Abstract
Previous studies have shown that dietary copper supplementation reversed heart hypertrophy induced by pressure overload in a mouse model. The present study was undertaken to understand the cellular basis of copper-induced regression of cardiac hypertrophy. Primary cultures of neonatal rat cardiomyocytes were treated with phenylephrine at a final concentration of 100 μM in cultures for 48 h to induce cellular hypertrophy. The hypertrophied cardiomyocytes were exposed to copper sulfate at a final concentration of 5 μM in cultures for additional 24 h. This copper treatment reduced the size of the hypertrophied cardiomyocytes, as measured by flow cytometry, protein content in cells, cell volume and cardiomyocyte hypertrophy markers including beta myosin heavy chain protein, skeletal alpha-actin, and atrial natriuretic peptide. Cell cycle analysis and cell sorting of p-histone-3 labeled cardiomyocytes indicated that cell division was not involved in the copper-induced regression of cardiomyocyte hypertrophy. Copper also inhibited PE-induced apoptosis, determined by a TUNEL assay. Because copper stimulates vascular endothelial growth factor (VEGF) production through activation of hypoxia-inducible transcription factor, an anti-VEGF antibody at a final concentration of 2 ng/ml in cultures was used and shown to blunt copper-induced regression of cell hypertrophy. Conversely, VEGF alone at a final concentration of 0.2 μg/ml reversed cell hypertrophy as the same as copper did. This study demonstrates that both copper and VEGF reduce the size of hypertrophied cardiomyocytes, and copper regression of cardiac hypertrophy is VEGF dependent.
Keywords: Copper, VEGF, cardiomyocytes, cell size, hypertrophy, primary cultures
Introduction
Previous studies have shown that dietary supplementation with physiologically relevant levels of copper (Cu) reversed pressure overload-induced cardiac hypertrophy in a mouse model along with a complete recovery of the contractile function of the heart [1]. Although the regression of cardiac hypertrophy by dietary copper supplementation in humans is unknown, a recent study in a small population of chronic heart failure patients has shown that dietary supplementation with micronutrients for 9 months increased left ventricle ejection, decreased left ventricle volume, and improved the quality of life [2]. Among the formulated micronutrients was Cu (1.2 mg/day), which has been considered to play an important role in the improvement of cardiac function [3].
Cardiac hypertrophy is coordinated with angiogenesis during the cardiac growth, and disruption of this coordination leads to cardiac dysfunction and heart failure [4,5]. Vascular endothelial growth factor (VEGF) is a critical growth factor for the regulation of angiogenesis and promotes blood vessel formation in the recovery of tissue injury [6,7]. During the transition from cardiac hypertrophy to heart failure, VEGF levels in the heart are suppressed [1,4]. Interestingly, the regression of cardiac hypertrophy induced by dietary Cu supplementation is VEGF-dependent; systemic administration of anti-VEGF antibody completely blocks Cu supplementation-induced regression of heart hypertrophy [1]. Because VEGF promotes blood vessel formation and the anti-VEGF antibody blocks the angiogenesis, the regression of heart hypertrophy was considered angiogenesis-dependent.
Although angiogenesis plays an important role in the regression of heart hypertrophy, the direct effect of Cu through VEGF on cardiomyocytes would make a critical contribution to the regression because cardiomyocytes contain VEGF receptors. To distinguish the contribution of the direct effect of Cu on cardiomyocytes to the regression of cardiac hypertrophy from the angiogenesis-dependent effect, the primary cultures of cardiomyocytes would provide a valuable model; the effect of blood vessel formation on the regression of cardiomyocyte hypertrophy can be excluded. Therefore, in the present study we used primary cultures of neonatal rat cardiomyocytes to examine the direct effect of Cu on the regression of cardiomyocyte hypertrophy produced by phenylepherine (PE) stimulation.
At the cellular level, cardiac hypertrophy involves the increases in the cell volume of individual cardiomyocytes. The regression of cardiac hypertrophy would thus involve the reduction in the size of the hypertrophied cardiomyocytes. However, the cellular basis of the regression of heart hypertrophy has not been determined. This also can be addressed using the primary cultures of neonatal rat cardiomyocytes. Through a thorough analysis of cell cycle and mitotic activities of the cardiomyocytes in cultures under different conditions in combination with the analysis of cell size, the contribution of the reduction in the size of individual hypertrophied cardiomyocytes to the regression of heart hypertrophy can be determined. Therefore, the present study has two purposes: (1) to determine the direct effect of Cu on the regression of cardiac hypertrophy and (2) to define the cellular basis of regression of cardiac hypertrophy. The results obtained demonstrated that Cu causes a direct reduction in the size of hypertrophied cardiomyocytes and this effect is VEGF dependent.
Materials and Methods
Cell culture
Primary cultures of neonatal rat cardiomyocytes were established according to a procedure published previously [8], with some modifications. Briefly, the hearts of 1-3 day-old Sprague-Dawley rats were minced and incubated overnight at 4 °C with trypsin in Hanks’ balanced salt solution (HBSS, Life Technologies Ltd, Carlsbad, CA). The tissue was then digested with collagenase (Worthington Biochemical Corporation, Lakewood, NJ) in L-15 media (Worthington Biochemical Corporation) at 37 °C for 70 min. The digests were resuspended in DMEM/Ham’s nutrient mixture F12 (1:1, vol/vol, Life Technologies Ltd) supplemented with antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin, MP Biomedicals, LLC, Solon, OH), 10% fetal bovine serum (FBS, Life Technologies Ltd) and 0.1 mM 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich, St. Louis, MO). The fibroblasts were removed by pre-incubation for 2 h at 37 °C. The cardiomyocytes in the suspension were collected and seeded in gelatin-coated plates containing DMEM/F12 media supplemented with FBS, BrdU and antibiotics for 24 h before subsequent experiments.
