Abstract
To investigate the targeting mechanism for proteins bound to the mammalian Lin-7 (mLin-7) PDZ domain, we created receptor protein chimeras composed of the carboxyl-terminal amino acids of LET-23 fused to truncated nerve growth factor receptor/P75. mLin-7 bound to the chimera with a wild-type LET-23 carboxyl-terminal tail (P75t-Let23WT), but not a mutant tail (P75t-Let23MUT). In Madin-Darby canine kidney (MDCK) cells, P75t-Let23WT localized to the basolateral plasma membrane domain, whereas P75t-Let23MUT remained apical. Furthermore, mutant mLin-7 constructs acted as dominant interfering proteins and inhibited the basolateral localization of P75t-Let23WT. The mechanisms for this differential localization were examined further, and, initially, we found that P75t-Let23WT and P75t-Let23MUT were delivered equally to the apical and basolateral plasma membrane domains. Although basolateral retention of P75t-Let23WT, but not P75t-Let23MUT, was observed, the greatest difference in receptor localization was seen in the rapid trafficking of P75t-Let23WT to the basolateral plasma membrane domain after endocytosis, whereas P75t-Let23MUT was degraded in lysosomes, indicating that mLin-7 binding can alter the fate of endocytosed proteins. Altogether, these data support a model for basolateral protein targeting in mammalian epithelial cells dependent on protein–protein interactions with mLin-7, and also suggest a dynamic role for mLin-7 in endosomal sorting.
INTRODUCTION
The establishment and maintenance of cell polarity requires efficient targeting mechanisms for the selective localization of proteins, and recent investigations have revealed evolutionarily conserved pathways in organisms as diverse as yeast, nematodes, insects, and mammals (Le Gall et al., 1995; Drubin and Nelson, 1996; Caplan, 1997; Ponting, 1997; Yeaman et al., 1999; Bilder et al., 2000). In higher organisms, elements of these pathways are essential to the function of polarized cells, such as neurons and epithelia, but are also found in less polarized cell types, such as fibroblasts (Craig and Banker, 1994; Keller and Simons, 1997; Cereijido et al., 1998). It has been difficult to determine the precise sequence of events leading to cell polarization, but it is generally believed that polarization is initiated as a response to spatial cues, such as cell adhesion, followed by the organization of the cytoskeleton, after which specific protein targeting acts to reinforce and/or maintain the polarized state (Yeaman et al., 1999).
Several general mechanisms for the localization of proteins have emerged, which can be separated into direct and indirect pathways (Mostov et al., 2000). The direct pathway includes mechanisms governing protein delivery to the plasma membrane as well as modes of retention at those sites. Indirect mechanisms include endosomal recycling and transcytosis. Many of these mechanisms rely upon protein–protein interactions directed by discrete signal peptides present on the targeted proteins themselves (Mellman and Warren, 2000; Mostov et al., 2000). For example, dihydrophobic repeats, tyrosine-containing peptides, and other motifs that form a structure referred to as a tight β-turn are found in the cytoplasmic domain of some basolateral proteins, whereas N-glycosylation of lumenal domains or glycophosphoinositol anchoring are often associated with apical targeting. Some of the effectors of protein targeting have also been tentatively identified, such as the μ1b clathrin adaptor associated with basolateral targeting of some proteins in epithelial cells (Folsch et al., 1999; Ohno et al., 1999), or the proteins of the exocyst complex, initially identified in yeast, which govern vesicle docking and fusion at the basolateral plasma membrane in mammalian cells (Grindstaff et al., 1998).
Much recent research has focused on proteins containing PDZ (PSD-95, Discs Large and Zona Occludens-1) domains, which bind to the carboxyl terminus of their target proteins (Songyang et al., 1997; Fanning and Anderson, 1998). Proteins containing PDZ domains are important during many stages of the establishment and maintenance of cell polarity, serving as scaffolds for the organization of protein complexes involving cytoskeletal proteins, adhesion molecules, and transmembrane receptors (Kim, 1997; Fanning and Anderson, 1998; Fanning and Anderson, 1999; Garner et al., 2000). Some examples include basolateral targeting of ErbB-2/Her-2 in mammalian epithelia by Erbin (Borg et al., 2000), organization of adherens junctions in Caenorhabditis elegans by LET-413 (Legouis et al., 2000), apical targeting in Drosophila sp. by Scribble (Bilder and Perrimon, 2000), and the clustering of ion channels and receptors at neuronal synapses by PSD-95 and other membrane-associated guanylate kinases (Kornau et al., 1997; Sheng and Wyszynski, 1997; Fanning and Anderson, 1999; Garner et al., 2000).
We have continued our investigation of the LIN-7/LIN-2/LIN-10 protein complex, originally identified as important for the basolateral localizaton of the receptor tyrosine kinase LET-23 in vulval precursor cells of C. elegans (Simske et al., 1996; Kaech et al., 1998). The mammalian orthologs to these C. elegans proteins, identified as mLin-7/VELIS/MALS, mLin-2/CASK, and mLin-10/X11/Mint, also form a protein complex in mammalian neuronal cells, but only mLin-7 and mLin-2 interact in cells of epithelial origin (Hata et al., 1996; Borg et al., 1998; Butz et al., 1998; Jo et al., 1999; Irie et al., 1999). The L27 domain in the amino-terminal half of mLin-7 binds to mLin-2/CASK, an interaction also required for the localization of mLin-7 to the basolateral membrane of Madin-Darby canine kidney (MDCK) cells (Doerks et al., 2000; Straight et al., 2000). The PDZ domain of mLin-7 binds to the carboxyl termini of two basolateral proteins, the receptor-tyrosine kinase LET-23 in C. elegans and the transporter protein BGT-1, found in mammalian kidney epithelial cells (Simske et al., 1996; Perego et al., 1999; Straight et al., 2000). The interaction of mLin-7 with BGT-1 seems to be involved in the basolateral retention of BGT-1, whereas other targeting signals are responsible for the initial delivery to the basolateral membrane (Perego et al., 1999).
Herein we describe our efforts to elucidate the mechanism by which interaction with the mLin-7 PDZ domain targets proteins to the basolateral plasma membrane domain of epithelial cells. Because known mLin-7 PDZ domain partners, such as BGT-1, are complex proteins possessing multiple intrinsic targeting signals, we sought to simplify our system. Therefore, we isolated the role of mLin-7 PDZ binding from extraneous basolateral targeting signals by creating receptor protein chimeras consisting of a truncated nerve growth factor receptor/P75 (NGFR/P75) fused to the carboxyl terminus of LET-23. These chimeras, in combination with mLin-7 mutant proteins, were used in the examination of several modes of direct and indirect protein targeting in MDCK cells. These assays revealed that interaction with the mLin-7 PDZ domain does not influence delivery of proteins to the plasma membrane, but does play a role in basolateral retention. Most intriguingly, however, our results suggest that mLin-7 also plays an active role in protein localization as a basolateral targeting effector within the sorting endosome to target proteins to the basolateral plasma membrane.
