Abstract
The accessory Sec system is a specialized transport system that exports serine-rich repeat (SRR) glycoproteins of Gram-positive bacteria. This system contains two homologues of the general secretory (Sec) pathway (SecA2 and SecY2) and several other essential proteins (Asp1 to Asp5) that share no homology to proteins of known function. In Streptococcus gordonii, Asp2 is required for the transport of the SRR adhesin GspB, but its role in export is unknown. Tertiary structure predictions suggest that the carboxyl terminus of Asp2 resembles the catalytic region of numerous enzymes that function through a Ser-Asp-His catalytic triad. Sequence alignment of all Asp2 homologues identified a highly conserved pentapeptide motif (Gly-X-Ser362-X-Gly) typical of most Ser-Asp-His catalytic triads, where Ser forms the reactive residue. Site-directed mutagenesis of residues comprising the predicted catalytic triad of Asp2 of S. gordonii had no effect upon GspB transport but did result in a marked change in the electrophoretic mobility of the protein. Lectin-binding studies and monosaccharide content analysis of this altered glycoform revealed an increase in glucosamine deposition. Random mutagenesis of the Asp2 region containing this catalytic domain also disrupted GspB transport. Collectively, our findings suggest that Asp2 is a bifunctional protein that is essential for both GspB transport and correct glycosylation. The catalytic domain may be responsible for controlling the glycosylation of GspB, while other surrounding regions are functionally required for glycoprotein transport.
INTRODUCTION
The accessory Sec system of Gram-positive bacteria is dedicated to the export of a family of glycosylated serine-rich repeat (SRR) proteins (30). These surface proteins are increasingly being recognized as important virulence factors for their respective pathogens. The SRR proteins GspB and Hsa of Streptococcus gordonii, together with SraP of Staphylococcus aureus, are platelet-binding adhesins that contribute to the pathogenesis of infective endocarditis (4, 36, 45). In addition, the SRR proteins of Streptococcus pneumoniae and group B streptococcus have been characterized as surface adhesins mediating microbial persistence within the lung and adhesion to brain microvascular endothelial cells, respectively, thereby instigating invasive disease (28, 35, 42).
Each SRR protein is encoded within an operon that also encodes components responsible for its glycosylation and transport to the cell surface. In Streptococcus gordonii, the accessory Sec system is comprised of SecA and SecY homologues (SecA2 and SecY2), suggesting that a Sec-like mechanism may be utilized for protein transport. Indeed, SecA2 has ATPase activity, and both SecA2 and SecY2 have domain organizations that match those of their Sec counterparts (6, 41). The remaining 5 components, termed Asps (accessory Sec proteins), share no significant sequence homology to any protein of known function, and it remains unclear how these Asps contribute toward the transport process.
Our studies in S. gordonii have shown that these Asps can interact with each other, SecA2, and GspB and that at least some of these interactions are essential for export. Asp3 and Asp2 can directly bind to unfolded regions of GspB, suggesting that these Asps may function in part as molecular chaperones to facilitate GspB trafficking out of the cell (46). In addition, we have shown that Asp3 can also bind to other members of the accessory Sec system (Asp1, Asp2, and SecA2), implicating Asp3 as a central component of the accessory system (34).
Glycosylation of GspB is carried out by four other proteins encoded within the same SRR operon. Modification of GspB is initiated by two glycosyltransferases (GtfA and GtfB), which catalyze the transfer of N-acetylglucosamine (GlcNAc) to the SRR regions of GspB (3, 39). Gly and Nss further add to the glycan composition of GspB, with Nss being responsible for glucose deposition (40, 48). Both GtfA and GtfB are present in all bacteria encoding an SRR operon (24), and their functions in GlcNAc deposition appear to be conserved (9, 26). The number and apparent type of glycosyltransferases accompanying GtfA and GtfB differ among SRR operons (24). Gly and Nss themselves can be either absent or encoded alongside other glycosyltransferases. Such variation in the number of encoded glycosyltransferases among SRR operons is thought to contribute to the glycan variations present in SRR proteins (40, 47).
Since the transport or glycosylation of GspB involves different sets of proteins, the two processes have been thought to be largely independent of one another. Although glycosylation of full-length GspB is essential for its stability, previous studies have not shown any overlap between these events. Indeed, some truncated, nonglycosylated forms of GspB are relatively stable in the absence of glycosylation and are efficiently exported by the accessory Sec system (34). These and other results have indicated that export does not require GspB glycosylation and have further suggested that the modification of GspB is independent of the transport process. In an attempt to gain a better understanding of Asp function, we conducted detailed sequence analyses of the accessory Sec components of Streptococcus gordonii. We now report that Aps2 shares structural homology to a group of enzymes that utilize a Ser-His-Asp catalytic triad for enzymatic function. Moreover, disruption of this catalytic triad results in the export of an aberrantly glycosylated form of GspB, suggestive of a role for Asp2 in glycosylation. Thus, Asp2 appears to be a bifunctional protein possessing a role in both the transport and the glycosylation of GspB.
MATERIALS AND METHODS
Strains and growth conditions.
All strains and plasmids used in this study are listed in Table 1. S. gordonii strains were grown in Todd-Hewitt broth (THB). Escherichia coli DH5α served as a host for cloning purposes. E. coli was grown at 37°C under aeration in Luria broth (LB). When appropriate, the following antibiotics were added to the media at the indicated concentrations, unless stated otherwise: ampicillin, 50 μg ml−1 for E. coli; chloramphenicol, 34 μg ml−1 for E. coli and 5 μg ml−1 for S. gordonii; kanamycin, 30 μg ml−1 for E. coli; erythromycin, 500 μg ml−1 for E. coli and 60 μg ml−1 for S. gordonii; spectinomycin, 50 μg ml−1 for E. coli and 100 μg ml−1 for S. gordonii.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strain | ||
| S. gordonii | ||
| M99 | Parent strain | 38 |
| PS614 | M99 Δasp2::spec | 39 |
| PS615 | M99 gspB::pB2061His6 Δasp2::spec | 39 |
| PS1243 | M99 gspB::pB736flagC Δasp2::spec | 34 |
| PS1740 | M99 ΔgspB | 5 |
| PS463 | PS1740 gspB::pB1060His6 | 5 |
| PS465 | PS1764 gspB::pB3029His6 | 5 |
| PS2749 | PS463 Δasp2::spec | This study |
| PS2750 | PS465 Δasp2::spec | This study |
| E. coli | ||
| DH5α | General cloning strain | Invitrogen |
| OverExpressC43(DE3) | F− ompT hsdSB(rB− mB−) gal dcm (DE3) C43 | Lucigen |
| Plasmids | ||
| pCDFDuet | E. coli T7 expression vector, Specr | Novagen |
| pET28b | E. coli T7 expression vector, Kanr | Novagen |
| pACYCDuet | E. coli T7 expression vector, Cmr | Novagen |
| pCDFNss | Vector expressing HANss, Specr | This study |
| pCDFGly | Vector expressing HAGly, Specr | This study |
| pCDFNss-Gly | Vector coexpressing HANss and HAGly, Cmr | This study |
| pETAsp2 | Vector expressing His6Asp2, Kanr | This study |
| pETAsp2[S362A] | Vector expressing His6Asp2[Ser362-Ala362], Kanr | This study |
| pGSTGspB1060 | Vector expressing GSTGspB1060, Cmr | This study |
| pGSTGspB1060-GtfA/B | Vector coexpressing GSTGspB1060, GtfA, and GtfB, Cmr | This study |
| pORF2K | E. coli cloning vector, ColE1 Cmr | 40 |
| pMSP3545 | Nisin expression vector | 8 |
| pMSP3545asp2 | asp2 in pMSP3545 | This study |
| pMSP3545asp2::tn104 | asp2::Tn98 in pMSP3545 | This study |
| pMSP3545asp2::tn152 | asp2::Tn105 in pMSP3545 | This study |
| pMSP3545asp2::tn332 | asp2::Tn237 in pMSP3545 | This study |
| pMSP3545asp2::tn347 | asp2::Tn311 in pMSP3545 | This study |
| pMSP3545asp2::tn349 | asp2::Tn340 in pMSP3545 | This study |
| pMSP3545asp2::tn414 | asp2::Tn345 in pMSP3545 | This study |
| pMSP3545asp2::tn422 | asp2::Tn358 in pMSP3545 | This study |
| pMSP3545asp2::tn452 | asp2::Tn391 in pMSP3545 | This study |
| pMSP3545asp2::tn452 | asp2::Tn450 in pMSP3545 | This study |
| pMSP3545asp2::tn452 | asp2::Tn466 in pMSP3545 | This study |
Ermr, erythromycin resistance; Ampr, ampicillin resistance; Kanr, kanamycin resistance; Cmr, chloramphenicol resistance; Specr, spectinomycin resistance.