Experimental procedure
Cell hypertrophy was induced by phenylephrine (PE, Sigma-Aldrich) at a final concentration of 100 μM for 48 h in serum-free media, following a procedure published previously [9]. At the end of the 48 h incubation, Cu in the form of copper sulfate was added directly to the cultures at a final concentration of 5 μM Cu element for additional 24 h incubation. At the end of Cu treatment or 72 h culturing, the cells were collected by trysinization and suspended in phosphate buffered saline (PBS) buffer, and counted using a hemocytometer. Protein content was measured using a Bradford method (Bio-Rad, Hercules, CA), and normalized by the cell number. For the flow cytometry analysis of cell size and cell proliferation, the harvested cells were subjected to fixation and incubation with different markers before analysis, as described below. To assess the role of VEGF in Cu regression of cell hypertrophy, we used monoclonal anti-VEGF antibody (R & D Systems, Inc., Minneapolis, MN) at a final concentration of 2 ng/ml in cultures. The antibody was added to the cultures 1 h prior to the addition of Cu in the cultures. The effect of VEGF was examined by adding recombinant rat VEGF164 (R & D Systems) at 0.2 μg/ml to substitute for Cu in the media.
The purity of cardiomyocytes in cultures was more than 95%, which was determined at the end of each experiment (48 h PE treatment and 24 h Cu treatment) using a flow cytometer. Briefly, cells were trypsinized, washed with PBS, and fixed in 75% ethanol at 4 °C for at least 18 h. Primary anti-α-sarcomeric actin antibody (Sigma-Aldrich) was diluted in PBS and incubated at room temperature for 1 h. Secondary fluorescein-conjugated goat anti-mouse IgM antibody (FITC, Southern Biotech, Temecula, CA) was used for immunofluorescence and incubated for 30 min at room temperature. The fluorescence distribution from 30,000 cells was captured and recorded using a CellQuest Acquisition program (Becton & Dickinson, Franklin Lakes, NJ). Data were analyzed using a FlowJo software (Tree Star, Inc., Ashland, OR), and the percentage of the α-sarcomeric actin labeled cardiomyocytes in the population was calculated.
Cell size determination
The cell volume was determined using a microslide field finder (Fisher Scientific, Pittsburgh, PA) following the procedure described previously [10]. Briefly, cardiomyocytes in cultures were trypsinized, suspended in PBS, and loaded onto the microslide field finder. The diameters of approximately 150 cells from each group were assessed and recorded. The cell volume was calculated using the equation for the volume of a sphere: V = (4/3) πr3, where V = cell volume, π= 3.14, and r = radius. In addition, the cell size was further determined by a flow cytometer as described previously [11]. Briefly, the cells were trypsinized, suspended in PBS, and directly passed through the flow cytometer. For each sample, 30,000 cells were recorded and analyzed through the forward scatter light (FSC), which is proportional to the cell size. The data were quantitatively analyzed using the FlowJo software.
Immunocytochemistry
To examine the morphological changes of cardiomyocytes in cultures after the treatment with PE and Cu, cells were cultured in an 8-well chamber slide and treated as described in the experimental procedure. After Cu treatment for 24 h, cells were fixed in 4% paraformaldehyde in PBS. After fixation, the culture chamber was incubated with monoclonal anti-α-sarcomeric-actin antibody (Sigma-Aldrich) followed by incubation with FITC-conjugated goat anti-mouse IgM [12]. Fluorescence was visualized by a phase contrast microscope (Nikon Instruments, Melville, NY). Images were acquired by a Nikon digital camera DXM 1200 (Nikon Instruments) using a Nikon ACT-1 software.
Cell cycle analysis
To analyze the distribution of cardiomyocytes in cell cycle under different treatment conditions, cells were trypsinized, washed with PBS, and fixed in 75% ethanol at 4 °C for at least 18 h. The cardiomyocytes were then incubated for 30 min in DNA staining buffer, which contains 200 μg/ml ribonuclease A (Sigma-Aldrich) and 5 μg/mL propidium iodide (PI, Sigma-Aldrich). The PI stained cells were analyzed using a flow cytometer. For each sample, 30,000 cells were captured and cells in different phases of cell cycle are expressed as a percentage of the total number of cells counted.
Assessment of mitosis
Determination of mitotic activities of cardiomyocytes under different treatments was done as described previously [13], with some modifications. Briefly, the cells were trypsinized, washed with PBS, and fixed in 75% ethanol at 4 °C for at least 18 h. Immunofluorescence staining with an Alexa Fluor 488 conjugated antibody to phosphorylated Histone-3 (Ser10, 1:10, Cell Signaling Technology, Inc., Danvers, MA), which is a mitosis-specific marker, was performed on the fixed cardiomyocytes. DNA staining buffer was added 30 min prior to detection using a flow cytometer. Mitotic index was determined by using the FlowJo software based on the data collection from 30, 000 cells per sample. The data were plotted in two-dimensional PI versus Alexa Fluor 488-p-Histone-3 (p-H3) fluorescence, and the percentage of the p-H3 stained cells in the cell population counted was calculated.