MATERIALS AND METHODS
DNA Constructs
The cloning of mouse mlin-7 cDNA, as well as the creation of the Myc epitope-tagged mLin-7 constructs (pRK5Myc-mLin-7WT, pRK5Myc-mLin-7N [amino acids 1–92] and pRK5Myc-mLin-7PDZ [amino acids 79–197]) used in this investigation, have been described previously (Borg et al., 1996, 1998; Straight et al., 2000). The cloning of human mlin-2 was described previously (Borg et al., 1998). The plasmid pcDNA-3HA-mLin-2 was created by inserting three influenza virus hemagglutinin epitopes (YPYDVPDYA) in frame with the amino terminus of mLin-2 that had been cloned into the pcDNA3 (Invitrogen, Carlsbad, CA) backbone. The plasmid pCMV5A-P75, which contains the full-length human NGFR/P75 (Johnson et al., 1986), was used as a template for polymerase chain reaction with the oligonucleotide primers P75RVRI (5′-GGAAGTCGAGCGATATCGCGGAATTCGGGCGATGGGGG-3′) and P75BamStopHind (5′-GCTGTTGGCTCCAAGCTTGTTTCAGGATCCGCTGTTCCACG-3′). This polymerase chain reaction product was subcloned into the EcoRI and HindIII sites of plasmid pcDNA3.1/Zeo(−) (Invitrogen) to create the truncated protein construct pP75t, which expresses amino acids 1–306 of the human NGFR/P75. Complimentary oligonucleotides encoding the carboxyl-terminal amino acids of C. elegans LET-23 and appropriate 5′ and 3′ overhangs suitable for subcloning into the BamHI and HindIII sites of pP75t were generated to create the chimeric constructs pP75t-Let23WT and pP75t-Let23MUT. The oligonucleotides used in the generation of pP75t-Let23WT were 5′-GATCCGTTCAATATGAAAATGAAGAAGTATCACAAAAGGAAACTTGTCTTTAAA-3′ and 5′-AGCTTTTAAAGACACGTTTCCTTTTGTGATACTTCTTCATTTTCATATTGAACG-3′. The oligonucleotides for the generation of pP75t-Let23MUT were 5′-GATCCGTTCAATATGAAAATGAAGAAGTATCACAAAAGGAAACTTGTTAAA-3′ and 5′-AGCTTTTAACACGTTTCCTTTTGTGATACTTCTTCATTTTCATATTGAACG-3′. The complimentary oligonucleotides were annealed, digested with appropriate restriction enzymes, and ligated in frame to pP75t for expression in mammalian cells. The correct sequence of all constructs was confirmed by automated sequencing at the University of Michigan DNA Sequencing Core.
Cell Culture and Transfection
Human embryonic kidney 293 (HEK293) and MDCK cells were grown in DMEM (Life Technologies, Grand Island, NY) containing 100 U/ml penicillin and 100 μg/ml streptomycin sulfate, supplemented with 10% fetal calf serum.
HEK293 cells, seeded the day before at 105/10-cm dish were transiently transfected with 5–10 μg of DNA by using calcium phosphate (Chen and Okayama, 1987). Forty-eight hours after transfection the cells were surface biotinylated (as described below for MDCK cells on filters, by using appropriately larger reagent volumes) before the collection of lysates. Clonal cell lines were derived from MDCK cells stably transfected with DNA by using FuGENE 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN) followed by 10–14 d of selection with Geneticin/G-418 (600 μg/ml active; Life Technologies) or Zeocin (200 μg/ml; Invitrogen). The Geneticin-resistant cell lines expressing Myc-tagged mLin-7 constructs (described in Straight et al., 2000) were also transfected with pP75t-Let23WT and pP75t-Let23MUT as described above.
For most experiments, MDCK cells were seeded at high density onto 12- or 24-mm Transwell membrane filters (0.4-μm pore size; Corning Costar, Cambridge, MA) and grown 5–7 d at confluence to form a polarized monolayer. The formation of properly polarized monolayers by the MDCK cell lines used in these studies was determined by immunofluorescence with anti-ZO-1 (Zymed, San Francisco, CA) and anti-E-cadherin (Sigma Chemical, St. Louis, MO), as well as by assessing monolayer integrity with a [3H]inulin permeability assay (Caplan et al., 1986).
Antibodies
The generation, affinity purification, and characterization of the anti-mLin-7 antibody (UM199) used in some applications were described in Straight et al. (2000). The generation and use of anti-mLin-2 antisera (UM195 and UM196) was described in Borg et al. (1998). The mouse hybridoma cell line ME20.4 was obtained from American Type Culture Collection (Rockville, MD) and injected into mice for the production of ascites fluid containing monoclonal antibody to NGFR/P75, which was used for immunoprecipitation and immunostaining. The anti-Myc monoclonal antibody (clone 9E10) was used for imunoprecipitation, immunostaining, and immunoblotting. The anti-hemagglutinin (HA) monoclonal antibodies 3F10 and 12CA5 (both obtained from Roche Molecular Biochemicals) were used for immunoprecipitation and immunoblot, respectively. Affinity-purified rabbit polyclonal anti-ZO-1 antibodies and rat anti-uvomorulin/E-cadherin monoclonal antibodies used for control immunostaining were purchased from Zymed and Sigma Chemical, respectively. Fluorochrome-conjugated secondary antibodies used in immunostaining procedures were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and Molecular Probes (Eugene, OR).
Immunostaining of MDCK Cells and Confocal Microscopy
For simple immunostaining, cells grown on 12- or 24-mm Transwell filters were washed with phosphate-buffered saline (PBS) and then fixed with 4% formaldehyde/PBS and permeabilized with 0.1% Triton X-100/PBS. After blocking 1 h with goat serum, the cells were incubated with primary antibodies diluted in 2% goat serum/PBS (affinity-purified anti-mLin-7 at 1:100, anti-Myc at 1:400, anti-ZO-1 at 1:400, and anti-E-cadherin at 1:1600, anti-P75 1:1000) in a humidified chamber for 2 h to overnight at 30°C. After washing three times with 2% goat serum/PBS, the cells were incubated with secondary antibodies coupled to fluorescein isothiocyanate (FITC), Cy3, Cy5, or Texas Red (diluted at 1:500 in 2% goat serum/PBS) for 2 h in a humidified chamber at 30°C. The specificity of all antibodies was determined from appropriate positive and negative control immunostaining.
Basolateral retention of chimera proteins was examined by secondary antibody capture. MDCK cell lines on 12-mm Transwell filters were incubated 1–2 h on ice with growth medium buffered with 25 mM HEPES, pH 7.2 and containing anti-P75 (5 μl of ascites/ml) at the basolateral side. Unbound antibody was removed by extensively washing with ice-cold wash buffer (DMEM, 5% bovine serum albumin, 25 mM HEPES, pH 7.2). Cells were then incubated at 37°C in growth medium buffered with 25 mM HEPES, pH 7.2 and containing goat anti-mouse IgG-Texas Red (5 μl/ml) at the apical side to capture antibody–protein complexes reaching the apical side. Cells were washed to remove unbound capture antibody, fixed and permeabilized, and then the remaining uncoupled primary antibody at the basolateral side was immunostained with goat anti-mouse IgG-FITC. Negative controls included cells that were not prebound with anti-P75 antibody.
To examine endocytic sorting of internalized chimera protein, MDCK cell lines on 12-mm Transwell filters were incubated 1–2 h in ice-cold growth medium buffered with 25 mM HEPES, pH 7.2 to prebind anti-P75 (5 μl of ascites/ml) to the apical side. Unbound antibody was removed by extensively washing with ice-cold wash buffer, and the cells were then incubated at 37°C in growth medium for 0–2 h to allow trafficking to occur. At the appropriate time, cells were fixed, permeabilized, and stained with secondary antibodies. Some filters were also costained for mLin-7 by using appropriate antibodies. Either nocodazole {20 μg/ml, methyl(5-[2[thienylcarbanyl]-1H-benzimidazol-2yl)carbamate}, chloroquine (25 μM), or leupeptin (10 μg/ml) were sometimes present during the course of the experiment. These reagents were acquired from Sigma Chemical.
For examination, all filters were cut from their plastic casing with a scalpel and mounted on glass slides with ProLong antifade reagent (Molecular Probes). Preliminary examination of immunostained cells was performed on an Olympus BX60 fluorescent microscope and digital images were taken with a SPOT charge-coupled device camera (Diagnostic Instruments, Los Angeles, CA). Confocal laser-scanning microscopy was performed at the Morphology and Image Analysis Core of the University of Michigan Diabetes Research Center by using a Nikon Diaphot 200 microscope paired with a Noran laser and InterVision software (Noran Instruments, Middleton, WI) on a Silicon Graphics workstation (IRIX version 6.5). Typically, 20–30 serial images were taken in 0.5-μm steps, beginning 1–2 μm below the focal plane of the bottom of the cell monolayer and proceeding upward to 1–2 μm above the top of the monolayer. Adobe Photoshop (Adobe Systems, San Jose, CA) was used to compress grayscales, overlay channels, and otherwise prepare the digital images for presentation.