DNA manipulations.
Routine molecular biology techniques for cloning, sequencing, and PCR amplification were performed as described previously (32). Chromosomal DNA was isolated from S. gordonii as described by Madoff et al. (25). Plasmid DNA was isolated from E. coli using miniprep columns (Qiagen). DNA restriction and modification enzymes were used according to the manufacturer's recommendations (NEB). E. coli cells were transformed following CaCl2 treatment (32), while S. gordonii was transformed by competence-induced transformation as described previously (5).
Site-directed mutagenesis.
Alanine replacement mutations within asp2 were made by a two-stage PCR procedure. For codon conversion to alanine, overlapping primers (Table 2) were used with either primer pETasp2-F (5′-GCATATGAAGATTCAAAAACATAAGGAA) or primer pETasp2-R (5′-CGTTCTCGAGTAACCATTTGACTCCTCTAAA) (underlining indicates NheI and XhoI restriction sites in pETasp2-F and pETasp2-R, respectively) to generate overlapping DNA fragments spanning the entire asp2 open reading frame. The two DNA fragments were combined for the second-stage PCR and then amplified using primers pETasp2-F and pETasp2-R. Amplified products were digested with the appropriate restriction enzymes and ligated into similarly digested pET28b. The confirmed asp2 mutants were subsequently cloned in pMSP3545. Sequence analysis was used to verify the correct mutation.
Table 2.
Oligonucleotides used for alanine-scanning mutagenesis of asp2
| Mutant | Direction | Mutagenic oligonucleotidea | Codon change |
|---|---|---|---|
| S318A | Sense | TGCCGATCAGCGATTAGACGG | TCT → GCC |
| Antisense | AATCGCTGATCGGCAATCAAAAGAAAAGGGC | ||
| S331A | Sense | GGTGCCGATGAATTAGAAGAAGG | AGC → GCC |
| Antisense | CTTCTAATTCATCGGCACCTAGATAAAAGACACC | ||
| S352A | Sense | TGCCGAACGAGAATTAATTCTATCC | TCT → GCC |
| Antisense | AATTCTCGTTCGGCAAAACCTAGTAATTCCATATG | ||
| S359A | Sense | CTAGCCGGTATATCCATGGGAAC | TCC → GCC |
| Antisense | ATATACCGGCTAGAATTAATTCTCGTTCAG | ||
| S362A | Sense | TATAGCCATGGGAACCTATG | TCC → GCC |
| Antisense | CCATGGCTATACCGGATAG | ||
| E451A | Sense | GGCTTACATGAAGGCCGAAGACTACGATCCGACAGC | GAA → GCC |
| Antisense | GATCGTAGTCTTCGGCCTTCATGTAAGCCAGACC | ||
| E452A | Sense | GCTTACATGAAGGAAGCCGACTACGATCCGACAGCT | GAA → GCC |
| Antisense | CGGATCGTAGTCGGCTTCCTTCATGTAAGCCAG | ||
| D453A | Sense | CATGAAGGAAGAAGCCTACGATCCGACAGCTTAC | GAC → GCC |
| Antisense | GCTGTCGGATCGTAGGCTTCTTCCTTCATGTAAGCC | ||
| H482A | Sense | CTTTCAGGGCGAGCTAATGACGATTCTAC | CAT → GCT |
| Antisense | GAATCGTCATTACGTCGCCCTGAAAGCC |
Sequences of mutagenic primers are shown in the 5′–3′ order, with altered codons shown in bold.
Construction of asp2 deletion strains.
The deletion of accessory sec genes from S. gordonii strains that express GspB736flag has been described previously (7). PS463 and PS465 are isogenic variants of the parent strain Streptococcus gordonii M99 that express His6-tagged GspB1070 (GspB1070His6) and GspB3029His6, respectively (5). Both of these strains were used to construct mutants having deletions of asp2 through nonpolar allelic exchange, as described previously (7).
Asp2 protein purification.
Plasmid pET28b (Novagen) was used for the synthesis of an N-terminally His6-tagged Asp2 fusion protein, which was constructed as follows. A DNA fragment containing the entire coding sequence of asp2 was amplified from S. gordonii strain M99 chromosomal DNA using Vent high-fidelity polymerase (NEB) with primers pETasp2-F and pETasp2-R (incorporating 5′ NheI and 3′ XhoI restriction sites, respectively). The resulting PCR product was cloned into pET28b, creating an in-frame insertion of a His6 tag at the N terminus, and pET28b was transformed into E. coli OverExpressC43(DE3) (Lucigen). The identity of the cloned DNA fragment was verified through restriction digestion analysis and DNA sequencing. For induction of His6-tagged Asp2 (His6Asp2) protein expression, E. coli was grown at 37°C in LB medium supplemented with kanamycin. At an optical density at 600 nm of 0.6, isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a final concentration of 1 mM, and cells were grown for a further 2 h at 25°C to induce expression. Induced cultures were pelleted and resuspended in lysis buffer (50 mM Na phosphate, pH 8, 150 mM NaCl, 10 mM imidazole, and 1% Triton X-100). Cells were lysed by sonication, and His6Asp2 protein was purified from the clarified lysates under native conditions by affinity chromatography, using Ni2+-nitrilotriacetic acid agarose (Qiagen). Purified Asp2 protein was reconstituted in storage buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, and 5% glycerol) and stored in aliquots at −80°C until required for use.
Hydrolase and esterase activity.
Hydrolase activity was determined as described by Gladden et al. (16). In brief, p-nitrophenyl-β-d-glucopyranoside at a final concentration of 1 mg ml−1 was mixed with 20 μg of purified Asp2 in 50 mM sodium acetate in a total reaction volume of 100 μl. The mixture was heated to 70°C and incubated for 1 h, and the reaction was quenched by adding 50 μl of cold 2% NaCO3. The release of p-nitrophenol was measured by recording the change of the absorbance at 405 nm.