Real-time quantitative RT-PCR
Total RNA was extracted from cardiomyocytes collected after 72 h culturing (48 h PE + 24 h Cu) using TRIzol reagent (Invitrogen, Carlsbad, California) according to the manufacturer’s instruction. RNA concentration was quantified with a Beckman Coulter DU 650 spectrophotometer (Pegasus Scientific, Frederick, MD). Complementary DNAs (cDNAs) were synthesized using a GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA) in PerkinElmer DNA Thermal Cycler 480. The amount of cDNA corresponding to 100 ng of RNA was amplified using a SYBR green PCR kit (Applied Biosystems) with the primer pairs of rat beta-myosin heavy chain (β-MHC), skeletal alpha-actin (α-SA), atrial natriuretic peptide (ANP), or GAPDH respectively. The primer sequences were designed as reported previously [14]. Real-time PCR reactions were performed, recorded, and analyzed by a 7500 Real Time PCR System (Applied Biosystems). Samples were amplified for 1 cycle at 50 °C for 2 min and 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and 60°C for 1 min. Results were calculated from the cycle threshold (Ct) value using the ΔΔCt method for quantification [15]. The expression of interested genes was normalized by GAPDH in each sample. Untreated group was set as control, a calibrator sample. Take β-MHC as an example, ΔΔCt=(Ct β-MHC-sample-CtGAPDH-sample)-(Ct β-MHC-control-CtGAPDH-control). The relative expression of the sample was then calculated using the formula 2−ΔΔCt. Thus, all the data were expressed as the fold-change of the control group that was 1. All the samples were measured in triplicate.
TUNEL (TdT-mediated dUTP nick-end labeling) Assay
Cardiomyocyte apoptosis was quantified by using an ApopTag apoptosis detection kit (Chemicon International, Inc., Temecula, CA) according to the manufacturer’s instruction with some modifications. Briefly, the cells were cultured and treated with PE for 48 h and Cu for additional 24 h in a Lab-Tek II chamber glass slide. At the end of 72 h culturing, the cells were fixed with 1% paraformaldehyde and permeabilized by 0.3% Triton X-100 in PBS. Then the culture slides were incubated with the reaction mixture containing TdT and digoxigenin-conjugated dUTP for 1 h at 37 °C, followed by an incubation with anti-digoxigenin peroxidase conjugate antibody, using 3,3′-diaminobenzidene as the chromagen. As a positive control, cells were treated with DNase 1 to introduce nicks in genomic DNA. For negative control, TdT enzyme was removed from the reaction mixture. The apoptotic index was calculated as the percentage of the TUNEL-positive cells in the total cell population by counting cells exhibiting brown nuclei among randomly selected 1000-1200 nuclei in each slide chamber and the data were obtained from triplicate chambers for each experiment.
Statistical analysis
Data were obtained from three separate experiments and are expressed as means ± S.E.M. A 2 × 2 factorial design was applied to all of the experiments with an exception of the experiment presented in Fig 7, which was a 2 × 2 × 2 factorial design, and the data were analyzed according to the experimental design. After a significant interaction was detected by the analysis of variance (ANOVA) based on the factorial design, the significance of the main effects was further determined. The level of significance was considered when P < 0.05.
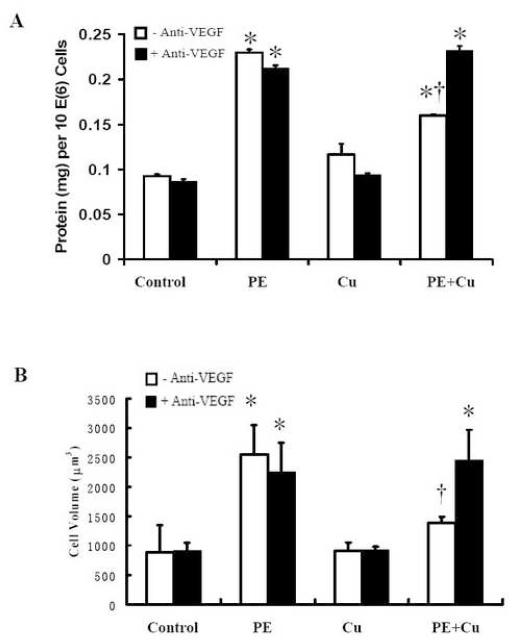
Figure 7.
The antagonistic effect of anti-VEGF antibody on Cu-induced reversal of cardiomyocyte hypertrophy. The cultures were treated with the anti-VEGF antibody at the final concentration of 2 ng/ml in the culture 30 min prior to the addition of CuSO4. A. Cellular Protein content. B. Cell volume. Each group of data were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. Values are means ± S.E.M. *, significantly different from control group and †, significantly different from PE-treated group (p < 0.05).