Lysis, Precipitation, and Immunoblot
Lysates for precipitation experiments were prepared from transfected HEK293 or MDCK cell lines grown on 10- or 15-cm tissue culture dishes as described (Borg et al., 1996). Briefly, cells were washed twice with cold PBS and lysed on ice in 1 ml of lysis buffer (50 mM HEPES [pH 7.5], 10% glycerol, 150 mM NaCl, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA) supplemented with 1 mM phenylmethylsulfonylfluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 100 mM NaF, 10 mM Na4P2O7, and 200 μM Na3VO4. The lysates were cleared by centrifugation at 16,000 × g for 20 min at 4°C to remove insoluble debris. For immunoprecipitation, 0.5 ml of lysate from HEK293 or 1.0 ml of lysate from MDCK was incubated with appropriate antibody (anti-Myc, 3–5 μl of ascites; anti-mLin-2, 5–10 μl of serum; anti-P75, 5–10 μl of ascites; anti-HA, 5 μl) overnight at 4°C. Protein G-Sepharose CL4B (for anti-HA; Zymed) or protein A-Sepharose CL4B (for all other antibodies; Zymed) and, for anti-P75 only, rabbit anti-mouse IgG (Sigma Chemical) were added and immune complexes were recovered after 1 h, and then washed three times with HNTG buffer (50 mM HEPES pH 7.5, 10% glycerol, 150 mM NaCl, 0.1% Triton X-100). An exception was made for Myc-mLin-7/P75t-Let23 complexes from transfected HEK293 cells, which were washed with a less stringent buffer containing only 50 mM HEPES pH 7.5 and 10% glycerol. Proteins were eluted by boiling in 1× SDS-sample buffer (with β-mercaptoethanol), separated by SDS-PAGE, and transferred to nitrocellulose. Precipitated proteins were detected by immunoblot with appropriate primary antibodies, followed by incubation with sheep anti-mouse Ig-horseradish peroxidase (HRP) (Amersham Pharmacia Biotech, Arlington Heights, IL) or protein A-horseradish peroxidase (Kirkegaard and Perry Laboratories, Gaithersburg, MD), as appropriate. The immunoreactive proteins were revealed by incubation with the Renaissance chemiluminescence reagent (PerkinsElmer Life Science Products, Boston MA), followed by exposure to X-OMAT Blue XB-1 autoradiography film (Eastman Kodak, Rochester, NY).
Cell-Surface Biotinylation
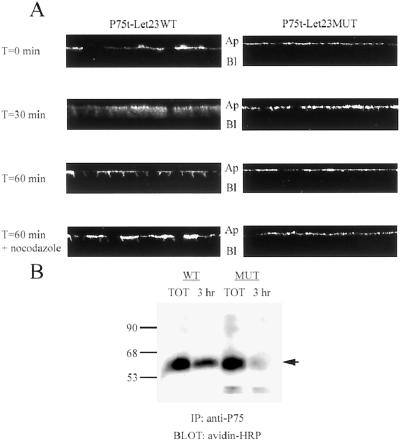
MDCK cell lines on 24-mmTranswell filters were selectively biotinlyated at either the apical or basolateral cell surface (Sargiacomo et al., 1989; Gottardi et al., 1995). Briefly, cells were washed with ice-cold Ringer's solution pH 9.0 and then incubated with freshly dissolved NHS-SS-biotin (300 μg/ml; Pierce, Rockford, IL) for 30 min on ice. The biotinylation reaction was quenched by extensively washing with Tris-saline. The cells were lysed in 1 ml of lysis buffer, precipitated with anti-P75, separated by SDS-PAGE, and transferred to nitrocellulose as described in the previous section. Biotinylated proteins were detected with avidin-horseradish peroxidase (Pierce) and chemiluminescence.
Alternatively, cells biotinylated apically were used for primary antibody capture to examine endocytic sorting. After quenching the biotinylation reaction, cells were incubated 3 h at 37°C in growth medium buffered with 25 mM HEPES, pH 7.2 and containing anti-P75 (10 μl of ascites/ml) at the basolateral side to capture biotinylated protein. After several washes with wash buffer (DMEM, 5% bovine serum albumin, 25 mM HEPES, pH 7.2) to remove unbound antibody, cells were lysed and the antibody-bound proteins were precipitated and prepared for detection with avidin-HRP as described above. Control precipitations were also performed with anti-P75 added directly to lysates to determine the total biotinylated chimera protein present.
35S-Labeling
To determine the site of initial delivery of chimera protein to the plasma membrane, MDCK cells on 24-mm Transwell filters were incubated 2 h in methionine-free DMEM (Life Technologies, Grand Island, NY) supplemented with 10% dialyzed fetal bovine serum. Cells were then pulse-labeled for 1 h at 37°C by the addition of 250 μCi/ml 35S-Easytag Express label (PerkinElmer Life Science Products). Unincorporated label was washed away and cells were incubated at 37°C for 3 h in complete growth medium containing anti-P75 (10 μl of ascites/ml) at either the apical or basolateral side. Unbound antibody was washed away and then cells were lysed and the antibody-bound proteins were precipitated as described above. Control precipitations were also performed with anti-P75 added directly to lysates to determine the total labeled chimera protein present. Precipitated proteins were separated by SDS-PAGE, and the gel was fixed in 30% methanol/10% acetic acid, soaked 30 min in Amplify (Amersham Pharmacia Biotech), and dried. Densitometry was performed using a Molecular Dynamics laser scanner model STORM860 and ImageQuant Software (Molecular Dynamics, Sunnyvale, CA). Each sample was prepared in triplicate (n = 3) so that SD from the means could be determined.
The rate of degradation of chimera protein was determined using a pulse-chase protocol. Briefly, MDCK cell lines grown to confluence on 10-cm tissue culture dishes were methionine-starved and pulse-labeled for 1 h with [35S]methionine as described in the delivery experiment above. Unincorporated label was washed away and cells were incubated at 37°C for 0–24 h in complete growth medium, after which the cells were lysed and proteins were precipitated with anti-P75 (10 μl of ascites) as described above. Precipitated proteins were separated by SDS-PAGE, the gel was fixed, soaked in Amplify, and dried. Densitometry was performed as described above.
RESULTS
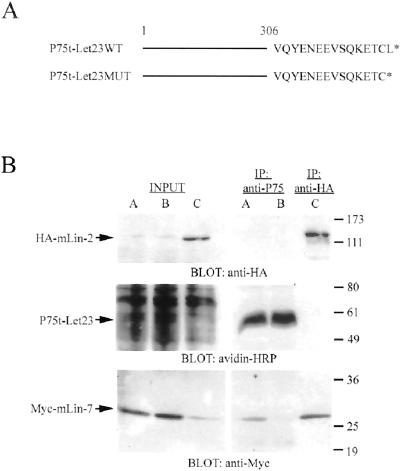
Figure 1A presents a schematic representation of the NGFR/P75-Let23 chimeras created for these investigations. The NGFR portion of these constructs was truncated at Ser306, five amino acids after the transmembrane domain, and thus lacks the entire cytoplasmic domain of the NGFR/P75. This truncation of NGFR/P75 prevents activation of a cell-signaling cascade, and the truncated receptor was previously shown to localize to the apical domain of MDCK cells (Le Bivic et al., 1991). Furthermore, NGFR/P75 is nonnative to MDCK cells, not expressed in epithelial cells, and antibodies to the extracellular domain are available. The coding region for the first 306 amino acids of the human NFGR/P75 was ligated to oligonucleotides encoding the carboxyl-terminal 15 amino acids of LET-23 to form the wild-type construct P75t-Let23WT. P75t-Let23MUT was also created, which contained a mutant carboxyl terminus that deleted the terminal leucine residue of LET-23, thus destroying the mLin-7 PDZ binding site. The coprecipitation of Myc-mLin-7 with P75t-Let23WT, but not with the mutant-tailed P75t-Let23MUT expressed at similar levels, clearly demonstrated the specificity of interaction of the wild-type chimera with mLin-7 (Figure 1B). The coprecipitation of Myc-mLin-7 with HA-mLin2 is shown as positive control.
Figure 1.