Esterase activity was determined by measuring the hydrolysis of the p-nitrophenyl (pNP) esters of acetate (C2), butyrate (C4), caproate (C6), caprylate (C8), caprate (C10), laurate (C12), myristate (C14), and palmitate (C16). pNP esters were dissolved in acetonitrile to give a stock concentration of 50 mM. In a total reaction volume of 200 μl, 0.1 mM pNP ester in 50 mM Tris HCl (pH 8) containing 4% ethanol was used as a substrate in a 96-well microtiter plate assay. Purified Asp2 and its variants were added to the mixture, and the reaction was allowed to proceed for 30 min at 30°C. The release of p-nitrophenol was measured by recording the change of the absorbance at 405 nm. A recombinant esterase from Bacillus stearothermophilus (Sigma) served as a positive control for esterase activity.
Coexpression studies.
Glutathione S-transferase (GST)-tagged GspB1060 (GSTGspB1060) was amplified from pGST-GspBS2L (39) and cloned within the first multiple-cloning site (MCS) of pACYCDuet. DNA encoding gtfA and gtfB was amplified as a single DNA fragment from pGEX-GspBS1-BR-G-04 (39) and cloned within the second MCS of pACYCDuet. DNA encoding nss or gly was cloned as an N-terminal hemagglutinin (HA) tag fusion (HAnss and HAgly, respectively) within the IPTG-inducible plasmid pCDFDuet, while asp2 was cloned as an N-terminal Flag tag fusion within pETDuet. E. coli OverExpressC43(DE3) cells were simultaneously transformed with the designated plasmid set, and recombinant colonies were selected on plates containing the appropriate antibiotics (ampicillin, 25 μg ml−1; chloramphenicol, 17 μg ml−1; spectinomycin, 25 μg ml−1). Coexpression of GSTGspB1060 with defined glycosylation and accessory Sec components was carried out as described previously (39). E. coli lysates were probed with biotinylated succinylated wheat germ agglutinin (sWGA) or anti-GST antibodies to identify GSTGspB1060.
Far Western blot analysis.
Culture medium containing nonglycosylated GspB736flag secreted from strain M99 was reconstituted in 4× LDS sample buffer (Invitrogen). Proteins were separated by SDS-PAGE (4 to 12% gradient) and transferred to nitrocellulose membranes. The membranes were blocked by incubating for 2 h in phosphate-buffered saline (PBS) containing 5% (wt/vol) skimmed milk at room temperature, followed by 2 h of incubation in PBS–0.05% Tween containing recombinant His6Asp2 protein (1 μM). Membranes were washed 3 times for 15 min in PBS–0.05% Tween, and bound His6Asp2 was detected with mouse anti-His6 antibody (GE Amersham). Membranes were developed as previously described.
Densitometry analysis.
To quantify differences in GspB transport, blots were incubated at room temperature (RT) with mouse anti-Flag antibody (Sigma), used at a concentration of 1:5,000 for 2 h, followed by another 90 min incubation with a 1:20,000 dilution of Alexa Fluor 680 anti-mouse IgG (LI-COR Biosciences). Immunoreactive bands were visualized using a LI-COR infrared imager (LI-COR Biosciences) at 700 nm. Band intensity was measured using Odyssey (version 3.0) software.
2D gel electrophoresis.
Fifteen milliliters of culture medium from logarithmically growing cells (containing comparable densities of bacteria [in numbers of CFU/ml]) was concentrated 10-fold with an Amicon-Ultra 15 centrifugal filter device (Millipore). Concentrated proteins were processed using a two-dimensional (2D) ReadyPrep kit (Bio-Rad) and suspended in 8 M urea, 2% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 0.2% Bio-Lyte 3/10 ampholytes, 50 mM dithiothreitol, and 0.002% bromophenol blue. Protein was hydrated onto pH 4 to 7 immobilized pH gradient strips (Bio-Rad) overnight and then focused for 30,000 V-h. Strips were then embedded into 3 to 8% gradient Tris-acetate SDS-polyacrylamide gels to resolve in the second dimension. Gels were either stained with Imperial protein stain (Thermo Scientific) or transferred to nitrocellulose for Western blot analysis.
Whole-cell lectin-binding assay.
The ability of S. gordonii strains to bind to a panel of lectins was assessed as described previously (3, 40). All lectins tested were biotinylated (Vector) and used at the following concentrations: WGA, 1 μg ml−1; concanavalin A (ConA) and sWGA, 2 μg ml−1; and Griffonia simplicifolia lectin (GSL-I, GSL-II) and Maackia amurensis lectin (Mal II), 10 μg ml−1.
Monosaccharide composition analysis.
Secreted GspB2064 (previously described as GspB194) was purified from strain M99 culture supernatants for monosaccharide content as previously described (3). Monosaccharide analysis of purified GspB2064 was performed by the Glycotechnology Core Resource at the University of California, San Diego.
Mutagenesis of asp2.
Random, in-frame insertions of Tn5 within asp2 were generated as described previously. In brief, an EcoRI-XhoI restriction fragment of asp2 was cloned into pBluescriptΔNotI. The resulting construct, pBluescriptKS-asp2, was subjected to EZ-Tn5 transposition, and the transposition mixture was used to transform E. coli. Colonies containing EZ-Tn5<KAN> insertions within asp2 were identified by PCR, and the kanamycin resistance (KAN) cassette was removed through NotI restriction digestion. The religated plasmid contained asp2 with a random 19-codon in-frame insertion. The mutated EcoRI-XhoI asp2 gene fragment was amplified using primers ORF3C5 and ORF3C2 (40), digested with BamHI and XbaI, and cloned into similarly digested pMSP3545asp2. The resulting plasmids were sequenced to confirm the presence of the EZ-Tn5 mutation and introduced into S. gordonii strain PS1243 (asp2 deletion [Δasp2] strain). Supernatants and protoplasts prepared from nisin-induced cells were collected and probed for secreted GspB736flag and Asp2 expression as described previously (7).
Bacterial binding to sialyl-T antigen.
S. gordonii strains were grown for 18 h, washed twice with Dulbecco's PBS (DPBS), sonicated briefly to disrupt aggregate cells, and then diluted to 1 × 107 ml−1. Fifty microliters of bacterial suspensions was then applied to wells of a NeutrAvidin-coated microtiter plate that had been coated with 20 μM biotinylated sialyl-T antigen (GlycoTech Corporation). After 2 h of incubation at room temperature under vigorous shaking, the unbound bacteria were removed by aspiration. Wells were washed three times with DPBS, and the bound bacteria were released by trypsin treatment (1 h incubation with 50 μl of 1 mg ml−1 trypsin at room temperature). The number of input and bound bacteria was determined by plating serial dilutions of the bacterial suspensions on sheep blood agar plates, and the binding was expressed as the percentage of the input bound to sialyl-T antigen. Results are reported as the mean ± standard deviation (SD; with n = 5). Differences in binding were compared by the unpaired t test.
RESULTS
The C-terminal region is important for Asp2-mediated export.
Although Asp2 is essential for GspB transport, its precise role in export is unknown. Traditional BLASTP analysis of the primary amino acid sequence has failed to identify significant sequence similarity to any protein of characterized function, except to other Asp2 homologues. To determine what features of Asp2 are necessary for GspB export, a series of random in-frame transposon insertions of asp2 was generated, and they were assessed for their ability to support GspB736flag secretion (GspB736flag is a truncated version of GspB that has the same requirements for transport as the native glycoprotein but lacks a cell wall-anchoring domain, enabling it to be freely secreted into the culture medium).