Results
Copper reversed PE-induced cell hypertrophy in primary cultures of neonatal rat cardiomyocytes
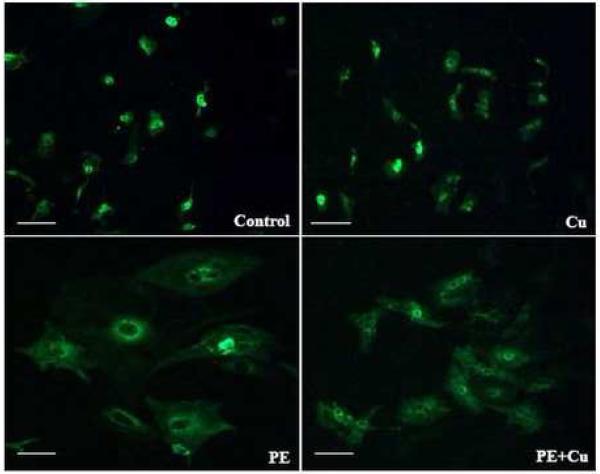
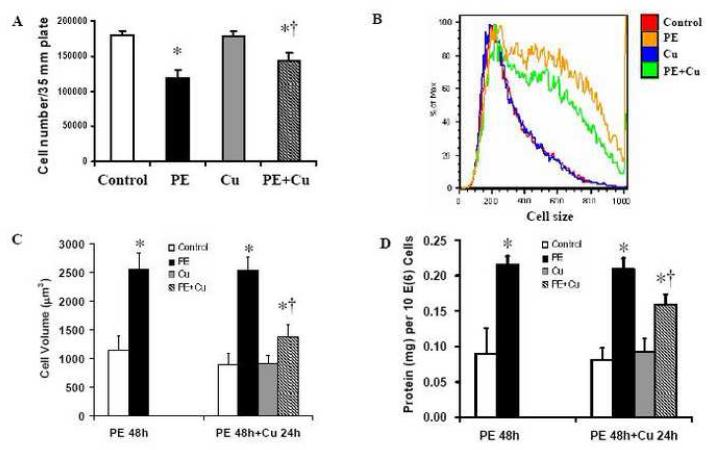
Cardiomyocyte hypertrophy was induced by PE, an α-adrenergic receptor agonist, at a final concentration of 100 μM in cultures for 48 h in serum-free media. These cells were then treated with Cu by a direct addition of CuSO4 into the cultures at a final concentration of 5 μM Cu element for additional 24 h. Morphological analysis showed a reduction in the size of the PE-induced hypertrophied cardiomyocytes, but not in the non-PE treated cardiomyocytes after Cu treatment (Fig 1). The cell number in the PE-treated cultures was less than that in the non-PE-treated controls. The cell number in Cu treated hypertrophied cardiomyocytes were more than that of PE-treated cultures (Fig 2A), suggesting that Cu might protect the cardiomyocytes from apoptosis or necrosis, or stimulate proliferation of the hypertrophied cardiomyocytes. The results presented in Fig 2B show that the cell size was significantly reduced by Cu treatment in the PE-induced hypertrophied cardiomyocytes measured by flow cytometry, which is consistent with the result obtained from a direct measurement of the cell volume by a microslide field finder (Fig 2C). In addition, Cu treatment significantly reduced the total protein content in the PE-pretreated cardiomyocytes (Fig 2D), further confirming Cu-induced regression of the PE-induced cardiomyocyte hypertrophy. However, Cu did not change the phenotype of non-PE-treated control cells, as examined by all of the parameters described above.
Figure 1.
Representative fluorescence microscopic images of cellular morphology by staining the cells with anti-α-sarcomeric actin antibody in primary cultures of neonatal rat cardiomyocytes. Cellular hypertrophy was induced by 100 μM PE for 48 h in serum-free media, and then the cultures were exposed to 5 μM CuSO4 for additional 24 h in the presence of PE. After fixation, cells were incubated with the anti-α-sarcomeric actin antibody followed by an incubation with fluorescein-conjugated goat anti-mouse IgM. 200X.
Figure 2.
PE-induced cell hypertrophy and its regression by Cu in primary cultures of neonatal rat cardiomyocytes. The induction of cellular hypertrophy and the treatment with Cu were as the same as that described for Figure 1. A. Cell number was counted using a hemocytometer after the cells were trypsinized and suspended in PBS at the end of Cu treatment. B. Analysis of cell size by flow cytometer. C. Analysis of cell volume after the Cu treatment for 24 h. Cell volume was calculated from cell diameter, which was determined by a microslide field finder. Approximately 150 cells from each group were randomly measured by the microslide field finder. D. The protein content in the cells measured by a Bradford method and normalized by cell number. Control (non-treated and incubated for 72 h), PE (PE treated for 48 + 24 h), Cu (Cu treatment during the last 24 h), and PE+Cu (PE treated for 48 h followed by Cu for 24 h). Each group of data were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. Values are means ± S.E.M. *, significantly different from control group and †, significantly different from PE-treated group (p < 0.05).
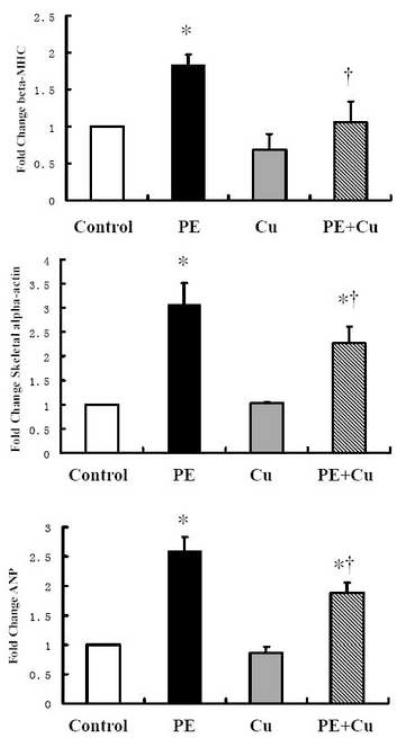
To further confirm the effect of Cu on PE-induced cardiomyocyte hypertrophy, changes in the markers of cardiomyocyte hypertrophy, including β-MHC, α-SA and ANP, were determined by a real-time RT-PCR analysis. The data presented in Fig 3 show that the increases in these marker proteins expression induced by PE treatment were significantly suppressed by Cu treatment, agreeing with the data obtained from morphological and protein content analysis.
Figure 3.
PE-induced changes in cellular hypertrophic markers and the effect of Cu addition in primary cultures of neonatal rat cardiomyocytes. The experimental condition was as the same as described above (Figure 2), and the experimental procedure the data acquisition and analysis were described in the Materials and Methods. Values are means ± S.E.M. *, significantly different from control group and †, significantly different from PE-treated group (p < 0.05).
