandium (Fig. 3A), the uppS86 mutation appears to be a reduction-of-function mutation. Based on the results of previous structural and mutational analyses of E. coli C55-PP synthase (6, 7, 10, 16), residue Thr86 (corresponding to Ser72 in E. coli C55-PP synthase) is located in the flexible loop, which is important for catalysis and substrate binding. Deletion of this residue in E. coli C55-PP synthase decreases the kcat value by 105-fold (6). Accordingly, it is possible that replacement of Thr86 with Ile increased the hydrophobicity of the loop, affecting the enzymatic activity. Interestingly, the corresponding residue in Saccharomyces cerevisiae and human dehydrodolichyl pyrophosphate synthases, which catalyze much longer chain elongations than bacterial C55-PP synthases, is originally Ile (9, 27). Although we have no experimental evidence at present, replacement of Thr86 with Ile might affect the chain length of the product.
It is notable that the uppS86 mutation facilitated amylase production (Table 3). We reported previously that amyE expression is activated in the presence of scandium at the late stationary phase, leading to increased production of amylase (12). The upps86 mutation conferring scandium resistance, however, did not affect the amyE expression level, suggesting that the observed effect of the uppS86 mutation was exerted at the posttranscriptional level. Thus, the mechanism by which amylase production was stimulated by the uppS86 mutation is different from that caused by the addition of scandium to the growth medium.
We also demonstrated the roles of BcrC and YubB in scandium resistance; BcrC contributed to resistance to scandium in B. subtilis (Fig. 3), while YubB had no significant effect on scandium resistance unless it was overproduced. As the relative expression of yubB at the transcriptional level was as high as that of bcrC (data not shown), YubB might be negatively regulated at the posttranscriptional level. It is also possible that the C55-PP phosphatase activity of YubB is much lower than that of BcrC. Further investigation is necessary to clarify the YubB function in B. subtilis.
Our results suggest that the accumulation of C55-PP resulted in increased susceptibility to rare earth elements. Although the mechanism by which rare earth elements inhibit the growth of B. subtilis remains unclear, accumulation of rare earth elements on the cell envelope may prevent biological function(s) essential for growth.
ACKNOWLEDGMENTS
This work was supported by a grant (to T.I.) from a KAKENHI number 22780078 Grant-in-Aid for Young Scientists (B) and by a grant (to K.O.) from the Effective Promotion of Joint Research of Special Coordination Funds (Ministry of Education, Culture, Sports, Science and Technology of Japan).
We are grateful to the Roche NimbleGen, Inc. (Madison, WI), for supporting the mutation search using the comparative genome sequencing technique.
Footnotes
Published ahead of print 17 August 2012
REFERENCES
- 1. Apfel CM, Takacs B, Fountoulakis M, Stieger M, Keck W. 1999. Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: cloning, expression, and characterization of the essential uppS gene. J. Bacteriol. 181: 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bayer ME, Bayer MH. 1991. Lanthanide accumulation in the periplasmic space of Escherichia coli B. J. Bacteriol. 173: 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernard R, El Ghachi M, Mengin-Lecreulx D, Chippaux M, Denizot F. 2005. BcrC from Bacillus subtilis acts as an undecaprenyl pyrophosphate phosphatase in bacitracin resistance. J. Biol. Chem. 280: 28852–28857 [DOI] [PubMed] [Google Scholar]
- 4. Beveridge TJ. 1989. Role of cellular design in bacterial metal accumulation and mineralization. Annu. Rev. Microbiol. 43: 147–171 [DOI] [PubMed] [Google Scholar]
- 5. Bouhss A, Trunkfield AE, Bugg TD, Mengin-Lecreulx D. 2008. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol. Rev. 32: 208–233 [DOI] [PubMed] [Google Scholar]
- 6. Chang SY, Chen YK, Wang AH, Liang PH. 2003. Identification of the active conformation and the importance of length of the flexible loop 72–83 in regulating the conformational change of undecaprenyl pyrophosphate synthase. Biochemistry 42: 14452–14459 [DOI] [PubMed] [Google Scholar]
- 7. Chen YH, Chen AP, Chen CT, Wang AH, Liang PH. 2002. Probing the conformational change of Escherichia coli undecaprenyl pyrophosphate synthase during catalysis using an inhibitor and tryptophan mutants. J. Biol. Chem. 277: 7369–7376 [DOI] [PubMed] [Google Scholar]
- 8. El Ghachi M, Bouhss A, Blanot D, Mengin-Lecreulx D. 2004. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J. Biol. Chem. 279: 30106–30113 [DOI] [PubMed] [Google Scholar]
- 9. Endo S, Zhang YW, Takahashi S, Koyama T. 2003. Identification of human dehydrodolichyl diphosphate synthase gene. Biochim. Biophys. Acta 1625: 291–295 [DOI] [PubMed] [Google Scholar]
- 10. Guo RT, et al. 2005. Crystal structures of undecaprenyl pyrophosphate synthase in complex with magnesium, isopentenyl pyrophosphate, and farnesyl thiopyrophosphate: roles of the metal ion and conserved residues in catalysis. J. Biol. Chem. 280: 20762–20774 [DOI] [PubMed] [Google Scholar]