A total of 10 mutations spanning the entire length of the coding sequence were produced within asp2 (Fig. 1). These mutant alleles were used to complement a strain M99 variant (PS1243) containing a deletion of asp2, and the resulting transformants were tested for the ability to support export of GspB736flag. Western blot analysis of the protoplast fraction of the complemented strains showed comparable expression levels of all the Asp2 variants (Fig. 1D). Export of GspB736flag was maintained in Asp2 containing insertions at amino acids (aa) 98, 105, 237, 311, 340, 391, and 466. Insertion at amino acid 391 appeared to have a minor effect upon transport, with a partial effect manifesting in an accumulation of GspB736flag within the protoplast. Interestingly, an insertion at amino acid 98 resulted in a GspB736flag with altered electrophoretic mobility, suggesting that Asp2 can affect the glycosylation of GspB. In contrast, export was abolished by insertions at amino acids 345, 358, and 450, where a clear accumulation of GspB736flag was seen within the protoplast fraction, confirming an export defect. Analysis of these Western blots by densitometry confirmed that insertion at amino acids 345, 358, and 450 resulted in a clear reduction in GspB736flag export to the culture medium, with a corresponding accumulation of the preprotein within the protoplast (Fig. 1C). Collectively, these findings suggest that the C-terminal end of Asp2 is essential for GspB secretion.
Fig 1.
Effects of Asp2 domain disruption upon the export of GspB736flag. (A) Schematic illustrating the locations of EZ-Tn5 insertions within asp2. (B) Western blot analysis of GspB736flag export by PS1243 derivative strains carrying in-frame insertions within asp2. Culture media (M) and protoplasts (P) were collected from exponentially growing strains and prepared as described in the Methods and Materials. (C) Densitometry analysis of GspB736flag levels within media or protoplasts. The y axis represents GspB736flag levels, based on band intensity analysis via LI-COR imaging. (D) Western blot detection of Asp2 and the in-frame insertion mutants. Proteins were separated by SDS-PAGE and subjected to Western blot analysis using anti-Flag and anti-His antibodies for GspB736flag and Asp2 detection, respectively.
Asp2 shares structural similarities to proteins possessing a Ser-His-Asp catalytic triad.
The finding that a particular domain of Asp2 was essential for GspB transport prompted us to analyze what determinants within this region may be responsible. To this end, we examined the predicted structural similarities of Asp2 to other proteins in the public databases. The tertiary structure prediction programs PHYRE2 (19), HHpred (37), Robetta (20), and SMART (22, 33) consistently identified a region within the C-terminal half of Asp2 resembling the catalytic region of numerous enzymes that utilize a Ser-His-Asp catalytic triad for catalytic activity. PHYRE2 identified the closest structural homologue as a copper-inducible hydrolase of Bacillus cereus (20% sequence identity with 98.1% confidence), followed by a 2,6-dihydropseudooxynictotine hydrolase from Arthrobacter nicotinovorans (12% sequence identity, 98.1% confidence) and a carboxylesterase of Mesorhizobium loti (12% sequence identity with 98% confidence). Enzymes possessing an identical catalytic triad were also identified as structural homologues from HHpred (Fig. 2) and Robetta analysis, with the esterase EstA of S. pneumoniae consistently being identified as a structural homologue to the C-terminal half of Asp2. In addition, protein domain analysis using the SMART algorithm identified an α/β hydrolase domain within aa 274 to 389 of Asp2, comparable to the region showing structural similarity to bacterial esterases. In combination, these findings suggest that the C terminus of Asp2 may possess enzymatic activity that involves a conserved Ser-His-Asp catalytic triad.
Fig 2.
Predicted structural homology analysis of Asp2. The predicted structural similarity of Asp2 to proteins with a solved crystal structure was assessed using the homology detection and structure prediction server HHpred (http://toolkit.tuebingen.mpg.de/hhpred). Proteins exhibiting structural similarity are shown aligned to the relevant region of Asp2 and are listed with their Protein Data Bank functional designation together with their species of origin.
Mutation of the catalytic triad affects the electrophoretic mobility of GspB but not transport.
Since Asp2 is required for GspB export, we first examined whether the predicted catalytic triad was important for the transport of the glycoprotein. To directly test this, we generated a series of alanine substitutions specifically targeting residues predicted to be essential for catalytic activity. Structural modeling of the carboxyl-terminal region of Asp2 identified residues Ser362, Glu452, and His482 as constituting a catalytic triad, when threaded on multiple enzymes (where Ser362 was found to reside within the putative catalytic GXSXG motif) (Fig. 3). To determine whether these residues did indeed constitute a catalytic triad, Ser362, Glut452, and His482 together with other candidate catalytic residues (Ser318, Ser331, Ser352, Ser359, Glu451, and Asp453) were replaced by alanine, which is known to eliminate catalytic activity (1, 43). Mutant alleles were cloned within the nisin-inducible expression plasmid and used to complement an asp2 deletion in strain PS1243.
Fig 3.
Predicted structure of the conserved catalytic region of Asp2. (A) Proposed structure of the carboxyl-terminal region (aa 227 to 494) of Asp2 based on homology modeling using the crystal structure of the known thioesterase of Vibrio harveyi. Spatial arrangement of the catalytic residues Ser362, Glu452, and His482. The diagram was generated using PyMOL software. (B) Amino acid alignment of the catalytic region among Asp2 homologues. The conserved GXSXG motif and Glu/Asp and His catalytic residues are highlighted in pink.
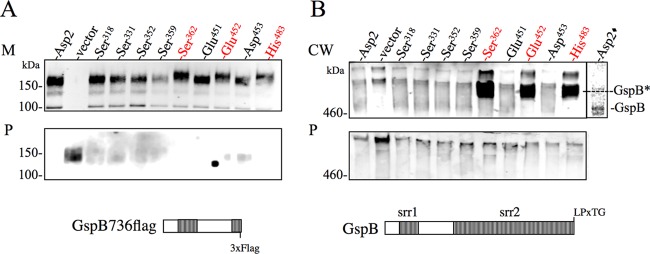
Complementation with His6asp2 in trans restored GspB736flag secretion into the culture medium, with little to no preprotein retention within the protoplasts (Fig. 4A). Similarly, all of the asp2 mutants tested also restored secretion of GspB736flag into the culture medium, indicating that replacement of these residues does not affect the export of GspB736flag. Interestingly, a slight change in electrophoretic mobility was observed in GspB736flag secreted from PS1243 complemented with the Ser362, Glu452, or His482 alanine replacement mutant (Fig. 4A).
Fig 4.
Effects of mutating the catalytic triad upon the export of GspB736flag and native GspB. Western blot analysis of GspB736flag (A) and native full-length GspB (B) export by S. gordonii strains carrying the designated serine-alanine substitutions within Asp2. Residues constituting the catalytic triad of Asp2 are highlighted in red. Culture media (M) and protoplasts (P) were collected from exponentially growing strains, while cell wall (CW) proteins were released by mutanolysin digestion, as described in the Materials and Methods. Proteins were separated by SDS-PAGE (3 to 8%) and analyzed by Western blotting, using anti-Flag antibody to detect GspB736flag and anti-GspB serum to detect native GspB. GspB*, the excessively glycosylated form of GspB due to Asp2 mutation; Asp2◆, an overexposure of native GspB detected with anti-GspB serum.