Differential binding of mLin-7 to the chimeric receptors. (A) Schematic representation of the NGFR/P75 chimeric receptor constructs. Amino acids 1–306 of the NGFR/P75 (truncated after the transmembrane domain) were fused to the carboxyl-terminal amino acids of LET-23 (shown in single letter code) to form the P75t-Let23WT construct. The P75t-Let23MUT construct deletes the final amino acid in the LET-23 carboxyl terminus, destroying the mLin-7 PDZ domain ligand. (B) HEK293 cells were transfected with plasmids as follows: lanes A, pRK5Myc-mLin-7 plus pP75t-Let23WT; lanes B, pRK5Myc-mLin-7 plus pP75t-Let23MUT; lanes C, pRK5Myc-mLin-7 plus pcDNA-3HA-mLin-2. Forty-eight hours after transfection, cells were surface biotinylated, and then lysates were collected for immunoprecipitation with anti-P75 or anti-HA antibodies, as indicated. Precipitated proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose, and the membrane was probed with avidin-HRP (to detect surface biotinylated P75t-Let23), anti-HA, or anti-Myc antibodies. The input lanes contain approximately one-tenth the amount of HEK293 lysate used in the precipitation experiments. The arrows indicate the relevant bands on the immunoblots. Relative molecular weight is shown to the right in kilodaltons.
After determining that our chimeras interacted with mLin-7 in the predicted manner, we examined the localization of these constructs in MDCK cell lines generated by transfection. Primary examination of the these cell lines by immunostaining for E-cadherin and ZO-1, proteins present at adherens junctions and tight junctions, respectively, showed that these cell lines were morphologically indistinguishable from parental MDCK cells, forming monolayers of tall, columnar epithelial cells (our unpublished results). Similarly, [3H]inulin permeability assays demonstrated the formation of intact tight junctions in these cell lines (our unpublished results).
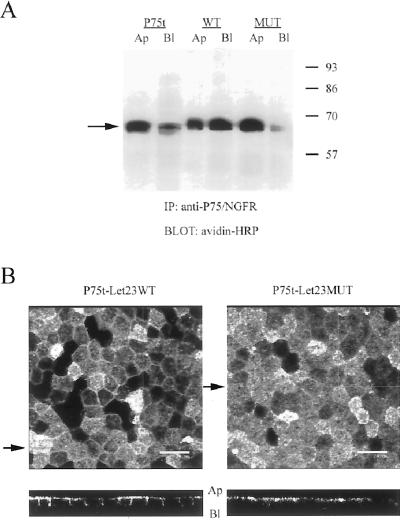
Previously, the NGFR/P75 truncated at Ser306 was reported to localize apically in MDCK cells (Le Bivic et al., 1991). Initial results obtained by cell-surface biotinylation of polarized monolayers of MDCK cell lines are shown in Figure 2A. Immunoprecipitation of NGFR/P75 from cells selectively biotinylated at either the apical or basolateral side confirmed the primarily apical distribution for the truncated receptor alone (P75t). This primarily apical distribution was not significantly changed by the addition of the mutant tail (P75t-Let23MUT), but the addition of the wild-type tail (P75t-Let23WT), which was able to bind mLin-7, resulted in a significant redistribution of the chimera to the basolateral side of the monolayer.
Figure 2.
Differential localization of the receptor chimeras correlated with their ability to bind to mLin-7. Shown in A are the results of a cell-surface biotinylation experiment to determine the steady-state localization of the P75t constructs. Stable MDCK cell lines expressing P75t, P75t-Let23WT, or P75t-Let23MUT were grown as confluent monolayers on Transwell polycarbonate filters and selectively labeled at the apical or basolateral side with biotin. Lysates were collected and immunoprecipitation performed with anti-P75 antibodies. The precipitates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed for biotinylated proteins by using avidin-HRP. Relative molecular weight is shown to the left in kilodaltons. An arrow indicates the relevant band. (B) Same cell lines used above were stained with anti-P75 antibodies and secondary antibodies coupled to fluorochromes, and then examined by confocal laser-scanning microscopy. The top panels show digital photomicrographs (the X-Y dimension of the Z-series), whereas the bottom panels show the X-Z dimension (Z-section). The arrows next to the top panels indicate the plane through which the Z-sections were taken. The apical (Ap) and basolateral (Bl) sides are indicated next to the Z-sections. Bars, 20 μm.
Similar basolateral redistribution of P75t-Let23WT was observed by examination of these cells via immunofluorescence (Figure 2B). Confocal microscopy showed P75t-Let23WT localized to both the apical and basolateral domains of MDCK cells, whereas P75t-Let23MUT was primarily restricted to the apical domain. Furthermore, the basolateral localization of P75t-Let23WT coincided with the immunolocalization of mLin-7 (our unpublished results). These experiments demonstrate that interaction with mLin-7 correlates with the significant redistribution of an apical receptor protein to the basolateral plasma membrane domain.
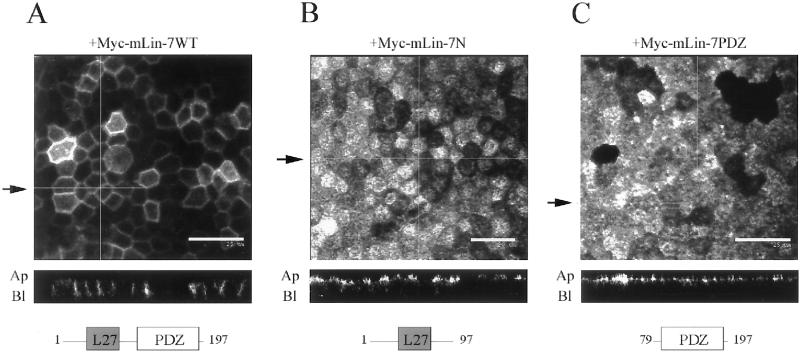
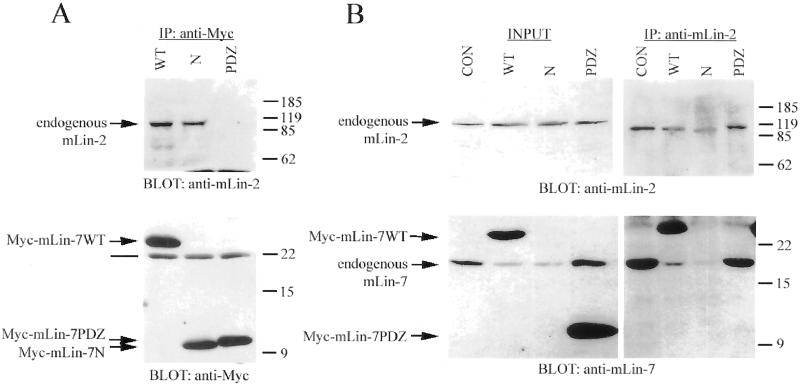
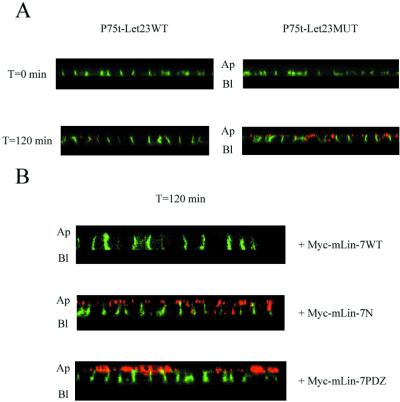
To support the hypothesis that binding to mLin-7 was responsible for this redistribution, the receptor chimeras were transfected into MDCK cell lines expressing mutant Myc-tagged mLin-7 constructs. The resulting cell lines were examined by confocal microscopy (Figure 3). The coexpression of Myc-mLin-7WT, the full-length mLin-7 protein, did not interfere with the basolateral distribution of P75t-Let23WT, and in fact seemed to further shift localization from apical to basolateral (Figure 3A). However, the Myc-mLin-7 mutant proteins Myc-mLin-7N and Myc-mLin-7PDZ acted as dominant interfering inhibitors of basolateral localization, resulting in a predominantly apical distribution of P75t-Let23WT (Figure 3, B and C). Myc-mLin-7N was able to bind to mLin-2 (Figure 4A) and competed with endogenous mLin-7 for binding to endogenous mLin-2 in this cell line (Figure 4B). This competition would likely result in the mislocalization of endogenous mLin-7 in these cells, because binding to mLin-2 is required for localization of mLin-7 to the basolateral surface (Straight et al., 2000). The overexpression of Myc-mLin-7N, as well as Myc-mLin-7WT, also appeared to decrease the expression of endogenous mLin-7 (Figure 4B), likely exacerbating the inhibitory effects of Myc-mLin-7N on mLin-7-mediated localization. Myc-mLin-7PDZ did not bind to mLin-2 (Figure 4A) but was expressed far in excess of the amount of endogenous mLin-7 present (Figure 4B). Previously, this mutant protein was shown to be distributed throughout the cytoplasm (Straight et al., 2000), conceivably interfering with the ability of P75t-Let23WT to bind endogenous mLin-7 and properly localize. The apical localization of P75t-Let23MUT was not significantly altered in cells expressing any of the Myc-tagged mLin-7 constructs (our unpublished results). These results reaffirm that interaction with full-length mLin-7 was required for the basolateral localization of P75t-Let23WT.
