Abstract
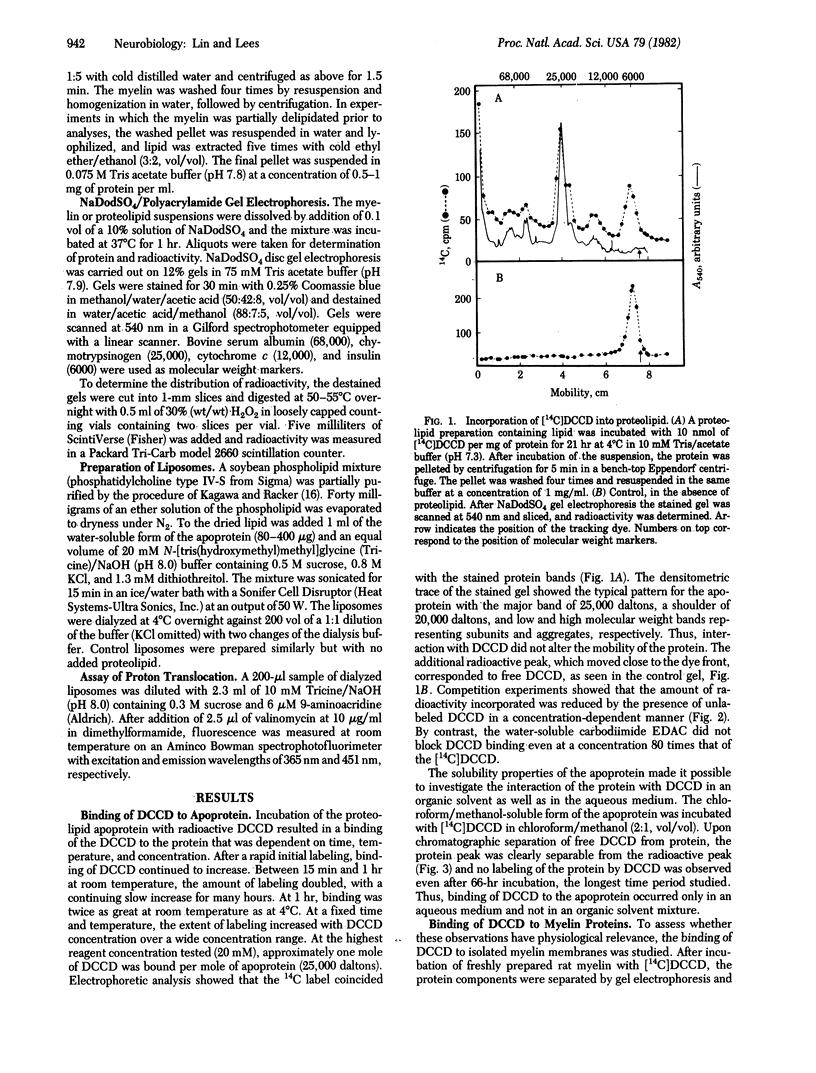
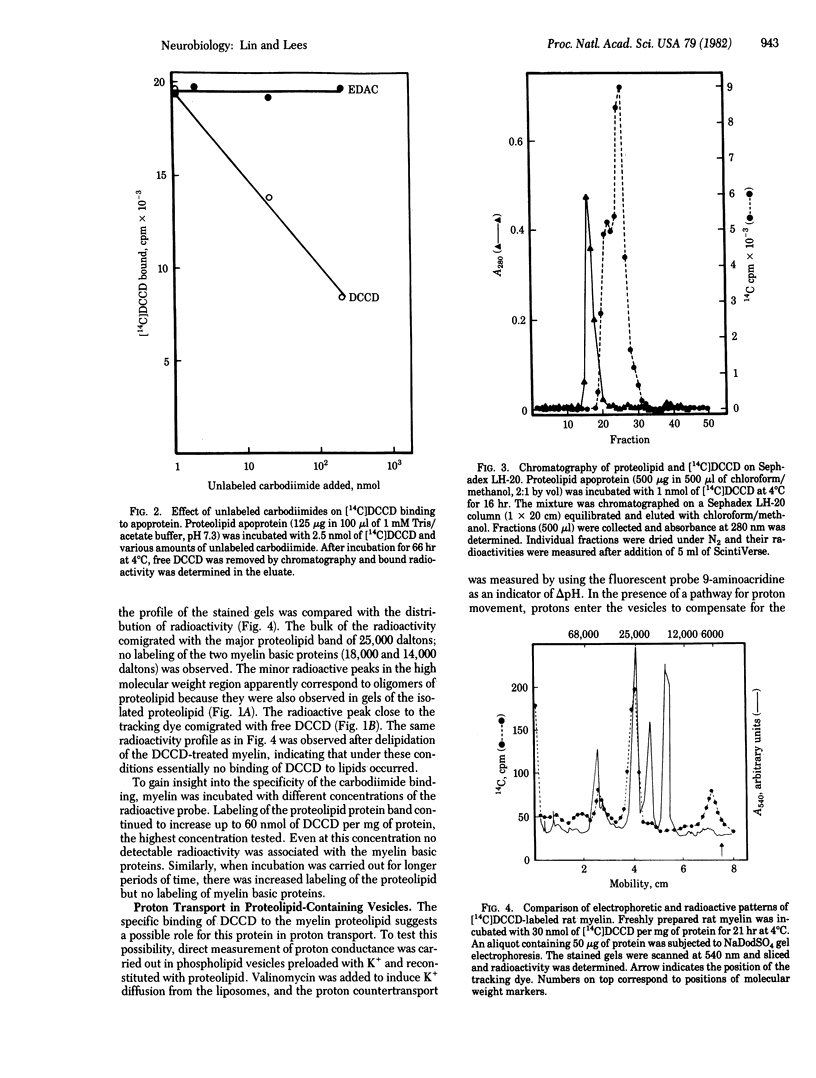
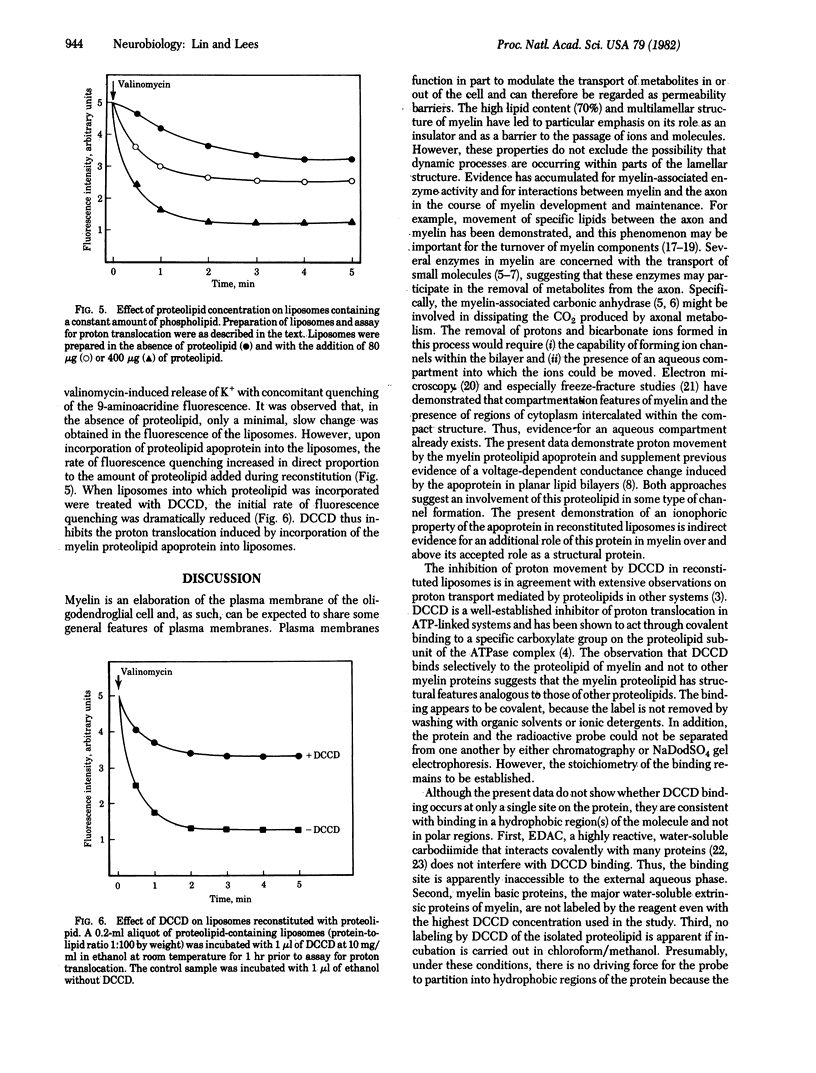
Dicyclohexylcarbodiimide (DCCD) is known to bind preferentially to a proteolipid subunit of proton-translocating systems and thereby to inhibit proton transport. In the present study we show that, in an aqueous medium, DCCD binds to the bovine white matter proteolipid apoprotein, the major protein of central nervous system myelin. The binding is dependent on time, temperature, and concentration and is not inhibited by the hydrophilic carbodiimide 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. By contrast, when the incubation is carried out in chloroform/methanol no labeling by DCCD is demonstrable. In isolated rat myelin, DCCD binds specifically to the proteolipid and not to the myelin basic proteins. Liposomes reconstituted with the myelin proteolipid apoprotein transport protons, as assayed by quenching of the fluorescence of 9-aminoacridine. Preincubation of proteolipid-containing liposomes with DCCD results in an inhibition of transport. These studies have important implications for a possible ionophoric function of the myelin proteolipid and for the occurrence of transport processes within myelin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cammer W., Fredman T., Rose A. L., Norton W. T. Brain carbonic anhydrase: activity in isolated myelin and the effect of hexachlorophene. J Neurochem. 1976 Jul;27(1):165–171. doi: 10.1111/j.1471-4159.1976.