We next examined the Asp2 mutants for their ability to export native full-length GspB. The Asp2 mutants were used to complement an asp2 deletion in strain PS614, which expresses full-length cell wall-anchored GspB. As was observed with GspB736flag export, complementation with wild-type (WT) Asp2 and all of the alanine replacement mutants restored GspB surface expression, as the protein was now readily detectable in the cell wall fraction. However, the GspB exported by strains expressing the Asp2 variants with substitutions in the catalytic triad (Ser362, Glu452, and His482) migrated with a higher molecular mass than the WT protein. Furthermore, GspB secreted from S. gordonii expressing these Asp2 variants was more readily detectable with anti-GspB antiserum, an antibody predominately reactive against the carbohydrate moieties present on native GspB (3, 40) (Fig. 4B). Collectively, these findings indicate that the catalytic triad present within Asp2 does not influence the export of GspB but is important for the posttranslational modification of the protein.
The Srr region is a site of Asp2 activity.
The observation that mutating the catalytic triad of Asp2 had a greater impact on the electrophoretic mobility of native GspB than the truncated variant GspB736flag suggested that the site of Asp2 activity was within the SRR2 region (a target site for glycosylation). This was consistent with our previous finding that Asp2 could bind the SRR regions directly (46). To define the regions of GspB affected by Asp2, we expressed a series of GspB C-terminal truncates differing in the length of their SRR2 region and assessed the effects of the S362A mutation upon export and electrophoretic mobility (Fig. 5).
Fig 5.

Asp2-dependent export of GspB truncates from S. gordonii strains. Western blot analysis of secreted GspB variants in culture media (M) or in protoplasts (P). S. gordonii Δasp2 strains were complemented with either native asp2 or asp2S362A. Proteins were separated by SDS-PAGE through 3 to 8% polyacrylamide gradient gels and subjected to Western blot analysis using anti-GspB antiserum.
We first examined by Western blotting the export of GspB1060, which has an SRR2 region that is 324 aa longer than that of GspB736flag. When expressed in the presence of native Asp2, GspB1060 was exported into the culture medium as a 268-kDa band, while export of this variant in an Asp2S362A background resulted in a form of GspB exhibiting a larger apparent mass (approaching 460 kDa), with no accumulation seen within protoplasts, suggesting that transport is not affected by this mutation. This slower migration pattern became more pronounced in exported GspB variants containing more of the SRR2 region (GspB2061 and GspB3029). Moreover, the size of GspB secreted by the catalytic mutant mirrored the size of nonexported GspB in the corresponding Δasp2 strain (data not shown). In combination, these findings suggest that the effect of altering the catalytic site of Asp2 becomes more apparent as the number of glycosylation sites (the SRR region) increases.
Mutations in the catalytic triad of Asp2 alter glycosylation of GspB.
Changes in the electrophoretic mobility of glycoproteins can often be associated with a change in glycan content. To directly test this possibility, we examined the glycosylation of full-length GspB expressed by M99 strain PS614 (an asp2 deletion strain) complemented with selected variants of Asp2. Immobilized bacteria were probed with a panel of lectins (ConA, WGA, sWGA, Mal II, GSL-I, and GSL-II) that recognize glycans present on GspB. As was seen for PS614 complemented with asp2, GspB expressed in the background of any of the asp2 mutants also exhibited detectable binding to all of the lectins tested (Fig. 6A), suggesting that glycan structures found in native GspB are still present in the Asp2 mutant strains. Interestingly, bacteria expressing GspB in an Asp2 background with the S-to-A change at amino acid 362 (Asp2S362A) had significantly higher levels of sWGA and GSL-II binding than WT Asp2 (P < 0.05). Since both lectins bind glucosamine, these findings indicated that the carbohydrate composition of GspB was altered, with higher levels of the monosaccharide being produced by the Asp2S362A variant. To confirm that this change in lectin binding by bacteria was due to GspB, we assessed lectin binding to a secreted form of GspB. M99 strain PS615 was complemented with either asp2 or the asp2 variants, and the culture supernatants were probed with sWGA to detect secreted GspB2061. A band with a molecular mass corresponding to that of GspB2061 was identified in the culture supernatants of cells complemented with asp2, asp2S318A, asp2S331A, asp2S352A, and asp2S359A, while sWGA failed to recognize any proteins secreted in PS615 transformed with the vector alone (Fig. 6B). Consistent with the whole-cell lectin-binding assay, the higher-molecular-mass form of GspB2061 from the strain complemented with asp2S362A (also previously recognized with anti-GspB; Fig. 5) bound considerably more sWGA than GspB2061 secreted from any other Asp2 background. These combined results suggest that mutation of the catalytic triad within Asp2 leads to a change in the carbohydrate content of GspB by either an increase in GlcNAc levels or a removal of the distal sugars that may mask GlcNAc.
Fig 6.
Impact of mutating the Asp2 catalytic triad on the glycosylation of GspB. (A) Binding of a panel of biotinylated lectins to immobilized S. gordonii strain PS614 expressing full-length GspB in the designated asp2 background. Levels of lectin binding were detected with a horseradish peroxidase-conjugated antibiotin antibody and expressed as means ± SDs of triplicate measurements. *, values that are significantly different (P < 0.05) from the value for PS614 (with asp2). (B) Lectin blot analysis of GspB2061 secreted by PS615 complemented with asp2 variants. Proteins in the culture media were separated by SDS-PAGE (3 to 8%) and probed with sWGA.
The Asp2S362A mutation leads to an excessive GlcNAc deposition.
To more directly characterize the change in GspB glycosylation resulting from the S362A mutation, we assessed the impact of this mutation on the monosaccharide content of GspB2061. We have previously characterized the monosaccharide composition of this secreted form of GspB and have shown that it is comparable to that of the native glycoprotein (3). GspB2061 exported in a WT Asp2 background predominantly contained glucose and GlcNAc, with minor amounts of N-acetylgalactosamine, galactose, fucose, and mannose (Table 3), consistent with previous reports. The same monosaccharides were also detected on GspB2061 when exported in an Asp2S362A background. However, the amount of GlcNAc liberated from this form of GspB was approximately 5-fold greater than that seen on native GspB2061, with minor changes in the amounts of galactose and fucose also detected (Table 3).
Table 3.
Monosaccharide associated with GspB2061 secreted from S. gordonii and S. gordonii Asp2S362A
| Monosaccharidea | Amt bound (nmol) |
|
|---|---|---|
| Asp2 | Asp2S362A | |
| GlcNAc | 0.4408 | 5.2101 |
| Glc | 1.3847 | 1.1639 |
| GalNAc | 0.191 | 0.1679 |
| Gal | 0.2347 | 0.4838 |
| Fucose | 0.0029 | 0.0296 |
| Mannose | 0.5624 | 0.5501 |
Monosaccharides bound to 2 μg of purified GspB2061.