Figure 3.
Myc-mLin-7 deletion mutants interfered with the basolateral localization of P75t-Let23WT. The localization of P75t-Let23WT in MDCK cell lines coexpressing Myc-tagged mLin-7 constructs is shown in A–C: P75t-Let23WT plus Myc-mLin-7WT (full-length mLin-7) (A), Myc-mLin-7N (amino acids 1–92), which contains the mLin-2 binding site (B), or Myc-mLin-7PDZ (amino acids 79–197), containing the PDZ domain (C). These cells were grown as confluent monolayers on Transwell filters, fixed and permeabilized, and then stained with anti-P75 antibodies and secondary antibodies coupled to fluorochromes. Examination was by confocal laser-scanning microscopy. The top panels show digital photomicrographs, whereas the bottom panels show Z-sections. The arrows next to the top panels indicate the plane through which the Z-sections were taken. The apical (Ap) and basolateral (Bl) sides are indicated next to the Z-sections. Bars, 20 (B) and 25 μm (A and C). Below each Z-section is a cartoon depicting the Myc-mLIn-7 construct that was coexpressed in these cell lines. The numbers refer to the amino acid positions, whereas the boxed regions represent identified protein interaction domains: L27, coiled-coil region that binds to mLin-2/CASK; PDZ, PDZ domain that binds to the carboxyl terminus of C. elegans Let-23.
Figure 4.
Myc-mLin7N competed with endogenous mLin-7 for binding to endogenous mLin-2 in MDCK cells. (A and B) Precipitation analysis of MDCK cell lines expressing Myc-tagged mLin-7 constructs (described in the legend to Figure 3). Triton lysates were collected for immunoprecipitation with the indicated antibodies. Precipitated proteins were separated by 15% SDS-PAGE, transferred to nitrocellulose, and the membrane was probed with antibodies as indicated. (A) Immunoprecipitation with anti-Myc antibodies and coprecipitation of endogenous mLin-2. Top panel was immunoblotted with anti-mLin-2 antiserum. Bottom panel was immunoblotted with anti-Myc antibodies. (B) Immunoprecipitation with anti-mLin-2 antiserum and coprecipitation of endogenous mLin-7. Top panels were immunoblotted with anti-mLin-2 antiserum. The exposure of the left top panel was three times that of the right top panel. Bottom panels were immunoblotted with purified anti-mLin-7 antibodies. Note that the anti-mLin-7 antibodies are primarily immunoreactive to the mLin-7 PDZ domain and do not significantly recognize the amino terminus of mLin-7. Lanes in A and B are CON, mock-transfected MDCK cells; WT, stably expresses Myc-mLin-7WT; N, stably expresses Myc-mLin-7N; and PDZ, stably expresses Myc-mLin-7PDZ. The INPUT lanes contain approximately one-tenth the amount of lysate used in the precipitations. Arrows indicate the relevant bands on the immunoblots. The line in A indicates the light chain of the antibody used in the precipitation. Relative molecular weight is shown to the right in kilodaltons.
Next, we wished to discern the means by which mLin-7 was acting to effect basolateral targeting. To this end, we performed experiments to examine three mechanisms of protein targeting: differential delivery to the plasma membrane, specific retention at membrane domains, and endocytic sorting or transcytosis. The delivery of newly synthesized chimera proteins was examined by capturing pulse-labeled proteins with anti-P75 antibodies at either the apical or basolateral side of MDCK cell monolayers. With this method, the delivery of P75t-Let23WT and P75t-Let23MUT to the apical and basolateral surfaces was not significantly different (Figure 5), indicating that the initial delivery of the chimeras was not influenced by their ability to bind mLin-7. These results indicated that the mLin-7 PDZ domain ligand does not appear to play a role in direct basolateral protein targeting, unlike many previously identified protein-targeting signals found on basolateral proteins.
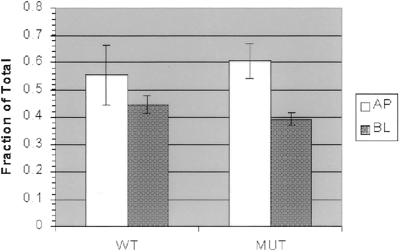
Figure 5.
mLin-7 binding did not influence delivery of receptor chimeras to the plasma membrane in a primary antibody capture experiment. MDCK cell lines expressing P75t-Let23WT (WT) or P75t-Let23MUT (MUT) were grown on Transwell filters, pulse-labeled with [35S]methionine for 1 h, and then chased for 3 h with complete growth medium containing antibody to P75 at either the apical (AP) or basolateral (BL) side to capture newly synthesized P75t-Let23 chimera. After washing to remove unbound antibody, the cells were lysed and the lysates subjected to precipitation with rabbit anti-mouse IgG and protein A-Sepharose. Precipitated proteins were separated by 10% SDS-PAGE and quantitated on a phosphorimager. Control precipitations were also performed to determine total labeled chimera protein. Delivery data is expressed as the fraction of captured P75t-Let23 divided by the total P75t-Let23. The experiment was performed in triplicate (n = 3) and the SDs are shown.
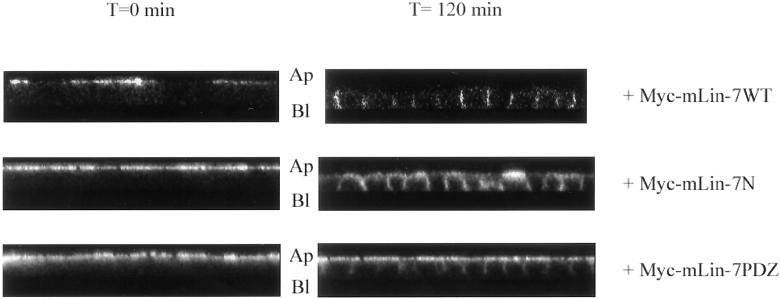
The ability of mLin-7 PDZ ligand to act as a basolateral retention signal has already been reported for the mLin-7 binding partner BGT-1 (Perego et al., 1999). We examined the role of basolateral retention by mLin-7 with our chimeras in a secondary antibody capture assay (Figure 6). Because surface biotinylation (Figure 2A) and initial delivery (Figure 5) experiments indicated that both P75t-Let23WT and P75t-Let23MUT cells lines had a population of receptors present at both the apical and basolateral sides, we chose to selectively label the receptors at the basolateral side and examine their stability of over time. Briefly, cells were chilled to 0°C to halt membrane trafficking and labeled on the basolateral side with anti-P75 antibodies, washed, and then warmed to 37°C in the presence of a secondary antibody conjugated to fluorochromes (capturing antibody) at the apical side. The cells used in these studies had intact tight junctions, consequently the passage of anti-P75 antibody, bound to chimeric protein, from the basolateral to the apical surface cannot occur by diffusion within the plasma membrane. Furthermore, control experiments with untransfected MDCK cells showed that anti-P75 antibodies did not significantly bind to the cell surface in the absence of antigen, nor was there significant nonspecific uptake of the antibody into these cells (our unpublished results). Therefore, the primary antibody bound to chimera protein must reach the apical surface and bind to the capturing antibody after endocytosis and intracellular transit. The latter is feasible because the anti-P75 antibody used in these studies remains stably bound to the extracellular domain of NGFR/P75 under the low pH conditions present in endocytic vesicles (Le Bivic et al., 1991). As seen in Figure 6A, P75t-Let23WT was tightly retained at the basolateral side, whereas P75t-Let23MUT was not and resulted in secondary antibody capture at the apical surface. In the absence of primary antibody, no significant capture antibody labeling was observed (our unpublished results), demonstrating the specificity of this assay.