Cu inhibition of PE-induced apoptosis
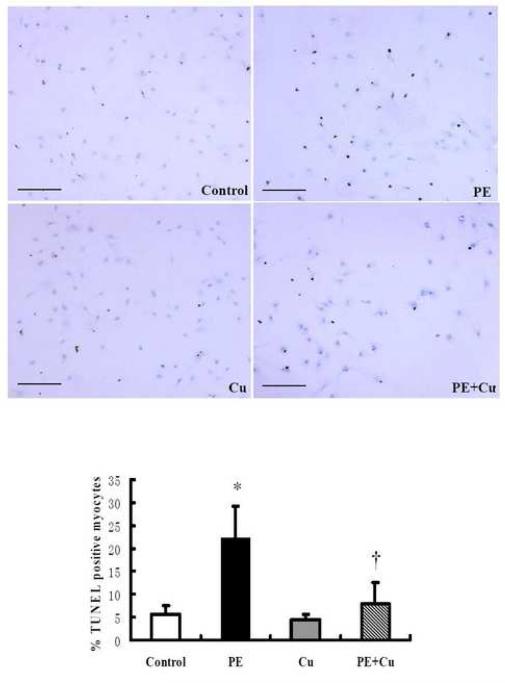
The determine whether or not the reduced number of cardiomyocytes in the PE treated cultures was due to apoptosis and the effect of Cu treatment on apoptosis, a TUNEL assay was performed to quantitatively analyze the apoptotic cells in cultures under different conditions. The data presented in Fig 4 show that PE caused a significant increase in the number of TUNEL positive cardiomyocytes and Cu treatment significantly inhibited the effect of PE treatment.
Figure 4.
PE-induced increase in TUNEL-positive cells (brown nuclei) and the effect of Cu addition in primary cultures of neonatal rat cardiomyocytes. Cells ere cultured in a Lab-Tek II chamber glass slide, following the same treatments described for Figure 2. The experimental procedure the data acquisition and analysis were described in the Materials and Methods. The image scale bar = Values are means ± S.E.M. *, significantly different from control group and †, significantly different from PE-treated group (p < 0.05).
Cu regression of cardiomyocyte hypertrophy did not involve promotion of cell proliferation
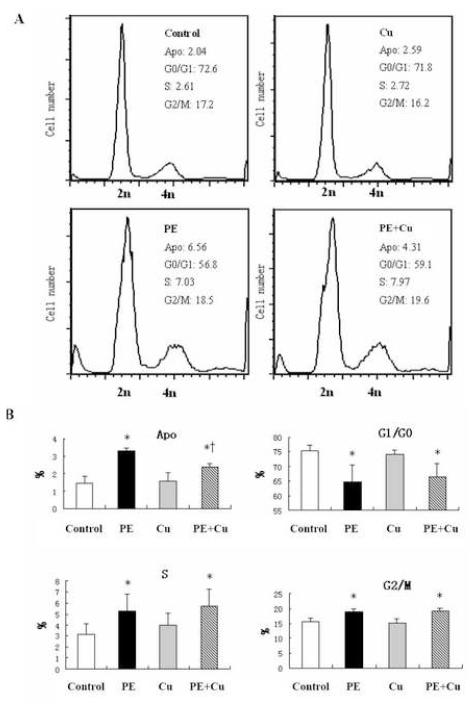
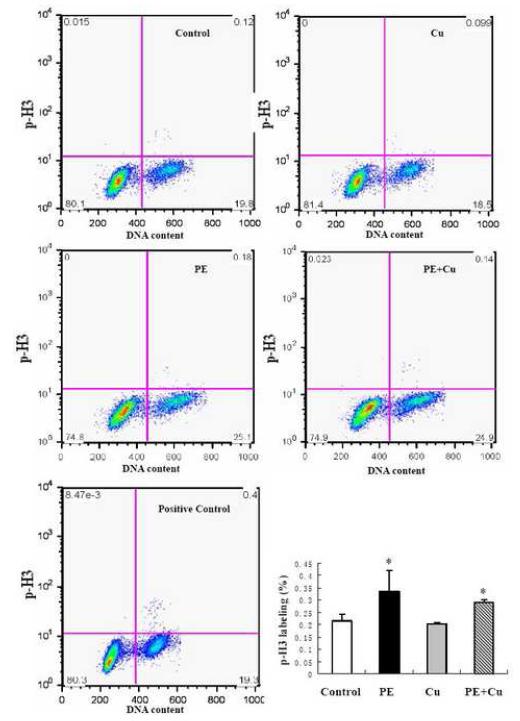
To determine whether or not Cu stimulates cardiomyocyte proliferation, we measured the cell cycle distribution of the cardiomyocytes in cultures under different conditions. FACS analysis of cells stained with PI was performed. The results presented in Fig 5 show that PE treatment increased the number of cells in the population with diminished DNA content, which is considered as the apoptotic cell population, and this increase was reduced by Cu treatment. Conversely, PE reduced the number of cells in the G0/G1 phase correspondingly and Cu significantly inhibited this reduction. PE also increased the percentage of cells in the S and G2/M phases, and Cu did not alter the effect of PE (Fig 5). To assess the mitotic activity of cardiomyocytes, an Alex Fluor 488-labled p-H3 antibody was incubated with the fixed cells and PI was added before FACS analysis. The plot of DNA content (PI-staining) versus p-H3 labeling and the quantitative analysis of the p-H3 labeled cells showed that although PE increased the number of p-H3-labeled cells, Cu did not change the effect of PE (Fig 6), thus excluding the contribution of cell division to Cu-induced regression of cardiomyocyte hypertrophy.
Figure 5.
Flow cytometry analysis of cell cycle. At the end of Cu treatment as described in Figure 1, the cells were collected, fixed, and incubated with PI as described in the Materials and Methods. The cells were then subjected to flow cytometry analysis and 30,000 cells in each sample were counted. A. Representative histograms demonstrating the distribution of cells in cell cycle under different experimental conditions. B. Quantitative analysis of the distribution of cells in cell cycle (% of cells in each phase relative to the total population). Apo: Cell population with diminished DNA content, referred to as apoptotic population; G1/G0: Cells in quiescent or early G1 phase, as defined with 2n DNA content; S: Cells in DNA synthesis phase, 4n DNA content; G2/M: G2 and Mitotic population. The labels for the treatment conditions are the same as described in Figure 2. Each group of data were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. Values are means ± S.E.M. *, significantly different from control group and †, significantly different from PE-treated group (p < 0.05).