This increase in the content of GlcNAc (a sugar of neutral charge) of GspB was further assessed through 2D electrophoretic analysis of secreted GspB2061. Nonglycosylated GspB has a predicted pI of 3.9, while the truncated variant GspB2061 has a predicted pI of 4.08, indicating that both are acidic proteins. Indeed, glycosylated GspB2061 was resolved over a pI range of 4 to 5, suggesting that its native glycan composition did not strongly influence the pI (Fig. 7). Furthermore, a shift from heterogeneity of native GspB2061 to an exclusive high-molecular-mass glycoform exported from the Asp2S362A variant, both at the same pI position, indicates a change in molecular mass and not net charge. These results, in combination with the lectin affinity analysis described above, demonstrate that the disruption of the catalytic triad within Asp2 leads to an increase in the GlcNAc content of GspB.
Fig 7.

Positional shift of secreted Gsp2061 due to asp2 mutation. M99 Δasp2 complemented with asp2S362A (A) or wild-type asp2 (B) was grown to mid-log phase, and the culture media were analyzed by 2D electrophoresis. Different glycoforms of secreted GspB2061 were detected using anti-GspB antiserum. Arrows, positional shift of native GspB2061 (boxed in green) to a larger variant glycoform (boxed in red) due to asp2 mutation. IEF, isoelectric focusing.
Asp2 does not exhibit direct hydrolase or esterase activity.
As mentioned above, structural similarity analysis indicated that Asp2 resembles a number of esterases and hydrolases that utilize a Ser-Asp-His triad for catalytic activity. This prompted us to examine directly whether Asp2 indeed possessed esterase or hydrolase activity. Recombinant His6Asp2 and His6Asp2S362A were purified to homogeneity, and each was assessed for enzymatic activity against pNP ester and hydrolase substrates. When tested against a panel of monoesters of various chain lengths (C2 to C16), neither His6Asp2 nor His6Asp2 S362A exhibited significant activity in comparison to that of a defined esterase from Bacillus stearothermophilus (Fig. 8A). Similarly, both His6Asp2 and His6Asp2S362A displayed low activity against the hydrolase substrate (pNP-N-acetyl-β-d-glucosaminide) (data not shown). Collectively, these findings suggest that under the conditions tested, Asp2 does not possess detectable esterase or hydrolase activity.
Fig 8.
Esterase activity of Asp2 and its influence upon other glycosylation components. (A) Relative activities of Asp2 and its catalytic mutant (Ser362) toward pNP esters with different carbon chain lengths. Release of p-nitrophenyl is shown relative to the activity of a known carboxyl esterase (Bacillus Est). Data shown are means ± SDs of triplicate measurements. (B) GST-GspB1061 glycosylation in E. coli. The presence (+) or absence (−) of individual components of the glycosylation machinery is indicated. E. coli lysates were separated by SDS-PAGE (3 to 8%), transferred to membranes, and probed with either ant-GST or sWGA.
Asp2 does not affect the activity of GtfA/GtfB, Nss, or Gly.
The observation that expression of the Asp2 catalytic mutant resulted in secretion of GspB with increased GlcNAc content suggested that Asp2 could either modify GspB directly or affect the activity of GtfA, GtfB, Gly, or Nss. Of note, GtfA and GtfB act synergistically to add the proximal GlcNAc sugar, while Gly and Nss add distal sugars to the final glycan composition of GspB (3, 40). To test these possibilities, we examined the impact of Asp2 on the glycosylation of the SRR regions of GspB, when expressed in conjunction with the four glycosylation proteins. We utilized an established in vivo glycosylation system where truncated forms of GspB undergo glycosylation in E. coli, when coexpressed with the above-described proteins (39). This system permits the specific coexpression of different glycan-modifying proteins in conjunction with a GspB variant, thereby permitting analysis of the specific contribution of each protein in glycosylation.
We first assessed the glycosylation of a GST-GspB variant encompassing the SRR1-BR and SRR2 regions of GspB1060 by GtfA/GtfB. As expected, little to no GspB was detected in cell lysates when expressed by itself (Fig. 8B). This is consistent with our previous findings, where any GspB variant with an SRR region longer than ∼100 aa is unstable if nonglycosylated (7). Coexpression with GtfA/GtfB resulted in the appearance of GspB that was recognized by both anti-GST and sWGA, with recognition by the latter indicative of modification with GlcNAc (Fig. 8B). Coexpression of GspB with either Nss or Gly failed to produce any detectable GspB (results identical to those shown in the first lane of Fig. 8B), as these enzymes require the deposition of GlcNAc to modify GspB (39, 40). Expression of all four glycosylation proteins resulted in a form of GspB with an apparent molecular mass that was larger than that seen with GtfA/GtfB alone, indicating that additional glycan modification had occurred. However, no changes in the electrophoretic mobility of these GspB glycoforms were evident in E. coli when collectively expressed with Asp2 or its catalytic mutant, suggesting that Asp2 does not directly affect the activity of GtfA/GtfB, Gly, or Nss on GspB. Moreover, no enzymatic cleavage of Gly and Nss was observed within E. coli lysates when coexpressed with Asp2 or its catalytic mutant (data not shown), suggesting that Asp2 does not directly process the enzymes mediating glycosylation. These findings are consistent with those of zymogram analysis of purified recombinant Asp2, where no protease activity was observed (data not shown). In aggregate, these data indicate that Asp2 does not directly affect the glycosylation activities of GtfA/GtfB, Gly, and Nss.
Incorrect glycosylation of GspB leads to a reduced binding to sialyl-T antigen.
The finding that GspB can be produced and exported as a different glycoform following Asp2 mutation raised the issue of whether this altered glycosylation had an impact on the biologic activity of the glycoprotein. One function of GspB is to mediate attachment to human platelets through the interaction of the nonglycosylated binding region to sialyl-T antigen, an O-linked saccharide found on the platelet membrane glycoprotein Ibα (GPIbα). To examine the impact of aberrant glycosylation on GspB function, we compared binding to immobilized sialyl-T antigen by strain PS614 complemented with asp2 and PS614 complemented with the asp2S362A variant. As expected, PS614 expressing WT asp2 exhibited high levels of binding to sialyl-T antigen compared with that of the asp2 deletion strain (Fig. 9). Interestingly, PS614 expressing the Asp2S362A mutation had significantly reduced binding to sialyl-T antigen, with levels being no higher than those seen with the asp2 deletion strain. These results indicate that the incorrect glycosylation of GspB can impair binding to its host receptor.
Fig 9.

Binding of S. gordonii strains to sialyl-T antigen. S. gordonii strain PS614 complemented with asp2, empty vector (Δasp2), or asp2S362A was assessed for binding to immobilized sialyl-T antigen. Binding is expressed as the percentage (mean ± SD) of input bacteria that remained bound to sialyl-T antigen after repeated washing of the wells. *, P < 0.05 compared with WT asp2.
DISCUSSION
The SRR glycoproteins are a family of adhesins that are increasingly recognized as important virulence factors expressed by Gram-positive bacteria. They are unique virulence determinants, in that glycosylated proteins are strikingly rare among Gram-positive bacteria, and they are exported to the cell surface through a dedicated transport system. Both glycosylation and export involve multicomponent processes. Studies within S. gordonii show that glycan production and assembly are mediated through 4 sugar-modifying enzymes (GtfA, GtfB, Nss, and Gly), while GspB transport is accomplished through the coordinated activity of a 7-component accessory Sec system. We have previously shown that GspB glycosylation occurs independently of export and that glycosylation is not required for transport by this pathway (7).