Figure 6.
mLin-7 retained P75t-Let23WT at the basolateral plasma membrane domain in a secondary antibody capture experiment. (A) MDCK cell lines expressing P75t-Let23WT or P75t-Let23MUT were grown on Transwell filters, chilled on ice, and then incubated for 1 h with HEPES-buffered growth medium containing anti-P75 at the basolateral side to bind P75t-Let23 at the cell surface. After washing to remove unbound antibody, the cells were warmed to 37°C in growth medium containing goat anti-mouse IgG-Texas Red conjugate (the capture antibody, shown in red) at the apical side only. At the time indicated to the left, the cells were washed to remove unbound antibody, fixed and permeabilized, stained with goat anti-mouse IgG-FITC conjugate (shown in green) to reveal anti-P75 not already bound to the Texas Red conjugate, and examined by confocal laser-scanning microscopy. The panels show Z-sections that have been contrast enhanced for presentation, and the apical (Ap) and basolateral (Bl) sides are indicated. (B) Similar to experiment described in A, but performed with MDCK cell lines coexpressing P75t-Let23WT with Myc-mLin-7WT, Myc-mLin-7N, or Myc-mLin-7PDZ (see Figure 3 for description of mLin-7 constructs). Only the 120-min time point is shown.
To confirm that basolateral retention of P75t-Let23WT was a function of mLin-7, cell lines coexpressing Myc-tagged mLin-7 constructs were examined with this technique (Figure 6B). Cells coexpressing Myc-mLin-7WT demonstrated efficient basolateral retention of P75t-Let23WT (Figure 6B, top). However, the mutant Myc-mLin-7 constructs Myc-mLin-7N and mLin-7PDZ again acted as dominant interfering inhibitors, and significant trafficking of P75t-Let23WT from basolateral to apical was observed by the capture of secondary antibody at the apical surface in these cells (Figure 6B, middle and bottom). The coexpression of these mutant mLin-7 constructs had no significant effect on P75t-Let23MUT (our unpublished results). These results clearly illustrate that the interaction with mLin-7 stabilized proteins with mLin-7 PDZ ligands at the basolateral plasma membrane.
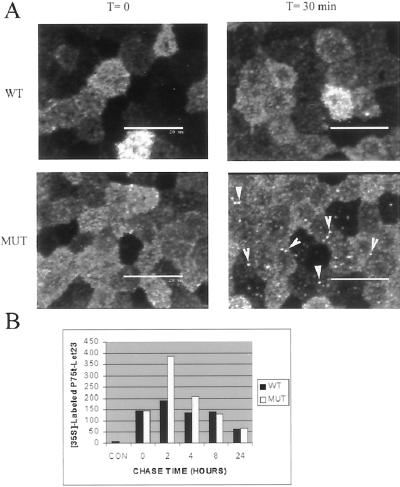
Still undetermined though was the specific mechanism by which P75-Let23WT sorted to the basolateral plasma membrane domain. Transcytosis and sorting during recycling of endocytosed proteins have both been observed in MDCK cells (Sheff et al., 1999; Leung et al., 2000; Mostov et al., 2000). We endeavored to determine whether mLin-7 acted during endocytic sorting to effect basolateral targeting, so we selectively labeled that portion of P75t-Let23WT or P75t-Let23MUT receptors at the apical surface and followed their localization over time (Figure 7). P75t-Let23WT labeled at the apical side of the MDCK cell monolayer by anti-P75 antibody (T = 0 min) rapidly traversed the cell (T = 30 min) to the basolateral side (T = 60 min) at 37°C, whereas P75t-Let23MUT remained apical after 60 min (Figure 7A). The inclusion of nocodazole during the assay inhibited the transit of P75t-Let23WT to the basolateral side (T = 60 min plus nocodazole), indicating that microtubules were required for this activity.
Figure 7.
P75t-Let23WT sorted to the basolateral plasma membrane domain after endocytosis from the apical surface. (A) To examine endocytic sorting, MDCK cell lines expressing P75t-Let23WT or P75t-Let23MUT were grown on Transwell filters, chilled on ice, and then incubated for 1 h with HEPES-buffered growth medium containing anti-P75 at the apical side to bind P75t-Let23 at the cell surface. After washing to remove unbound antibody, the cells were warmed to 37°C in growth medium. At the time indicated to the left, the cells were fixed and permeabilized, stained with goat anti-mouse IgG-FITC conjugate, and examined by confocal laser-scanning microscopy. Nocodazole (20 μg/ml) was added to the incubations and washes for the samples indicated. The panels show Z-sections that have been contrast enhanced for presentation, and the apical (Ap) and basolateral (Bl) sides are labeled. (B) Biotinylation-antibody capture: MDCK cell lines expressing P75t-Let23WT (WT) or P75t-Let23MUT (MUT) were grown on Transwell filters, labeled at the apical side with biotin, and then incubated for 3 h with growth medium containing anti-P75 at the basolateral side to capture biotinylated P75t-Let23 chimera. After washing to remove unbound antibody, the cells were lysed and the lysates subjected to precipitation with rabbit anti-mouse IgG and protein A-Sepharose. The precipitate was separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed for biotinylated proteins with avidin-HRP. Control precipitations were also performed to determine total biotinylated P75t-Let23 (TOT). Relative molecular weight is shown to the left in kilodaltons. An arrow indicates the relevant band.
To demonstrate that this phenomenon was not directed by the binding of antibody to P75t-Let23WT, the proteins on the apical side were also labeled with biotin, and labeled chimera protein was collected from the basolateral side with anti-P75 antibody. As seen in Figure 7B, biotinylated P75t-Let23WT was readily detected at the basolateral side, whereas P75t-Let23MUT was not. Altogether, the results of the experiments in Figure 7 argued that mLin-7 was acting at the level of the sorting endosome to deliver proteins from the apical to the basolateral membrane.
To confirm the role of mLin-7 in this endocytic sorting phenomenon, we again used cells coexpressing the Myc-tagged mLin-7 mutants in the assay described previously for Figure 7A. As seen in previous experiments, Myc-mLin-7N and Myc-mLin-7PDZ acted as dominant interfering inhibitors, significantly impairing the rate of apical-to-basolateral trafficking of P75t-Let23WT (Figure 8, middle and bottom). The expression of Myc-mLin-7WT did not inhibit the trafficking (Figure 8, top), and none of the Myc-mLin-7 constructs had significant effect on P75t-Let23MUT in this assay (our unpublished results). These results reinforce the role of mLin-7 as an effector of apical-to-basolateral targeting in the sorting endosome.
Figure 8.
Overexpression of Myc-mLin-7 deletion mutants interfered with the basolateral sorting of P75t-Let23WT. These panels depict the results of an endocytosis experiment similar to that described in Figure 7A, but performed with MDCK cell lines coexpressing P75t-Let23WT with Myc-mLin-7WT, Myc-mLin-7N, or Myc-mLin-7PDZ (see Figure 3 for description of mLin-7 constructs). The panels show Z-sections at time 0 and 120 min, and the apical (Ap) and basolateral (Bl) sides are indicated.
Finally, if P75t-Let23WT sorted to the basolateral plasma membrane after endocytosis due to its ability to interact with mLin-7, what was the fate of P75t-Let23MUT? P75t-Let23MUT appeared to be stably associated with the apical plasma membrane in the assay described in Figure 8A, although the intensity of the staining decreased over time (our unpublished results). This observation, combined with the instability of P75t-Let23MUT at the basolateral side (Figure 6A), led to the hypothesis that P75t-Let23MUT was degraded after endocytosis. When the endocytosis assay was performed in the presence of chloroquine, a weak base that served as an inhibitor of vesicle acidification, brightly stained intracellular vesicles accumulated in P75t-Let23MUT, but not P75t-Let23WT (Figure 9A). Examination of the Z-series revealed that these vesicles primarily resided in the apical cytoplasm. Similar results were observed with leupeptin, an inhibitor of lysosomal hydrolases, suggesting that the vesicles in which P75t-Let23MUT was accumulating were lysosomes (our unpublished results). Also, intracellular vesicles accumulated after internalization of P75t-Let23MUT, but not P75t-Let23WT, from the basolateral side as well when either chloroquine or leupeptin was present (our unpublished results). These results suggest that P75t-Let23MUT may be targeted to lysosomes for degradation after endocytosis.