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., Koshland D. E., Jr Reaction of tyrosine residues in proteins with carbodiimide reagents. Biochim Biophys Acta. 1968 Jun 26;160(2):272–274. doi: 10.1016/0005-2795(68)90102-5. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., Triplett R. B. Reaction of carbodiimides with protein sulfhydryl groups. Biochim Biophys Acta. 1970 Mar 31;200(3):564–566. doi: 10.1016/0005-2795(70)90112-1. [DOI] [PubMed] [Google Scholar]
- Chan D. S., Lees M. B. Gel electrophoresis studies of bovine brain white matter proteolipid and myelin proteins. Biochemistry. 1974 Jun 18;13(13):2704–2712. doi: 10.1021/bi00710a008. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951 Aug;191(2):807–817. [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Forman D. S., Ledeen R. W. Axonal transport of gangliosides in the goldfish optic nerve. Science. 1972 Aug 18;177(4049):630–633. doi: 10.1126/science.177.4049.630. [DOI] [PubMed] [Google Scholar]
- Gould R. M. Inositol lipid synthesis localized in axons unmyelinated fibers of peripheral nerve. Brain Res. 1976 Nov 19;117(1):168–174. doi: 10.1016/0006-8993(76)90569-2. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Miller J. A., Ledeen R. W., Haley J., Specht S. C. Axonal transport of phospholipid in goldfish optic system. Exp Neurol. 1975 Feb;46(2):261–281. doi: 10.1016/0014-4886(75)90134-x. [DOI] [PubMed] [Google Scholar]
- Hirano A., Dembitzer H. M. A structural analysis of the myelin sheath in the central nervous system. J Cell Biol. 1967 Aug;34(2):555–567. doi: 10.1083/jcb.34.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lees M. B., Paxman S. Modification of the Lowry procedure for the analysis of proteolipid protein. Anal Biochem. 1972 May;47(1):184–192. doi: 10.1016/0003-2697(72)90291-6. [DOI] [PubMed] [Google Scholar]