The roles of the Asps in this specialized export system remain elusive. Asp2 is one of the least-characterized components of the accessory Sec system. We identified a serine-based catalytic triad within the C-terminal end of the protein, a motif that forms the catalytic site for a diverse group of enzymes, including proteases, lipases, hydrolases, and esterases (2, 14, 31), mutation of which led to an aberrantly glycosylated but exported form of GspB. This altered glycosylation was due to an increase in GlcNAc content, suggesting that the enzymatic activity of Asp2 was either directly or indirectly involved in the glycosylation of GspB. In addition, mutation of these key catalytic residues did not affect the ability of Asp2 to support GspB export, indicating that this catalytic activity is dispensable for transport.
The precise substrate and catalytic step mediated through the enzymatic activity of Asp2 remains to be elucidated. However, our studies showed that Asp2 did not influence the activity of Gly or Nss or the ability of GtfA and GtfB to initiate glycosylation. These findings are consistent with the possibility that Asp2 may directly modify glycosylated GspB. Protein glycosylation, involving numerous glycan-modifying components, generally employs a glycan-processing or -trimming step to ensure export of a correct glycoform (17, 18, 21). Loss in trimming of eukaryotic glycoproteins is associated with hyperglycosylation (10, 17, 27), as was seen in GspB following Asp2 mutation. Interestingly, glycoside hydrolases (which cleave glycosidic linkages to release a sugar moiety) are associated with trimming and typically function through a serine-based catalytic triad (15). Although the native glycan structure present on GspB has yet to be determined, it is possible that Asp2 may function in a similar role toward maintaining a particular glycoform of GspB.
Asp2 alone failed to exhibit detectable enzymatic activity against a panel of synthetic esters and hydrolase substrates, suggesting that the catalytic activity seen in S. gordonii requires additional cofactors to be reproduced in vitro. Of note, we have previously shown that Asp3 can mediate multiple protein-protein interactions among the accessory Sec components (34). Thus, it is possible that Asp2 forms a multimeric protein complex with one or more of these transport proteins that facilitates its catalytic activity. Precedent for this can be seen in the glycosylation machinery utilized within the Golgi apparatus, where a conserved oligosaccharyl transferase complex (COG) is responsible for N-linked protein glycosylation. Such complex formation not only enables colocalization of the glycosylation components, so that they may function in sequence, but also serves as a means to control the activity of each enzyme individually (11, 12). The contributory role of other accessory Sec components toward the catalytic activity of Asp2 is being vigorously pursued.
Interestingly, complex formation between homologues of the Asps in other SRR pathogens has also been described to be important for the glycosylation of SRR proteins. In Streptococcus parasanguinis, disruption of an interaction between Gap1 and Gap3 (Asp1 and Asp3 homologues, respectively) results in the export of a high-molecular-mass form of Fap1 (the SRR protein of S. parasanguinis) (23, 29, 44). It has been proposed that this high-molecular-mass variant represents an intermediate Fap1 glycoform and that these proteins (with Gap1 proposed to be a possible glycosyltransferase) contribute toward the final glycosylation state of Fap1 (23). Asp1-Asp3 binding has also been identified in S. gordonii, although no effect upon GspB glycosylation was evident following disruption of this interaction (34). However, the ability of Asp3 to bind both Asp1 and Asp2 is consistent with the possibility that a larger protein complex may be required for effective GspB glycosylation.
Although the catalytic mutants of Asp2 (S362A, E452A, and H489A) were still able to efficiently export GspB (albeit an aberrantly glycosylated form), several insertions surrounding the catalytic triad were found to negatively impact GspB transport. We have previously shown that Asp2 can directly bind the SRR region of GspB (46). None of the catalytic mutants lost the ability to bind GspB (data not shown). However, if this protein interaction is necessary for GspB transport, it is a distinct possibility that the residues lining the catalytic site may be responsible for preprotein binding. Moreover, if GspB is indeed a substrate for Asp2, the binding of GspB near or at its catalytic site would promote efficient enzymatic activity. Alternatively, if Asp2 promotes a stable Asp-based complex, it could also indirectly affect GspB export if one or more of its binding partners have a direct role upon GspB transport (as has been speculated for Asp3 [34]).
The observation that GspB can be expressed as a different glycoform with greater glycan deposition suggests that there are control mechanisms in place for GspB to maintain a particular glycosylation state. Furthermore, the significant reduction in strain M99 binding to sialyl-T antigen following aberrant glycosylation of GspB strongly suggests that maintenance of a particular GspB glycosylation state is physiologically relevant toward M99 binding to platelets. Indeed, excessive glycosylation could foreseeably lead to a conformational change of GspB or occlude its binding region, thereby preventing platelet adhesion. These findings support the concept that excessive glycosylation of an SRR protein affects its physiological function. Indeed, the ability to modulate glycosylation of GspB could serve as a means to regulate bacterial adhesin. Of note, Mistou et al. demonstrated that alterations in glycosylation of the SRR protein Srr1 of Streptococcus agalactiae resulted in impaired bacterial adhesion and reduced virulence (26). These findings further demonstrate the necessity of linking the processes of glycosylation and protein transport in the biogenesis of an effective virulence factor.
Finally, the findings presented here show that a specific region within Asp2 is required to support GspB export while also influencing its glycosylation state. Indeed, Asp2 appears to be a novel bifunctional protein, acting as a mediator of GspB transport while also modulating its glycosylation. Although we have previously shown that the processes of GspB transport and glycosylation are separable, our findings presented here suggest that there is a convergence of the two pathways and that Asp2 may be responsible for the successful coordination of these two activities. Moreover, since the maintenance of a correct glycoform appears to be essential for SRR protein function, it would be advantageous to link the two processes. The contributory roles of other accessory Sec members toward GspB glycosylation are now being explored.
ACKNOWLEDGMENTS
This study was supported by the U.S. Department of Veteran Affairs, the Northern California Institute for Research and Education, and grants R01 AI41513 and R01 AI057433 from the National Institutes of Health.
We thank Oren Rosenberg for help with the modeling of Asp2.
Footnotes
Published ahead of print 10 August 2012
REFERENCES
- 1. Amada K, Haruki M, Imanaka T, Morikawa M, Kanaya S. 2000. Overproduction in Escherichia coli, purification and characterization of a family I.3 lipase from Pseudomonas sp. MIS38. Biochim. Biophys. Acta 1478:201–210 [DOI] [PubMed] [Google Scholar]
- 2. Arpigny JL, Jaeger KE. 1999. Bacterial lipolytic enzymes: classification and properties. Biochem. J. 343(Pt 1):177–183 [PMC free article] [PubMed] [Google Scholar]
- 3. Bensing BA, Gibson BW, Sullam PM. 2004. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J. Bacteriol. 186:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bensing BA, Lopez JA, Sullam PM. 2004. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha. Infect. Immun. 72:6528–6537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bensing BA, Sullam PM. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081–1094 [DOI] [PubMed] [Google Scholar]
- 6. Bensing BA, Sullam PM. 2009. Characterization of Streptococcus gordonii SecA2 as a paralogue of SecA. J. Bacteriol. 191:3482–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bensing BA, Takamatsu D, Sullam PM. 2005. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 58:1468–1481 [DOI] [PubMed] [Google Scholar]
- 8. Bryan EM, Bae T, Kleerebezem M, Dunny GM. 2000. Improved vectors for nisin-controlled expression in Gram-positive bacteria. Plasmid 44:183–190 [DOI] [PubMed] [Google Scholar]
- 9. Bu S, et al. 2008. Interaction between two putative glycosyltransferases is required for glycosylation of a serine-rich streptococcal adhesin. J. Bacteriol. 190:1256–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damme M, et al. 2010. Impaired lysosomal trimming of N-linked oligosaccharides leads to hyperglycosylation of native lysosomal proteins in mice with alpha-mannosidosis. Mol. Cell. Biol. 30:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Graffenried CL, Bertozzi CR. 2004. The roles of enzyme localisation and complex formation in glycan assembly within the Golgi apparatus. Curr. Opin. Cell Biol. 16:356–363 [DOI] [PubMed] [Google Scholar]
- 12. Dempski RE, Jr, Imperiali B. 2002. Oligosaccharyl transferase: gatekeeper to the secretory pathway. Curr. Opin. Chem. Biol. 6:844–850 [DOI] [PubMed] [Google Scholar]
- 13. Reference deleted.