Figure 6.
Flow cytometry analysis of cell mitotic activity. At the end of Cu treatment as described in Figure 1, the cells were collected, fixed, and incubated with polyclonal p-H3 (Ser10) antibody (Alexa Fluor 488 conjugate) and with PI as described in the Materials and Methods. The cells were then subjected to flow cytometry analysis and 30,000 cells in each sample were counted. Representative histograms demonstrating the percentage of p-H3 stained cells under different experimental conditions. DNA content was assessed by PI staining. Quantitative analysis of the p-H3 stained cells among the total population was shown in the bar graph. The labels for the treatment groups are the same as described in Figure 2. Data were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. Values are means ± S.E.M. *, significantly different from control group (p < 0.05).
Cu-induced reduction in cell size was VEGF-dependent
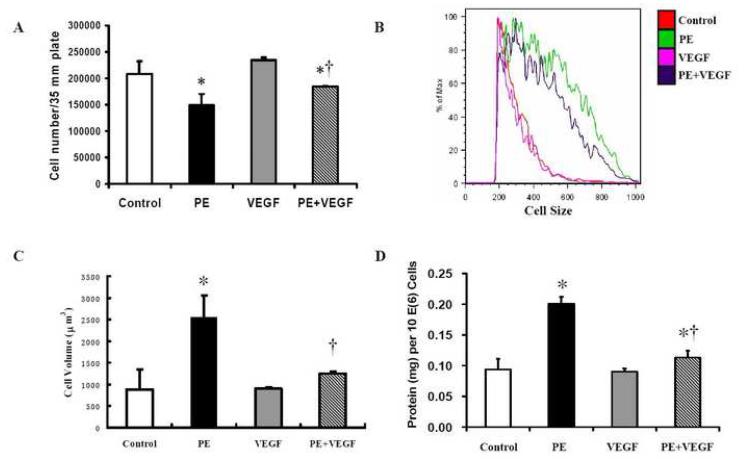
Previous studies in vivo have shown that dietary Cu supplementation-induced regression of hypertrophic cardiomyopathy was partially mediated by VEGF-dependent angiogenesis [1]. To determine the role of VEGF in the direct effect of Cu on cardiomyocyte hypertrophy, an anti-VEGF antibody at a final concentration of 2 ng/ml was added before the addition of 5 μM Cu element in the PE-treated cultures. The results presented in Fig 7 show that the addition of the anti-VEGF-antibody completely blocked the regression of cardiomyocyte hypertrophy by Cu treatment. To further define the involvement of VEGF in the regression of cell hypertrophy, VEGF alone at a final concentration of 0.2 μg/ml was added to the cultures of hypertrophied cardiomyocytes to replace Cu. The results showed that VEGF mimicked the effect of Cu and reduced the hypertrophied cell size, cell volume, and the total cellular protein content as the same as 5 μM Cu (Fig. 8). The same VEGF treatment did not alter the cell size, volume, or protein content of non-PE-treated cardiomyocytes. Therefore, the Cu-induced reduction in cardiomyocyte hypertrophy was VEGF-dependent.
Figure 8.
VEGF-induced regression of cardiomyocyte hypertrophy. VEGF at a final concentration of 0.2 μg/ml was added into the cultures after the treatment with PE for 48 h. The VEGF treatment lasted for 24 h before the cells were collected for analysis. A. Cell number counted using a hemocytometer. B. Cell size analyzed by flow cytometer. C. Cell volume. D. Cellular protein content. The experimental condition and procedure, and the label for each treatment were as the same as described in Figure 2. Each group of data were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. Values are means ± S.E.M. *, significantly different from control group and †, significantly different from PE-treated group (p < 0.05).
Discussion
The results presented here demonstrated that Cu addition causes regression of cellular hypertrophy of neonatal rat cardiomyocytes in primary cultures. The level of Cu added in the cultures is relevant to that supplemented in diet, which causes regression of pressure-overload hypertrophic cardiomyopathy in a mouse model [1]. This in vitro study thus recapitulates in vivo observation. However, there are fundamental differences between the in vitro and the in vivo models. The reduction in the size of hypertrophied cardiomyocytes was directly observed in the in vitro model. One of the concerns in the in vivo model is that the regression of heart hypertrophy may involve division of the hypertrophied cardiomyocytes, however, the present study demonstrated that Cu treatment does not cause proliferation of the hypertrophied cardiomyocytes. Therefore, the reduction in the size of hypertrophied cardiomyocytes would make a significant contribution to the regression of heart hypertrophy, although this may not exclude the possibility of cell division of hypertrophied cardiomyocytes in vivo. Another difference between the in vitro and the in vivo models is that the role of angiogenesis in reduction in the size of hypertrophied cardiomyocytes can be excluded in the in vitro model. Thus, the results here demonstrated that Cu induced regression of cardiomyocyte hypertrophy is VEGF-dependent but angiogenesis-independent.
We have recently reported that VEGF production in the heart was decreased in the chronic pressure overload mouse model and Cu supplementation increased myocardial VEGF production through activation of hypoxia-inducible factor 1 transcription factor. Consequently, systemic administration of anti-VEGF antibody blunted Cu supplementation-induced regression of hypertrophic cardiomyopathy [1]. One of the effects of the anti-VEGF antibody is the suppression of Cu-induced angiogenesis, to which VEGF is the crucial regulator. It has been shown that disruption of coordinated heart hypertrophy and angiogenesis contributes to the transition to heart failure [4,16,17]. It is well known that the stimulation of cell growth and proliferation is the major, if not exclusive, action of VEGF. However, the result that Cu supplementation-induced regression of heart hypertrophy is VEGF-dependent challenges this general belief. It is possible that VEGF functions in reduction in the size of hypertrophied cardiomyocytes to maintain their physiological function. If proven, this will be a newly identified biological function of VEGF and will bring up a new perspective of VEGF biology and medicine.