Figure 9.
P75t-Let23MUT was sorted to a degradative pathway after internalization. (A) Endocytosis of chimera proteins from the apical side as described in Figure 7, except that 25 μM chloroquine, a weak base used to counter vesicle acidification, was included throughout the course of the experiment. The initial localization of prebound anti-P75 is shown (T = 0 min). After 30 min at 37°C (T = 30 min), intracellular vesicular staining was evident in P75t-Let23MUT (MUT), but not in P75t-Let23WT (WT). Arrows highlight some vesicles. Bars, 25 μm. (B) Amount of P75t-Let23WT (WT) and P75t-Let23MUT (MUT) remaining over time after pulse-labeling with [35S]methionine was determined by immunoprecipitation with anti-P75 antibody. Chase time in hours is shown on the x-axis, and the amount of labeled protein remaining (determined by densitometry) is shown on the y-axis. Negative control precipitation by using anti-Myc antibodies at T = 0 h is indicated as CON.
This hypothesis was supported by the relative degradation rates of the chimeras. After 35S-labeling, the amount of mature, radiolabeled chimeric receptor remaining over time was collected by precipitation with anti-P75 antibodies and measured by densitometry (Figure 9B). The apparent increase in labeling of mature receptor between T = 0 h and T = 2 h was due to the maturation of radiolabeled, immature receptor present at T = 0 (our unpublished results). Extrapolation from the data returned a rate of degradation for P75t-Let23MUT (T1/2= 15 h) almost twice that of P75t-Let23WT (T1/2= 27 h). Therefore, the inability of P75t-Let23MUT to interact with mLin-7 results in not only instability at the plasma membrane but also in a relatively high rate of degradation.
DISCUSSION
We have created receptor chimeras that differentially localized in MDCK cells in a manner that correlates with their ability to bind mLin-7 (Figures 1 and 2). Coexpression of the dominant interfering Myc-mLin-7 mutants Myc-mLin-7N and Myc-mLin-7PDZ provided proof that mLin-7 was responsible for the shift in steady-state localization of P75t-Let23WT from apical to basolateral (Figure 3). The inhibitory effects of Myc-mLin-7N and Myc-mLin-7PDZ were likely due to the ability of these deletion constructs to compete with endogenous mLin-7 for binding to endogenous mLin-2, or to interfere with the binding of P75t-Let23WT to endogenous mLin-7, respectively (Figure 4).
Our data argue against the involvement of mLin-7 in the selective delivery of proteins from the Golgi to specific plasma membrane domains, but support basolateral retention of those proteins that bind mLin-7 (Figures 5 and 6). Previously, Perego et al. (1997) showed that the carboxyl terminus of BGT-1, which includes a mLin-7 PDZ ligand, contained a basolateral sorting determinant. Subsequently, it was shown that the deletion of the mLin-7 PDZ ligand from the carboxyl-terminal tail of BGT-1 adversely affected the stability of BGT-1 at the basolateral side of MDCK cells (Perego et al., 1999). In that study, BGT-1 that was unable to bind to mLin-7 was redirected from the plasma membrane into intracellular vesicles. However, the basolateral localization of LET-23 in vulval precursor cells of C. elegans may involve mechanisms beyond simple retention. In lin-10 and lin-7 mutant animals, LET-23 is mislocalized to the apical domain (Simske et al., 1996; Whitfield et al., 1999), which suggests a defect in specific trafficking to the basolateral plasma membrane domain.
A possible explanation for the findings in C. elegans comes from our data. We found that, in addition to acting as a basolateral retention mechanism, mLin-7 binding also regulates the sorting of internalized protein to the basolateral plasma membrane or, in the absence of mLin-7 binding, to a degradative pathway (Figures 7–9). The three fates of endocytosed proteins are segregated by discrete compartments: fluid phase markers and components destined for degradation in lysosomes are set aside in the early endosome; the common recycling endosome is responsible for recycling back to the surface of origin; and the subapical recycling compartment governs transcytosis to the opposite surface (Luton and Mostov, 1999; Leung et al., 2000; Mostov et al., 2000). The formation of the common recycling endosome and the transfer to the subapical recycling compartment both require microtubule networks (Luton and Mostov, 1999; Leung et al., 2000).
Apical internalization of P75t-Let23WT resulted in trafficking to the basolateral side in a process that required microtubules (Figure 7A, bottom), indicating the requirement for the common recycling endosome and/or the subapical recycling compartment in this activity. The Myc-mLin-7 mutants Myc-mLin-7N and Myc-mLin-7PDZ again interfered with the activity of endogenous mLin-7, and slowed, but did not abolish, trafficking of P75tLet23WT from apical to basolateral (Figure 8), indicating that the trafficking was dependent on mLin-7. Although the mutant proteins were efficiently expressed (Figure 4A), the amount relative to endogenous mLin-7 might not have been sufficient to completely prevent interaction with endogenous proteins. A further indication that relative protein stoichiometry may be important to this process comes from the fact that apical-to-basolateral sorting was not complete for P75t-Let23WT (Figure 7A), unless excess full-length mLin-7 was present (Figure 8, top). It is possible that receptors reaching the basolateral side may not remain there due to an insufficient amount of mLin-7 or its binding partners.
Finally, the presence of P75t-Let23MUT in intracellular vesicles after treatment with chloroquine or leupeptin was consistent with segregation of P75t-Let23MUT to lysosomes, and was corroborated by the increased rate of degradation for P75t-let23MUT relative to P75t-Let23WT (Figure 9). These results parallel the activity of the PDZ-domain containing protein EBP50 on the trafficking of the β2 andrenergic receptor (Cao et al., 1999). Binding of EBP50 to the carboxyl terminus of the β2 andrenergic receptor resulted in recycling of the receptor to the plasma membrane, whereas inhibition of this binding resulted in lysosomal degradation of endocytosed receptor.
In summary, our results suggest a unique dynamic role, as well as a previously identified static role, for the mLin-7 PDZ domain in basolateral protein targeting. The following model is proposed to accommodate these two distinct roles: 1) Proteins that bind mLin-7 may or may not be delivered to the basolateral plasma membrane via interaction with mLin-7 or other effector proteins. 2) Upon internalization, mislocalized proteins at the apical side are actively sorted to the basolateral side through binding to mLin-7 in the endosomal pathway. 3) Finally, once target proteins reach the basolateral side, they may then be selectively retained by their ability to bind mLin-7.
Similar dual roles in protein targeting have been identified for PSD-95, a MAGUK superfamily member related to mLin-2 (Fanning and Anderson, 1998; Fanning and Anderson, 1999), which functions as a scaffold to anchor Shaker-type K+ channels and N-methyl-d-aspartate-type glutamate receptors at the synapses of neurons via PDZ domain interactions (Kornau et al., 1997; Sheng and Wyszynski, 1997; Craven and Bredt, 1998). Recently, El-Husseini et al. (2000) demonstrated that PSD-95 was also a vesicular protein that migrated dependent on microtubules, implying that PSD-95 was not merely a static cytoskeletal element, but may also participate dynamically in postsynaptic ion channel clustering.
Several important questions remain: First, can a specific compartment be defined for the activity of mLin-7 during sorting? Are the mLin-7 binding partners, mLin-2 or any of the related PALS (Proteins Associated with Lin Seven; [Kamberov et al., 2000]), also present within the sorting system, or do they simply act as anchors at the plasma membrane? Previously published data showed that mLin-7 and mLin-2 coprecipitate in both membrane-bound and cytosolic fractions of epithelial cell lysates (Straight et al., 2000), indicating that pools of this protein complex exist in the cytoplasm and might represent the portion present within the sorting pathway. Logically, this compartment should also contain P75t-Let23WT, but not P75t-Let23MUT, providing a convenient marker for isolation in future experiments.