- 14. Ekici OD, Paetzel M, Dalbey RE. 2008. Unconventional serine proteases: variations on the catalytic Ser/His/Asp triad configuration. Protein Sci. 17:2023–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garbe J, Collin M. 2012. Bacterial hydrolysis of host glycoproteins—powerful protein modification and efficient nutrient acquisition. J. Innate Immun. 4:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gladden JM, et al. 2011. Glycoside hydrolase activities of thermophilic bacterial consortia adapted to switchgrass. Appl. Environ. Microbiol. 77:5804–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hebert DN, Foellmer B, Helenius A. 1995. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell 81:425–433 [DOI] [PubMed] [Google Scholar]
- 18. Hurtado-Guerrero R, Dorfmueller HC, van Aalten DM. 2008. Molecular mechanisms of O-GlcNAcylation. Curr. Opin. Struct. Biol. 18:551–557 [DOI] [PubMed] [Google Scholar]
- 19. Kelley LA, Sternberg MJ. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371 [DOI] [PubMed] [Google Scholar]
- 20. Kim DE, Chivian D, Baker D. 2004. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 32:W526–W531 doi:10.1093/nar/gkh468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larkin A, Imperiali B. 2011. The expanding horizons of asparagine-linked glycosylation. Biochemistry 50:4411–4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Letunic I, Doerks T, Bork P. 2009. SMART 6: recent updates and new developments. Nucleic Acids Res. 37:D229–D232 doi:10.1093/nar/gkn808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, et al. 2008. A conserved domain of previously unknown function in Gap1 mediates protein-protein interaction and is required for biogenesis of a serine-rich streptococcal adhesin. Mol. Microbiol. 70:1094–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lofling J, Vimberg V, Battig P, Henriques-Normark B. 2011. Cellular interactions by LPxTG-anchored pneumococcal adhesins and their streptococcal homologues. Cell. Microbiol. 13:186–197 [DOI] [PubMed] [Google Scholar]
- 25. Madoff LC, Michel JL, Gong EW, Kling DE, Kasper DL. 1996. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc. Natl. Acad. Sci. U. S. A. 93:4131–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mistou MY, Dramsi S, Brega S, Poyart C, Trieu-Cuot P. 2009. Molecular dissection of the secA2 locus of group B Streptococcus reveals that glycosylation of the Srr1 LPXTG protein is required for full virulence. J. Bacteriol. 191:4195–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moremen KW. 2002. Golgi alpha-mannosidase II deficiency in vertebrate systems: implications for asparagine-linked oligosaccharide processing in mammals. Biochim. Biophys. Acta 1573:225–235 [DOI] [PubMed] [Google Scholar]
- 28. Orihuela CJ. 2009. Role played by psrP-secY2A2 (accessory region 34) in the invasive disease potential of Streptococcus pneumoniae. J. Infect. Dis. 200:1180–1181 [DOI] [PubMed] [Google Scholar]
- 29. Peng Z, et al. 2008. Identification of critical residues in Gap3 of Streptococcus parasanguinis involved in Fap1 glycosylation, fimbrial formation and in vitro adhesion. BMC Microbiol. 8:52 doi:10.1186/1471-2180-8-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rigel NW, et al. 2009. The accessory SecA2 system of mycobacteria requires ATP binding and the canonical SecA1. J. Biol. Chem. 284:9927–9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ro HS, Hong HP, Kho BH, Kim S, Chung BH. 2004. Genome-wide cloning and characterization of microbial esterases. FEMS Microbiol. Lett. 233:97–105 [DOI] [PubMed] [Google Scholar]
- 32. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 33. Schultz J, Milpetz F, Bork P, Ponting CP. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. U. S. A. 95:5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seepersaud R, Bensing BA, Yen YT, Sullam PM. 2010. Asp3 mediates multiple protein-protein interactions within the accessory Sec system of Streptococcus gordonii. Mol. Microbiol. 78:490–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shivshankar P, Sanchez C, Rose LF, Orihuela CJ. 2009. The Streptococcus pneumoniae adhesin PsrP binds to keratin 10 on lung cells. Mol. Microbiol. 73:663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siboo IR, Chambers HF, Sullam PM. 2005. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect. Immun. 73:2273–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soding J, Biegert A, Lupas AN. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33:W244–W248 doi:10.1093/nar/gki408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sullam PM, Valone FH, Mills J. 1987. Mechanisms of platelet aggregation by viridans group streptococci. Infect. Immun. 55:1743–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takamatsu D, Bensing BA, Sullam PM. 2004. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J. Bacteriol. 186:7100–7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takamatsu D, Bensing BA, Sullam PM. 2004. Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol. Microbiol. 52:189–203 [DOI] [PubMed] [Google Scholar]
- 41. Takamatsu D, Bensing BA, Sullam PM. 2005. Two additional components of the accessory sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J. Bacteriol. 187:3878–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Sorge NM, et al. 2009. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J. Infect. Dis. 199:1479–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weadge JT, Clarke AJ. 2007. Neisseria gonorrhoeae O-acetylpeptidoglycan esterase, a serine esterase with a Ser-His-Asp catalytic triad. Biochemistry 46:4932–4941 [DOI] [PubMed] [Google Scholar]
- 44. Wu H, Bu S, Newell P, Chen Q, Fives-Taylor P. 2007. Two gene determinants are differentially involved in the biogenesis of Fap1 precursors in Streptococcus parasanguis. J. Bacteriol. 189:1390–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiong YQ, Bensing BA, Bayer AS, Chambers HF, Sullam PM. 2008. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb. Pathog. 45:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yen YT, Seepersaud R, Bensing BA, Sullam PM. 2011. Asp2 and Asp3 interact directly with GspB, the export substrate of the Streptococcus gordonii accessory Sec system. J. Bacteriol. 193:3165–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou M, Wu H. 2009. Glycosylation and biogenesis of a family of serine-rich bacterial adhesins. Microbiology 155:317–327 [DOI] [PubMed] [Google Scholar]
- 48. Zhou M, Zhu F, Dong S, Pritchard DG, Wu H. 2010. A novel glucosyltransferase is required for glycosylation of a serine-rich adhesin and biofilm formation by Streptococcus parasanguinis. J. Biol. Chem. 285:12140–12148 [DOI] [PMC free article] [PubMed] [Google Scholar]