- 11. Inaoka T, Kasai K, Ochi K. 2001. Construction of an in vivo nonsense readthrough assay system and functional analysis of ribosomal proteins S12, S4, and S5 in Bacillus subtilis. J. Bacteriol. 183: 4958–4963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inaoka T, Ochi K. 2011. Scandium stimulates the production of amylase and bacilysin in Bacillus subtilis. Appl. Environ. Microbiol. 77: 8181–8183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inaoka T, Satomura T, Fujita Y, Ochi K. 2009. Novel gene regulation mediated by overproduction of secondary metabolite neotrehalosadiamine in Bacillus subtilis. FEMS Microbiol. Lett. 291: 151–156 [DOI] [PubMed] [Google Scholar]
- 14. Itaya M, Kondo K, Tanaka T. 1989. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 17: 4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawai K, Wang G, Okamoto S, Ochi K. 2007. The rare earth, scandium, causes antibiotic overproduction in Streptomyces spp. FEMS Microbiol. Lett. 274: 311–315 [DOI] [PubMed] [Google Scholar]
- 16. Ko TP, et al. 2001. Mechanism of product chain length determination and the role of a flexible loop in Escherichia coli undecaprenyl-pyrophosphate synthase catalysis. J. Biol. Chem. 276: 47474–47482 [DOI] [PubMed] [Google Scholar]
- 17. Li X, Lindahl L, Sha Y, Zengel JM. 1997. Analysis of the Bacillus subtilis S10 ribosomal protein gene cluster identifies two promoters that may be responsible for transcription of the entire 15-kilobase S10-spc-α cluster. J. Bacteriol. 179: 7046–7054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moriwaki H, Koide R, Yoshikawa R, Warabino Y, Yamamoto H. 12 June 2012, posting date. Adsorption of rare earth ions onto the cell walls of wild-type and lipoteichoic acid-defective strains of Bacillus subtilis. Appl. Microbiol. Biotechnol. doi:10.1007/s00253-012-4200-3 [DOI] [PubMed] [Google Scholar]
- 19. Moriya S, et al. 1998. A Bacillus subtilis gene-encoding protein homologous to eukaryotic SMC motor protein is necessary for chromosome partition. Mol. Microbiol. 29: 179–187 [DOI] [PubMed] [Google Scholar]
- 20. Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67: 686–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohashi Y, Ohshima H, Tsuge K, Itaya M. 2003. Far different levels of gene expression provided by an oriented cloning system in Bacillus subtilis and Escherichia coli. FEMS Microbiol. Lett. 221: 125–130 [DOI] [PubMed] [Google Scholar]
- 22. Ohki R, et al. 2003. A bacitracin-resistant Bacillus subtilis gene encodes a homologue of the membrane-spanning subunit of the Bacillus licheniformis ABC transporter. J. Bacteriol. 185: 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raetz CR, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71: 635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reeves PR, et al. 1996. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 4: 495–503 [DOI] [PubMed] [Google Scholar]
- 25. Rogers HJ, Synge C, Woods VE. 1980. Antibacterial effect of scandium and indium complexes of enterochelin on Klebsiella pneumoniae. Antimicrob. Agents Chemother. 18: 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rogers HJ, Woods VE, Synge C. 1982. Antibacterial effect of the scandium and indium complexes of enterochelin on Escherichia coli. J. Gen. Microbiol. 128: 2389–2394 [DOI] [PubMed] [Google Scholar]
- 27. Sato M, Fujisaki S, Sato K, Nishimura Y, Nakano A. 2001. Yeast Saccharomyces cerevisiae has two cis-prenyltransferases with different properties and localizations. Implication for their distinct physiological roles in dolichol synthesis. Genes Cells 6: 495–506 [DOI] [PubMed] [Google Scholar]
- 28. Stone KJ, Strominger JL. 1971. Mechanism of action of bacitracin: complexation with metal ion and C55-isoprenyl pyrophosphate. Proc. Natl. Acad. Sci. U. S. A. 68: 3223–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Storm DR, Strominger JL. 1973. Complex formation between bacitracin peptides and isoprenyl pyrophosphates. The specificity of lipid-peptide interactions. J. Biol. Chem. 248: 3940–3945 [PubMed] [Google Scholar]
- 30. Takahashi Y, Yamamoto M, Yamamoto Y, Tanaka K. 2010. EXAFS study on the cause of enrichment of heavy REEs on bacterial cell surfaces. Geochim. Cosmochim. Acta 74: 5443–5462 [Google Scholar]
- 31. Tanaka Y, Hosaka T, Ochi K. 2010. Rare earth elements activate the secondary metabolite-biosynthetic gene clusters in Streptomyces coelicolor A3(2). J. Antibiot. (Tokyo) 63: 477–481 [DOI] [PubMed] [Google Scholar]
- 32. van Heijenoort J. 2001. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat. Prod. Rep. 18: 503–519 [DOI] [PubMed] [Google Scholar]
- 33. Wicken AJ, Knox KW. 1980. Bacterial cell surface amphiphiles. Biochim. Biophys. Acta 604: 1–26 [DOI] [PubMed] [Google Scholar]
- 34. Wurm M. 1951. The effect of lanthanum on growth and metabolism of Streptococcus faecalis R. J. Biol. Chem. 192: 707–714 [PubMed] [Google Scholar]