It is also possible that the binding of the mLin-7 PDZ domain to target proteins is a regulated event. For example, the phosphorylation of the carboxyl terminus of the β2 andrenergic receptor disrupts binding to the PDZ domain of EBP50, acting as a switch between basolateral recycling and lysosomal degradation of the receptor protein (Cao et al., 1999). Furthermore, many steps within the trafficking of transport vesicles involve Rho- or Rab-GTPase proteins (Leung et al., 1999; Sheff et al., 1999; Ellis and Mellor, 2000; Jou et al., 2000) and phosphatidylinositol 3-kinase is known to modulate vesicle fusion (Patki et al., 1997; Simonsen et al., 1998; Lawe et al., 2000). mLin-7 or mLin-2 might interact with a critical effector protein of one or more of these regulatory events. The answers to these questions will have to await the identification of other partners of this protein complex.
ACKNOWLEDGMENTS
We thank Moses Chao for providing the pCMV5A-P75 plasmid DNA. We are also grateful to Thomas Komorowski, manager of the University of Michigan Diabetes Research Center Morphology and Image Analysis Core (National Institutes of Health Grant 5-P60-DK20572), for assistance with confocal microscopy, and Elizabeth Smith at the University of Michigan Hybridoma Core for the production of anti-P75 ascites. B.M. is an investigator of the Howard Hughes Medical Institute. Support for this work was also received from the National Institutes of Health Cellular Biotechnology Training Program grant GM-08353 (to D.K.).
REFERENCES
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors [see comments] Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble [see comments] Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adelaide J, Margolis B, Birnbaum D. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol. 2000;2:407–414. doi: 10.1038/35017038. [DOI] [PubMed] [Google Scholar]
- Borg JP, Ooi J, Levy E, Margolis B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol Cell Biol. 1996;16:6229–6241. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg JP, Straight SW, Kaech SM, de Taddeo-Borg M, Kroon DE, Karnak D, Turner RS, Kim SK, Margolis B. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J Biol Chem. 1998;273:31633–31636. doi: 10.1074/jbc.273.48.31633. [DOI] [PubMed] [Google Scholar]
- Butz S, Okamoto M, Sudhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Caplan MJ. Membrane polarity in epithelial cells: protein sorting and establishment of polarized domains. Am J Physiol. 1997;272:F425–F429. doi: 10.1152/ajprenal.1997.272.4.F425. [DOI] [PubMed] [Google Scholar]
- Caplan MJ, Anderson HC, Palade GE, Jamieson JD. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986;46:623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Valdes J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998;60:161–177. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- Doerks T, Bork P, Kamberov E, Makarova O, Muecke S, Margolis B. L27, a novel heterodimerization domain in receptor targeting proteins Lin-2 and Lin-7. Trends Biochem Sci. 2000;25:317–318. doi: 10.1016/s0968-0004(00)01599-1. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Craven SE, Chetkovich DM, Firestein BL, Schnell E, Aoki C, Bredt DS. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000;148:159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S, Mellor H. Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 2000;10:85–88. doi: 10.1016/s0962-8924(99)01710-9. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. PDZ domains and the formation of protein networks at the plasma membrane. Curr Top Microbiol Immunol. 1998;228:209–233. doi: 10.1007/978-3-642-80481-6_9. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. Protein modules as organizers of membrane structure. Curr Opin Cell Biol. 1999;11:432–439. doi: 10.1016/S0955-0674(99)80062-3. [DOI] [PubMed] [Google Scholar]
- Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells [see comments] Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signaling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Dunbar LA, Caplan MJ. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol. 1995;268:F285–F295. doi: 10.1152/ajprenal.1995.268.2.F285. [DOI] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie M, Hata Y, Deguchi M, Ide N, Hirao K, Yao I, Nishioka H, Takai Y. Isolation and characterization of mammalian homologues of Caenorhabditis elegans lin-7: localization at cell-cell junctions. Oncogene. 1999;18:2811–2817. doi: 10.1038/sj.onc.1202652. [DOI] [PubMed] [Google Scholar]
- Jo K, Derin R, Li M, Bredt DS. Characterization of MALS/Velis-1, -2, and -3: a family of mammalian LIN-7 homologs enriched at brain synapses in association with the postsynaptic density-95/NMDA receptor postsynaptic complex. J Neurosci. 1999;19:4189–4199. doi: 10.1523/JNEUROSCI.19-11-04189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao M. Expression and structure of the human NGF receptor. Cell. 1986;47:545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Jou TS, Leung SM, Fung LM, Ruiz WG, Nelson WJ, Apodaca G. Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1. Mol Biol Cell. 2000;11:287–304. doi: 10.1091/mbc.11.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Whitfield CW, Kim SK. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 1998;94:761–771. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamberov E, Makarova O, Roh M, Liu A, Karnak D, Straight S, Margolis B. Molecular cloning and characterization of Pals, proteins associated with mLin-7. J Biol Chem. 2000;275:11425–11431. doi: 10.1074/jbc.275.15.11425. [DOI] [PubMed] [Google Scholar]
- Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Kim SK. Polarized signaling: basolateral receptor localization in epithelial cells by PDZ-containing proteins. Curr Opin Cell Biol. 1997;9:853–859. doi: 10.1016/s0955-0674(97)80088-9. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- Lawe DC, Patki V, Heller-Harrison R, Lambright D, Corvera S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization. J Biol Chem. 2000;275:3699–3705. doi: 10.1074/jbc.275.5.3699. [DOI] [PubMed] [Google Scholar]
- Le Bivic A, Sambuy Y, Patzak A, Patil N, Chao M, Rodriguez-Boulan E. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol. 1991;115:607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall AH, Yeaman C, Muesch A, Rodriguez-Boulan E. Epithelial cell polarity: new perspectives. Semin Nephrol. 1995;15:272–284. [PubMed] [Google Scholar]
- Legouis R, Gansmuller A, Sookhareea S, Bosher JM, Baillie DL, Labouesse M. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat Cell Biol. 2000;2:415–422. doi: 10.1038/35017046. [DOI] [PubMed] [Google Scholar]
- Leung SM, Rojas R, Maples C, Flynn C, Ruiz WG, Jou TS, Apodaca G. Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA. Mol Biol Cell. 1999;10:4369–4384. doi: 10.1091/mbc.10.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung SM, Ruiz WG, Apodaca G. Sorting of membrane and fluid at the apical pole of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:2131–2150. doi: 10.1091/mbc.11.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luton F, Mostov KE. Transduction of basolateral-to-apical signals across epithelial cells: ligand-stimulated transcytosis of the polymeric immunoglobulin receptor requires two signals. Mol Biol Cell. 1999;10:1409–1427. doi: 10.1091/mbc.10.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- Patki V, Virbasius J, Lane WS, Toh BH, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego C, Bulbarelli A, Longhi R, Caimi M, Villa A, Caplan MJ, Pietrini G. Sorting of two polytopic proteins, the gamma-aminobutyric acid and betaine transporters, in polarized epithelial cells. J Biol Chem. 1997;272:6584–6592. doi: 10.1074/jbc.272.10.6584. [DOI] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Villa A, Longhi R, Kaech SM, Frohli E, Hajnal A, Kim SK, Pietrini G. PDZ-mediated interactions retain the epithelial GABA transporter on the basolateral surface of polarized epithelial cells. EMBO J. 1999;18:2384–2393. doi: 10.1093/emboj/18.9.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP. Evidence for PDZ domains in bacteria, yeast, and plants. Protein Sci. 1997;6:464–468. doi: 10.1002/pro.5560060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M, Lisanti M, Graeve L, Le Bivic A, Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989;107:277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Wyszynski M. Ion channel targeting in neurons. Bioessays. 1997;19:847–853. doi: 10.1002/bies.950191004. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion [see comments] Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Simske JS, Kaech SM, Harp SA, Kim SK. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell. 1996;85:195–204. doi: 10.1016/s0092-8674(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Straight SW, Karnak D, Borg JP, Kamberov E, Dare H, Margolis B, Wade JB. mLin-7 is localized to the basolateral surface of renal epithelia via its NH(2) terminus. Am J Physiol Renal Physiol. 2000;278:F464–F475. doi: 10.1152/ajprenal.2000.278.3.F464. [DOI] [PubMed] [Google Scholar]
- Whitfield CW, Benard C, Barnes T, Hekimi S, Kim SK. Basolateral localization of the Caenorhabditis elegans epidermal growth factor receptor in epithelial cells by the PDZ protein LIN-10. Mol Biol Cell. 1999;10:2087–2100. doi: 10.1091/mbc.10.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]