- Lees M. B., Sakura J. D., Sapirstein V. S., Curatolo W. Structure and function of proteolipids in myelin and non-myelin membranes. Biochim Biophys Acta. 1979 Aug 20;559(2-3):209–230. doi: 10.1016/0304-4157(79)90002-9. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973 Oct;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Reiss D. S., Lees M. B., Sapirstein V. S. Is Na + ATPase a myelin-associated enzyme? J Neurochem. 1981 Apr;36(4):1418–1426. doi: 10.1111/j.1471-4159.1981.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Sapirstein V. S., Lees M. B. Purification of myelin carbonic anhydrase. J Neurochem. 1978 Aug;31(2):505–511. doi: 10.1111/j.1471-4159.1978.tb02665.x. [DOI] [PubMed] [Google Scholar]
- Sebald W., Machleidt W., Wachter E. N,N'-dicyclohexylcarbodiimide binds specifically to a single glutamyl residue of the proteolipid subunit of the mitochondrial adenosinetriphosphatases from Neurospora crassa and Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Feb;77(2):785–789. doi: 10.1073/pnas.77.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman G., Folch-Pi J. Rotatory dispersion and circular dichroism of brain "proteolipid" protein. J Neurochem. 1970 May;17(5):597–605. doi: 10.1111/j.1471-4159.1970.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Ting-Beall H. P., Lees M. B., Robertson J. D. Interactions of Folch-Lees proteolipid apoprotein with planar lipid bilayers. J Membr Biol. 1979 Dec 12;51(1):33–46. doi: 10.1007/BF01869342. [DOI] [PubMed] [Google Scholar]