There are two critical questions that need to be addressed before a mechanistic approach to the function of VEGF in reducing the size of hypertrophied cardiomyocytes should take place. First, can hypertrophied cardiomyocytes reduce their size in response to an appropriate stimulation? Second, is this reduction in the size of hypertrophied cardiomyocytes directly related to VEGF? The results obtained here unambiguously addressed these two questions.
We took advantage of the primary cultures of neonatal rat cardiomyocytes to address these two questions simultaneously. Because we directly treated the cardiomyocytes in cultures, the influence of angiogenesis or blood vessels was excluded. The reduction in the size of the hypertrophied cardiomyocytes could be easily observed and analyzed with high purity of cardiomyocytes in cultures. There are other types of cells in cultures, particularly fibroblast cells that may confound the experimental results. To eliminate the potential influence of these cells, we not only used a differentially culturing procedure to remove fibroblast cells before culturing cardiomyocytes, but also used BrdU to inhibit the proliferation of the remaining fibroblasts in cultures, which has been reported previously [18]. To further ensure the high purity of cardiomyocytes in cultures, we used an anti-α-sarcomeric actin antibody to specifically label cardiomyocytes. At the end of each experiment, we used a flow cytometry cell sorting procedure to estimate the proportion of cardiomyocytes in the population of each culture. We have found that more than 95% were cardiomyocytes in the cultures, so that the influence of fibroblasts and other cell types was minimized. Therefore, the effect of Cu addition on the regression of cell hypertrophy was addressed in cardiomyocytes.
The hypertrophied cardiomyocytes were exposed to 5 μM CuSO4 (the final concentration in the cultures) to examine the effect of Cu on regression of cardiomyocyte hypertrophy. In addition, the DMEM/F12 media contain 1.3 μg/L of Cu, which would add 0.02 μM Cu to the final concentration in the cultures. The level of Cu selected in this study was derived from the calculation of in vivo Cu supplementation used in our previous studies (20 ppm Cu in diet). This is the level of DRI for Cu (0.9 mg/day) plus Cu supplementation (2 mg/day) for humans. It should note that many studies in cultured cells use high levels of Cu, ranging from 100 to 500 μM CuCl2 or CuSO4 [19-21].
The data obtained here demonstrated that the hypertrophied cardiomyocytes indeed reduced their size under an appropriate stimulation. For the cell size analysis, we used three different parameters; a direct measurement and calculation of the cell volume from cardiomyocytes collected from cultures through trypsinization, flow cytometry analysis of the cell size, and the measurement of cellular protein content. These measurements provided a consistent assessment of the reduction in the size of the PE-treated cardiomyocytes in cultures treated with relevant level of Cu. We also observed that the same Cu treatment did not change the size of cardiomyocytes without pre-treatment with PE in cultures.
The reduction in the size of cardiomyocytes in the Cu-treated cultures might result from a division of the hypertrophied cardiomyocytes, the daughter cells becoming smaller. We did observe that in the Cu treated cultures had a high number of cells than in the PE-treated only cultures. But the TUNEL assay and the cell cycle analysis indicated that the higher number of cells in the Cu-treated cultures mostly likely resulted from inhibition of PE-induced cell death. Furthermore, the analysis of p-H3-labled cells demonstrated that Cu did not cause proliferation of hypertrophied cardiomyocytes. Therefore, Cu caused a direct reduction in the size of hypertrophied cardiomyocytes.
Cu regression of cardiomyocyte hypertrophy is VEGF dependent because the anti-VEGF antibody blocked and VEGF alone mimicked the Cu regression of cardiomyocyte hypertrophy. This VEGF-mediated effect is angiogenesis-independent because the primary culture condition eliminated the influence of blood vessel formation. This is an important finding. VEGF has been extensively studied regarding its effect on cell growth and proliferation. In the cardiomyocytes, VEGF also is required for cell growth [22-24]. Studies in vivo have shown that a decoy VEGF receptor blocked cardiac growth induced by Akt1 activation [4,16]. Therefore, VEGF would have a dual function: stimulating cardiomyocyte growth under stress condition and reducing the size in the hypertrophied cardiomyocytes to improve their physiological function. In addition, other studies in vivo have demonstrated that VEGF prevents apoptosis and preserves contractile function in hypertrophied heart [25].
This novel observation that VEGF reduces the size of hypertrophied cardiomyocytes suggests a new biological function of VEGF. This newly identified action of VEGF in the heart is angiogenesis-independent. The main VEGF receptors (VEGFRs) comprise a family of closely related receptor tyrosine kinases consisting of three members, VEGFR-1 (Flt-1), VEGFR-2 (KDR or Flk-1), and VEGFR-3 (Flt-3). The studies of these receptors have been focused on their role in endothelial cells and blood vessel formation. Activation of the VEGFR-2 receptor by VEGF in cells devoid of VEGFR-1 results in a mitogenic response, while the activation of VEGFR-1 in cells lack of VEGFR-2 does not induce cell proliferation [26,27]. These results indicate that the signaling transduction cascades induced by VEGFR-1 and -2 are different, although the molecular basis for this difference is unknown. It has also been observed that VEGF promoted the binding and phosphorylation of the Shc and Nyc adaptors, Grb-2 binding, and MAP kinase activation in porcine aortic endothelial cells expressing recombinant VEGFR-2. In contrast, MAP kinase was not activated by VEGF in cells expressing recombinant VEGFR-1 [28]. These differential activities between VEGFR-1 and -2 in the endothelial cells suggest that the signaling transduction cascades induced by these receptors in the cardiomyocytes would also be different. This scenario becomes an important direction for our future studies.
Cu stimulation of VEGF production has been observed in other type of cells, such as keratinocytes [29]. In the present study, we used isolated cardiomyocytes in cultures, which eliminated the interaction between cardiomyocytes and other cell types, to which Cu would have significant effects on their VEGF production as well as other biological function. This becomes a major limitation of the present study because it did not address the effect of other cells and the extracellular matrix on regression of heart hypertrophy, which may play the major role in vivo. Another limitation of the present study is that the use of neonatal rat cardiomyocytes, which are different from adult cardiomyocytes in vivo. Whether or not Cu can reduce the size of hypertrophied adult cardiomyocytes need to be studied specifically under in vivo conditions in the adult mouse model.
In summary, the present study demonstrates that Cu at relevant levels of in vivo experimental conditions reverses cardiomyocyte hypertrophy induced by PE stimulation. The reduction in the size of hypertrophied cardiomyocytes is VEGF-dependent. Future studies need to focus on the mechanistic insights into VEGF signaling pathways leading to cell size reduction.
Acknowledgements
This study was supported in part by NIH grants HL63760 and HL59225 (to YJK) and YJK is a Distinguished University Scholar of the University of Louisville.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, et al. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med. 2007;204:657–66. doi: 10.1084/jem.20061943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Witte KKA, Nikitin NP, Parker AC, von Haehling S, Volk H-D, Anker SD, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238–44. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- [3].Klevay LM. Heart failure improvement from a supplement containing copper. Eur Heart J. 2006;27:117. doi: 10.1093/eurheartj/ehi634. [DOI] [PubMed] [Google Scholar]
- [4].Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Walsh K, Shiojima I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J Clin Invest. 2007;117:3176–9. doi: 10.1172/JCI34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–84. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–8. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- [8].Hoffmann P, Richards D, Heinroth-Hoffmann I, Mathias P, Wey H, Toraason M. Arachidonic acid disrupts calcium dynamics in neonatal rat cardiac myocytes. Cardiovasc Res. 1995;30:889–98. [PubMed] [Google Scholar]
- [9].Siddiqui RA, Shaikh SR, Kovacs R, Stillwell W, Zaloga G. Inhibition of phenylephrine-induced cardiac hypertrophy by docosahexaenoic acid. J Cell Biochem. 2004;92:1141–59. doi: 10.1002/jcb.20135. [DOI] [PubMed] [Google Scholar]
- [10].Zhou Y, Jiang Y, Kang YJ. Copper inhibition of hydrogen peroxide-induced hypertrophy in embryonic rat cardiac H9c2 cells. Exp Biol Med. 2007;232:385–9. [PubMed] [Google Scholar]
- [11].Yoshioka J, Prince RN, Huang H, Perkins SB, Cruz FU, MacGillivray C, et al. Cardiomyocyte hypertrophy and degradation of connexin43 through spatially restricted autocrine/paracrine heparin-binding EGF. Proc Natl Acad Sci U S A. 2005;102:10622–7. doi: 10.1073/pnas.0501198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barron M, Gao M, Lough J. Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative. Dev Dyn. 2000;218:383–93. doi: 10.1002/(SICI)1097-0177(200006)218:2<383::AID-DVDY11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [13].Ikezoe T, Yang J, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, et al. A novel treatment strategy targeting Aurora kinases in acute myelogenous leukemia. Mol Cancer Ther. 2007;6:1851–7. doi: 10.1158/1535-7163.MCT-07-0067. [DOI] [PubMed] [Google Scholar]
- [14].Tsujita Y, Muraski J, Shiraishi I, Kato T, Kajstura J, Anversa P, et al. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2006;103:11946–51. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–9. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- [16].Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure-overload. Hypertension. 2006;47:887–93. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Friehs I, Moran AM, Stamm C, Choi YH, Cowan DB, McGowan FX, et al. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. Ann Thorac Surg. 2004;77:2004–11. doi: 10.1016/j.athoracsur.2003.11.003. [DOI] [PubMed] [Google Scholar]
- [18].Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–16. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- [19].Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood. 2005;105:4613–9. doi: 10.1182/blood-2004-10-3980. [DOI] [PubMed] [Google Scholar]
- [20].Ahmed Z, Idowu BD, Brown RA. Stabilization of fibronectin mats with micromolar concentrations of copper. Biomaterials. 1999;20:201–9. doi: 10.1016/s0142-9612(98)00015-5. [DOI] [PubMed] [Google Scholar]
- [21].Hu GF. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem. 1998;69:326–35. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [22].Shimojo N, Jesmin S, Zaedi S, Otsuki T, Maeda S, Yamaguchi N, et al. Contributory role of VEGF overexpression in endothelin-1-induced cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H474–81. doi: 10.1152/ajpheart.00922.2006. [DOI] [PubMed] [Google Scholar]
- [23].Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, et al. P53-induced inhibition of HIF-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–8. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- [24].Shyu KG, Liou JY, Wang BW, Fang WJ, Chang H. Carvedilol prevents cardiac hypertrophy and overexpression of hypoxia-inducible factor-1 and vascular endothelial growth factor in pressure-overloaded rat heart. J Biomed Sci. 2005;12:409–20. doi: 10.1007/s11373-005-3008-x. [DOI] [PubMed] [Google Scholar]
- [25].Friehs I, Barillas R, Vasilyev NV, Roy N, McGowan FX, del Nido PJ. Vascular endothelial growth factor prevents apoptosis and preserves contractile function in hypertrophied infant heart. Circulation. 2006;114(1 Suppl):I290–5. doi: 10.1161/CIRCULATIONAHA.105.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995;10:135–47. [PubMed] [Google Scholar]
- [27].Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–95. [PubMed] [Google Scholar]
- [28].Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–7. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- [29].Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, Hunt TK, et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol. 2002;282:1821–7. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]